Highlight:
In developing transfer cells, inward-directed deposition of wall ingrowth papillae into the cytoplasm occurs independently of, but through, depletion zones in the cortical microtubule array created by cytosolic Ca2+ plumes.
Key words: Cortical microtubule arrays, cytosolic calcium, localized cell wall deposition, seed, transfer cell, wall ingrowth papillae.
Abstract
Trans-differentiation to a transfer-cell morphology is characterized by the localized deposition of wall ingrowth papillae that protrude into the cytosol. Whether the cortical microtubule array directs wall ingrowth papillae formation was investigated using a Vicia faba cotyledon culture system in which their adaxial epidermal cells were spontaneously induced to trans-differentiate to transfer cells. During deposition of wall ingrowth papillae, the aligned cortical microtubule arrays in precursor epidermal cells were reorganized into a randomized array characterized by circular depletion zones. Concurrence of the temporal appearance, spatial pattern, and size of depletion zones and wall ingrowth papillae was consistent with each papilla occupying a depletion zone. Surprisingly, microtubules appeared not to regulate construction of wall ingrowth papillae, as neither depolymerization nor stabilization of cortical microtubules changed their deposition pattern or morphology. Moreover, the size and spatial pattern of depletion zones was unaltered when the formation of wall ingrowth papillae was blocked by inhibiting cellulose biosynthesis. In contrast, the depletion zones were absent when the cytosolic calcium plumes, responsible for directing wall ingrowth papillae formation, were blocked or dissipated. Thus, we conclude that the depletion zones within the cortical microtubule array result from localized depolymerization of microtubules initiated by elevated cytosolic Ca2+ levels at loci where wall ingrowth papillae are deposited. The physiological significance of the depletion zones as a mechanism to accommodate the construction of wall ingrowth papillae without compromising maintenance of the plasma membrane–microtubule inter-relationship is discussed.
Introduction
As part of their differentiation, numerous plant cell types deposit specialized cell walls that relate to their final function. Included among these cell types are transfer cells that trans-differentiate from many cell types, conferring on them an enhanced capacity for membrane exchange of nutrients. This functional state is achieved through the deposition of extensive ingrowth wall labyrinths that act as scaffolds to support an amplified surface area of plasma membrane enriched in nutrient transporters (Offler et al., 2003). Ingrowth walls with a reticulate morphology comprise a uniform wall, deposited inward of the original primary wall, and from which wall ingrowth papillae arise at loci (McCurdy et al., 2008; Andriunas et al., 2013). Depending on the species and cell type, the papillae then branch and fuse, resulting in a fenestrated layer of wall material and, by repeating this sequence of wall deposition, form a multi-layered wall labyrinth (Talbot et al., 2001). Such a multi-layered ingrowth wall labyrinth, polarized to their outer periclinal wall, characterizes the abaxial epidermal transfer cells of Vicia faba cotyledons. In contrast, their adaxial epidermal cells do not form transfer cells in vivo. However, when cotyledons are placed in culture on a Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) lacking growth substances (Offler et al., 1997), their adaxial epidermal cells spontaneously and rapidly (within hours; Wardini et al., 2007) trans-differentiate into transfer cells that are morphologically (Talbot et al., 2001) and functionally (Farley et al., 2000) equivalent to their abaxial counterparts. This study reports the use of this cotyledon culture system to investigate whether microtubule organization plays a role in directing the construction of wall ingrowth papillae during transfer-cell trans-differentiation.
Plant cell walls are composites of many polymers, which are assembled and organized in a myriad of different configurations (Carpita, 2012). Microtubules, together with actin filaments, have key roles in cell wall construction, but as yet these are not fully understood (McKenna et al., 2014). Microtubules typically assemble at the cell cortex, forming patterns ranging from co-aligned (parallel) to randomly organized arrays (Shaw, 2013). Early models of microtubule/cellulose microfibril interactions suggested a direct relationship between the spatial organization of cortical microtubules (CMTs) and alignment of cellulose microfibrils (Green, 1962; Ledbetter and Porter, 1963). While more recent studies (Himmelspach et al., 2003; Sugimoto et al., 2003; Paradez et al., 2006) have demonstrated that, in growing cells, cellulose microfibrils can maintain their parallel trajectories when CMTs are depolymerized, the alignment theory remains strongly supported by new evidence. For instance, an anchor protein, POM2/CSI1, binds cellulose synthases, CMTs, and, potentially, the plasma membrane, and has been shown to ensure tracking of cellulose synthase complexes along CMTs for ordered cellulose microfibril deposition (Bringmann et al., 2012; Lei et al., 2013).
During cell differentiation, CMT positioning controls the patterning of deposition of specialized and localized walls, for example secondary walls in xylem tracheary elements (Oda et al., 2010) and thickened inner walls of guard cells (Kong et al., 2010). In these examples, reorganization of CMTs through interaction with specific plasma membrane proteins located in microdomains provides the template for localized cell wall deposition. This evidence, combined with the dependence of wall ingrowth formation on depositing a cellulose scaffold (Talbot et al., 2007a ), suggests that changes in the interaction between cellulose synthases and CMTs may well direct construction of wall ingrowth papillae at right angles to the original primary wall of transfer cells. The spiral intertwined cellulose microfibrils of the cellulose scaffold (Talbot et al., 2007a ) are consistent with cellulose synthase complexes being constrained at the tips of wall ingrowth papillae (see also model in McCurdy et al., 2008). In this case, the role for CMTs would differ fundamentally from that in tip growing systems of root hairs and pollen tubes. Here, CMTs are excluded from the growing tip (Mendrinna and Persson, 2015) at which cellulose microfibrils are deposited in a randomized pattern (Akkerman et al., 2012). While the role of actin is not the focus of this study, it is likely to be responsible for trafficking secretory vesicles containing cellulose synthases to, but not constraining cellulose synthases at, the tips of developing wall ingrowth papillae, a role equivalent to that in tip growing cells (Mendrinna and Persson, 2015).
Coupling of cellulose synthase complexes and CMTs to achieve cellulose microfibril deposition at right angles to the original wall would require modification of the CMT array. Consistent with this proposition is the reported concurrent CMT reorganization with ingrowth wall construction during transfer-cell trans-differentiation of placental cells of Lilium longifolium (Singh et al., 1999) and of abaxial epidermal cells of V. faba cotyledons (Bulbert et al., 1998). In the latter case, as wall ingrowth papillae deposition commenced, CMTs became polarized to the site of ingrowth wall construction before being randomized to surround developing wall ingrowth papillae. However, the precise role of the modified CMT array in directing the pattern of localized wall ingrowth papillae deposition remains unclear.
The results of this study confirmed that the aligned CMT array is reorganized to a randomized array during trans-differentiation of epidermal cells. The changes not apparent from earlier studies are that circular depletion zones appeared in the randomized CMT array. The temporal and spatial pattern of these depletion zones equated with that of deposition of wall ingrowth papillae. However, the latter remained unchanged when CMTs were depolymerized or stabilized, negating a regulatory role for CMTs in directing deposition of wall ingrowth papillae. Interestingly, manipulating cellulose biosynthesis and cytosolic Ca2+ concentration during trans-differentiation to a transfer-cell morphology established that cytosolic Ca2+ plumes, known to define loci for wall ingrowth papillae formation (Zhang et al., 2015), were responsible for localized depolymerization of CMTs to form the depletion zones within the randomized CMT arrays.
Materials and methods
Plant growth conditions
V. faba L. cv. Fiord plants were raised under controlled environmental conditions according to Zhou et al. (2010).
Cotyledon culture
Excised cotyledons were cultured aseptically on either a solid or liquid form of MS medium adjusted to 300 mOsmol kg–1 using betaine. The medium pH was adjusted to 5.8 with 1M KOH or HCl prior to sterilization. For cultures examining changes across 24h of trans-differentiation, a liquid MS medium was used. For mid- and long-term culture periods (i.e. 3 and 6 d), solid MS medium with 0.8% aqueous agar, 100mM sucrose and 62.5mM l-asparagine was used to ensure a sufficient supply of sugar and amino acids to support ongoing cell activities. Sister cotyledon pairs were divided between MS medium with and without specified pharmacological agents. For all culture periods specified above, the excised cotyledons were first cultured at 4 °C for 4h to allow the pharmacological agent to be taken up by the epidermal cells. Thereafter, transfer-cell trans-differentiation was initiated by transferring the cotyledon cultures to 26 °C for the specified period examined. Each pharmacological agent was applied in MS medium at a concentration that did not impact cell viability as verified by staining tissue sections of cultured cotyledons with 0.1% (w/v) tetrazolium blue for 20min. For the two longer culture periods, cotyledons were transferred to fresh plates daily.
Visualizing cortical microtubules and cell walls using confocal laser-scanning microscopy
V. faba cotyledons were fixed for 3h at room temperature in 4% (w/v) paraformaldehyde, 0.1% (w/v) glutaraldehyde, 2mM CaCl2 and 5mM dithiothreitol buffered in 50mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), pH 7.0. After fixation, cotyledons were washed three times for 10min each in PBS and excess liquid was removed. Epidermal strips were peeled from the adaxial surface of each cotyledon (Dibley et al., 2009) and used to immunolabel CMT arrays as follows. The epidermal peels were transferred to a 96-well plate and blocked for 1h in 250 μl PBS with 1% (w/v) bovine serum albumin (PBS/BSA) at room temperature. After blocking, peels were incubated overnight at 4 °C with a mouse monoclonal anti-α-tubulin antibody (Sigma-Aldrich, USA), diluted 1:100 in PBS/BSA, washed six times for 10min each in PBS with 0.2% (v/v) Tween 20 (PBS/T) and incubated for a further 3h at room temperature in secondary antibody, goat anti-rat IgG conjugated to Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA) diluted 1:100 in PBS/T. Secondary antibody was removed by six 10min washes with PBS/T. Some epidermal peels were post-stained with filtered 0.5% (w/v) aqueous Congo red (Sigma, Australia) for 1min to visualize cell walls. Peels were mounted on glass slides in an anti-fading buffer containing 180 μl of Mowiol 4-88 with 20 μl of 0.1% (w/v) phenylenediamine.
An Olympus FV1000 confocal laser-scanning microscope (CLSM) with diode-pumped solid-state lasers, combined with an acousto-optic tunable filter laser combiner, was used to visualize the adaxial epidermal cells. A 473nm laser (15 mW, power set to 20%) with a 510–550nm emission filter set was used to visualize CMT fluorescence with the gain of the photomultiplier tube set to 600V. For Congo red fluorescence, a 559nm laser (15 mW, power set to 20%) was selected, with the emission filter and photomultiplier gain set to 600–660nm and 700V, respectively. Using cell wall fluorescence as a reference point, the focal plane for imaging was positioned 200–300nm inward of the inner surface of the uniform wall. A ×60 oil-immersion lens (numerical aperture 1.25) was used.
Visualizing cytosolic calcium
Cultured cotyledons were loaded with a membrane-permeable Ca2+-sensitive dye, Oregon Green BAPTA-1 acetoxymethyl ester (OGB-1) at a concentration of 20 µM, for 3h at 4 °C. The loaded cotyledons were then incubated on liquid MS medium for 2h at 26 °C to allow cleavage of the Oregon Green ester by cytosolic esterases, hence trapping the membrane-impermeant Oregon Green dye in the cell cytosol (Zhang et al., 2015). Transverse hand-cut sections were then prepared and stained with 0.1% (w/v) tetrazolium blue (prepared in PBS buffer containing 100mM sucrose) for 20min to identify viable cells for microscope observations. Thereafter, the tissue sections were counterstained with 0.1% (w/v) Calcofluor White for 30 s to outline cell walls of adaxial epidermal cells, before being mounted in 200 µl of PBS buffer containing 100mM sucrose and visualized using an Olympus FV1000 CLSM. A 405nm UV laser with a 440–490nm emission filter set was used to visualize Calcofluor white fluorescence, while a 473nm laser with a 510–550nm emission filter set was used to visualize OGB-1 fluorescence.
Visualizing wall ingrowth papillae by electron microscopy
Scanning electron microscopy (SEM)
Protoplasts of cotyledon adaxial epidermal peels (Dibley et al., 2009) were removed by washing in 2% (v/v) NaOCl for 3h at room temperature with hourly changes followed by three 10min washes in dH2O to remove the bleach. Peels then were placed in small steel cages and dehydrated at 4 °C through a 10% step-graded ethanol/dH2O series, changed at 30min intervals from 10% up to 100% ethanol. After holding in 100% ethanol at least overnight, peels were critical-point dried with liquid CO2 in a critical-point drier (Balzers Union, Liechtenstein). The dried peels were orientated outer face down on sticky carbon tabs to reveal the cytoplasmic face of their outer periclinal cell walls. Samples were sputter coated with gold to a thickness of 20nm in a sputter-coating unit (SPI Suppliers, USA) and viewed at 15kV with a Philips XL30 scanning electron microscope. Cells were scored for the presence/absence of wall ingrowth papillae. Cell numbers induced to form wall ingrowth papillae were expressed as a percentage of total cells scored (130–150 cells for each replicate cotyledon). From these data, the mean percentage of cells containing wall ingrowth papillae, or the mean percentage of cells at specified stages of wall ingrowth papillae development, were computed as described by Zhou et al. (2010).
Transmission electron microscopy (TEM)
Cotyledons were cut into 2×2×1mm blocks and fixed in 3% (v/v) glutaraldehyde and 4% (w/v) paraformaldehyde with 10mM sucrose in 50mM PIPES (pH 6.8) for 4h on ice, followed by post-fixation overnight at 4 °C in 1% (w/v) osmium tetroxide (ProSciTech, Qld, Australia) in 50mM PIPES buffer. Tissue was dehydrated in ethanol (10% steps), infiltrated, and embedded in LR White resin. Ultrathin (70nm thick) sections collected on Formvar-coated nickel 1nm slot grids were stained with saturated uranyl acetate and lead citrate and viewed with a JEOL 1200 EX II electron microscope.
Statistical analyses
Treatment effects on cell percentages with wall ingrowth papillae and CMT distribution patterns were analysed by performing paired t-tests to determine the statistical significance of treatments. All image analysis was performed with ImageJ v.1.31 (http://rsb.info.nih.gov/ij). Microtubule angles were measured from confocal images relative to the assumed long axis of a cell using the ‘angle’ tool. Five hundred angles were measured for each replicate (five images per replicate, 100 measurements per image). For CMT arrays, images taken by Olympus FluoView FV1000 CLSM were converted and analysed in FV10-ASW 4.0 viewer software and all converted images were cropped in Adobe® Photoshop CS2 to give an appropriate size.
Results
Microtubules are reorganized during wall ingrowth papillae formation
To investigate whether CMT organization changed during trans-differentiation of cotyledon epidermal cells to a transfer-cell morphology, V. faba cotyledons were cultured for 24h. The cytoplasmic face of the outer periclinal wall of epidermal cells in adaxial peels of the cultured cotyledons was viewed to assess wall ingrowth papillae formation, and peels were immunolabelled to visualize the spatial organization of their CMT arrays.
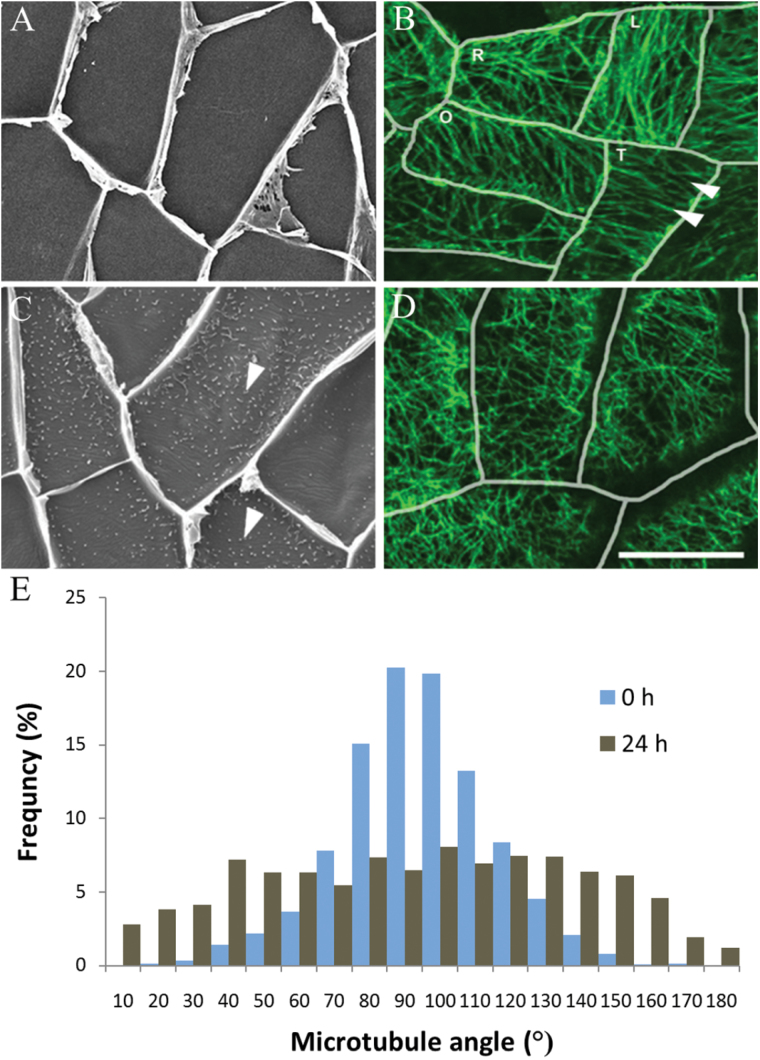
In freshly harvested (t=0h) cotyledons, where wall ingrowth papillae formation had not commenced (Fig. 1A), CMTs in most adaxial epidermal cells were organized into parallel arrays aligned either parallel or transverse to the long axis of each cell or occasionally more obliquely (Fig. 1B). In cotyledons cultured for 24h, cells exhibited extensive numbers of developing wall ingrowth papillae (Fig. 1C) and CMTs in most cells were randomized (Fig. 1D). To quantify this change in CMT organization, the angles of CMTs relative to the long axis of each cell were measured. In epidermal cells of freshly harvested cotyledons (t=0h), the percentage frequency of CMT angle was distributed normally around a modal value perpendicular to the long axis of each cell (Fig. 1E). In contrast, after 24h of cotyledon culture, CMT angle was distributed more evenly across a 180° plane (Fig. 1E), indicating that randomization of CMTs occurred during wall ingrowth papillae formation.
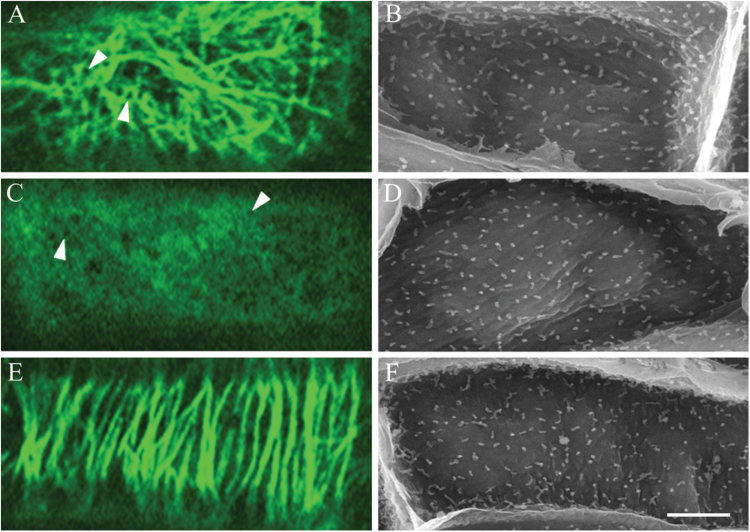
Fig. 1.
Changes in CMT organization during wall ingrowth papillae formation in adaxial epidermal cells of V. faba cotyledons cultured for 24h. (A–D) Adaxial epidermal peels from freshly harvested (0h) (A, B) or cotyledons cultured for 24h (C, D). Wall ingrowth papillae were visualized by viewing the cytoplasmic face of the outer periclinal wall of cells by SEM (A, C) or epidermal peels were immunolabelled with anti-α-tubulin and IgG–Alexa Fluor 488 conjugate to visualize CMT organization by CLSM (B, D). In freshly harvested cotyledons, CMT arrays (B, arrowheads) in their adaxial epidermal cells were mostly aligned in parallel arrays either transverse to the long (L) or short (T) axis of each epidermal cell, or in an oblique pattern to the long axis (O) (see E). In a small number of cells, the CMT array was organized randomly (R). After 24h of cotyledon culture, in which wall ingrowth papillae had been deposited in most cells (C, arrowheads), CMTs were randomly organized (D) (see E). Bar, 20 μm. (E) Angles of CMTs relative to the long axis of the cell expressed as the percentage frequency of total CMT angles measured.
Three distinct CMT arrays are evident during wall ingrowth papillae formation
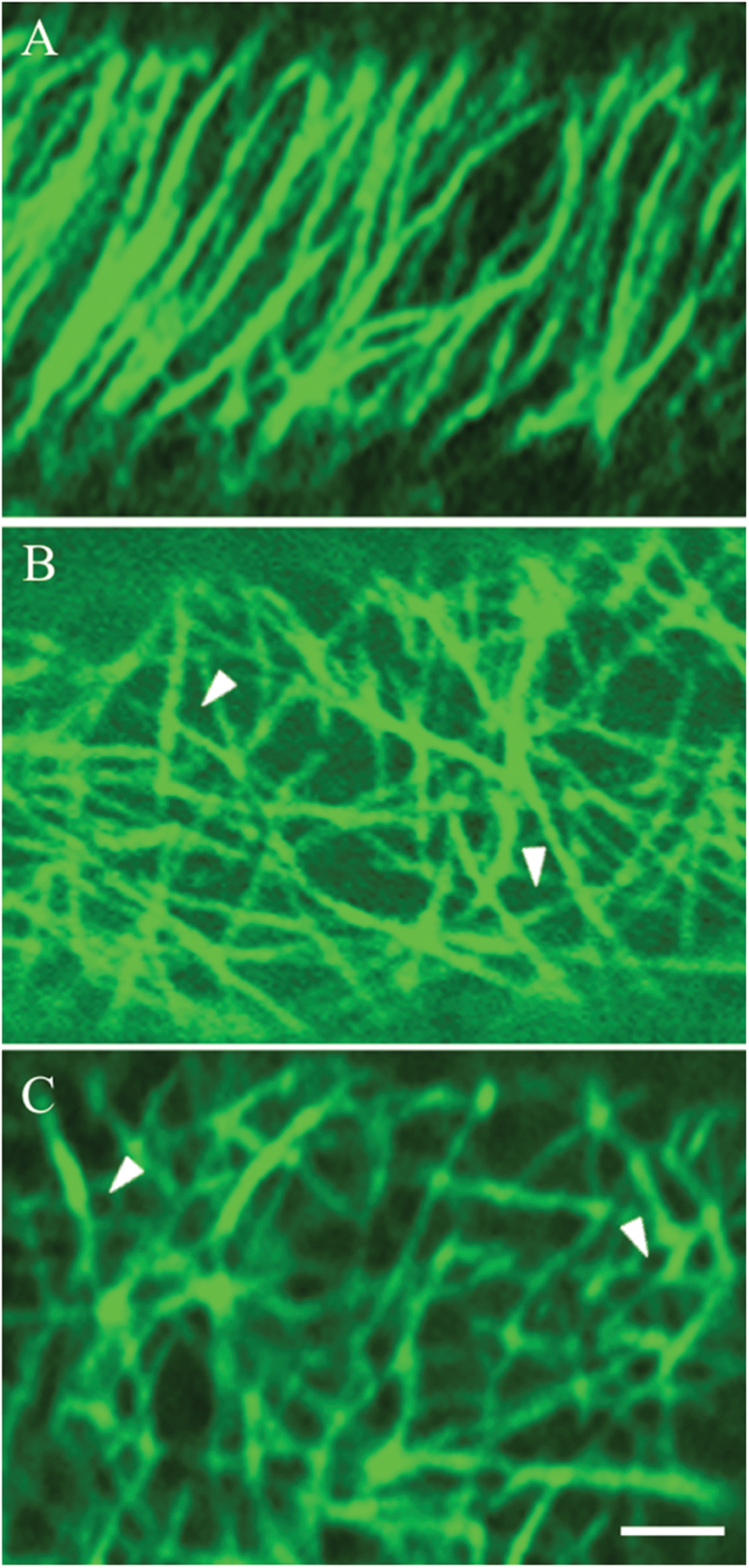
Three different CMT arrays were identified to occur across 24h of cotyledon culture. These have been defined as ‘organized’, ‘randomized’, and ‘randomized with depletion zones’ (Fig. 2). ‘Organized’ arrays are composed of parallel thick CMT bundles characteristic of those found in expanding plant cells (Fig. 2A; see also Deinum and Mulder, 2013). In ‘randomized’ arrays, criss-crossing bundles of CMTs formed polygonal gaps in the CMT array (Fig. 2B). The ‘randomized with depletion zones’ arrays were composed of small circular depletion zones (terminology adopted from Oda et al., 2010; Hardham, 2013). These depletion zones were surrounded by a possible combination of fine fragmented CMTs and tubulin monomers sometimes appearing like a collar (Fig. 2C). The striking difference between the last two CMT arrays (Fig. 2B, C) was that the fluorescence signal appeared to surround the circular depletion zones (Fig. 2C), whereas polygonal gaps were formed in the randomized array as a result of more linearly but randomly organized CMTs (Fig. 2B).
Fig. 2.
Confocal images of three categories of CMT arrays observed in adaxial epidermal cells of V. faba cotyledons cultured for 24h. CMT arrays immunolabelled with anti-α-tubulin and IgG–Alexa Fluor 488 conjugate. (A) ‘Organized’: parallel arrays of thick CMT bundles. (B) ‘Randomized’: an array defined by thick, strongly labelled CMT bundles arranged in a random network with distinctive polygonal gaps (arrowheads) in the network. (C) ‘Randomized with depletion zones’: an array composed of circular depletion zones surrounded by a possible combination of fine fragmented CMTs and tubulin monomers sometimes appearing like a collar (arrowheads). Bar, 2.5 μm.
Temporal appearance of the ‘randomized with depletion zones’ CMT array and dimensions of ‘depletion zones’ correlate with those of wall ingrowth papillae
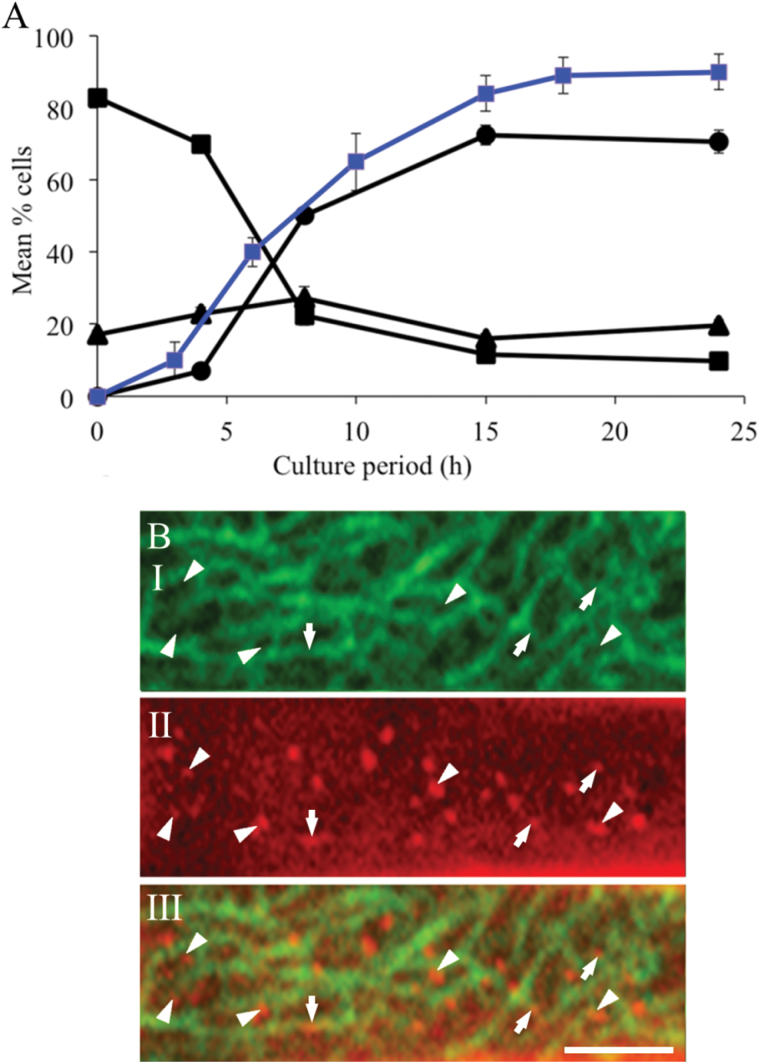
To establish the temporal progression of the three CMT arrays (Fig. 2) in adaxial epidermal cells during wall ingrowth papillae formation, cotyledons were cultured for 0, 4, 8, 15, and 24h and the percentage of epidermal cells displaying each category of CMT array was determined (Fig. 3A). Prior to culture, over 80% of the epidermal cells displayed an ‘organized’ CMT array. By 4h of culture, cells with an ‘organized’ array were reduced to 70% as CMTs became ‘randomized’, and in a small percentage of cells, CMT arrays with ‘randomized with depletion zones’ were observed (Fig. 3A). By 8h of culture, a rapid decline in cells exhibiting ‘organized’ arrays to 20% was mirrored by an increase to 55% in cells displaying the ‘randomized with depletion zones’ CMT array (Fig. 3A). Thereafter, percentages of cells exhibiting the three categories of CMT arrays reached steady-state levels by 15h of cotyledon culture (Fig. 3A). Most significantly, the temporal appearance of the ‘randomized with depletion zones’ CMT array correlated strongly (r 2>0.95) with that of wall ingrowth papillae (Wardini et al., 2007) across 24h of cotyledon culture (Fig. 3A).
Fig. 3.
Temporal and spatial relationships between categories of CMT arrays and wall ingrowth papillae in adaxial epidermal cells of cultured V. faba cotyledons. (A) Temporal pattern of changes in the percentages of cells exhibiting organized (squares), randomized (triangles), and randomized with depletion zones (circles) CMT arrays across 24h of cotyledon culture. Data are means±SE from four replicate cotyledons with a minimum of 100 cells recorded for each cotyledon. Data from Wardini et al. (2007) on the percentage of epidermal cells with wall ingrowth papillae is superimposed (blue line). (B) Spatial relationship between circular depletion zones in the ‘randomized with depletion zones’ CMT array and wall ingrowth papillae in cells of cotyledons cultured for 24h. (I) CMTs immunolabelled with anti-α-tubulin and IgG–Alexa Fluor 488 conjugate. (II) Cell walls stained with Congo red. (III) Digital overlay of labelling of CMT array and cell wall staining. Circular depletion zones (I) intensely stained wall ingrowth papillae (II) and their co-localization (III) indicated by arrowheads. Arrows indicate shorter wall ingrowth papillae and their overarching CMTs. Bar, 2 μm.
The spatial relationship between the ‘randomized with depletion zones’ CMT array and wall ingrowth papillae was evaluated as follows. Epidermal peels of cotyledons cultured for 24h were immunolabelled to visualize CMT arrays and co-stained with Congo red. The latter stain preferentially binds cellulose (Meloan and Puchtler, 1978), permitting visualization of wall ingrowth papillae (Zhang et al., 2015). Regions of intense Congo red staining (Fig. 3B, panel II) were found to co-localize with depletion zones in the CMT array (Fig. 3B, panel III), and in overlays, these represented 72±5% (n=10 overlays) of the total number of wall ingrowth papillae observed. The remaining 28% of wall ingrowth papillae appeared less strongly stained and were overarched by CMTs. This is consistent with these wall ingrowth papillae being shorter (<300 vs 500nm in length with the focal plane set between 200 and 300nm; see Materials and methods) and not yet extended through the CMT array (Fig. 3B, panel III). Additionally, the diameters of the depletion zones (488±9nm; n=800) were commensurate with those of the Congo red regions (500±19nm; n=800) and the basal regions of wall ingrowth papillae (430±8nm; n=240) measured from transmission electron micrographs. The 14% larger diameters of depletion zones and Congo red regions compared with diameters of wall ingrowth papillae probably resulted from a combination of tissue shrinkage during preparation for transmission electron microscopy and flaring of the fluorescent signal seen in confocal images. Collectively, these observations led to the hypothesis that wall ingrowth papillae formation is regulated by CMT network reorganization into a randomized array characterized by depletion zones within which the wall ingrowth papillae are located (Fig. 3B).
Depolymerizing or stabilizing CMT arrays does not alter ingrowth wall papillae formation
The causality of the relationship between CMT reorganization and wall ingrowth papillae formation was tested by culturing cotyledons in the presence or absence of 20 µM oryzalin to depolymerize (Bajer and Molè-Bajer, 1986) or 5 µM taxol to stabilize (Schiff and Horwitz, 1980) the CMTs.
Effects over 24h of cotyledon culture
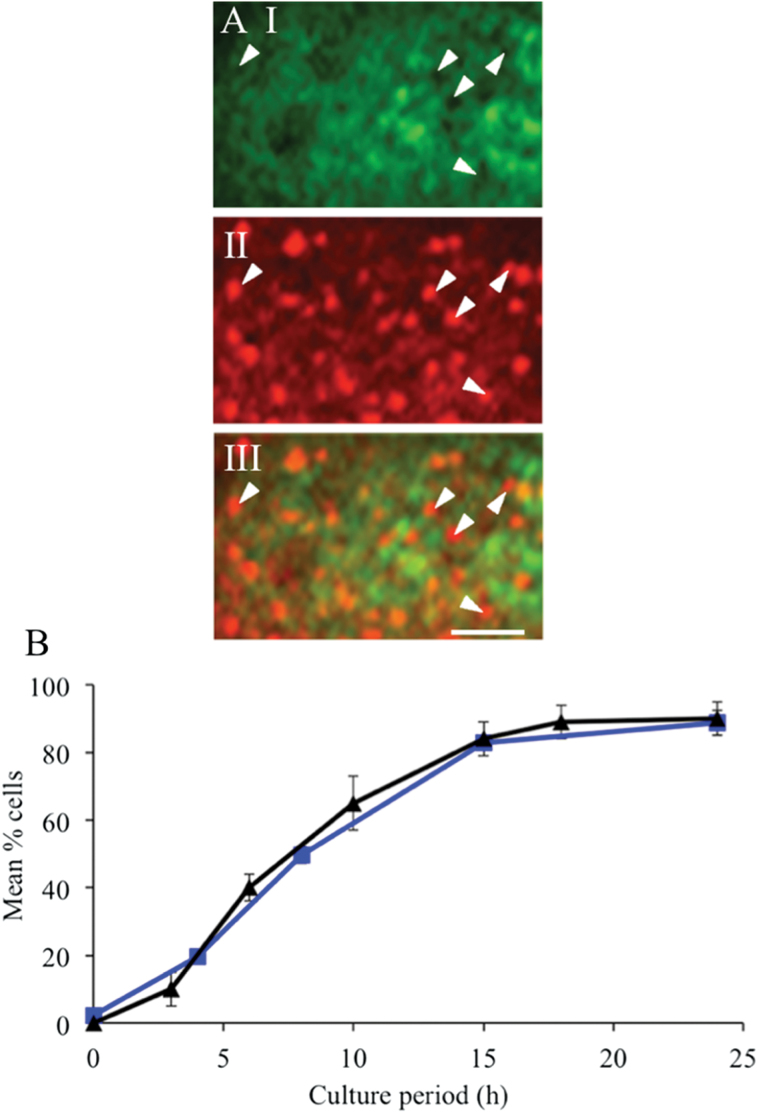
After culturing cotyledons at 4 °C for 4h in the presence of oryzalin (t=0h), the CMT arrays were disrupted completely in over 80% of epidermal cells and ‘partially disrupted’ in the remaining cells (Supplementary Fig. S1, available at JXB online). After transferring oryzalin-treated cotyledons to 26 °C for 4h in the continued presence of oryzalin, CMTs were completely disrupted in 95% of cells (Supplementary Fig. S2E, F, available at JXB online) and remained so throughout the 24h culture period (Supplementary Fig. S2H, I, K, L, N, O). Thus, throughout the phase of wall ingrowth papillae deposition, the CMTs were disrupted. However, the anti-tubulin fluorescence appeared patchy, indicating that tubulin was aggregated (Figs 4A, panel I, and 5C, and Supplementary Fig. S2F, I, L, O).
Fig. 4.
Effect of oryzalin on the spatiotemporal relationships of CMT arrays with wall ingrowth papillae in adaxial epidermal cells of cultured V. faba cotyledons. (A) Spatial relationship between circular depletion zones in the depolymerized aggregated α-tubulin fluorescence signal and wall ingrowth papillae in cells of cotyledons cultured for 24h. (I) Immunolabelled α-tubulin. (II) Congo red staining. (III) Overlay of the two channels. Circular depletion zones and wall ingrowth papillae are indicated by arrowheads. Bar, 2 μm. (B) Percentage of epidermal cells exhibiting circular ‘depletion zones’ within depolymerized tubulin (squares) and wall ingrowth papillae (triangles) (from Wardini et al., 2007) across 24h of cotyledon culture. Results are means±SE derived from four replicate cotyledons with a minimum of 100 cells scored in each replicate.
Of most interest to this study was the appearance of depletion zones within this patchy fluorescence (Figs 4A, panel I, and 5C and Supplementary Fig. S2I, L, O). These depletion zones were structurally similar, and had equivalent diameters, to those of the ‘randomized with depletion zones’ CMT array in epidermal cells of cotyledons cultured on MS medium alone (475 vs 488nm, respectively) (Fig. 2C; c.f. Fig. 4A, panel I). Co-staining immunolabelled epidermal peels of 24h cultured and oryzalin-treated cotyledons with Congo red established that these depletion zones co-localized with regions of intense Congo red staining (Fig. 4A, panel III). A similar pattern was detected for the depletion zones of cells of 24h cultured control cotyledons (Fig. 4A; c.f. Fig. 3B). Significantly, the temporal appearance of these depletion zones strongly correlated with that of wall ingrowth papillae (Fig. 4B; r 2=0.99) and of the depletion zones of control cotyledons (Fig. 3A; r 2>0.95). Moreover, the percentage of cells of oryzalin-treated cotyledons exhibiting wall ingrowth papillae was equivalent to that of the controls (Fig. 4B), as were wall ingrowth papillae densities (Table 1) and their morphology (Fig. 5D vs B). An interesting observation for which currently we have no explanation is that in 13.5±1.6% of cells of oryzalin-treated cotyledons, 1.5% of wall ingrowth papillae in these cells exhibited an aberrant organization. This small percentage of aberrant wall ingrowth papillae was apparent after 24h of culture and persisted in cotyledons cultured for 3 and 6 d (Supplementary Fig. S3, available at JXB online)
Table 1.
Effect of CMT polymerization state, presence of cytosolic Ca2+ plumes, and cellulose biosynthesis on formation of depletion zones in CMT arrays and wall ingrowth papillae in adaxial epidermal cells of V. faba cotyledons
Cotyledons were cultured for 24h in liquid MS medium in the absence/presence of oryzalin, taxol, BAPTA, DCB, and Eosin Yellow. Data are means±SE of 400 cells from four replicate cotyledons exposed to each treatment. For wall ingrowth papillae (WIP) density, data are means±SE of 100 cells from four replicate cotyledons.
| Treatment | Cells with depletion zones (%) | Cells with WIPs | Density of WIPs per 100 μm2 |
|---|---|---|---|
| Control | 81.2±3.3 | 87.9±4.1 | 35.2±2.6 |
| Oryzalin (20 μM) | 82.7±3.4 | 86.3±6.8 | 39.4±4.2 |
| Taxol (5 μM) | 2.5±0.3 | 88.0±4.7 | 35.9±1.8 |
| BAPTA (600 μM) | 16.1±2.6 | 24.3±2.0 | 3.3±0.5 |
| DCB (5 μM) | 78.1±1.0 | 19.5±2.3 | 3.5±0.6 |
| Eosin Yellow (0.5 μM) | 1.3±0.4 | 17.6±1.4 | 1.7±0.7 |
Fig. 5.
Effect of oryzalin and taxol on CMT array and wall ingrowth papillae formation in adaxial epidermal cells of cultured V. faba cotyledons. Confocal images of CMTs immunolabelled with anti-α-tubulin and IgG–Alexa Fluor 488 conjugate (A, C, E) and SEM images of the cytoplasmic face of the outer periclinal wall to visualize wall ingrowth papillae (B, D, F) of epidermal cells of cotyledons cultured for 24h in MS medium alone (A, B) or 24h in the presence of, 20 µM oryzalin (C, D) or 5 µM taxol (E, F). In the presence of oryzalin, CMT arrays were depolymerized and then tubulin aggregated and exhibited depletion zones (arrowheads in C). In contrast, CMT arrays were stabilized in the presence of taxol (E) retaining an alignment comparable to those located in freshly harvested cotyledons (c.f. Fig. 2A). Bar, 10 µm.
CMTs in most trans-differentiating epidermal cells of cotyledons cultured on MS medium containing taxol were maintained as ‘organized’ arrays (Fig. 5E) with only 2.5% of cells exhibiting a ‘randomized with depletion zones’ array (Table 1). These data indicated that randomization of CMT arrays during the 24h culture period was slowed significantly, if not stalled, in taxol-treated cotyledons. However, control and taxol-treated cotyledons exhibited identical percentages of cells with wall ingrowth papillae (Table 1), as well as comparable wall ingrowth papillae densities (Table 1) and morphologies (Fig. 5F vs B). These results demonstrated that stabilization of CMT arrays did not affect the formation of wall ingrowth papillae.
Effects over 3 and 6 d of cotyledon culture
Wall ingrowth deposition in cotyledon epidermal cells involves sequential deposition of wall ingrowth papillae that branch and fuse to form a fenestrated layer of wall material. This sequence of deposition is repeated to form a multiple-layered ingrowth wall (Talbot et al., 2001). To test if CMTs were required for the later stages of ingrowth wall formation, cotyledons were cultured for 4h at 4 °C in the absence/presence of either oryzalin or taxol, before culturing for 3 or 6 d at 26 °C in the absence/presence of the drug.
For cotyledons cultured on MS medium alone, the CMT array in over 95% of adaxial epidermal cells was randomized at 3 d (Table 2) and visualized as a diffuse fluorescence (Fig. 6A). Within this fluorescence, depletion zones equivalent to those of 24h cultured cotyledons (Fig. 5A) were evident, consistent with new papillae arising from the most recently formed fenestrated layer of the ingrowth wall (Fig. 6B). As the number of fenestrated layers increased from two to five with the longer (6 d) culture period (Table 2), the intensity of the fluorescence decreased (data not shown). In the presence of oryzalin, CMTs were completely depolymerized, and depletion zones, similar to those of control cells, were evident in the diffuse tubulin fluorescence by 3 d of culture (Fig. 6C) and remained unchanged over the longer period (data not shown). In contrast, in the majority of taxol-treated cells, thick bundles of CMTs were aligned perpendicular to the longitudinal axis of the epidermal cells (Fig. 6E) with this CMT organization being retained at 6 d (data not shown). For both oryzalin- and taxol-treated cotyledons, the percentages of cells with a fenestrated wall labyrinth (Table 2, Fig. 6D, F vs B), and the number of fenestrated layers forming the labyrinth (Table 2), were identical to that of the control at both 3 and 6 d of cotyledon culture. This finding indicated that polymerization or stabilization of CMT arrays had no discernible effect on fenestration of the ingrowth wall.
Table 2.
Long-term effect of oryzalin or taxol on CMT organization and ingrowth wall formation in adaxial epidermal cells of V. faba cotyledons
Cotyledons were cultured for 3 or 6 d on MS medium in the absence/presence of these pharmacological reagents. Data are means±SE of 400 cells from four replicate cotyledons exposed to each treatment. For layers of fenestrated wall ingrowth (WI), data are means±SE of 100 cells from four replicate cotyledons.
| Culture time | Treatment | Cells with depletion zones (%) | Cells with fenestrated WI (%) | Number of WI layers |
|---|---|---|---|---|
| 3 d | Control | 97.2±2.4 | 83.8±5.4 | 2.8±0.1 |
| Oryzalin (20 μM) | 98.5±1.1 | 81.4±2.1 | 2.8±0.2 | |
| Taxol (5 μM) | 15.4±1.2 | 81.1±2.2 | 2.7±0.1 | |
| 6 d | Control | 97.0±0.7 | 95.7±1.6 | 4.7±0.2 |
| Oryzalin (20 μM) | 99.1±1.2 | 96.2±2.2 | 4.9±0.3 | |
| Taxol (5 μM) | 36.7±1.4 | 97.7±1.3 | 4.6±0.2 |
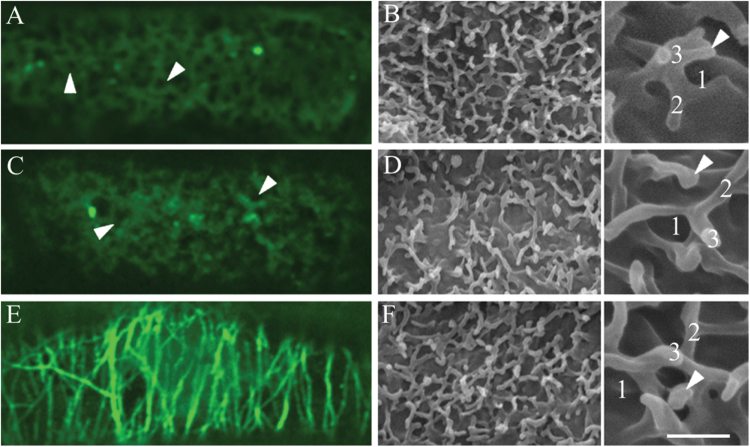
Fig. 6.
Effect of prolonged depolymerization or stabilization of CMT arrays on the formation of the ingrowth wall labyrinth in adaxial epidermal cells of V. faba cotyledons. Confocal images of immunolabelling with anti-α-tubulin and IgG–Alexa Fluor 488 conjugate (A, C, E) and SEM images of the cytoplasmic face of the ingrowth wall labyrinth (B, D, F) of epidermal cells of cotyledons cultured for 3 d on MS medium alone (A, B) or in the presence of 20 µM oryzalin (C, D) or 5 µM taxol (E, F). Note that in the control (A), the fluorescence is diffuse and there are some depletion zones (arrowheads). In oryzalin-treated cells (C), the tubulin fluorescence appeared to be aggregated in places, with depletion zones (arrowheads) visible, while in cells of the taxol-treated cotyledons (E), thick microtubule bundles were aligned perpendicular to their longitudinal axis. The ingrowth walls in (B), (D), and (F) were identical, being comprised of three fenestrated layers (number of layers labelled in inserts of B, D, F) with wall ingrowth papillae (arrowheads) arising from the most recently deposited fenestrated layer. Bar, 10 µm (A–F); 2 µm, inserts.
Cytosolic Ca2+ regulates the spatiotemporal relationship between CMT depletion zones and deposition of wall ingrowth papillae
While the above results precluded a regulatory role for CMTs in wall ingrowth papillae deposition, CMT arrays were reorganized and depletion zones appeared in the CMT network during the first 24h of trans-differentiation of epidermal cells to a transfer-cell morphology (Figs 1 and 2). Furthermore, there was a correlation between the temporal appearance (Figs 3A and 4B), spatial localization (Figs. 3B and 4A), and the dimensions of these depletion zones and those of wall ingrowth papillae in both control and oryzalin-treated cotyledons. Given this close association, we explored the inter-relationship between appearance of the depletion zones within the CMT arrays, deposition of wall ingrowth papillae, and cytosolic Ca2+ plumes that define loci for wall ingrowth papillae deposition (Zhang et al., 2015). Cotyledons were cultured for 24h in the presence of 600 µM 1,2-bis(o-aminophenoxy)ethane- N,N,N’,N’-tetraacetic acid (BAPTA), a Ca2+ chelator (Oiki et al., 1994), 5 µM 2,6-dichlorobenzonitrile (DCB), a cellulose biosynthesis inhibitor (Montezinos and Delmer, 1980), or 0.5 µM Eosin Yellow, a Ca2+-ATPase inhibitor (De Michelis et al., 1993).
In the presence of BAPTA, which inhibits the formation of cytosolic Ca2+ plumes and wall ingrowth papillae (Fig. 7A; Zhang et al., 2015), no depletion zones occurred in the randomized CMT array (Fig. 7B) in 84 % of the epidermal cells (Table 1). This percentage equated to that of cells without wall ingrowth papillae (Table 1). In contrast, in the presence of DCB, depletion zones were evident in the randomized CMT array (Fig. 7D) in 78% of cells (Table 1). Assessing wall ingrowth papillae formation by SEM (Fig. 7C) established that deposition of wall ingrowth papillae had been substantially inhibited by DCB (Table 1; see also Talbot et al. 2007a ). However, generation of the cytosolic Ca2+ signal (Zhang et al., 2015) was unaffected by DCB (Supplementary Fig. S4B vs A, available at JXB online). These data suggested that the cytosolic Ca2+ plumes, and not deposition of wall ingrowth papillae, were responsible for forming the depletion zones within the CMT arrays. This conclusion was tested further under conditions where the cytosolic Ca2+ plumes were dissipated but elevated cytosolic concentrations were retained by culturing cotyledons in the presence of Eosin Yellow (Zhang et al., 2015). While CMT arrays were partially depolymerized, no depletion zones were evident in these arrays (Fig. 7F) and the percentage of cells with wall ingrowth papillae was significantly decreased (Fig. 7E, Table 1). Collectively, these findings were consistent with the cytosolic Ca2+ plumes being responsible for the observed depletion zones that characterized the ‘randomized with depletion zones’ array.
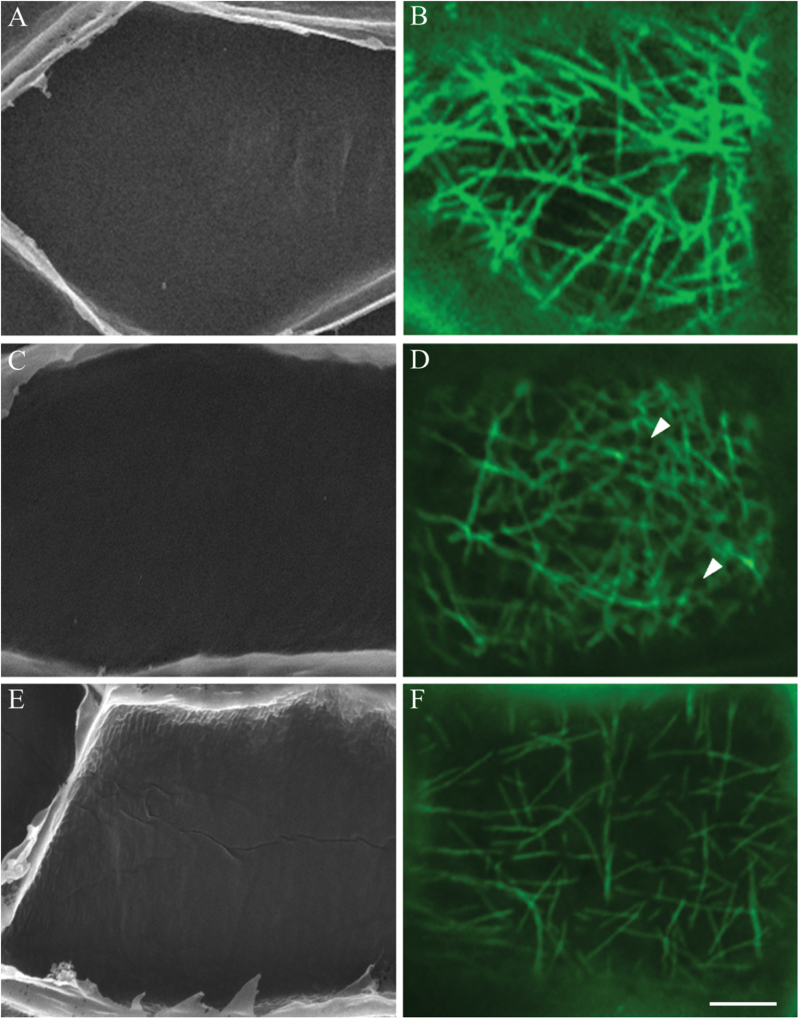
Fig. 7.
Effect of manipulating wall ingrowth papillae deposition and cytosolic Ca2+ on reorganization of CMT arrays in adaxial epidermal cells of cultured V. faba cotyledons. Representative SEM images of the cytoplasmic face of the outer periclinal wall (A, C, E) and confocal images of CMTs immunolabelled with anti-α-tubulin and IgG–Alexa Fluor 488 conjugate (B, D, F) of cells of cotyledons cultured for 24h in the presence of 600 µM of the Ca2+ chelator BAPTA (A, B), 5 µM of the cellulose biosynthesis inhibitor DCB (C, D) or 0.5 µM of the Ca2+ ATPase inhibitor Eosin Yellow (E, F). In the presence of BAPTA, wall ingrowth papillae formation was inhibited (A) and the CMT arrays, while randomized, were devoid of localized depletion zones (B). In contrast, when wall ingrowth papillae formation was inhibited in the presence of DCB (C), randomized CMT arrays exhibited circular depletion zones (D, arrowheads). Dissipation of localized Ca2+ plumes with Eosin Yellow resulted in a partially, but uniformly, depleted CMT array (F) and inhibition of wall ingrowth papillae formation (E). Bar, 5 µm.
Discussion
CMT reorganization during ingrowth wall formation results in a randomized CMT array with depletion zones
When V. faba cotyledons are placed in culture, their adaxial epidermal cells are induced spontaneously to undergo trans-differentiation to a transfer-cell morphology. A cohort of the epidermal cells immediately enters a phase of division (Dibley et al., 2009). During this developmental phase, the parallel-aligned cortical arrays of microtubules present in the precursor epidermal cells were rapidly reorganized into a randomized CMT array (Figs 2B and 3A). In contrast to trans-differentiating epidermal cells, microtubule randomization in nematode-induced giant transfer cells was confined to those located in the cytoplasm, with CMTs remaining dense and filamentous (Kyndt et al., 2013). Remodelling of cytoplasmic microtubules of giant cells is mediated by MAP65-3 (Caillaud et al., 2008) and is associated with forming mitotic spindles and phragmoplasts, supporting a partial cytokinesis (Kyndt et al., 2013).
As trans-differentiation of the epidermal cells progressed, circular depletion zones devoid of, but surrounded by, a possible combination of fine fragmented CMTs and tubulin monomers appeared within the randomized CMT arrays (Figs 1D and 2C), and by 6 d of culture, the tubulin fluorescence was diffuse (Fig. 6A). Depletion of CMT networks commonly occurs as cell wall deposition is completed (Sugimoto et al., 2000). However, in the trans-differentiating epidermal cells, wall deposition continued for at least 6 d (Table 1), in the absence of recognizable microtubules (Fig. 6A). Of greater interest was a temporal increase in the proportion of trans-differentiating epidermal cells exhibiting circular depletion zones within their randomized CMT arrays (Fig. 3A). Microtubule-depletion zones have been observed in other physiological contexts. These include remodelled CMT arrays of cells developing into tracheary elements (Oda et al., 2010) and as part of the defence response to invading pathogenic fungi or oomycetes. In the latter case, microtubule-depleted zones form in host cells immediately beneath the hyphal/appressorial contact sites (Hardham, 2013). Significantly, in both these systems the size of the depletion zone is consistent with the structure it defines. In differentiating tracheary elements, depletion zones and pits are ~5–10 µm in diameter (Oda et al., 2010) while depletion zones of epidermal cells of Arabidopsis cotyledons resulting from mechanical stimulation that mimics fungal infection are 20 µm in diameter (Hardham et al., 2008).
The temporal appearance of microtubule-depletion zones in CMT arrays of the trans-differentiating epidermal cells correlated with that exhibited by wall ingrowth papillae deposition (Fig. 3A). This finding, together with their comparable dimensions and most significantly their spatial inter-relationship (Fig. 3B), suggested that, during their construction, each wall ingrowth papilla extends into the cytoplasm through a microtubule-depletion zone. This relationship between microtubule-depletion zones and localized cell wall deposition differs from that reported for differentiating tracheary elements. In tracheary elements, the positioning of ‘pits’ in the secondary wall is due to exclusion of cellulose synthases by the microtubule depolymerizing enzyme MIDD1 (Oda et al., 2010), which is guided to the cell wall by GTP-bound ROP11 (Oda and Fukuda, 2012, 2013a , b ). Greater functional homology of the transfer-cell microtubule-depletion zones is shared with the defence response of plant cells to pathogenic fungi/oomycetes. Here, the pathogen-targeted cell deposits a localized wall papilla within the microtubule-depletion zone that impedes ingress of the pathogen into the host cytoplasm (Hardham, 2013).
CMT array reorganization does not regulate wall ingrowth papillae deposition
Our results showed that, although there is a close spatiotemporal inter-relationship between cortical microtubules and wall ingrowth papillae, CMT arrays exerted no regulatory influence over either the pattern of papillae initiation (Table 1) or their morphology (Fig. 5D, F). This was demonstrated by depolymerizing or stabilizing microtubules by exposure of the trans-differentiating epidermal cells to oryzalin or taxol, respectively. Similarly, prolonged exposure to these drugs for up to 6 d did not affect deposition of the ingrowth wall labyrinth (Table 2, Fig. 6D, F). These responses differed from those reported for nematode giant cells. In giant cells, microtubule depolymerization with oryzalin reduced giant cell size, and stabilization with taxol slowed rates of giant cell initiation (de Almeida Engler et al., 2004). These latter responses appeared to result from microtubule depolymerization/stabilization blocking nuclear division (de Almeida Engler et al., 2004; Caillaud et al., 2008). The absence of these responses in epidermal cells trans-differentiating to a transfer-cell morphology suggests that cell division exerts minimal influence over developmental events leading to deposition of wall ingrowth papillae. Similarly, construction of wall papillae to oppose invasion by pathogenic fungi/oomycetes was unaffected by a depolymerized or stabilized CMT array (Hardham, 2013).
Construction of flange wall ingrowths also exhibits a close relationship with microtubules whereby parallel arrays of microtubule bundles and cellulose microfibrils co-localize in xylem nodal transfer cells of Triticum aestivum and basal endosperm transfer cells of Zea mays (Talbot et al., 2007b ) in a pattern comparable to that found for microtubule-regulated secondary wall deposition (Baskin, 2001). Whether CMTs function in flange ingrowth wall deposition as for secondary wall thickenings remains to be determined.
Cytosolic Ca2+ plumes are responsible for depletion zones within the CMT array
We were surprised to discover that circular depletion zones appeared within the aggregated tubulin resulting from depolymerized CMTs in cells cultured for 24h. The temporal appearance of these zones (Fig. 4B) and their dimensions equated with those of the depletion zones of control epidermal cells. Moreover, their spatial organization mirrored that of wall ingrowth papillae (Fig. 4A) in an identical configuration to that found for control epidermal cells (Fig. 4A; c.f. Fig. 3B). These findings raised the question as to the mechanism responsible for creating the CMT depletion zones. Broad possibilities included a mechanical force, generated by the inward-directed construction of wall ingrowth papillae into the cytosol, displacing the CMT array. Alternatively, the depleted-microtubule zones could be created by a mechanism that causes localized CMT depolymerization as described for developing tracheary elements (Oda and Fukuda, 2013a, b ) or host cells invaded by pathogenic fungi/oomycetes (Hardham, 2013). For this scenario, CMT depolymerization would need to be closely co-ordinated in a spatiotemporal manner with construction of the wall ingrowth papillae. This could be achieved, for example, by a localized force on the plasma membrane imposed by each extending wall ingrowth papillae being transduced by a mechanosensitive mechanism to elicit a signal cascade leading to a localized depolymerization/remodelling of the CMT array (Hardham et al., 2008).
To test these possibilities, construction of wall ingrowth papillae was inhibited by blocking cellulose biosynthesis with DCB (Table 1, Fig. 7C; see also Talbot et al., 2007a ). Under these conditions, microtubule-depletion zones still formed in the CMT network (Fig. 7D). This finding clearly dispels the concept that microtubule-depletion zones were formed as a result of an imposed mechanical force generated by the inward extension of developing wall ingrowth papillae displacing microtubules or on the plasma membrane to initiate a mechanosensitive signal cascade (Hardham, 2013). The possibility that wall ingrowth papillae deposition depended upon creation of localized zones of microtubule depolymerization, as found for formation of pits in differentiating tracheary elements (Oda et al., 2010; Oda and Fukuda, 2012, 2013a , b ), was also excluded as depolymerization of the CMT network by oryzalin (Fig. 5C) did not affect construction or patterning of wall ingrowth papillae (Table 1, Fig. 5D).
Plumes of cytosolic Ca2+ determine the loci at which wall ingrowth papillae are constructed in trans-differentiating epidermal cells (Zhang et al., 2015). This raised the possibility that cytosolic Ca2+ plumes could provide a physiochemical environment to post-translationally regulate the catalytic activity of a microtubule-associated protein or proteins that function(s) to destabilize microtubules. In this context, we found that formation of the localized microtubule-depletion zones in epidermal cells was a Ca2+-dependent phenomenon, as evidenced by their appearance in DCB-treated cells being dependent on the presence of plumes of elevated cytosolic Ca2+ (Zhang et al., 2015; see Fig. 7D vs B, Suppementary Fig. S3 and Table 1). Moreover, consistent with this conclusion was the observed loss of microtubule-depletion zones (Fig. 7F) under conditions where an elevated cytosolic Ca2+ concentration was sustained but the cytosolic Ca2+ plumes were dissipated by inhibiting Ca2+ efflux from the cytosol by an Eosin Yellow block of Ca2+-ATPase activity (Zhang et al., 2015). Interestingly, this treatment resulted in a less dense CMT array composed of a randomly orientated meshwork of fine microtubules suggestive of partial microtubule depolymerization (Fig. 7F). The identity(ies) of the proposed Ca2+-dependent microtubule-destabilizing protein or proteins are not known. In other systems, microtubule-destabilizing protein25 (MDP25) has been shown to bind to and depolymerize microtubules following its Ca2+-dependent dissociation from the plasma membrane and release into the cytosol in senescing leaves (Keech et al., 2010), elongating hypocotyls (Li et al., 2011) and pollen tubes (Qin et al., 2014). However, none of these examples describe formation of localized microtubule-depletion zones. This would rely on a mechanism to constrain the lateral spread of MDP25 through the cytosol as described for the formation of microtubule-depletion zones in differentiating tracheary elements (Oda and Fukuda, 2013b ). To the best of our knowledge, localized depolymerization of CMTs by elevated cytosolic Ca2+ to form depletion zones within CMT arrays has not been reported previously.
Possible role(s) of the microtubule-depletion zones in trans-differentiating transfer cells
Our findings raise the question of whether microtubule-depletion zones serve roles other than regulating patterns of cell wall deposition as found for differentiating tracheary elements (Oda et al., 2010; Oda and Fukuda, 2012, 2013a , b ). A hint that this may be the case is derived from the discovery that certain species-specific host/pathogen combinations, by blocking microtubule depolymerization/reorientation, lower resistance to pathogen invasion despite wall papillae forming beneath the pathogen contact site (Hardham, 2013).
One obvious possibility for transfer-cell development is that co-localization of microtubule-depletion zones with extending wall ingrowth papillae provides an unimpeded route for the papillae to grow through the CMT array into the cytosol. If this were not the case, the extending papillae, with centres of 1.4 µm and lengths of 500nm (Zhang et al., 2015), could displace the CMT array 500nm into the cytoplasm away from contact with most of the plasma membrane lining the outer periclinal ingrowth wall. Avoiding this outcome by extension through depletion zones provides an uninterrupted contact for the CMT array with the plasma membrane lining the shanks of wall ingrowth papillae as well as cell wall regions between adjacent papillae. Organized in this way, the CMT array could play a key role in positioning transporters and thus facilitating sym-/apoplasmic exchange of nutrients and defence molecules across the plasma membrane abutting the ingrowth wall. Both of these phenomena represent core functions of transfer cells (Offler et al., 2003).
As suggested for deposition of a wall papillae plug beneath pathogen contact sites (Hardham, 2013), we conclude that CMTs and cellulose synthase complexes are uncoupled (Paradez et al., 2006) at the tips of developing wall ingrowth papillae. However, in contrast to other uncoupled systems such as tip growth (Mendrinna and Persson, 2015) the cellulose synthase complexes are constrained to the tips of developing wall ingrowths as they extrude whorls of cellulose microfibrils arranged perpendicular to the underlying uniform wall (Talbot et al., 2007a ). The mechanism constraining these cellulose synthase complexes is currently unknown but is likely to be a Ca2+-dependent process as shown by the plumes of elevated cytosolic calcium being essential for wall ingrowth formation (Zhang et al., 2015).
Supplementary data
Supplementary data are available at JXB online
Supplementary Fig. S1. Effect of the MT-disrupting drug oryzalin on CMT arrays in adaxial epidermal cells of cultured V. faba cotyledons.
Supplementary Fig. S2. Effect of the MT-disrupting drug oryzalin on CMT organization in adaxial epidermal cells of cultured V. faba cotyledons.
Supplementary Fig. S3. Aberrant wall ingrowth papillae in oryzalin-treated epidermal cells of cultured V. faba cotyledons
Supplementary Fig. S4. Effect of the cellulose biosynthesis inhibitor DCB on the generation of a polarized Ca2+ signal in adaxial epidermal cells of cultured V. faba cotyledons.
Acknowledgements
The conscientious efforts of Joseph Enright in ensuring a continuous supply of healthy experimental plant material are appreciated. This work was supported by the Australian Research Council-Discovery Project scheme (DP0664626 to JWP, DWMcC and CEO, and DP130101396 to JWP and CEO). H-MZ acknowledges the support of a UoN RHD scholarship. The authors declare no competing interests.
Glossary
Abbreviations:
- BAPTA
1,2-bis(o-aminophenoxy)ethane- N,N,N’,N’-tetraacetic acid
- BSA
bovine serum albumin
- CLSM
confocal laser-scanning microscope
- CMT
cortical microtubule
- DCB
2,6-dichlorobenzonitrile
- MS
Murishige and Skoog
- OGB-1
Oregon Green BAPTA-1 acetoxymethyl ester
- PBS
phosphate buffered saline
- PIPES
piperazine-N,N′-bis (2-ethanesulfonic acid)
- SE
standard error
- SEM
scanning electron microscope
- TEM
transmission electron microscopy.
References
- Akkerman M, Franssen-Verheijen MA, Immerzeel P, Hollander LN, Schel JH, Emons AM. 2012. Texture of cellulose microfibrils of root hair cell walls of Arabidopsis thaliana, Medicago truncatula, and Vicia sativa . Journal of Microscopy 247, 60–67. [DOI] [PubMed] [Google Scholar]
- Andriunas FA, Zhang HM, Xia X, Patrick JW, Offler CE. 2013. Intersection of transfer cells with phloem biology-broad evolutionary trends, function, and induction. Frontiers in Plant Science 4, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajer AS, Molè-Bajer J. 1986. Reorganization of microtubules in endosperm cells and cell fragments of the higher plant Haemanthus in vivo. Journal of Cell Biology 102, 263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI. 2001. On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma 215, 150–171. [DOI] [PubMed] [Google Scholar]
- Bringmann M, Landrein B, Schudoma C, Hamant O, Hauser MT, Persson S. 2012. Cracking the elusive alignment hypothesis: the microtubule-cellulose synthase nexus unraveled. Trends in Plant Science 17, 666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulbert MW, Offler CE, McCurdy DW. 1998. Polarized microtubule deposition coincides with wall ingrowth formation in transfer cells of Vicia faba L. cotyledons. Protoplasma 201, 8–16. [Google Scholar]
- Caillaud MC, Abad P, Favery B. 2008. Cytoskeleton reorganization, a key process in root-knot nematode-induced giant cell ontogenesis. Plant Signaling & Behaviour 3, 816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC. 2012. Progress in the biological synthesis of the plant cell wall: new ideas for improving biomass for bioenergy. Current Opinion in Biotechnology 23, 330–337. [DOI] [PubMed] [Google Scholar]
- de Almeida Engler J, Van Poucke K, Karimi M, De Groodt R, Gheysen G, Engler G, Gheysen G. 2004. Dynamic cytoskeleton rearrangements in giant cells and syncytia of nematode-infected roots. The Plant Journal 38, 12–26. [DOI] [PubMed] [Google Scholar]
- De Michelis MI, Carnelli A, Rasi-Caldogno F. 1993. The Ca-pump of the plasma membrane of Arabidopsis thaliana: characteristics and sensitivity to fluorescein derivatives. Botanica Acta 106, 20–25. [Google Scholar]
- Deinum EE, Mulder BM. 2013. Modelling the role of microtubules in plant cell morphology. Current Opinion in Plant Biology 16, 688–692. [DOI] [PubMed] [Google Scholar]
- Dibley SJ, Zhou Y, Andriunas FA, Talbot MJ, Offler CE, Patrick JW, McCurdy DW. 2009. Early gene expression programs accompanying trans-differentiation of epidermal cells of Vicia faba cotyledons into transfer cells. New Phytologist . 182, 863–877. [DOI] [PubMed] [Google Scholar]
- Farley SJ, Patrick JW, Offler CE. 2000. Functional transfer cells differentiate in cultured cotyledons of Vicia faba seeds. Protoplasma 214, 102–117. [Google Scholar]
- Green PB. 1962. Mechanism for plant cellular morphogenesis. Science 138, 1404–1405. [DOI] [PubMed] [Google Scholar]
- Hardham AR, Takemoto D, White RG. 2008. Rapid and dynamic subcellular reorganization following mechanical stimulation of Arabidopsis epidermal cells mimics responses to fungal and oomycete attack. BMC Plant Biology 8, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardham AR. 2013. Microtubules and biotic interactions. The Plant Journal 75, 278–289. [DOI] [PubMed] [Google Scholar]
- Himmelspach R, Williamson RE, Wasteneys GO. 2003. Cellulose microfibril alignment recovers from DCB-induced disruption despite microtubule disorganization. The Plant Journal 36, 565–575. [DOI] [PubMed] [Google Scholar]
- Keech O, Pesquet E, Gutierrez L, Ahad A, Bellini C, Smith SM, Gardestrom P. 2010. Leaf senescence is accompanied by an early disruption of the microtubule network in Arabidopsis . Plant Physiology 154, 1710–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Z, Hotta T, Lee YR, Horio T, Liu B. 2010. The γ-tubulin complex protein GCP4 is required for organizing functional microtubule arrays in Arabidopsis thaliana . The Plant Cell 22, 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyndt T, Vieira P, Gheysen G, de Almeida-Engler J. 2013. Nematode feeding sites: unique organs in plant roots. Planta 238, 807–818. [DOI] [PubMed] [Google Scholar]
- Ledbetter MC and Porter KR. 1963. A “microtubule” in plant cell fine structure. Journal of Cell Biology 19, 2239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Li S, Du J, Bashline L, Gu Y. 2013. Cellulose synthase INTERACTIVE3 regulates cellulose biosynthesis in both a microtubule-dependent and microtubule-independent manner in Arabidopsis . The Plant Cell 25, 4912–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang X, Qin T, Zhang Y, Liu X, Sun J, Zhou Y, Zhu L, Zhang Z, Yuan M, Mao T. 2011. MDP25, a novel calcium regulatory protein, mediates hypocotyl cell elongation by destabilizing cortical microtubules in Arabidopsis . The Plant Cell 23, 4411–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy DW, Patrick JW, Offler CE. 2008. Wall ingrowth formation in transfer cells: novel examples of localized wall deposition in plant cells. Current Opinion in Plant Biology 11, 653–661. [DOI] [PubMed] [Google Scholar]
- McKenna JF, Tolmie AF, Runions J. 2014. Across the great divide: the plant cell surface continuum. Current Opinion in Plant Biology 22, 132–140. [DOI] [PubMed] [Google Scholar]
- Meloan SN, Puchtler H. 1978. Demonstration of amyloid with Mesitol WLS-Congo Red: application of a textile auxiliary to histochemistry. Histochemistry 58, 163–166. [DOI] [PubMed] [Google Scholar]
- Mendrinna A, Persson S. 2015. Root hair growth: it’s a one way street. F1000Prime Reports 7, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montezinos D, Delmer DP. 1980. Characterization of inhibitors of cellulose synthesis in cotton fibers. Planta 148, 305–311. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio-essays with tobacco tissue cultures. Physiologia Plantarum 15, 473–497. [Google Scholar]
- Oda Y, Fukuda H. 2012. Initiation of cell wall pattern by a Rho- and microtubule-driven symmetry breaking. Science 337, 1333–1336. [DOI] [PubMed] [Google Scholar]
- Oda Y, Fukuda H. 2013. a Spatial organization of xylem cell walls by ROP GTPases and microtubule-associated proteins. Current Opinion in Plant Biology 16, 743–748. [DOI] [PubMed] [Google Scholar]
- Oda Y, Fukuda H. 2013. b The dynamic interplay of plasma membrane domains and cortical microtubules in secondary cell wall patterning. Frontiers in Plant Science 4, 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Iida Y, Kondo Y, Fukuda H. 2010. Wood cell-wall structure requires local 2D-microtubule disassembly by a novel plasma membrane-anchored protein. Current Biology 20, 1197–1202. [DOI] [PubMed] [Google Scholar]
- Offler CE, Liet E, Sutton EG. 1997. Transfer cell induction in cotyledons of Vicia faba L. Protoplasma 200, 51–64. [Google Scholar]
- Offler CE, McCurdy DW, Patrick JW, Talbot MJ. 2003. Transfer cells: cells specialized for a special purpose. Annual Review of Plant Biology 54, 431–454. [DOI] [PubMed] [Google Scholar]
- Oiki S, Yamamoto T, Okada Y. 1994. A simultaneous evaluation method of purity and apparent stability constant of Ca-chelating agents and selectivity coefficient of Ca-selective electrodes. Cell Calcium 15, 199–208. [DOI] [PubMed] [Google Scholar]
- Paradez A, Wright A, Ehrhardt DW. 2006. Microtubule cortical array organization and plant cell morphogenesis. Current Opinion in Plant Biology 9, 571–578. [DOI] [PubMed] [Google Scholar]
- Qin T, Liu X, Li J, Sun J, Song L, Mao T. 2014. Arabidopsis microtubule-destabilizing protein 25 functions in pollen tube growth by severing actin filaments. The Plant Cell 26, 325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff PB., Horwitz SB. 1980. Taxol stabilizes microtubules in mouse fibroblast cells. Proceedings of the National Academy of Sciences, USA 77, 1561–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SL. 2013. Reorganization of the plant cortical microtubule array. Current Opinion in Plant Biology 16, 693–697. [DOI] [PubMed] [Google Scholar]
- Singh S, Lazzaro MD, Walles B. 1999. Microtubule organization in the differentiating transfer cells of the placenta in Lilium spp. Protoplasma 207, 75–83. [Google Scholar]
- Sugimoto K, Himmelspach R, Williamson RE, Wasteneys GO. 2003. Mutation or drug-dependent microtubule disruption causes radial swelling without altering parallel cellulose microfibril deposition in Arabidopsis root cells. The Plant Cell 15, 1414–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Williamson RE, Wasteneys GO. 2000. New techniques enable comparative analysis of microtubule orientation, wall texture, and growth rate in intact roots of Arabidopsis . Plant Physiology 124, 1493–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot MJ, Franceschi VR, McCurdy DW, Offler CE. 2001. Wall ingrowth architecture in epidermal transfer cells of Vicia faba cotyledons. Protoplasma 215, 191–203. [DOI] [PubMed] [Google Scholar]
- Talbot MJ, Wasteneys GO, McCurdy DW, Offler CE. 2007. b Deposition patterns of cellulose microfibrils in flange wall ingrowths of transfer cells indicate clear parallels with those of secondary wall thickenings. Functional Plant Biology 34, 307–313. [DOI] [PubMed] [Google Scholar]
- Talbot MJ, Wasteneys GO, Offler CE, McCurdy DW. 2007. a Cellulose synthesis is required for deposition of reticulate wall ingrowths in transfer cells. Plant Cell Physiology 48, 147–158. [DOI] [PubMed] [Google Scholar]
- Wardini T, Wang XD, Offler CE, Patrick JW. 2007. Induction of wall ingrowths of transfer cells occurs rapidly and depends upon gene expression in cotyledons of developing Vicia faba seeds. Protoplasma 231, 15–23. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Imtiaz MS, Laver DR, McCurdy DW, Offler CE, van Helden DF, Patrick JW. 2015. Polarized and persistent Ca2+ plumes define loci for wall ingrowth papillae formation in transfer cells. Journal of Experimental Botany 66, 1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Andriunas FA, Offler CE, McCurdy DW, Patrick JW. 2010. An epidermal-specific ethylene signal cascade regulates trans-differentiation of transfer cells in Vicia faba cotyledons. New Phytologist 185, 931–943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.