Salt-induced expressions of iron superoxide dismutases (FSD2 and FSD3) are regulated by MKK5 that interacts with MEKK1 via the MKK5-MPK6-coupled signalling cascade.
Keywords: Arabidopsis, FSD, iron superoxide dismutase, mitogen-activated protein kinase kinase 5, mitogen-activated protein kinase 6, salt stress
Abstract
Superoxide dismutases (SODs) are involved in plant adaptive responses to biotic and abiotic stresses but the upstream signalling process that modulates their expression is not clear. Expression of two iron SODs, FSD2 and FSD3, was significantly increased in Arabidopsis in response to NaCl treatment but blocked in transgenic MKK5-RNAi plant, mkk5. Using an assay system for transient expression in protoplasts, it was found that mitogen-activated protein kinase kinase 5 (MKK5) was also activated in response to salt stress. Overexpression of MKK5 in wild-type plants enhanced their tolerance to salt treatments, while mkk5 mutant exhibited hypersensitivity to salt stress in germination on salt-containing media. Moreover, another kinase, MPK6, was also involved in the MKK5-mediated iron superoxide dismutase (FSD) signalling pathway in salt stress. The kinase activity of MPK6 was totally turned off in mkk5, whereas the activity of MPK3 was only partially blocked. MKK5 interacted with the MEKK1 protein that was also involved in the salt-induced FSD signalling pathway. These data suggest that salt-induced FSD2 and FSD3 expressions are influenced by MEKK1 via MKK5MPK6-coupled signalling. This MAP kinase cascade (MEKK1, MKK5, and MPK6) mediates the salt-induced expression of iron superoxide dismutases.
Introduction
Reactive oxygen species (ROS) have long been known to be harmful to many cellular processes, however, a transient increase in ROS can also act as a signal that mediates the regulation of various cellular activities. Examples include responses to biotic or abiotic stresses, cell death, stomatal movement or root hair development (Gill and Tuteja, 2010; Acharya et al., 2013; Suzuki et al., 2013). As a first line of defence against ROS, the enzyme superoxide dismutase (SOD) catalyses the initial step in the AsadaHalliwell pathway in chloroplasts and converts superoxide to hydrogen peroxide and molecular oxygen (Bowler et al., 1994; Allen, 1995). SOD enzymes are classified into three groups according to their metal co-factor: iron SOD (FeSOD), manganese SOD (MnSOD), and copper-zinc SOD (Cu-Zn SOD) and are located in different cellular compartments (McKersie et al., 2000).
There is growing evidence to suggest that SODs and other anti-oxidative enzymes are closely associated with plant biotic or abiotic stress tolerance, although the signalling processes involved in stress-induced gene expression of SODs are largely unknown (Miller et al., 2008, van Breuseqem et al., 2008; Li et al., 2013; Xing et al., 2013). Overexpressing some SODs has been demonstrated to enhance diverse stress tolerances, such as to low temperature and high light (McKersie et al., 2000; Huang et al., 2012) and other abiotic stresses (Gill and Tuteja, 2010). A decrease in MnSOD leads to reduced root growth and affects tricarboxylic acid cycle flux and mitochondrial redox homeostasis (Morgan et al., 2008). SaFeSOD cloned from Sonneratia alba, a highly salt tolerant mangrove tree, is expressed in leaf, stem, flower, fruit, and root tissues with the highest expression in leaf tissues (Wang et al., 2013). Under nitrogen supplemented conditions, overexpression of either SOD protein, especially FeSOD, conferred significant tolerance against oxidative stress (Raghavan et al., 2011). Nevertheless, the signalling processes of stress-induced gene expression of SODs are still largely unknown (McKersie et al., 2000; Miller et al., 2008; van Breusegem et al., 2008).
Several lines of evidence suggest that the MAPK signalling cascade is, at several levels, involved in plant biotic or abiotic stress-induced signalling and indeed, most plant MAPKs investigated to date have been linked to stress responses (Xing et al., 2008, 2013; Ding et al., 2013; Li et al., 2013; Sheikh et al., 2013). In studies with alfalfa (Medicago sativa), stress-induced MAPKK (SIMKK) was isolated in a yeast two-hybrid interaction screen using the MAP kinase SIMK as a bait and the specific activation of SIMK by SIMKK was subsequently observed upon salt stress in protoplasts, supporting the function of SIMKK as an upstream activator of SIMK in this pathway (Kiegerl et al., 2000). A complete Arabidopsis MAPK signalling cascade pathway (MEKK1, MKK4/MKK5, and MPK3/6) has been identified and shown to be involved in the induction of expression of the WRKY22/WRKY29 transcription factors by the bacterial protein flagellin (Asai et al., 2002). Moreover, Arabidopsis MKK2 has been shown to be activated by MEKK1 and to increase freezing and salt tolerance by activating its direct targets MPK4 and MPK6, as well as the expression of other stress-induced marker genes (Teige et al., 2004). Previous reports have also shown that hydrogen peroxide-mediated stress signalling requires MAP kinase cascades (Kovtun et al., 2000; Kumar and Klessig, 2000; Samuel et al., 2000; Zhang et al., 2006; Xing et al., 2008), suggesting an important role for MAPK signalling in the generation of ROS and detoxification.
The involvement of Arabidopsis MKK5 in the MAP kinase cascade was suggested by its response to flagellin-induced signalling (Asai et al., 2002). Furthermore, Arabidopsis MKK5 has been shown to be involved in hydrogen peroxide-mediated cell death and oxidative stress (Ren et al., 2002; Xing et al., 2013). Taken together, these findings suggest that Arabidopsis MKK5 might be involved in both abiotic and biotic stress signalling. However, the exact roles and detailed mechanisms of MKK5-mediated signalling remain unknown.
To elucidate the potential role of MAPK signalling cascades in salt stress responses, an Arabidopsis protoplast transient expression system was used, in which transcription of FeSOD genes is induced by NaCl, allowing the roles of MAPK cascade components to be systematically evaluated. Using this system, MEKK1 was identified via the MKK5MPK6-coupled signalling associated with salt-induced FeSOD expression. These data suggest that this signalling pathway functions in response to salt stress and could potentially be utilized to enhance salt tolerance in crops.
Materials and methods
Plant materials and stress treatments
Arabidopsis thaliana (L.) plants were kept in a growth chamber at 222 C with 16h light and 8h dark and a relative humidity of 90%. RNAi gene-silenced plants of MKK5 and plants from MKK5-RNAi lines, MKK57, named mkk5 were generated and chosen for this study (Xing et al., 2013). For NaCl treatment of germination, wild-type and mutant seedlings growing on MS agar plates (MS complete medium with 30g/l sucrose and 7g/l agar) were transferred onto filter paper saturated with 150mM NaCl and the plants were incubated under light for various times. For NaCl treatment of protoplasts, isolated protoplasts from Arabidopsis leaves were subjected to a final NaCl concentration of 150mM for 10min.
Plasmid construction
-glucuronidase-tagged reporter vector construction
For construction of the vector containing -glucuronidase (GUS), GUS was substituted for GFP in the pHBT95-GFP vector by using the NcoI and NotI restriction sites. This vector was named pHBT95-GUS. The promoter regions of the iron superoxide dismutase (FSD)1, FSD2, and FSD3 genes were amplified by PCR from Arabidopsis (Col-0) genomic DNA (FSD1: forward primer, 5-ATATGGTTT ACCCATCTTAATTT-3, reverse primer, 5-TCTTTGTAATTG AAGCTGCACATT-3; FSD2: forward primer, 5-TAAAATTAA AACATTAAATTATAT-3, reverse primer, 5-CTTCACTCAAAG CGTTACTGATTAT-3, and FSD3: 5-AGTTCCTCCCACTGT TGTCGTCA-3, 5-TAGGTAAGATGATTAAATCGACAG-3) and were substituted for the cauliflower mosaic virus (CaMV) 35S promoter using the XhoI and ApaI sites, to yield pFSD1-GUS, pFSD2-GUS, and pFSD3-GUS. The pMPK3-GUS, pMPK4-GUS, pMPK6-GUS, and pMKK5-GUS vectors were constructed using the same method.
Effector vector construction
Arabidopsis MAPKK cDNAs were: MKK2 (At4g29810), MKK4 (At1g51660), and MKK5 (At3g21220). The MAPKs were MPK2 (At1g59580), MPK3 (At3G45640), MPK4 (At4g01370), MPK6 (At2g43790), MPK7 (At2g18170), and MPK9 (At3g18040). All cDNAs were amplified by PCR from Arabidopsis (Col-0 or mutants), cloned into the pENTR-TOPO cloning vector (Invitrogen, Carlsbad, CA, USA) and verified by sequencing. PCR products were inserted into the pGWB5-DHA vector containing a double HA epitope tag, the 35S promoter, and the NOS terminator.
Total RNA extraction, semi-quantitative reverse transcription-PCR and northern blot analysis
Seedlings (mutant and wild-type plants) were grown for 2 weeks on MS agar plates under continuous light and then treated with NaCl as described above. Total RNA was extracted using the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) according to the manufacturers instructions and stored at 80 C until further use. Total RNA was used as a template for first-strand cDNA synthesis using the SuperScript II First-Strand Synthesis System for reverse transcription (RT)-PCR (Invitrogen). The PCR volume was 25 l, containing 100ng of each primer, 2mM each dNTP, 0.5 l cDNA, and 0.75 units of Taq DNA polymerase (Invitrogen), and the reactions were run in a PTC-100 Programmable Thermal Controller (MJ Research, Watertown, MA, USA). PCR products were separated on 1.5% agarose gels.
For northern blot analysis, 15 g of total RNA was fractionated on a 1.0% formaldehyde-containing agarose gel with an RNA molecular weight marker (Promega Corp., Madison, WI, USA) and blotted onto a nylon membrane (Hybond-N, Amersham Pharmacia Biotech, Aylesbury, UK) overnight at room temperature. Equal loading of RNA was confirmed using ethidium bromide staining of the gel prior to transfer. Probes were labelled with [-32P] dCTP using a random-primed DNA labelling kit (Megaprime, Amersham) and hybridization was performed according to the manufacturers recommendations.
Protoplasts preparation and transformation
Arabidopsis protoplasts were isolated using a modified protocol from Abel and Theologis (1994). Leaves from 4-week old Arabidopsis plants were washed in distilled water and incubated with an enzyme solution containing 1% Cellulase R10, 0.25% Macerozyme R10 (both from Yakult, Tokyo, Japan), and 0.4M mannitol for about 34h at 23 C with gentle shaking (50rpm). The protoplast suspension was filtered through a net with a pore diameter of 150 M. After centrifugation (60 g, 2min), the supernatant was discarded and the cells were re-suspended in washing solution and washed twice in washing buffer containing Magma solution. The concentration of protoplasts was determined and the viability of the cells was verified by staining with FDA (fluoresce in diacetate) and subsequently by fluorescent microscopy (Zeiss Axioskop) (Nunberg and Thomas, 1993). Plasmid DNA solutions were adjusted with 0.5M mannitol and mixed with 5105 protoplasts. After addition of an equal volume of PEG 6000 solution, the suspension was incubated at room temperature for 10min and washed with washing and incubation (WI) solution before being re-suspended in WI solution. The transformed cells were incubated in the dark (23 C, 30rpm, overnight) and the suspensions were divided into different tubes, treated with NaCl solution and incubated for the appropriate times.
Assay for transient GUS activity
The suspensions of protoplasts were centrifuged (60 g, 2min) and washed with 0.5M mannitol. Pellets were dissolved in 105 l extraction buffer and extracts were vortexed and kept at 80 C for at least 1h or in liquid nitrogen for several minutes. GUS activity was quantified according to Ftterer et al. (1990) and a modified protocol for suppressing endogenous GUS activity (Kosugi et al., 1990). The protein concentration was measured according to the manufacturers instructions (Bio-Rad, Hercules, CA, USA) with BSA as a standard. GUS activity was measured in the supernatant after adding the substrate 4-methyl-umbelliferyl--d-glucuronide (MUG). MUG was hydrolysed by GUS protein into 4-methyl-umbelliferone or 7-hydroxy-4-methyl-cumarin (4-MU) and glucuronide, and GUS activity determined in a dark micro-titre plate (Nunc GmbH & Co. KG, Wiesbaden, Germany) using the HTS 7000 plus Bioassay Reader (emission, 460nm; excitation, 365nm). GUS activity was normalized according to the expression derived from pHBT95-GUS with no fusion protein (average 204 pmol of 4-MU produced per minute per microgram of protein obtained from six independent experiments).
Expression and purification of GST fusion proteins
Full-length Arabidopsis MKK5, MPK3, MPK4, and MPK6 cDNAs were obtained using RT-PCR, cloned into the pENTR-TOPO cloning vector (Invitrogen) and sequenced. The Escherichia coli Strain BL-21 codon plus (Stratagene, LA Jolla, CA, USA) was transformed with the expression constructs, which was prepared by subcloning the genes into pGEX-6P-1 vector (Amersham Pharmacia Biotech). Growth of bacteria and isolation of recombinant GST fusion protein was as described in Matsuoka et al. (2002).
Protein extraction, immunoprecipitation and kinase activity assay
The following steps were carried out at 4 C unless otherwise stated. Plant tissues (3:1 buffer volume:fresh weight) were homogenized with a pestle and mortar in 100mM Tris-HCl buffer (pH 8.0) containing 2mM EDTA, 5mM DTT, 10% glycerol, 1mM phenylmethylsulphonyl fluoride (PMSF), and 0.3 M aprotinin. The homogenate was filtered through four layers of muslin cloth and centrifuged at 12,000 g for 40min. The supernatant was desalted with a Sephadex G-25 column equilibrated with buffer suitable for the individual enzymes. The desalted supernatants were stored in aliquots at 80 C. The protein concentration was determined using the protein assay kit (Bio-Rad) with BSA as a standard.
Protein extracts (0.5mg) were incubated with 50 l antibody at 4 C overnight. Protein G-agarose beads (50 l) were added and incubated for 2h at 4 C. The proteinantibody complex on the beads was collected and washed three times in ice-cold PBS and before re-suspension in protein sample buffer.
The coding regions of MPK3, MPK4, and MPK6 were cloned into the pGEX-6P-1 vector and expressed as GST fusion proteins in BL21 codon plus E. coli cells (see above). Kinase inactive GST-MPK fusion proteins were generated by exchanging a conserved lysine reside in the ATP binding domains to methionine and arginine using the Quick Change kit (Stratagene). The point mutations were performed as described by Teige et al. (2004). Inactive GST-MPK (2 g) was incubated in 20 l of kinase reaction buffer (50mM Tris, pH 7.5, 10mM MgCl2, 1mM DTT, 0.1mM ATP, and 8 Ci of 32P-ATP) with immunoprecipitated GST fused to MKK5 from protoplasts. Kinase reactions were stopped after 30min by adding 4 l SDS loading buffer and heating for 5min at 95 C. Reaction products were analysed by SDS-PAGE, autoradiography, and Coomassie brilliant blue R250 staining.
In-gel kinase assays were performed essentially as described by Katou et al. (1999) with some modifications. Briefly, samples (20 g) of total protein or immunoprecipitate from 400 g of total protein were separated on a 10% SDS-polyacrylamide gel polymerized in the presence of 0.25mg/ml bovine brain myelin basic protein (Sigma). After electrophoresis, SDS was removed by washing the gel in buffer (25mM Tris-HCl pH 8.0, 0.1mM Na3VO4, 5mM NaF, 0.5mM DTT, 0.5mg/ml BSA, and 0.1% Triton X-100) three times (30min each) at room temperature. After 1h denaturation in a denaturing buffer containing guanidine, the kinases were allowed to renature overnight at 4 C with five changes of renaturing buffer (25mM Tris, pH 7.5, 1mM DTT, 0.1mM Na3VO4, and 5mM NaF). The phosphorylation of myelin basic protein was performed in 30ml reaction buffer (25mM Tris, pH 7.5, 0.1mM Na3VO4, 12mM MgCl2, 2mM EGTA, and 1mM DTT) with a pre-reaction for 30min, then 0.2 M ATP and 50 Ci of 32P-ATP in reaction buffer was added and the reactions incubated at room temperature for 6090min. The gel was then transferred into washing buffer at room temperature for at least 6h with six changes of buffer. Finally, the gel was dried on filter paper and autoradiographed.
Immunocomplex kinase assays
The method for kinase assays has been described by Xing et al. (2008, 2013). Kinase inactive MPK6-GST protein (1 g) immunoprecipitated from Arabidopsis protoplast using anti-GST was incubated with MKK5 immunoprecipitated from Arabidopsis seedlings using anti-MKK5 in the kinase reaction mixture [20 l of kinase reaction buffer containing 50mM Tris (pH 7.5), 1mM DTT, 10mM MgCl2, 0.1mM ATP, and 9.7103Bq of 32P-ATP] for 30min at room temperature. The reaction was stopped after 30min by adding 4 l of SDS loading buffer and heating for 5min at 95 C. Reaction products were analysed by autoradiography after SDS-PAGE. MPK3-GST, MPK4-GST, and MPK6-GST fusion proteins were generated by exchanging a conserved lysine residue in the ATP binding domains to methionine and arginine using the Quick Change kit (Stratagene). The point mutations for MPK6 were K92M and K93R (Teige et al., 2004).
Generation of MKK5 overexpressing plants
Full-length Arabidopsis MKK5 cDNA was obtained by RT-PCR, cloned into the pENTR-TOPO cloning vector (Invitrogen) and sequenced. After the LR reaction, MKK5 cDNA was inserted into the pGWB5-DHA vector, and the vector named pGWB5-DHA-MKK5. Transgenic Arabidopsis plants expressing the DHA-tagged MKK5 under control of the CaMV 35S promoter were generated using the floral dip method (Clough and Bent, 1998) and either Col-0 wild-type plants or mkk5 plants. Transformed plants were selected for growth on kanamycin containing media. Second generation plants were used for experiments.
Determination of O2 production and SOD activity in Arabidopsis leaves
O2 production in Arabidopsis leaves of treated and control plants was determined according to the method of Able et al. (1998) by monitoring the reduction of 3-[1-(phenylamino-carbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzenesulphonic acid hydrate (XTT) in the presence of O2. Leaves (1g) were frozen in N2, ground, and then homogenized with 5ml of 50mM TRIS-HCl buffer (pH 7.5) and centrifuged at 5000 g for 10min. The reaction mixture of 1ml contained 50 g of supernatant proteins, 50mM TRIS-HCl buffer (pH 7.5), and 0.5mM XTT. The reduction of XTT was determined at 470nm for 5min. Corrections were made for the background absorbance in the presence of 50 units SOD. O2 production rate was calculated using an extinction coefficient of 2.16104 M1 cm1. SOD (EC 1.12.1.11) was measured in the leaves as previously described (Xing et al. 2013).
Yeast two-hybrid interactions
Yeast two-hybrid interactions were performed using the ProQuestTM Two-Hybrid System (Invitrogen) according to the manufacturers instructions.
Results
Salt-Induced FeSODs in Arabidopsis
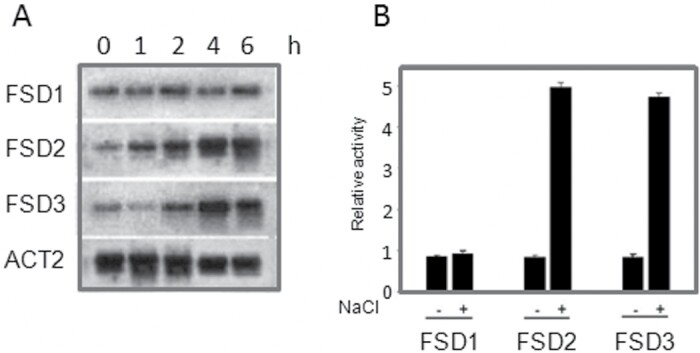
Salt-induced expression of FSD genes in Arabidopsis was investigated by northern blot analysis using RNA extracted from leaves. Treatment of plants with 150mM NaCl led to a significant increase in the expression of FSD2 and FSD3, with FSD2 responding earlier than FSD3. The FSD2 gene transcript increased within 1h of treatment, peaked at 4h, and remained high until 6h, while FSD3 transcription was induced within 2h and remained high until 6h. In contrast, FSD1 transcript level was not altered by NaCl treatment (Fig.1A).
Fig. 1.
FSD1, FSD2, and FSD3 expression following NaCl treatment. (A) Northern blot analysis of FSD1, FSD2, and FSD3 expression. Arabidopsis tissues from different points of the time course were used for RNA gel blot analysis. Total RNA was extracted from wild-type plants without stress treatment (control) or subjected to 150mM NaCl, and incubated for different times. The actin gene ACT2 was used as a loading control. (B) Relative GUS activity, driven by the FSD1, FSD2, or FSD3 promoters, showed the response to NaCl. The FSD1, FSD2, and FSD3 promoters were fused to GUS and tested for their response to NaCl in a protoplast transient expression assay. All experiments were repeated at least three times with similar results.
To further characterize FeSOD gene activation, the promoters of FSD1, FSD2, and FSD3 (1.2kb before the ATG) were fused to the -glucuronidase reporter gene (GUS) and tested for response to NaCl in transiently transfected protoplasts. Consistent with the northern blot data obtained with the endogenous genes, the FSD2 and FSD3 promoters but not those of FSD1 were activated by NaCl (Fig. 1B). Although the FSD1 gene was expressed, it was not induced by NaCl. An earlier report indicated that FSD1 transcription is under the control of a circadian clock (Kliebenstein et al., 1998); however, these results did not show that FSD2 or FSD3 are controlled by a circadian clock mechanism (data not shown).
Salt-induced FSD signalling operates through MKK5
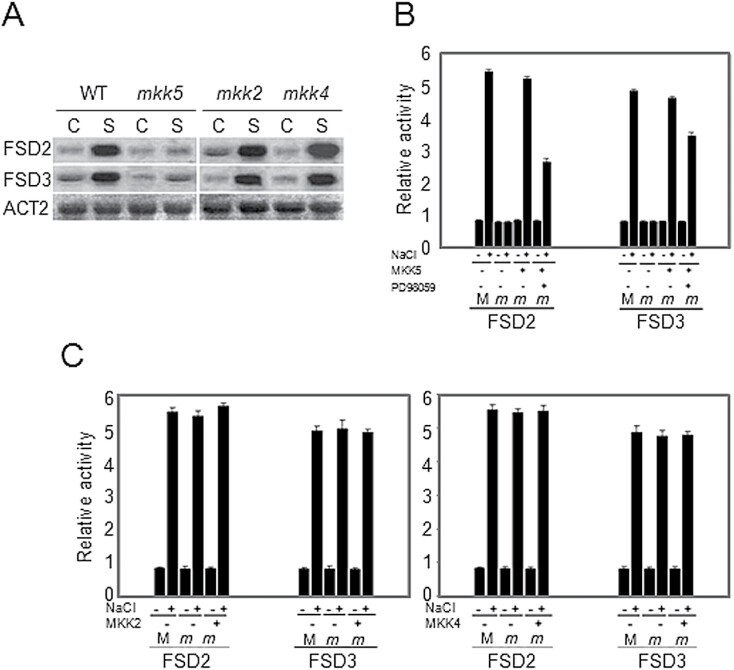
In a previous study all the 10 known mutants of the MAPKK family in Arabidopsis were screened for downstream signalling components (Xing et al., 2007, 2008, 2013). These MAPKK mutants were tested for FSD gene expression under salt stress in this study. Interestingly, the salt-activated gene expression of FSD2 and FSD3 that is apparent in wild-type plants was absent in the MKK5-RNAi plants (mkk5) but still present in MKK4-RNAi lines (mkk4). MKK4 is classified in the same subfamily as MKK5 and has the highest degree of DNA sequence homology to MKK5. Although MKK2 has also been shown to respond to NaCl-induced salt stress (Teige et al., 2004), the NaCl-induced increases of FSD2 and FSD3 transcripts were not blocked in MKK2 mutant, mkk2 plants (Fig. 2A). These results suggest that MKK5 is a strong candidate for mediating the salt-induced FSD expression.
Fig. 2.
Involvement of MKK5 in salt stress responses. RNA gel blot analysis of FSD2 and FSD3 transcript levels in response to salt stress in wild-type Arabidopsis plants, or the mkk2, mkk4, and mkk5 mutants. Total RNA was extracted from wild-type (WT) or mutant plants without stress treatment (control, C) or subjected to NaCl (150mM) for 4h (S). The actin gene ACT2 was used as a loading control. (B) Relative GUS activity driven by FSD2 or FSD3 promoter, respectively, showed the response of FSD2 and FSD3 to NaCl. The promoters of the FSD2 and FSD3 genes were fused to the GUS reporter gene and tested for their response to NaCl in transiently transformed protoplasts from wild-type plants or the mkk5 mutants. The MKK5 genes were cloned and co-transformed into protoplasts of the mkk5 mutant. Transformed protoplasts were pre-incubated 1h with 5 M PD98059 before treatment with 150mM NaCl. Protoplasts were isolated from wild-type (M) and the mkk5 mutant (m) leaves. (C) Relative GUS activity driven by FSD2 or FSD3 promoter, respectively, showed the response of FSD2 and FSD3 to NaCl. The promoters of the FSD2 and FSD3 genes were fused to the GUS reporter gene and tested for their response to NaCl in transiently transformed protoplasts from wild-type plants or the mkk2 and mkk4 mutants. The MKK2 and MKK4 genes were cloned and co-transformed into protoplasts of the mkk2 or mkk4 mutant, respectively. Protoplasts were isolated from wild-type (M) and mutant (m) leaves. All experiments were repeated at least three times with similar results.
To determine whether the FSD2 and FSD3 promoters were activated by salt through MKK5 signalling, protoplasts isolated from mkk5 leaves were transiently transfected with a pFSD2-GUS and a pFSD3-GUS reporter construct. In the mkk5 protoplasts, neither of the two promoters was activated by NaCl (Fig. 2B), although transient expression of wild-type MKK5 restored NaCl-induced activation of the promoters in the mkk5 mutant protoplasts (Fig. 2B). Consistent with these results, PD98059, a broad-spectrum MAPK kinase inhibitor, partially impaired the ability of NaCl to activate the FSD2 and FSD3 promotersmost notably that of FSD2 (Fig. 2B).
It was observed that the FSD2 and FSD3 promoters were activated by salt in a protoplast assay using protoplasts isolated from leaves of the mkk2 and mkk4 RNAi lines. Co-transformation of MKK2 or MKK4 with FSD2 or FSD3 promoters into protoplasts from mkk2 or mkk4 mutant leaves, respectively, showed no difference in activation patterns compared with wild-type (Fig. 2C). Together these results suggest that neither salt-activated MKK2 nor MKK4, which is homologous to MKK5, is involved in salt-induced FSD signalling.
The involvement of specific MAPKs in NaCl-induced FSD signalling
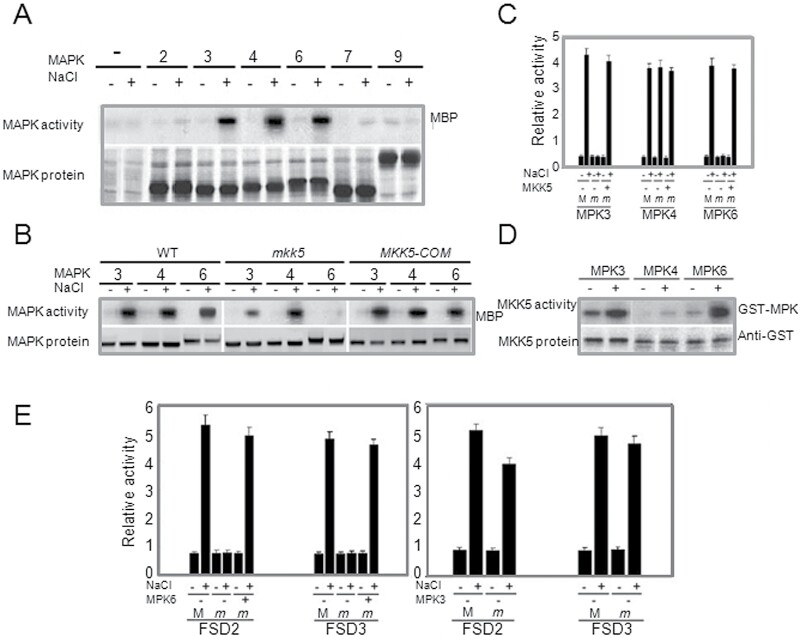
To determine which MAPKs are involved in salt-induced FSD signalling, six MAPKs were targeted that collectively represent four of the five MAPK subfamilies, and which may exhibit distinct functions based on sequence homology analysis (Mizoguchi et al., 2000, Tena et al., 2000; Hamel et al., 2006). The coding sequences of these MAPKs were each introduced into the pHBT95 vector in frame with the coding sequence of a GST, transiently expressed in protoplasts, immunoprecipitated with an anti-GST antibody, and tested for in vitro MAP kinase activity. MPK3, MPK4, and MPK6 all showed strong activation following NaCl treatment (Fig. 3A), while MPK7 showed a small activation but an overall weak expression. MPK2 and MPK9 did not show an altered expression (Fig. 3A).
Fig. 3.
NaCl activates MPK3 and MPK6 through MKK5. (A) MAPK activation with or without NaCl 10min and expression of each MAPK. (B) Salt-triggered activation of MPK3, MPK4, and MPK6 in Arabidopsis. Kinetics of MPK3, MPK4, and MPK6 activation were measured in wild-type and mkk5 mutant plants in response to salt stress. MPK3, MPK4, and MPK6 were immunoprecipitated from leaf cell extracts of salt-induced plants. MPK activity was measured in immunocomplex kinase assays using myelin basic protein (MBP) as a substrate and the levels of MPK3, MPK4, and MPK6 proteins were measured by western blot analysis. (C) Relative GUS activity driven by the MPK3, MPK4, or MPK6 promoter, respectively, showed MPK3, MPK4, and MPK6 response to NaCl in transiently transformed protoplasts of wild-type (M) and mkk5 mutant (m). The MKK5 genes were cloned and co-transformed into protoplasts of the mkk5 mutant. (D) In vitro phosphorylation of MPK3, MPK4, and MPK6 by active MKK5. GST-tagged MKK5 was immunoprecipitated from Arabidopsis protoplasts before and 10min after salt stress treatment. Immunoprecipitated MKK5 was subsequently used for phosphorylation of recombinant kinase inactive GST-MPK3, GST-MPK4, and GST-MPK6, respectively. Phosphorylation of MPKs was analysed by autoradiography after SDS-PAGE. MKK5 protein was detected using a GST antibody. (E) Relative GUS activity driven by the FSD2 and FSD3 promoters showed the FSD2 and FSD3 response to NaCl in transient expression assays using protoplasts from wild-type (M) or mpk3 and mpk6 mutants (m). The MKK5 genes were cloned and co-transformed into protoplasts of the mkk5 mutant. All experiments were repeated at least three times with similar results.
To investigate the molecular mechanism of MKK5 action in salt-induced FSD signalling, MPK3, MPK4, and MPK6 activation was analysed in wild-type and mkk5 plants. NaCl activation of MPK3 and MPK6, which belong to the same subfamily, but not MPK4, in wild-type, decreased in the mkk5 mutant protoplasts unless functional MKK5 was co-expressed in the mkk5 mutant (Fig. 3B). The activation of MPK6 was absent in mkk5 mutant protoplasts, whereas the activation of MPK3 was decreased, suggesting that MPK3 is not regulated to the same extent by MKK5 in salt-induced FSD signalling. To investigate whether the MPK3, MPK4, and MPK6 genes were activated transcriptionally in wild-type and the mkk5 mutant, the promoters of MPK3, MPK4, and MPK6 genes were fused to GUS and tested for their response to NaCl in transiently transfected protoplasts of wild-type and the mkk5 mutant. Consistent with the MAPK activity results, NaCl activation of MPK3 and MPK6 promoters, but not the MPK4 promoter, was blocked in mkk5 and restored when co-expressed with MKK5 (Fig. 3C). Together, these results suggest that MPK3 and MPK6, but not MPK4, are involved in MKK5-mediated salt-induced FSD signalling and that another signal is required to regulate NaCl-induced MPK4 signalling.
To investigate the phosphorylation targets of MKK5 in vitro, recombinant kinase inactive GST fusion proteins of MPK3, MPK4, and MPK6 were expressed and purified. MKK5 was expressed under the control of the 35S CaMV promoter, immunoprecipitated from transiently transformed protoplasts and tested for its ability to phosphorylate MPK3, MPK4, and MPK6 in vitro after activation by salt stress for 10min. Consistent with the above results, both MPK3 and MPK6, but not MPK4, were phosphorylated by MKK5 (Fig. 3D).
To directly test the role of MPK3 and MPK6 in salt-induced FSD signalling, protoplasts isolated from mpk3 or mpk6 mutant leaves were transiently transformed with the FSD2-GUS and FSD3-GUS reporter constructs. In the mpk6 mutant protoplasts, neither of the two promoters was activated by NaCl and the relative GUS activity of the two promoters was restored when co-expressing MPK6 in mpk6 mutant protoplasts. In contrast, the two promoters maintained the same activated level in the mpk3 mutant protoplasts as the wild-type (Fig. 3E). Thus, it appears that while the salt-induced expression of FSD2 and FSD3 requires MPK6, it does not require MPK3, even though both of them can be activated by MKK5.
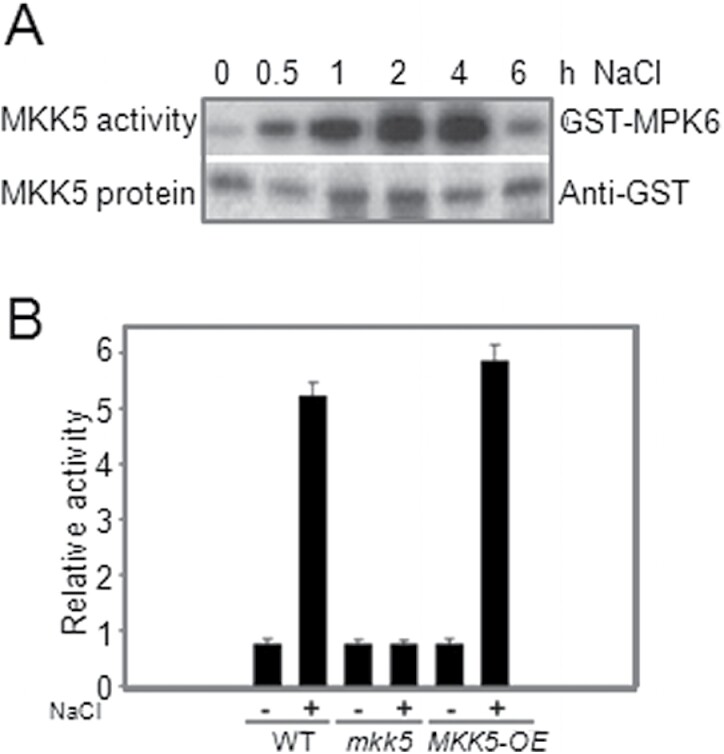
NaCl activation of MKK5
Salt activation of the FSD2 and FSD3 promoters was not observed in mkk5 protoplasts, so it was examined whether NaCl could activate MKK5 in Arabidopsis leaves. MKK5 activity was present from 0.5 to 6h after treatment and then dramatically decreased to basal levels (Fig. 4A). To confirm these results, MKK5-overexpressing plants (MKK5-OE) were constructed, and, consistent with the results using protoplasts, NaCl activation of the MKK5 promoter was absent in mkk5 but increased in MKK5-OE plants (Fig. 4B). These studies also demonstrated that the Arabidopsis protoplast transient expression system can offer a rapid and reliable tool for studying NaCl-induced FSD signalling based on early gene transcription.
Fig. 4.
Activation of MKK5 by salt stress. (A) MKK5 activity was determined after transient expression in plant cells upon salt stress. GST epitope-tagged MKK5 was immunoprecipitated from Arabidopsis protoplasts following NaCl (150mM) treatments for 10min. MKK5 kinase activity was determined by in vitro kinase assays using kinase inactive GST-MPK6 as a substrate. (B) Relative GUS activity driven by the MKK5 promoter in wild-type, mkk5, and MKK5-overexpressing plants induced by NaCl. The MKK5 promoter was fused to GUS and tested for its response to NaCl in transient expression assays using protoplasts. All experiments were repeated at least three times with similar results.
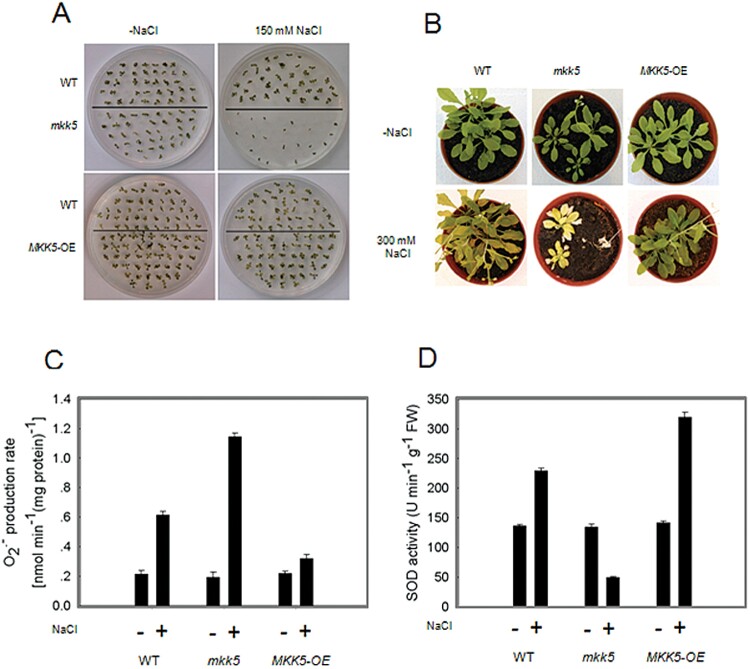
MKK5-overexpressing plants exhibit salt tolerance phenotypes
The MKK5-OE plants showed no obvious phenotype under normal conditions, but large differences when they were stressed with salt treatments (Fig. 5B). To analyse salt tolerance of the MKK5-OE and mkk5 plants, their germination efficiency on salt-containing media was evaluated. The mkk5 plants germinated at a much lower rate than either wild-type or MKK5-OE, while the latter showed a slightly improved ability to germinate on salt-containing media (Fig. 5A). A quantification of the differences in germination of the different lines on salt-containing media confirmed the qualitative analysis: the germination rate of the mkk5 plants was only 50% of that of wild-type. As shown in Fig. 5B, MKK5-OE plants exhibited increased salt tolerance compared with wild-type and the mkk5 plants, so while wild-type and MKK5-overexpressing plants survived the salt stress conditions, the mkk5 plants were sensitive to salt stress. Notably, the increased superoxide production was relatively much lower in MKK5-overexpressing plants than in the mkk5 and wild-type plants, and SOD activity was also higher in MKK5-overexpressing plants (Fig. 5C, D). Similar results were obtained with 10 independent MKK5-OE lines in at least three independent assays. Taken together, these results confirm the importance of MKK5 in conferring salt stress tolerance.
Fig. 5.
Phenotypic analysis of and overexpressing plants. (A) The salt-sensitive phenotype of MKK5-RNAi plants, mkk5, was investigated by germination assays of wild-type [Col-0 (WT)], mkk5, and MKK5 overexpressing plants on agar plates, with 0mM or 150mM NaCl. Seeds were sterilized, stratified, and plated. Germination was visually determined after10 d. (B) Salt-sensitive phenotype of mkk5 plants and salt tolerance of MKK5 overexpressing lines. (C) The production of superoxide in seedlings response to salt stress (300mM NaCl) for 10 d. (D) SOD activities in seedlings of wild-type, mkk5, and MKK5-OE response to salt stress (300mM NaCl) for 10 d. All experiments were repeated at least three times with similar results.
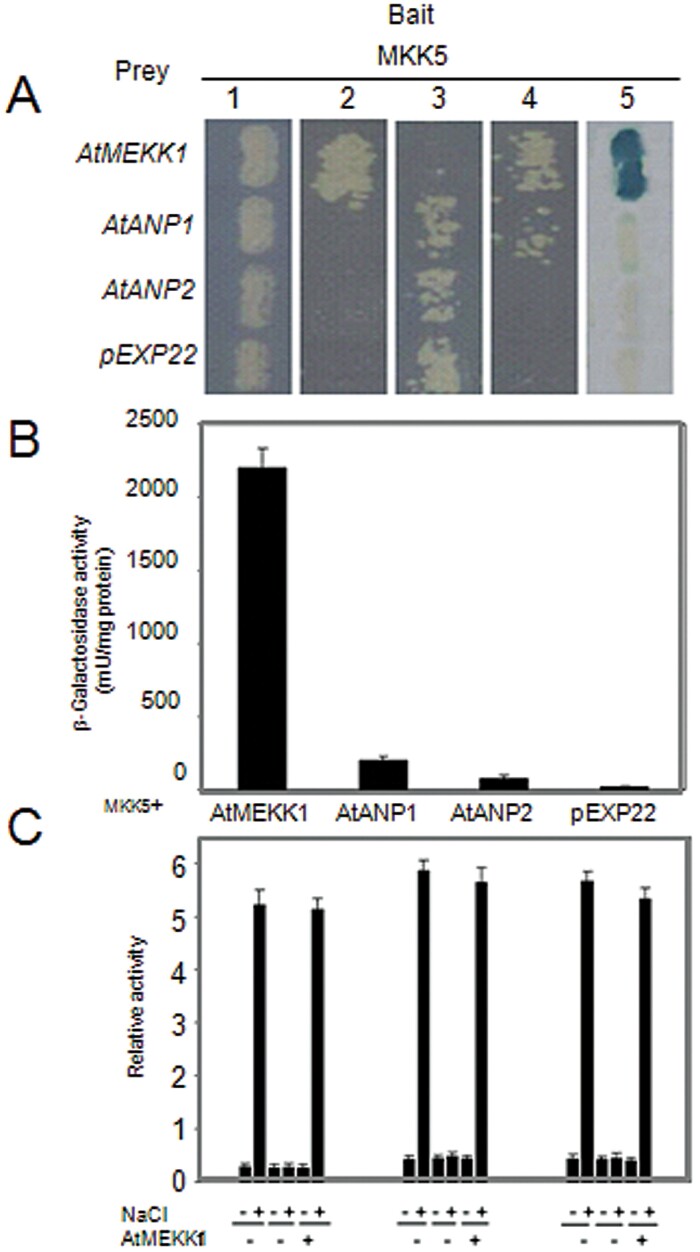
MEKK1 interacts with MKK5
The MEKK1 gene has previously been shown to be upregulated by both cold and salt stresses (Mizoguchi et al., 1997; Teige et al., 2004) and it has also been shown that the MEKK1MKK4/5MPK3/6 module acts downstream of the flagellin receptor FLS2 and upstream of the WRKY22 and WRKY29 transcription factors (Asai et al., 2002). Having established that MKK5 is involved in salt-induced FSD signalling, it was hypothesized that MKK5 might interact with MEKK1 under salt stress conditions. It was examined whether MKK5 and MEKK1 interact physically using the yeast two-hybrid system. Yeast strains containing pEXP-MKK5 were used as a bait, and MEKK1, ANP1, and ANP2 were chosen as candidates for upstream MKK5 action, since they have been shown to be activated by oxidative stress (Kovtun et al., 2000; Krysan et al., 2002). As can be seen in Fig. 6A, MEKK1 interacted strongly with MKK5 but not with ANP1 or ANP2. The coloured product produced in the assay appeared and this was confirmed by a quantitative -galactosidase assay (Fig. 6B). Finally, it was tested whether MKK5, the transcript levels of FSD2, and FSD3 were affected in wild-type and the mekk1 mutant, by fusing their promoters to GUS and testing their response to NaCl in transiently transfected protoplasts of wild-type and mekk1 mutant. Consistent with the results from the yeast two-hybrid assay, the activation of MKK5, FSD2, and FSD3 promoters were all arrested in the mekk1 mutant (Fig. 6C), suggesting that MEKK1 acts upstream of MKK5 and is involved in NaCl-induced FSD signalling.
Fig. 6.
MKK5 specifically interacts with MEKK1. (A) MKK5 strongly interacted with MEKK1 in the yeast two-hybrid system. Yeast strains containing pEXPTM32-MKK5 as bait and pEXPTM22-MEKK1 as prey were grown on the following medium to screen for transformants: 1, SC medium lacking Leu and Trp for 48h; 2, SC medium lacking Leu, Trp, and Ura for an additional 48h to select the transformants; 3, SC medium lacking Leu and Trp and adding 0.2% of 5-fluoroorotic acid (5-FOA) for an additional 48h to select the cells containing interacting proteins; 4, SC medium lacking Leu, Trp, and His and adding 100mM 3-Amino-1,2,4-Triazole (3AT) for an additional 48h to confirm the interaction; 5, YPAD medium 48h, then an X-Gal assay was performed on the membrane to confirm the results. The pEXPTM22 empty prey vector was used as negative control. (B) Quantitative analysis of -galactosidase activity of the yeast strains in liquid culture showing the interaction between MKK5 and MEKK1. Values are means of data from at least three independent experiments. (C) Relative GUS activity driven by MKK5, FSD2, or FSD3 promoters, respectively, showed the response to NaCl in transiently transfected protoplasts of wild-type (M) or mekk1 (m) plants. The MEKK1 genes were cloned and co-transformed into protoplasts of the mekk1 mutant. All experiments were repeated at least three times with similar results.
Discussion
Salt stress can modulate the activity of the antioxidant system (Khan et al., 2012), and several studies have demonstrated that antioxidant enzyme activities and levels of antioxidant compounds are increased in certain plants in response to salt stress (Meneguzzo et al., 1999; Shalata et al., 2001). As the first line of defence against ROS in plant cells, SODs react with the superoxide radical to produce H2O2 (Scandalios, 1997). SODs have been extensively studied for many years and growing evidence suggests that they respond to many abiotic stresses and that SOD overexpressing plants have an enhanced tolerance of abiotic stresses (Miller et al., 2008, van Breusegem et al., 2008; Li et al., 2013; Xing et al., 2013). This study was focused on FeSODs and analysing their response to salt stress. Of the three FeSODs from Arabidopsis, FSD2 and FSD3, but not FSD1, were strongly regulated by salt stress. Possibly as a consequence of circadian regulation, the mRNA abundance of FSD1 was not very stable but was normally higher than that of FSD2 or FSD3 (Fig. 1A). Different FeSODs respond to stresses in various ways, suggesting complex mechanisms for regulating the expression of the SOD gene family, involving substantial signalling pathways crosstalk.
The specific ROS sensors that process and translate associated stresses have yet to be identified (Andreasson and Ellis, 2010). However, the crosstalk among ROS signalling, ROS scavenging enzymes, and MAPK cascades is complex and elaborate. There is evidence from several systems for the interaction of MAPKs with ROS (Samuel and Ellis, 2002; Pitzschke and Hirt, 2006; Xing et al., 2008, 2013). The MAPK signalling pathway, as one of the major signalling cascades, plays a crucial role in diverse cellular functions, and particularly the redox-regulated processes involved in cellular metabolism (Davletova et al., 2004; Joo et al., 2005; Pitzschke and Hirt, 2006; Xing et al., 2008, 2013). In some studies, H2O2 is required for the activation of ZmMPK5 in maize leaves (Ding et al., 2009; Lin et al., 2009). ZmCPK11 induces the activation of ZmMPK5 in ABA signalling by increasing the production of H2O2 (Ding et al., 2013). Cai et al. (2014) found overexpression of ZmMKK1 alleviated ROS accumulation by maintaining high activities of ROS scavenging enzymes such as SOD, POD, CAT, and APX. Similarly, ZmMPK17 and ZmMKK4 transgenic tobacco plants have enhanced osmotic stress tolerance through high ROS scavenging ability (Kong et al., 2011;).
Although both MAPK signalling cascades and antioxidant systems are involved in biotic and abiotic stress, it is not clear how or if they interact. In a systematic screen of the 10 members of the Arabidopsis MAPKK family, it was found that transcription of the NaCl-activated FSD2 and FSD3 was significantly blocked in MKK5-RNAi plantsmkk5but not in mkk4the MKK4-RNAi plants, which belongs to the same subfamily as MKK5. In the flagellin-induced pathway of the MEKK1MKK4/MKK5MPK3/MPK6 module, MKK4 and MKK5 are involved in the same process of regulating the downstream WRKY22 and WRKY29 transcription factors (Asai et al., 2002). MKK4 and MKK5 are paralogous MKKs acting upstream of the MPK3/MPK6, and play a key role in mediating many different stress signals and in plant development (Andreasson and Ellis, 2010). It is very interesting that MKK5 and MKK4 show different responses to salt stress, while MKK4 was not involved in the regulation of FSD (Fig. 2A). The present study reveals that MKK4 and MKK5 may operate in different pathways and that only MKK5 is involved in NaCl-induced salt stress. The regulation of MKK5 on FSD2/3 seems to be specific. The function of MKK4 in salt stress, in contrast to MKK5, was investigated, and the salt tolerance in MKK4-RNAi plants tested. The MKK4-RNAi line did not show similar phenotypes as MKK5-RNAi plants under salt stress (data not shown). Miles et al. (2009) also showed that partial suppression of MKK5 expression in Arabidopsis is sufficient to induce ozone hypersensitivity, which would indicate that MKK4 and MKK5 are not fully redundant. However, it is still possible that the roles of MKK4 and MKK5 within the salt stress signalling network are redundant because there is no experimental evidence of a double mutant at present.
The NaCl-induced MAPK cascade leading to the activation of Arabidopsis MPK3, MPK4, and MPK6 is reminiscent of the activation of MPK4 and MPK6 by SIMK, under salt stress (Kiegerl et al., 2000). Teige et al. (2004) identified a salt-induced Arabidopsis MAPKK, MKK2, which is capable of activating MPK4 and MPK6. However, as shown in Fig 3A, these data further revealed an unexpected activation of MPK3. It was also shown that although MPK3, MPK4, and MPK6 are involved in salt stress responses, only MPK3 and MPK6 are regulated by MKK5 (Fig. 3C). In contrast to the activation of MPK4 and MPK6 by MKK2 following salt stress, the regulation of MPK3 and MPK6 by MKK5 is more complex, involving different MAPK signalling pathways. The MKK5MPK3/MPK6 pathway is similar to the flagellin-induced MAPK signalling pathway, MKK4/MKK5MPK3/MPK6 (Asai et al., 2002). The existence of a crosstalk between the different stress signals is suggested, and that MKK5, as an important component of MAPKs signalling cascades, plays a key role in plant responses to biotic and abiotic stresses. The MAPK family involves comprehensive protein interactions and different MAPKs may function to different stresses (Andreasson and Ellis, 2010). Together these data demonstrate that MKK5 is a key signal transducer in MAPKs signalling cascades involved in regulating different processes in response to multiple divergent stresses.
Supplementary data
Supplementary material is available at JXB online.
Figure S1. Original images of RNA gel blots used for preparation of Figure 1A. (A) FSD1 expression. (B) FSD2 expression. (C) FSD3 expression. (D) Actin gene ACT2 was used as loading control.
Figure S2. Original images of RNA gel blots used for preparation of Figure 2A. (A) FSD2 expression. (B) FSD3 expression. (C) Actin gene ACT2 was used as loading control.
Figure S3. Original images of MAPK activity analysis used for preparation of Figure 3A. (A) MAPK activity of control, MPK2 and MPK3. (B) MAPK activity of MPK4, MPK6, MPK7 and MPK9.
Figure S4. Original images of MAPK activity analysis used for preparation of Figure 3B. (A) MAPK activity of MPK3, MPK4 and MPK6 in WT. (B) MAPK activity of MPK3, MPK4 and MPK6 in mkk5 mutant. (C) MAPK activity of MPK3, MPK4 and MPK6 in MKK5-OE plants.
Figure S5. Original images of MKK5 activity analysis used for preparation of Figure 4A. (A) MKK5 activity. (B) Western Blot analysis of GST epitope-tagged MKK5.
Acknowledgements
This work was supported by the National Basic Research Program of China (973 project, 2012CB114300); Natural Science Foundation of China Major Research Plan (91317307); Hong Kong Research Grants Council (CUHK 473611, 473512 and 474313, AoE/M-05/12); Shenzhen Overseas Talents Innovation & Entrepreneurship Funding Scheme (The Peacock Scheme); The Beijing Natural Science Foundation and Scientific Research Key Program of the Beijing Commission of Education (KZ20130020018); The Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality (IDHT20140509); and Beijing University of Agriculture funding to improve research quality (GZL2015006).
Glossary
Abbreviations:
- CaMV
cauliflower mosaic virus
- FeSOD
iron SOD
- FSD
iron superoxide dismutase
- GUS
-glucuronidase
- MAPK
mitogen-activated protein kinase
- MAPKK
MAPK kinase
- 4-MU
4-methyl-umbelliferone or 7-hydroxy-4-methyl-cumarin
- MUG
4-methyl-umbelliferyl--D-glucuronide
- ROS
reactive oxygen species
- RT-PCR
reverse transcription-PCR
- SIMK
stress-induced MAPKK
- SIMKK
stress-induced MAPKK
- SOD
superoxide dismutase
- XTT
3-[1-(phenylamino-carbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzenesulphonic acid hydrate.
References
- Abel S, Theologis A. 1994. Transient transformation of Arabidopsis leaf protoplasts: a versatile experimental system to study gene expression. The Plant Journal 5, 421–428.. [DOI] [PubMed] [Google Scholar]
- Able AJ, Guest DI, Sutherland MW. 1998. Use of a new tetrazolium-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of Phytophthora parasitica var. nicotianae. Plant Physiology 117, 491–499.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya BR, Jeon BW, Zhang W, Assmann SM. 2013. Open stomata (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells. New Phytologist 200, 1049–1063.. [DOI] [PubMed] [Google Scholar]
- Allen RD. 1995. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiology 107, 1049–1054.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson E, Ellis B. 2010. Convergence and specificity in the Arabidopsis MAPK nexus. Trends in Plant Science 15, 106–113.. [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. 2002. MAP kinase signaling cascade in Arabidopsis innate immunity. Nature 415, 977–983.. [DOI] [PubMed] [Google Scholar]
- Bowler C, Van Camp W, Van Montagu M, Inz D. 1994. Superoxide dismutase in plants. Critical Reviews in Plant Science 13, 199–218.. [Google Scholar]
- Cai G, Wang G, Wang L, Pan J, Liu Y, Li D. 2014. ZmMKK1, a novel group A mitogen-activated protein kinase kinase gene in maize, conferred chilling stress tolerance and was involved inpathogen defense in transgenic tobacco. Plant Science 214, 57–73.. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743.. [DOI] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang HJ, Zhong SQ, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R. 2004. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17, 268–281.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding HD, Zhang XH, Xu SC, Sun LL, Jiang MY, Zhang AY, Jin YG. 2009. Induction of protection against paraguat-induced oxidative damage by abscisic acid in maize leaves is mediated through mitogen-activated protein kinase. Journal of Integrated Plant Biology 51, 961–972.. [DOI] [PubMed] [Google Scholar]
- Ding Y, Cao J, Ni L, Zhu Y, Zhang A, Tan M, Jiang M. 2013. ZmCPK11 is involved in abscisic acid-induced antioxidant defence and functions upstream of ZmMPK5 in abscisic acid signalling in maize. Journal of Experimental Botany 64, 871–884.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ftterer J, Gordon K, Sanfacon H, Bonneville JM, Hohn T. 1990. Positive and negative control of translation by the leader sequence of cauliflower mosaic virus pregenomic 35S RNA. EMBO Journal 9, 1697–1707.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48, 909–930.. [DOI] [PubMed] [Google Scholar]
- Hamel LP, Nicole MC, Sritubtim S. 2006. Ancient signals: comparative genomics of plant MAPK and MAPKK gene families. Trends in Plant Science 11, 192–198.. [DOI] [PubMed] [Google Scholar]
- Huang CH, Kuo WY, Weiss C, Jinn TL. 2012. Copper chaperone-dependent and independent activation of three copper-zinc superoxide dismutase homologs localized in different cellular compartments in Arabidopsis. Plant Physiology 158, 737–746.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JH, Wang SY, Chen JG, Jones AM, Fedoroff NV. 2005. Different signalling and cell death roles of heterotrimeric G protein and subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17, 957–970.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katou S, Senda K, Yoshioka H, Doke N, Kawakita K. 1999. A 51kDa Protein Kinase of Potato Activated with Hyphal Wall Components from Phytophthorainfestans. Plant Cell Physiology 40, 825–831.. [Google Scholar]
- Khan MN, Siddiqui MH, Mohammad F, Naeem M. 2012. Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 27, 210–218.. [DOI] [PubMed] [Google Scholar]
- Kiegerl S, Cardinale F, Siligan C. 2000. SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress-induced MAPK, SIMK. Plant Cell 12, 2247–2258.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, Monde R, Last RL. 1998. Superoxide dismutase in Arabidopsis: an eclectic enzyme family with disparate regulation and protein localization. Plant Physiology 118, 637–650.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Sun L, Zhou Y, Zhang M, Liu Y, Pan J, Li D. 2011. ZmMKK4 regulates osmotic stress through reactive oxygen species scavenging in transgenic tobacco. Plant Cell Reports 30, 2097–2104.. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y, Nakajima K, Arai Y. 1990. An improved assay for glucuronidase in transformed cells: methanol almost completely suppresses a putative endogenous fl-glucuronidase activity. Plant Science 70, 133–140.. [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J. 2000. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proceedings of the National Academy of Sciences.USA 97, 2940–2945.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan PJ, Jester PJ, Gottwald JR, Sussman MR. 2002. An Arabidopsis mitogen-activated protein kinase kinase kinase gene family encodes essential positive regulators of cytokinesis. Plant Cell 14, 1109–1120.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Klessig DF. 2000. Differential induction of tobacco MAP kinases by the defense signals nitric oxide, salicylic acid, ethylene, and jasmonic acid. Molecular Plant-Microbe Interaction 13, 347–351.. [DOI] [PubMed] [Google Scholar]
- Li G, Peng X, Wei L, Kang G. 2013. Salicylic acid increases the contents of glutathione and ascorbate and temporally regulates the related gene expression in salt-stressed wheat seedlings. Gene 529, 321–325.. [DOI] [PubMed] [Google Scholar]
- Lin F, Ding H, Wang J, Zhang H, Zhang A, Zhang Y, Tan M, Dong M, Jiang M. 2009. Positive feedback regulation of maize NADPH oxidase by mitogen-activated protein kinase cascade in abscisic acid signalling. Journal of Experimental Botany 60, 3221–3238.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka D, Nanmori T, Sato K, Fukami Y, Kikkawa U, Yasuda T. 2002. Activation of AtMEK1, an Arabidopsis mitogen-activated protein kinase kinase, in vitro and in vivo: analysis of active mutants expressed in E. coli and generation of the active form in stress response in seedlings. The Plant Journal 29, 637–647.. [DOI] [PubMed] [Google Scholar]
- Mckersie BD, Murnaghan J, Jones KS, Bowley SR. 2000. Iron-superoxide dismutase expression in transgenic alfalfa increases winter survival without a detectable increase in photosynthetic oxidative stress tolerance. Plant Physiology 122, 1427–1437.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneguzzo S, Navari-Izzo F, Izzo R. 1999. Antioxidative responses of shoots and roots of wheat to increasing NaCl concentrations. Journal of Plant Physiology 155, 274–280.. [Google Scholar]
- Miles GP, Samuel MA, Ellis BE. 2009. Suppression of MKK5 reduces ozone-induced signal transmission to both MPK3 and MPK6 and confers increased ozone sensitivity in Arabidopsis thaliana. Plant Signal Behavior 4, 687–692.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Shulaev V, Mittler R. 2008. Reactive oxygen signaling and abiotic stress. Physiologia Plantarum 133, 481–489.. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Ichimura K, Shinozaki K. 1997. Environmental stress response in plants: the role of mitogen-activated protein kinases. Trends in Biotechnology 15, 15–19.. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Ichimura K, Yoshida R, Shinozaki K. 2000. MAP kinase cascades in Arabidopsis: their roles in stress and hormone responses. In: Hirt H, ed. Results and problems in cell differentiation: MAP kinases in Plant Signal Transduction . Heidelberg: Springer, 29–38.. [DOI] [PubMed] [Google Scholar]
- Morgan M.J., Lehmann M., Schwarzlander 2008. Decrease in manganese superoxide dismutase leads to reduced root growth and affects tricarboxylic acid cycle flux and mitochondrial redox homeostasis. Plant Physiology 147, 101–114.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunberg AN, Thomas TL. 1993. Transient analysis of gene expression in plant cells. In: Glick B, Thompson J, eds. Methods in plant molecular biology and biotechnology , Vol. 9. BocaRaton: CRC Press, 147–154.. [Google Scholar]
- Pitzschke A, Hirt H. 2006. Mitogen-Activated Protein Kinases and Reactive Oxygen Species Signaling in Plants. Plant Physiology 141, 351–356.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan PS, Rajaram H, Apte SK. 2011. Nitrogen status dependent oxidative stress tolerance conferred by overexpression of MnSOD and FeSOD protein in Anabaena sp. Strain PCC7120. Plant Molecular Biology 77, 407–417.. [DOI] [PubMed] [Google Scholar]
- Ren D, Yang H, Zhang S. 2002. Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. Journal of Biological Chemistry 277, 559–565.. [DOI] [PubMed] [Google Scholar]
- Samuel MA, Miles GP, Ellis BE. 2000. Ozone treatment rapidly activates MAP kinase signalling in plants. The Plant Journal 22, 367–376.. [DOI] [PubMed] [Google Scholar]
- Samuel MA, Ellis BE. 2002. Double jeopardy: both overexpression and suppression of a redox-activated plant mitogen-activated protein kinase render tobacco plants ozone sensitive. Plant Cell 14, 2059–2069.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios JG. 1997. Molecular genetics of superoxide dismutase in plants. In: Scandalios JG, ed. Oxidative stress and the molecular biology of antioxidant defense . New York: Cold Spring Harbor Laboratory Press, 527–568.. [Google Scholar]
- Shalata A, Mittova V, Volokita M, Guy M, Tal M. 2001. Response of the cultivated tomato and its wild salt-tolerant relative Lycopersiconpennellii to salt-dependent oxidative stress: the root antioxidative system. Physiologia Plantarum 112, 487–494.. [DOI] [PubMed] [Google Scholar]
- Sheikh AH, Raghuram B, Jalmi SK, Wankhede DP, Singh P, Sinha AK. 2013. Interaction between two rice mitogen activated protein kinases and its possible role in plant defense. BMC Plant Biology 13, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Salazar C. 2013. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25, 3553–3569.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, Shinozaki K, Dangl J, Hirt H. 2004. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Molecular Cell 15, 141–152.. [DOI] [PubMed] [Google Scholar]
- van Breuseqem F, Bailey-Serres J, Mittler R. 2008. Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiology 147, 978–984.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wu Q, Zhang Z, Chen S, Zhou R. 2013. Cloning, expression, and characterization of iron superoxide dismutase in Sonneratiaalba, a highly salt tolerant mangrove tree. Protein Journal 32, 259–265.. [DOI] [PubMed] [Google Scholar]
- Xing Y, Jia WS, Zhang JH. 2007. AtMEK1 mediates stress-induced gene expression of CAT1 catalase by triggering H2O2 production in Arabidopsis. Journal of Experimental Botany 58, 2969–2981.. [DOI] [PubMed] [Google Scholar]
- Xing Y, Jia WS, Zhang JH. 2008. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. The Plant Journal 54, 440–451.. [DOI] [PubMed] [Google Scholar]
- Xing Y, Cao QQ, Zhang Q, Qin L, Jia WS, Zhang JH. 2013. MKK5 regulates high light-induced gene expression of Cu/Zn superoxide dismutase 1 and 2 in Arabidopsis. Plant and Cell Physiology 54, 1217–1227.. [DOI] [PubMed] [Google Scholar]
- Zhang AY, Jiang MY, Zhang JH, Tan M, Hu XL. 2006. Mitogen-activated protein kinase is involved in Abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiology 141, 475–487.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.