Highlight
The Rolled and Erect Leaf 1 (REL1) gene is a novel component controlling brassinosteroid signalling-associated leaf morphogenesis and leaf angle in Oryza sativa.
Key words: BiFC, BR, erect leaf, rice, rolled leaf, yeast two hybrid.
Abstract
Leaf morphology, particularly in crop, is one of the most important agronomic traits because it influences the yield through the manipulation of photosynthetic capacity and transpiration. To understand the regulatory mechanism of leaf morphogenesis, an Oryza sativa dominant mutant, rolled and erect leaf 1 (rel1) has been characterized. This mutant has a predominant rolled leaf, increased leaf angle, and reduced plant height phenotype that results in a reduction in grain yield. Electron microscope observations indicated that the leaf incurvations of rel1 dominant mutants result from the alteration of the size and number of bulliform cells. Molecular cloning revealed that the rel1 dominant mutant phenotype is caused by the activation of the REL1 gene, which encodes a novel unknown protein, despite its high degree of conservation among monocot plants. Moreover, the downregulation of the REL1 gene in the rel1 dominant mutant restored the phenotype of this dominant mutant. Alternatively, overexpression of REL1 in wild-type plants induced a phenotype similar to that of the dominant rel1 mutant, indicating that REL1 plays a positive role in leaf rolling and bending. Consistent with the observed rel1 phenotype, the REL1 gene was predominantly expressed in the meristem of various tissues during plant growth and development. Nevertheless, the responsiveness of both rel1 dominant mutants and REL1-overexpressing plants to exogenous brassinosteroid (BR) was reduced. Moreover, transcript levels of BR response genes in the rel1 dominant mutants and REL1-overexpressing lines were significantly altered. Additionally, seven REL1-interacting proteins were also identified from a yeast two-hybrid screen. Taken together, these findings suggest that REL1 regulates leaf morphology, particularly in leaf rolling and bending, through the coordination of BR signalling transduction.
Introduction
In plant, leaf development is a complex process comprising cell division and expansion, axis determination, and tissue differentiation and specification (Moulia, 2000). In terms of Oryza sativa (rice), the leaf is polarized along the adaxial–abaxial axis (Itoh et al., 2005). Moderate leaf rolling can maximize the rice yield through more efficient photosynthesis and reduced transpiration (Lang et al., 2004; Zhang et al., 2009; Zou et al., 2011), as well as by increasing stomatal resistance, reducing water loss, and the resulting erection of the leaf blade (King et al., 1996; Moulia, 2000; Sakamoto et al., 2006). Therefore, how to increase crop productivity and yield through manipulating the adaxial and abaxial cells has become an important issue in agriculture (Zou et al., 2011).
Advances in research suggest that alternation of bulliform cells arranged on the adaxial epidermis of the leaf leads to adaxial or abaxial rolling of mature leaves (Botwright et al., 2005; Fujino et al., 2008). Recent studies demonstrated that hypodermis cells were involved in leaf rolling in higher plants as well (Kadioglu and Terzi, 2007). To date, more than 10 rice leaf rolling–associated genes have been reported to be involved in abaxial and adaxial polarity establishment (Zou et al., 2011). For example, mutation of ROLLED LEAF 9 (RL9)/SHALLOT-LIKE1 caused an extreme rolled leaf phenotype by impairing programmed cell death of abaxial mesophyll cells (Yan et al., 2008; Zhang et al., 2009). Notably, most of the leaf rolling mutants exhibited an abaxially rolled leaf phenotype, such as abaxially curled leaf 1(acl1) and acl2 (Li et al., 2010). However, there are some regulators involved in regulation of the adaxially rolled leaf, such as ADAXIALIZED LEAF1 and Rice outermost cell-specific gene 5 (Hibara et al., 2009; Zou et al., 2011). In addition, other regulators have also been well characterized. For example, Narrow and Rolled Leaves 1 participates in regulating leaf morphology through coordinating the regulation of CONSTITUTIVELY WILTED1/ NARROW LEAF7, RL9, and OsAGO7 (Wu et al., 2010).
Another feature of leaf morphology is the leaf bending at the lamina joint, which results from the unequal elongation that occurs between the adaxial and abaxial cells (Itoh et al., 2005). Accumulating evidence has illustrated that brassinosteroids (BRs) play a pivotal role in leaf bending (Bishop and Yokota, 2001; Hong et al., 2004; Sakamoto et al., 2006; Tanaka et al., 2009; Tong et al., 2009; Tong et al., 2012; Zhang et al., 2012). For example, depleted rice BR receptor (OsBRI1) mutants exhibit a predominant erect leaf phenotype (Yamamuro et al., 2000). Similarly, suppression of OsBZR1 results in the erect leaf phenotype (Hong et al., 2004; Sakamoto et al., 2006; Bai et al., 2007). Intriguingly, LEAF and TILLER ANGLE INCREASED CONTROLLER (LIC) regulate leaf bending through inhibition of the transcription of OsBZR1, by binding to its promoter (Zhang et al., 2012). Dwarf and Low-Tillering (DLT) is another newly identified gene participating in leaf morphology. Tong and associates showed that OsBZR1 mediated leaf morphology through the repression of DLT by binding BR-response element in its promoter (Tong et al., 2009). Further research indicated that OsBIN2 also participated in establishment of leaf morphology through the interaction with DLT (Tong et al., 2012). However, the regulatory mechanism of BR-mediated leaf morphology in rice still needs to be further elucidated.
Here, a novel gene, REL1, has been characterized. REL1 encodes an unknown protein and plays a positive role in leaf rolling and bending. Detailed analyses indicated that leaf rolling of rel1 mutants was caused by the altered profile of bulliform cells. The results also suggest that REL1 may regulate leaf bending by coordinated expression of BR-associated genes. In addition, seven REL1-interacting proteins were identified through a yeast two-hybrid screen.
Materials and methods
Plant materials and growth conditions
Rice cultivar ‘Zhonghua 11’ was used as the wild type. All rice seeds in this study were propagated in the paddy field in Guangzhou, China. For laboratory work, rice plants were grown in a greenhouse under a 16-h-light/8-h-dark cycle at 30°C. No significant differences were observed when rel1 mutants were grown in the greenhouse compared to the paddy field.
Electron microscopy
The young leaf was prepared as a semithin cross-section, and the samples were fixed by 2% (v/v) OsO4 in phosphate buffer, air-pumped for 4–48h, and then transferred to 4°C overnight. The fixed solution was then discarded, and the samples were washed three times with 0.1M phosphate buffer (pH 7.3). Samples were dehydrated in a graded ethanol series and then submerged in glycomethacrylate solution (GMA to 95% ethanol at 1:1) at 4°C overnight, and then transferred to pure GMA at room temperature for 2–3h or 4°C overnight. Finally, the samples were embedded and the corresponding strips spliced for electron microscopy (HITACHI S-3000N electron microscope) and photographed. A paraffin cross-section assay was performed as previously described (Li et al., 2010) with minor modifications. Briefly, 2-week-old leaf samples were fixed with 50% formalin–acetic acid–alcohol solution and then stored at 4°C overnight. Subsequently, the samples were dehydrated in a graded ethanol series and the ethanol then substituted with a graded isoamyl acetate series. Afterwards, the samples were transferred to xylene and then embedded in paraffin. Strips of paraffin slices (2–3 μm) were spread at 42°C on a hot platform overnight. The slices were stained using 1% Toluidine Blue O at 37°C for 5min, and dried at 37°C after washing with water. After removal of paraffin using xylene, the slices were sealed for observation under the microscope (HITACHI S-3000N electron microscope) and photos captured.
Analysis of the T-DNA insertion locus in rel1 mutant
Thermal asymmetric interlaced polymerase chain reaction (PCR) was used to isolate the flanking sequence of T-DNA (Liu and Whittier, 1995). Nested primers of the T-DNA right border and the degenerated primers were TR1, TR2, TR3, and AD2-4. Primers for testing of the T-DNA inserting locus were rel1-39208 and TDNA7777 for the right site and rel1-72833 and TDNA10622 for the left. Sequences of the primers are supplied in Supplementary Table S1.
Plasmid construction and rice transformation
To generate the REL1 antisense construct, a 294bp fragment from the REL1 coding region to the 3′-untranslated region was amplified from rice cultivar ‘Zhonghua 11’ (wild type) cDNA templates using their corresponding primer pairs listed in Supplementary Table S1, and then the fragment was cloned into the binary vector pCAMBIA1301-Ubinos. To produce the REL1 overexpression transgenic plants, the full-length REL1 coding sequences was amplified from cDNA derived from rice ‘Zhonghua 11’ using their corresponding primer pairs listed in the Supplementary Table S1. After confirmation by DNA sequencing, each amplified sequence was cloned into the binary vector pCAMBIA1301-Ubinos for antisense or overexpression of REL1. The final constructs were electroporated into Agrobacterium tumefaciens strain EHA105 for rice.
In respect to the GUS assay, a 2545bp DNA fragment immediately upstream of the start codon of REL1 was amplified by using the corresponding specific primers (see Supplemental Table S1). The resulting REL1:GUS reporter gene was introduced into the wild-type plant by A. tumefaciens-mediated transformation. Transgenic plants were selected on Hygromycin medium and T3 transgenic plants were used to analyse GUS activity. To generate the REL1-green fluorescent protein (GFP) construct to examine subcellular localization, the pCAMBIA1300-35S-REL1-GFP construct was produced by the corresponding primers listed in Supplementary Table S1.
Histological GUS assay
The GUS activity analysis was made by using a standard protocol (Jefferson et al., 1987). Briefly, transgenic plant tissues were incubated in X-Gluc buffer (0.1mol L−1 K2HPO4 (pH 7.0), 0.1mol L−1 KH2PO4 (pH 7.0), 5 mmol L−1 K3Fe (CN)6, 5 mmol L−1 K4Fe(CN)6•3H2O, 0.1% Triton X-100, 20% methanol, 1mg mL−1 X-Gluc) at 37°C for 2 hours. The samples were cleared of chlorophyll by dehydration with ethanol. The stained samples were photographed using a Cannon digital camera and stereoscope (OLYMPUS SZX12).The stained stamen was sliced using resin sections (Leica Historesin) and analysed by light microscopy (OLYMPUS BX51).
BR response assay
The lamina joint assay using the micro-drop method was performed as described previously (Tong et al., 2009) with minor modifications. Briefly, 100nM L−1, 10nM L−1, and 1 µM L−1 of 24-epibrassinolide (epiBL) dissolved in ethanol was respectively spotted onto the top of lamina after 10 days’ germination and 3 days’ growth at 30°C. Images were taken after 3 days’ incubation, and the angles of lamina joint bending were measured.
RNA extraction and quantitative real-time PCR
Total RNA was extracted using the RNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s instructions. The first strand of cDNA was synthesized using TransScript First-Strand cDNA Synthesis SuperMix (TransGen Biotech) and quantitative real-time polymerase chain reaction (qRT-PCR) was performed as previously described (Tong et al., 2009). The relative expression level of a target gene was normalized to that of rice ACTIN1. All primers used in qRT-PCR are listed in Supplementary Table S1.
Confocal microscopy
To identify the subcellular localization of REL1, Agrobacterium strains separately harbouring pCAMBIA1300-35S-REL1-GFP and pt-rb CD3-1000 plasmids were used to transiently co-express REL1 and pt-rb CD3-1000 in the rice protoplast as previously described (Zhang et al., 2011). The subcellular localization of REL1 and pt-rb CD3-1000 was investigated by confocal microscopy systems. Briefly, GFP fluorescence images were taken using 488-nm laser excitation and the emission was collected via a 525-nm filter. The mCherry images of the pt-rb CD3-1000 were taken using a 561-nm laser.
Results
Characterization of the rice rel1 mutants
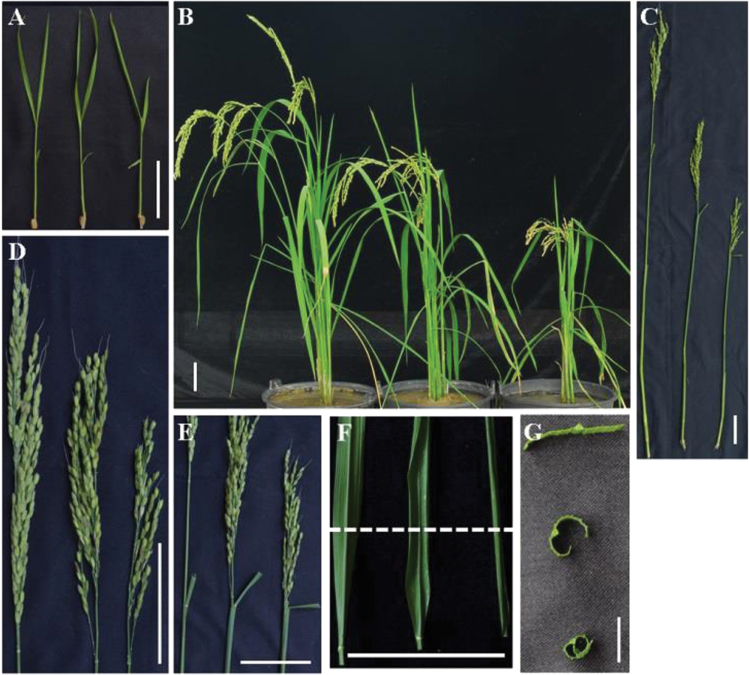
To identify genes regulating leaf morphology or architecture in rice, a genetic screen was performed with T-DNA insertion lines in rice cultivar ‘Zhonghua 11’ (wild-type plant). A mutant with the most obvious defects in leaf morphology from seedling to the mature stage was then isolated. This mutant was named rolled and erect leaf 1 (rel1). The rel1 was a dominant mutant (see details below), which did not show any detectable difference compared to wild type during the early growth stage (Fig. 1A). At later growth stages, the rel1 mutants differed from wild type, showing dwarfism and shortened panicles (Fig. 1B–D). Statistical analyses confirmed that the plant height, panicle length, and numbers of tiller were significantly reduced in rel1 mutants (Supplementary Fig. S1A–C). The most striking phenotype was the leaf adaxial rolling and bending (Fig. 1E–G). To extensively evaluate the leaf rolling, natural and maximum leaf width were measured and the rolling index (natural width versus maximum width) was used as an indicator for leaf rolling. The maximum width of leaves in wild type and the rel1 mutant was not different (Supplementary Fig. S1D). However, the natural width of leaves in rel1 was significantly reduced (Supplementary Fig. S1E), leading to a reduction in rolling index (Supplementary Fig. S1F). These results indicate that the rolling of the rel1 mutant resulted from changes in the natural width of the leaf rather than changes in the maximum width.
Fig. 1.
Phenotype of rel1 mutant. (A) Seedling phenotype of wild type (left), rel1 heterozygote (middle), and homozygote (right). Seeds were germinated at 28°C for 3 days and then transferred to a paddy field; 10-day-old plants are shown. Bar = 10cm. (B) Mature plant phenotype of wild type (left), rel1 heterozygote (middle), and homozygote (right). (C–H) The developmental defects in rel1 were observed in plant height (C), panicle length (D), leaf bending (E), and leaf rolling (F and G). Bar = 10cm. B–F, wild type, rel1 heterozygote, and homozygote from left to right, respectively. G, wild type, rel1 heterozygote, and homozygote from top to bottom, respectively.
As another predominant feature of rel1 mutant, leaf bending was measured according to the previously described method (Tong et al., 2009). Leaf bending of rel1 mutants was approximately 90 degrees, whereas leaf bending in wild type was only 15 degrees (Supplementary Fig. S1G). There were additional defects in the grain of the rel1 mutant, such as in the grain shape and weight (Fig. 1H; Supplementary Fig. S1H–J). Taken together, these results suggest that rel1 mutation caused abnormal development in rice, particularly in the leaf morphology.
Cell structure of rel1 leaf is altered
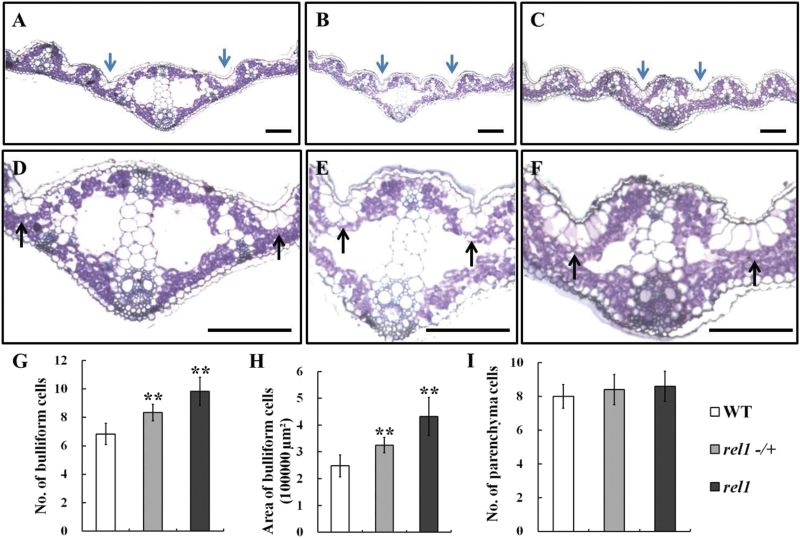
Generally, the adaxial rolling phenotype of mature leaves may be caused by the altered profiles of special kinds of bulky cells on the adaxial epidermis in the leaf, such as the parenchyma and bulliform cells (Botwright et al., 2005; Fujino et al., 2008). Therefore, it was assumed that the rolled leaf phenotype of rel1 might also be related to changes in the special bulky cells. To test this possibility, cross-sections of mature leaves were observed under an electron microscope. There was no difference between parenchyma cells in wild type and the rel1 mutant (Fig. 2A–C), whereas the there were discernible differences in the profiles of bulliform cells among the wild type, rel1 heterozygote (rel1−/+), and rel1 plants (Fig. 2A–C). Enlarged views of the bulliform cells demonstrated significant differences in numbers and size of bulliform cells between wild type and rel1 (Fig. 2D–F). Statistical results indicated that both numbers and size of bulliform cells in rel1 mutant were increased (Fig. 2G, H). Therefore, it was concluded that the leaf adaxial rolling phenotype of rel1 mutants was caused by the altered profiles of bulliform cells.
Fig. 2.
Cell structure is altered in rel1 mutant. (A–C) Electron microscopy of leaf in wild type (A), rel1 heterozygote (B), and rel1 homozygote (C). One-month-old leaves were used in this observation. (D–F) Enlarged view of cell structure of wild type (D), rel1 heterozygote (E), and rel1 homozygote (F). Bulliform cells are indicated by arrows and bar = 1cm (A to F). (G–I) Statistical analysis of bulliform cell number (G), bulliform cell size (H), and parenchyma cell number (I). More than 10 samples were investigated, and the significances are indicated by the asterisks at P < 0.05 (G and I).
Molecular cloning of REL1
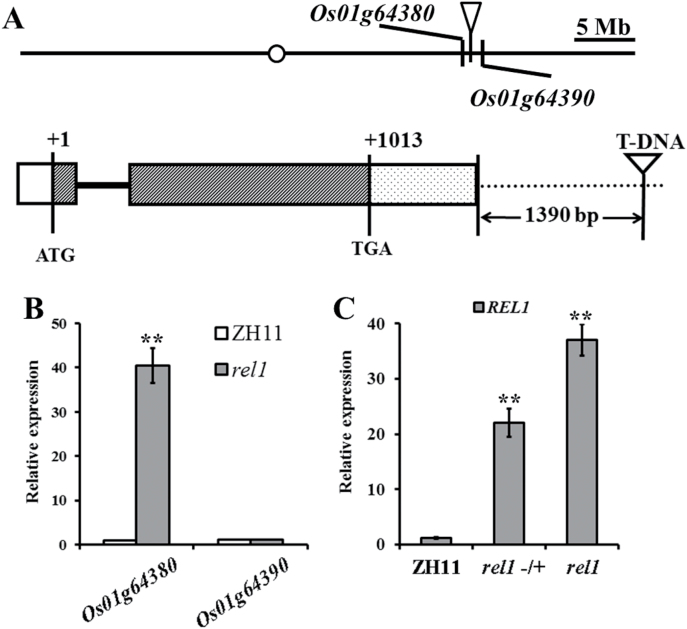
Genetic analysis by backcrossing the rel1 mutant with wild type indicated that the REL1 gene was controlled by a single dominant gene, because the segregation of the rel1-like, medium, and wild-type phenotype was approximate 1:2:1 (χ2 = 0.789). The genomic DNA fragment flanking the T-DNA insertion site in the rel1 mutant was isolated using thermal asymmetric interlaced PCR. A BLAST search with the flanking sequence indicated that the T-DNA insertion site was 1390bp from the stop codon of Os01g64380 and 7743bp from the start codon of Os01g64390 (Fig. 3A), suggesting that the rel1 phenotype might be caused by an alteration of one of these genes. Co-segregation of Os01g64380 or Os01g64390 with rel1-like phenotype was then examined in more than 100 F2 individuals. Using special primers (Supplementary Table S1 and Supplementary Fig. S2), the rel1 phenotype was found to fully co-segregate with Os01g64380. In addition, qRT-PCR analysis demonstrated that the transcript level of Os01g64380 was dramatically elevated in rel1, while that of Os01g64390 remained unchanged, as compared to wild type (Fig. 3B). Moreover, the higher expression level of Os01g64380 was also detected in rel1 heterozygous plants compared to wild type, and the highest expression was shown in the rel1 homozygote (Fig. 3C). These results suggest that the phenotype of rel1 might be caused by the elevated expression of Os01g64380.
Fig. 3.
Molecular cloning of the REL1 gene. (A) Location of T-DNA insertion and the schematic structure of REL1. The putative translation start is referred to as +1. ATG, start codon. TGA, stop codon. 5′ UTR, exon, intron, and 3′ UTR are indicated by the white box, black box, solid line, and dotted box, respectively. (B) Relative expression levels of Os01g64380 and OS01g64390 were determined by qRT-PCR. (C) Relative expression of Os01g64380 was determined by qRT-PCR in wild type, rel1 heterozygote, and rel1 homozygote. Total RNA was extracted from 1-month-old leaves of corresponding plants (B and C). This experiment was repeated more than three times with similar results, and the values are means ± SD of three biological repeats.
According to the annotation in the Rice Genome Annotation Project website (http://rice.plantbiology.msu.edu/), REL1 comprises two exons and one intron and encodes an unknown protein (Fig. 3A). Interestingly, REL1 protein is highly conserved in monocot plants (Supplementary Fig. S3). Therefore, it is suggested that REL1 is a functional unknown protein that is only present in monocot plants.
Knockdown of REL1 in the rel1 mutant restores the wild-type phenotype
To verify the identity of REL1, a REL1 antisense construct driven by the ubiquitin promoter was produced and introduced into rel1 mutants via A. tumefaciens-mediated transformation. Eight independent lines (REL1i) were obtained and all of them displayed a similar phenotype to the wild type. Among the eight transgenic lines, REL1i-2 and RELi-3 were selected for the following studies owing to the most significant repression of REL1 (Fig. 4H). Detailed analyses showed that the plant architecture and leaf morphology of the rel1 mutant phenotype, including plant height and panicle length, as well as leaf bending and rolling, were restored in both REL1i-2 and RELi-3 lines (Fig. 4A–G). Interestingly, knockdown of REL1 in wild type did not show any detectable mutant phenotype (Supplementary Fig. S4A–D). This might be owing to the low expression level of endogenous REL1 in wild type (Supplementary Fig. S4E). Taken together, these results indicate that REL1 was the target gene and played a positive role in leaf rolling and bending in rice.
Fig. 4.
Knockdown of REL1 in rel1 restored the defects of rel1 mutant. (A) Seedling phenotype of wild type (left), REL1i plants (REL1i-2 and REL1i-3, the middle two plants), and rel1 mutant (right). Two-week-old plants are shown. Bar = 10cm. (B) Mature plant phenotype of wild type (left), REL1i plants (REL1i-2 and REL1i-3, the middle two plants), and rel1 mutant (right). (C–) The defect phenotype of rel1 was restored by knockdown of REL1 for plant height (C), panicle height (D), leaf bending (E), and leaf rolling (F and G). Bar = 10cm. C–F, wild type, REL1i-2, REL1i-3, and rel1 mutant from left to right, respectively. G, wild type, REL1i-2, RELIi-3 and rel1from top to bottom, respectively. (H) Relative expression levels of REL1 in wild type, rel1 mutant, and REL1 antisense plants. One-month-old leaves were used for the qRT-PCR analysis, and the values are means ± SD of three biological repeats.
Overexpression of REL1 leads to a rel1-like phenotype
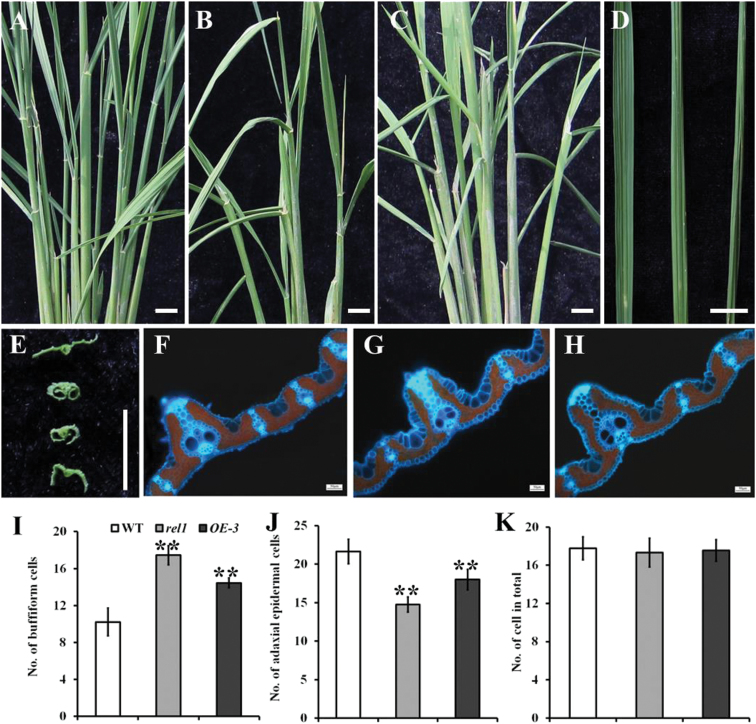
To further investigate whether activating REL1 would induce a rel1-like phenotype, a REL1 overexpression construct driven by the ubiquitin promoter was produced and introduced into wild type via A. tumefaciens-mediated transformation. Six independent transgenic lines (OE) were obtained, and all of them exhibited upregulated trends of REL1 expression (Supplementary Fig. S5A). Because the OE-3 line showed the highest expression among the transgenic lines (Supplementary Fig. S5A), it was selected for the following studies. Consistent with the expression levels, OE-3 plants displayed almost the same phenotype as the rel1 mutant, including fewer tillers (Fig. 5A–C) and rolled leaf phenotype (Fig. 5D, E). Erect leaf and dwarfism were also observed in OE-3 plants (Supplementary Fig. S5B–F). The numbers of bulliform cell were increased in OE-1 in comparison with the wild type (Fig. 5F–5I). Statistical analysis confirmed the significant reduction of the numbers of adaxial epidermal cells in rel1 and OE-3 (Fig. 5J). However, the numbers of parenchyma cells in rel1 and OE-3 leaves were similar to those in wild-type leaves (Fig. 5K). Taken together, it is concluded that activated REL1 induces the rel1-like phenotype and triggers cell division and expansion of bulliform cells.
Fig. 5.
Overexpressing REL1 results in a rel1-like phenotype. (A–C) Leaf morphology of wild type (A), rel1 mutant (B), and the REL1 overexpressing plant (OE-3, C) at tiller stage. Two-month-old plants are shown. (D) Leaf phenotype of wild type (left), rel1 (middle), and OE-3 (right). (E) Rolled leaf phenotype of wild type, rel1, OE-3, and OE-4 from top to bottom. (F–H) The cell structure of leaves in wild type (F), rel1 (G), and OE-3 (H). One-month-old leaves were used in this experiment. (I–K) Statistical analysis of bulliform cell number (I), bulliform cell size (J), and parenchyma cell number (K) in wild type, rel1, and OE-3. More than 10 samples were investigated, and the significances are indicated by the asterisks with P < 0.05 (I and J). Bar = 10cm (A to E).
Expression pattern of REL1
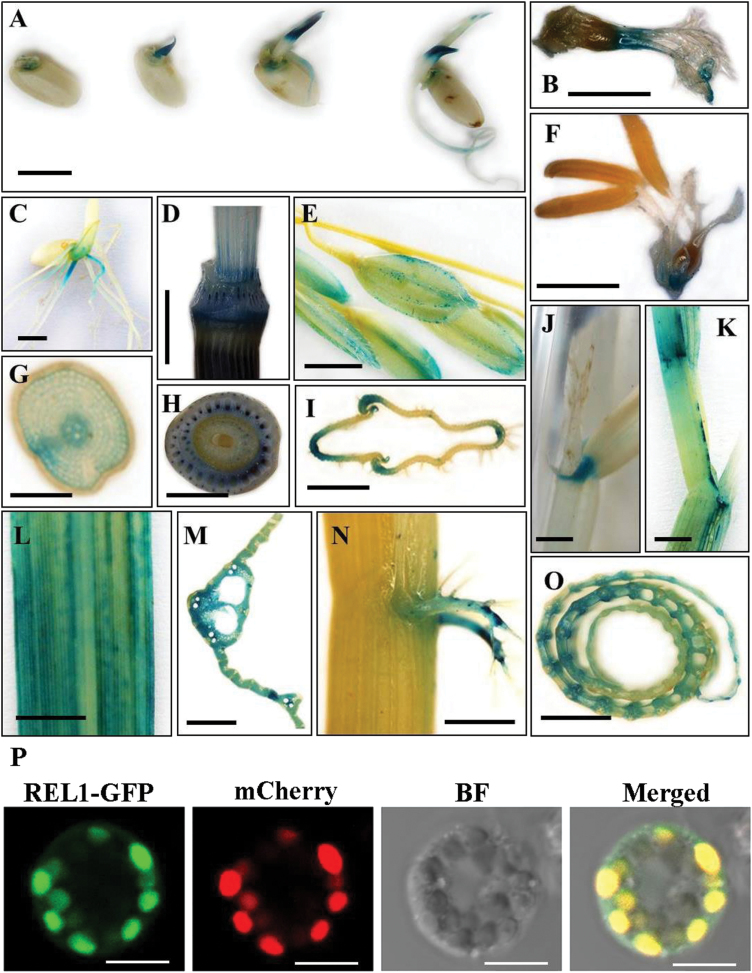
To elucidate the expression pattern of REL1, the 2545bp endogenous promoter of REL1 was fused with GUS and the resulting construct was then introduced into the wild type. GUS staining results demonstrated that REL1 was specifically expressed in shoot apical meristem during seed germination (Fig. 6A). In the inflorescence, the GUS signals of REL1 were stained in the stamens but not in the pistil (Fig. 6B, F). Interestingly, REL1 was also expressed in the elongation zone of roots at the first leaf stage (Fig. 6C), and further detected in the epidermis and endodermis (Fig. 6G). At the heading stage, REL1 was subsequently observed in the stem (Fig. 6D), and GUS staining was specifically detected in the endodermis (Fig. 6H). At the mature stage, there was an obvious GUS signal of REL1 on the spikelet hull (Fig. 6E), in which REL1 was only found in a portion of the lemmas and paleas (Fig. 6I). Further investigations showed that REL1 was constitutively expressed in the leaf during growth and development (Fig. 6J, K, L), particularly in the ligule, leaf sheath, and vascular (Fig. 6K, M, O). REL1 was also expressed in the lamina joint (Fig. 6N). The GENEVESTIGATOR tool was also used to analyse the expression pattern of REL1. As shown in Supplementary Fig. S6, REL1 was constitutively expressed in the root, leaf, and inflorescence, and especially accumulated in the mature leaf, which is consistent with the GUS staining results.
Fig. 6.
Expression pattern of REL1. (A) GUS staining of seedling. The signals were revealed by promoter–GUS fusion analysis in transgenic plants. (B–F) GUS activity was observed in the stamen (B), root (C), stem (D), spikelet (E), and inflorescence (F). (G–I) Cross-section of C, D, and E reveals the specific pattern of REL1. (J–O) GUS signals were observed in ligule (J), sheath and blade (K), mature leaf (L), cross view of mature leaf (M), lamina joint (N), and cross view of culm (O). A to O, bar = 1cm. GUS staining was performed in more than three independent lines with similar patterns. (P) Transient co-expression of REL1-GFP fusion protein and a plastid marker pt-rb CD3-1000 (Nelson et al., 2007) in rice protoplasts revealed that REL1 is mainly located in the plastid. The pt-rb CD3-1000 was visualized by mCherry fluorescence. BF, bright field. Bars = 10 µm.
To explore the subcellular localization of REL1 protein, REL1 was fused in-frame to the GFP gene. The resulting REL1-GFP transgenes, under the control of the Cauliflower mosaic virus 35S (35S) promoter, was transiently co-expressed with a plastid marker pt-rb CD3-1000 (Nelson et al., 2007) in rice protoplasts. Results indicated that REL1-GFP localized in the plastid (Fig. 6P). Taken together, it appears that REL1 is constitutively expressed in various tissues and stages and encodes a plastid-localized protein.
Expression of BR-associated genes is altered in rel1 mutant
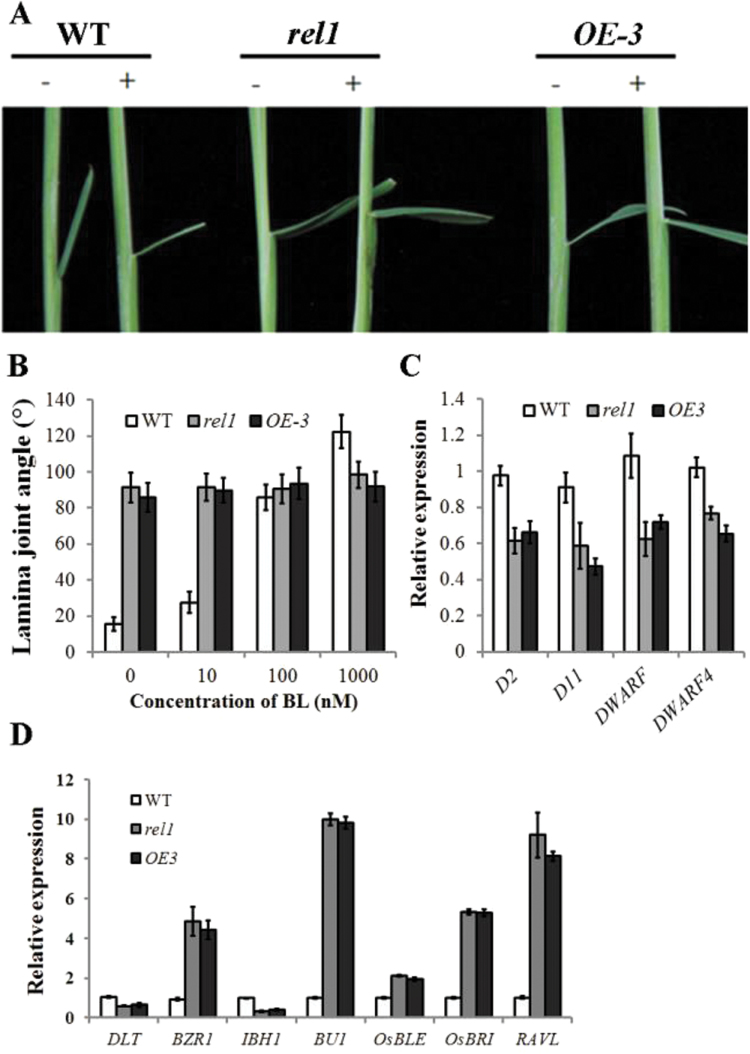
BR plays a vital role in plant growth and development, especially in leaf morphology. BR-deficient mutants usually exhibit a rolled and/or erect leaf phenotype (Bishop and Yokota, 2001; Hong et al., 2004). Considering the rolled and erect leaf phenotype present in the rel1 mutant, the question was asked whether the BR pathway is eliminated in rel1. To address this issue, the BR response of the rel1 mutant was examined by a lamina inclination assay as described previously (Tong et al., 2009). As expected, the wild-type plant was hypersensitive to BR hormone and showed the erect leaf phenotype after BR treatment (Fig. 7A). Notably, BL treatment induced a lamina joint angle in wild type of above 120 degrees at a concentration of 1000nM (Fig. 7B). By contrast, there was no change in the lamina joint angle of either rel1 or OE-3 with any concentration of BL treatment (Fig. 7A, B), indicating that rel1 and OE-3 were insensitive to the BR. qRT-PCR analysis of BR-associated genes was performed to evaluate the BR response to rel1. The D2, D11, and DWARFs have been well characterized as BR biosynthetic genes in both Arabidopsis and rice (Choe et al., 2001; Hong et al., 2003; Tanabe et al., 2005; Kim et al., 2006). Results showed that all of them were slightly downregulated in the rel1 and OE-3 lines (Fig. 7C). These results suggest that REL1 was not tightly associated with BR biosynthesis. Accumulating evidence implicates that inhibition of OsBZR1 leads to erect leaf phenotype, whereas OsBZR1-mediated leaf morphology is controlled by DLT (Tong et al., 2009). Surprisingly, the qRT-PCR analysis demonstrated that OsBZR1 was significantly upregulated in rel1 and OE-3, with ~5-fold changes (Fig. 7D), whereas DLT was repressed in rel1 and OE-3, along with another downstream regulator IBH1 (Fig. 7D). In addition, other BR signalling genes, including BU1, OsBRI, and RAVL, were significantly upregulated (Fig. 7D). Taken together, it is proposed that REL1 coordinates the expression of BR signalling-related genes to regulate leaf morphology.
Fig. 7.
BR response of REL1. (A) Phenotype of wild type, rel1, and OE-3 with or without BR treatment. Two-week-old plants were used in the micro-drop treatments. The BR treatment was performed as previously described (Tong et al., 2009). Bar = 10cm. (B) Quantification of lamina joint bending angle in wild type, rel1, and OE-3 with various concentration of BL treatment. Bars indicate standard deviation (n = 20). (C) Relative expression of BR biosynthetic genes D2, D11, DWARF4, and DWARF in wild type, rel1, and OE3. (D) Relative expression of BR signalling genes in wild type, rel1, and OE3, respectively. C and D, the values are the mean ± SD with three biological replicates.
Identification of REL1-interacting proteins by a yeast two-hybrid screen assay
To further explore the biological function of REL1, a yeast two-hybrid screen assay was performed to identify REL1-interacting proteins (RIPs). In total, 51 positive clones were obtained. However, only seven were confirmed to encode annotated proteins. Among these RIPs, both RIP1 and RIP2 encode zinc finger proteins, and the RIP3 protein belongs to an expansin-like A subfamily, while RIP4, RIP5, RIP6, and RIP7 encode hydrolase, oxidase, thaumatin and an unknown protein, respectively (Table 1). To further verify the relationship between REL1 and RIPs, knockdown of RIPs in the rel1 mutant background was performed. RIP3 protein was selected as a representative case, because a study in Nicotiana tabacum has implicated that upregulation of an expansin protein could induce the formation of leaf curvature (Sloan et al., 2009). Phenotypic analyses on the rip3 rel1 double mutants illustrated that knockdown of RIP3 in rel1 could rescue the rel1 phenotype, including plant height, leaf rolling, and leaf bending (Supplementary Fig. S7). Therefore, it is proposed that RIPs, particularly RIP3, are involved in regulating rice development through interplay with REL1.
Table 1.
List of REL1-interacting proteins from yeast 2-hybrid screen
| Name | Gene locus | Gene annotationa |
|---|---|---|
| RIP1 | LOC_Os06g17410 | dof zinc finger protein |
| RIP2 | LOC_Os10g30850 | zinc finger protein |
| RIP3 | LOC_Os03g04020 | Expansin-like A subfamily |
| RIP4 | LOC_Os01g71340 | glycosyl hydrolase |
| RIP5 | LOC_Os06g37150 | putative L-ascorbate oxidase |
| RIP6 | LOC_Os12g43380 | thaumatin-like protein |
| RIP7 | LOC_Os03g16950 | uncharacterized protein |
a Gene annotation was obtained from the GRAMENE or NCBI database.
Discussion
Leaves plays a vital role in photosynthesis, respiration, and transpiration for plant growth and development (Govaerts et al., 1996). Establishment of moderate leaf rolling is considered an important agronomic strategy in rice that can increase photosynthesis and reduce transpiration (Lang et al., 2004; Zhang et al., 2009; Zou et al., 2011), thereby increasing rice yield. In the present study, a dominant mutant, named rel1, was characterized. This mutant displayed a rolled and erect leaf, dwarfism, and small grain phenotype, eventually resulting in a reduction in grain yield. Overexpression of REL1 resulted in a rel1-like phenotype, indicating that REL1 positively participates in regulating leaf morphology. The results also suggested that REL1 regulates leaf bending through coordination of the BR pathway.
Generally, leaf rolling is induced by water loss from bulliform cells on the leaf upper epidermis in rice (O’Toole J and Cruz, 1980), suggesting that the number and density of bulliform cells may affect the extent of leaf rolling. However, it has been demonstrated that water loss from the adaxial subepidermal sclerenchyma and mesophyll also contributes to leaf rolling, and leaf rolling can also occur in leaves lacking bulliform cells (Shields, 1951). In the present study, rel1 mutants displayed abaxially rolled leaves, which resulted from the increased number and size of bulliform cells on the abaxial side of leaf blades (Fig. 2). Bulliform cells in rel1 were larger than those in the wild type, which may alter the mechanical properties of the leaf abaxial surface. Knockdown of REL1 expression in a rel1 background restored a wild-type phenotype (Fig. 4). Consistent with the activation of REL1 in rel1 mutants, overexpression of REL1 in wild type also resulted in a rel1-like phenotype (Fig. 5), indicating the positive role of REL1 in controlling leaf rolling via the regulation of cell division and expansion of bulliform cells. However, this positive role of REL1 in regulating leaf morphology needs to be further understood.
A bioinformatics analysis suggested that REL1 is specific to monocot plants because the REL1 protein homologies were only found in monocots such as rice, Zea mays, and Sorghum bicolor (Supplementary Fig. S3). In addition, BLAST analysis indicated that there was no relevant domain or motif present in REL, suggesting that REL1 encodes a functional unknown protein that only exists in monocot species. Considering the leaf architecture and morphology of monocot plants, it can be speculated that the function of these REL-like proteins may share some degree of conservation in leaf development in monocotyledonous plants, although some differentiations might also have occurred.
BR has been shown to participate in plant growth and development, especially in determining leaf morphology. BR-deficient mutants always exhibit a dwarf, erect, and rolled leaf phenotype (Bishop and Yokota, 2001; Hong et al., 2004). Results here indicated that BR biosynthesis-related genes were slightly reduced in the rel1 and OE-3 lines (Fig. 7), suggesting that REL1 likely is not associated with BR biosynthesis. However, OsBZR1 and DLT, which control bending of the lamina joint in rice (Tanaka et al., 2009; Tong et al., 2009; Tong et al., 2012), were induced and repressed, respectively, in rel1 and OE-1 (Fig. 7). These findings differed from the previous discovery that erect leaf phenotype results from the inhibition of BZR1 (Hong et al., 2004; Sakamoto et al., 2006; Bai et al., 2007). Additionally, other BR signalling genes, including BU1, OsBRI, and RAVL, were upregulated in the rel1 and OE3 lines (Fig. 7).Therefore, it is tempting to further investigate how REL1 coordinates the BR signalling genes to regulate leaf morphology in rice.
Because there is little knowledge about the biological function of REL1, a yeast two-hybrid was used to screen the RIPs. Only seven candidates were identified and confirmed. Among the corresponding seven RIPs, three were of more interest, RIP1 and RIP2 encoding two zinc finger proteins, and RIP3 encoding an expansin-like protein. Some regulators of leaf morphology have previously been identified as zinc finger protein. For example, the LIC gene encoding a zinc finger protein is involved in the regulation of leaf morphology (Zhang et al., 2012). Preliminary results here indicated that knockdown of RIP1 or RIP2 in rel1 would rescue the defect leaf phenotype of rel1 (data not shown). Therefore, it is proposed that both RIP1 and RIP2 may be involved in regulating leaf morphology through their interaction with REL1. Expansin proteins were initially identified as cell wall proteins capable of promoting the extension of plant tissue in vitro (McQueen-Mason et al., 1992). Studies in tobacco demonstrated that induction of expansin proteins could result in leaf curvature (Sloan et al., 2009). In the present study, the relationship between REL1 and RIP3 was further verified by knockdown of RIP3 in the rel1 mutant background, and a phenotypic analysis demonstrated that this knockdown elevated the expression of RIP3 in rel1 and could restore the wild-type phenotype (Supplementary Fig. S7). These results suggest that REL1 interacts with RIPs, particularly RIP3, to regulate leaf development. However, how REL1 interplays with these RIPs in regulating leaf development and other traits still remains to be further elucidated.
In summary, a novel important regulator, REL1, involving in regulating leaf morphology has here been characterized. Results indicate that REL1 positively controls leaf rolling and bending, and may coordinate BR-associated genes to regulate leaf morphology. In addition, a yeast 2-hybrid screen revealed seven REL-interacting proteins, which may improve our understanding of the role of REL1 in regulating other traits in rice, such as the control of tiller, plant height, and grain shape.
Supplementary data
Supplementary material is available at JXB online.
Supplemental Fig. S1. Statistical analysis of agronomic traits in rel1 mutants.
Supplemental Fig. S2. Genotyping of wild type, rel1 heterozygote, and rel1 homozygote mutants.
Supplemental Fig. S3. Alignment of REL1-like proteins among monocot plants.
Supplemental Fig. S4. Knockdown of REL1 in wild type did not result in a mutant phenotype.
Supplemental Fig. S5. Overexpression of REL1 leads to a rel1-like phenotype.
Supplemental Fig. S6. Expression pattern of REL1 in various tissues and stages.
Supplemental Fig. S7. Knockdown of RIP3 in rel1 is capable of rescuing rel1 mutant phenotype.
Supplemental Table S1. Primers used in this study.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (30571145 and 31071389) and the Natural Science Foundation of Guangdong Province (10151064201000024).
Reference
- Bai MY, Zhang LY, Gampala SS, Zhu SW, Song WY, Chong K, Wang ZY. 2007. Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice. Proceedings of the National Academy of Sciences of the United States of America 104, 13839–13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Yokota T. 2001. Plants steroid hormones, brassinosteroids: current highlights of molecular aspects on their synthesis/metabolism, transport, perception and response. Plant & Cell Physiology 42, 114–120. [DOI] [PubMed] [Google Scholar]
- Botwright TL, Rebetzke GJ, Condon AG, Richards RA. 2005. Influence of the gibberellin-sensitive Rht8 dwarfing gene on leaf epidermal cell dimensions and early vigour in wheat (Triticum aestivum L.). Annals of Botany 95, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Fujioka S, Noguchi T, Takatsuto S, Yoshida S, Feldmann KA. 2001. Overexpression of DWARF4 in the brassinosteroid biosynthetic pathway results in increased vegetative growth and seed yield in Arabidopsis . Plant Journal 26, 573–582. [DOI] [PubMed] [Google Scholar]
- Fujino K, Matsuda Y, Ozawa K, Nishimura T, Koshiba T, Fraaije MW, Sekiguchi H. 2008. NARROW LEAF 7 controls leaf shape mediated by auxin in rice. Molecular Genetics and Genomics 279, 499–507. [DOI] [PubMed] [Google Scholar]
- Govaerts YM, Jacquemoud S, Verstraete MM, Ustin SL. 1996. Three-dimensional radiation transfer modeling in a dicotyledon leaf. Applied Optics 35, 6585–6598. [DOI] [PubMed] [Google Scholar]
- Hibara K, Obara M, Hayashida E, Abe M, Ishimaru T, Satoh H, Itoh J, Nagato Y. 2009. The ADAXIALIZED LEAF1 gene functions in leaf and embryonic pattern formation in rice. Developmental Biology 334, 345–354. [DOI] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Matsuoka M. 2004. Brassinosteroids and rice architecture. Journal of Pesticide Science 29, 184–188. [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Umemura K, Uozu S, Fujioka S, Takatsuto S, Yoshida S, Ashikari M, Kitano H, Matsuoka M. 2003. A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome P450. The Plant Cell 15, 2900–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh J, Nonomura K, Ikeda K, Yamaki S, Inukai Y, Yamagishi H, Kitano H, Nagato Y. 2005. Rice plant development: from zygote to spikelet. Plant & Cell Physiology 46, 23–47. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. The EMBO Journal 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadioglu A, Terzi R. 2007. A dehydration avoidance mechanism: leaf rolling. The Botanical Review 73, 290–302. [Google Scholar]
- Kim HB, Kwon M, Ryu H, Fujioka S, Takatsuto S, Yoshida S, An CS, Lee I, Hwang I, Choe S. 2006. The regulation of DWARF4 expression is likely a critical mechanism in maintaining the homeostasis of bioactive brassinosteroids in Arabidopsis . Plant Physiology 140, 548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MJ, Vincent JFV, Harris W. 1996. Curling and folding of leaves of monocotyledons – a strategy for structural stiffness. New Zealand Journal of Botany 34, 411–416. [Google Scholar]
- Lang Y, Zhang Z, Gu X, Yang J, Zhu Q. 2004. Physiological and ecological effects of crimpy leaf character in rice (Oryza sativa L.) II. Photosynthetic character, dry mass production and yield forming. Zuo Wu Xue Bao 30, 883–887. [Google Scholar]
- Li L, Shi ZY, Li L, Shen GZ, Wang XQ, An LS, Zhang JL. 2010. Overexpression of ACL1 (abaxially curled leaf 1) increased bulliform cells and induced abaxial curling of leaf blades in rice. Molecular Plant 3, 807–817. [DOI] [PubMed] [Google Scholar]
- Liu YG, Whittier RF. 1995. Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25, 674–681. [DOI] [PubMed] [Google Scholar]
- McQueen-Mason S, Durachko DM, Cosgrove DJ. 1992. Two endogenous proteins that induce cell wall extension in plants. The Plant Cell 4, 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulia B. 2000. Leaves as shell structures: double curvature, auto-stresses, and minimal mechanical energy constraints on leaf rolling in grasses. Journal of Plant Growth Regulation 19, 19–30. [DOI] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenfuhr A. 2007. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant Journal 51, 1126–1136. [DOI] [PubMed] [Google Scholar]
- O’Toole JC, Cruz RT. 1980. Response of leaf water potential, stomatal resistance, and leaf rolling to water stress. Plant Physiology 65, 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Morinaka Y, Ohnishi T, et al. 2006. Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nature Biotechnology 24, 105–109. [DOI] [PubMed] [Google Scholar]
- Shields LM. 1951. The Involution Mechanism in Leaves of Certain Xeric Grasses . Delhi: International Society of Plant Morphologists. [Google Scholar]
- Sloan J, Backhaus A, Malinowski R, McQueen-Mason S, Fleming AJ. 2009. Phased control of expansin activity during leaf development identifies a sensitivity window for expansin-mediated induction of leaf growth. Plant Physiology 151, 1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S, Ashikari M, Fujioka S, et al. 2005. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. The Plant Cell 17, 776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Nakagawa H, Tomita C, et al. 2009. BRASSINOSTEROID UPREGULATED1, encoding a helix-loop-helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice. Plant Physiology 151, 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Liu L, Jin Y, Du L, Yin Y, Qian Q, Zhu L, Chu C. 2012. DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice. The Plant Cell 24, 2562–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Jin Y, Liu W, Li F, Fang J, Yin Y, Qian Q, Zhu L, Chu C. 2009. DWARF AND LOW-TILLERING, a new member of the GRAS family, plays positive roles in brassinosteroid signaling in rice. Plant Journal 58, 803–816. [DOI] [PubMed] [Google Scholar]
- Wu C, Fu Y, Hu G, Si H, Cheng S, Liu W. 2010. Isolation and characterization of a rice mutant with narrow and rolled leaves. Planta 232, 313–324. [DOI] [PubMed] [Google Scholar]
- Yamamuro C, Ihara Y, Wu X, Noguchi T, Fujioka S, Takatsuto S, Ashikari M, Kitano H, Matsuoka M. 2000. Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. The Plant Cell 12, 1591–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Yan CJ, Zeng XH, Yang YC, Fang YW, Tian CY, Sun YW, Cheng ZK, Gu MH. 2008. ROLLED LEAF 9, encoding a GARP protein, regulates the leaf abaxial cell fate in rice. Plant Molecular Biology 68, 239–250. [DOI] [PubMed] [Google Scholar]
- Zhang C, Xu Y, Guo S, Zhu J, Huan Q, Liu H, Wang L, Luo G, Wang X, Chong K. 2012. Dynamics of brassinosteroid response modulated by negative regulator LIC in rice. PLoS Genetics 8, e1002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GH, Xu Q, Zhu XD, Qian Q, Xue HW. 2009. SHALLOT-LIKE1 is a KANADI transcription factor that modulates rice leaf rolling by regulating leaf abaxial cell development. The Plant Cell 21, 719–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Su J, Duan S, et al. 2011. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou LP, Sun XH, Zhang ZG, Liu P, Wu JX, Tian CJ, Qiu JL, Lu TG. 2011. Leaf rolling controlled by the homeodomain leucine zipper class IV gene Roc5 in rice. Plant Physiology 156, 1589–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.