Abstract
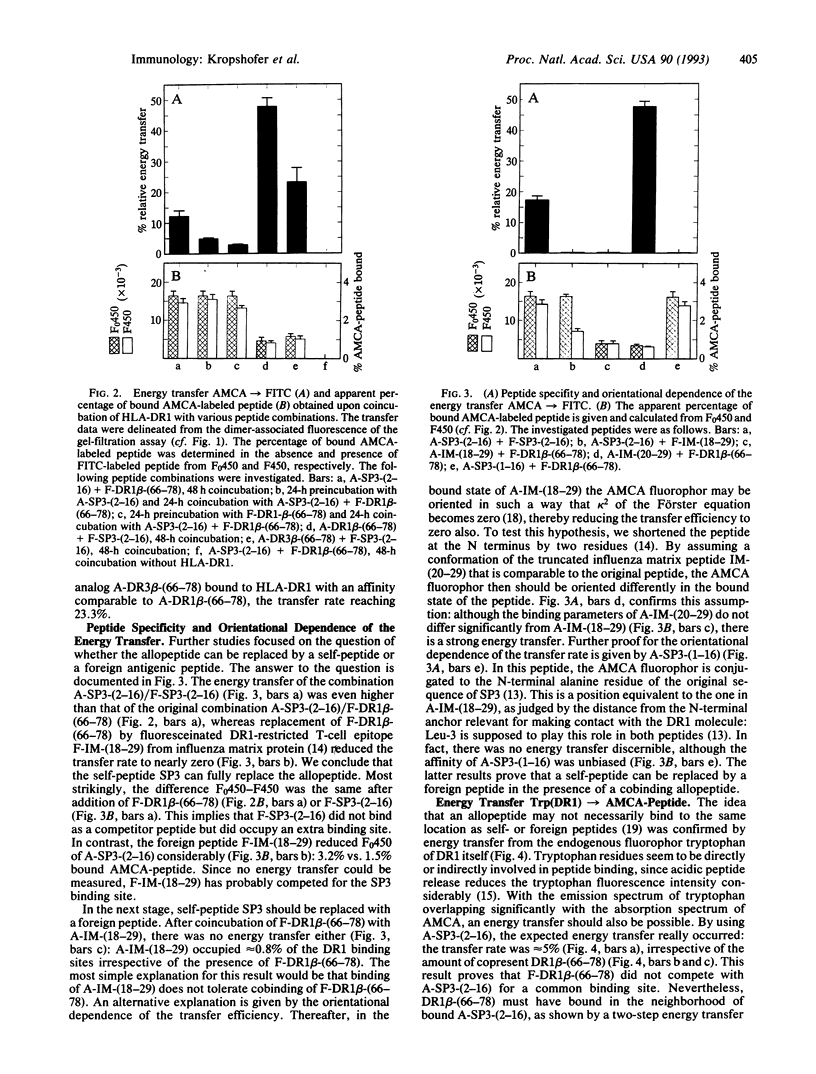
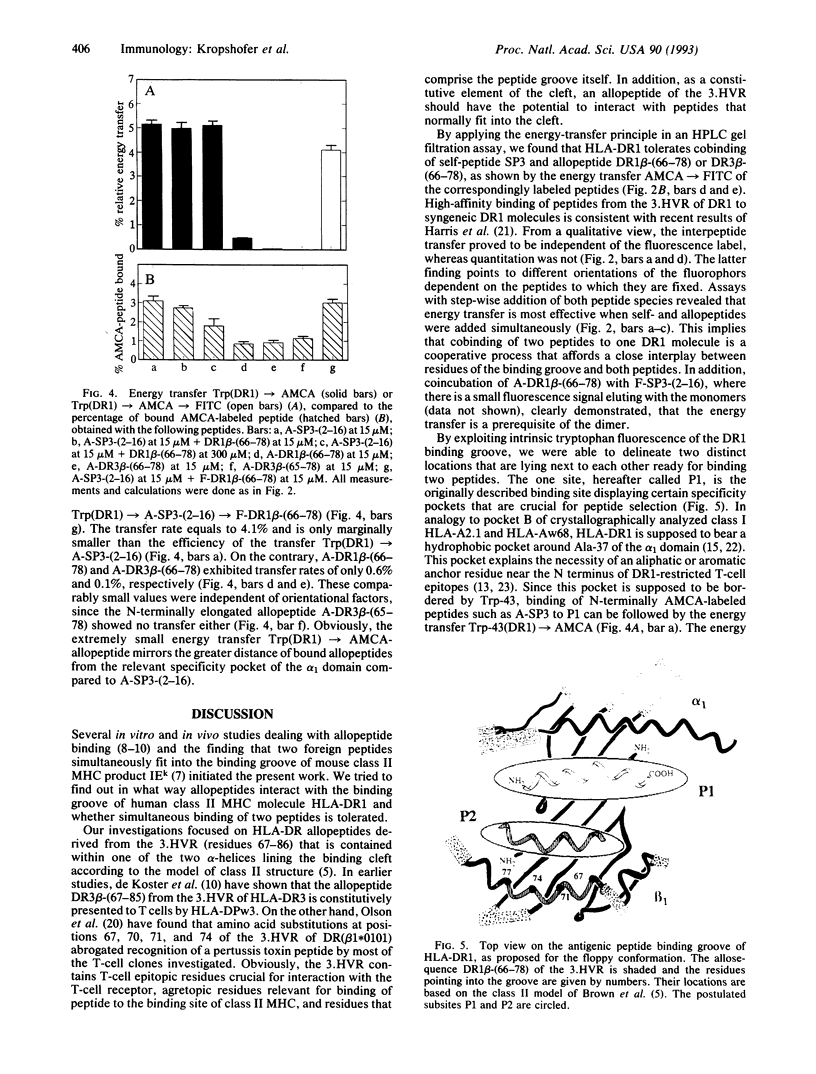
Purified human class II major histocompatibility antigen HLA-DR1 was subjected to high-performance gel filtration with fluorescence detection to investigate simultaneous binding of two classes of peptides: the N-terminally fluoresceinated allopeptides fluorescein isothiocyanate (FITC)-conjugated DR1 beta-(66-78) and FITC-conjugated DR3 beta-(66-78), derived from the third hypervariable region of the beta chain of DR1 and DR3, respectively, and the DR1-associated self-peptide SP3, carrying the fluorophor 7-amino-4-methyl-coumarin-3-acetic acid (AMCA) at the N terminus. By analyzing the dimer-associated fluorescence signals, we measured an interpeptide energy transfer AMCA-->FITC that proved to be peptide-specific: it did not occur after replacement of the allopeptide by the DR1-restricted peptide IM-(18-29) from influenza matrix protein, whereas it was restored by SP3, due to the high homology of SP3 and allopeptide. Transfer analyses with truncated AMCA-SP3 and AMCA-IM-(18-29) are consistent with Leu-3 being a common anchor residue of both peptides that allows an interaction with the hydrophobic specifity pocket around Ala-37 of the alpha 1 domain. This interaction is mirrored by the intrinsic fluorescence of neighboring Trp-43: we found the protein-peptide transfer Trp(DR1)-->AMCA with AMCA-SP3 but with none of the allopeptides. Since each energy transfer affords close proximity of two fluorophors, the following picture emerges: self- or foreign peptides bind to the DR1 binding cleft by occupation of previously described specificity pockets. Simultaneously, allopeptides of the third hypervariable region or homologous peptides may occupy a cryptic binding site by displacing the beta 1-helix that normally lines the binding groove. Thus, the described complexes raise additional possibilities for the molecular basis of auto- or alloreactivity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal B., Manickasundari M., Fraga E., Singh B. T cells that recognize peptide sequences of self MHC class II molecules exist in syngeneic mice. J Immunol. 1991 Jul 15;147(2):383–390. [PubMed] [Google Scholar]
- Bhayani H., Paterson Y. Analysis of peptide binding patterns in different major histocompatibility complex/T cell receptor complexes using pigeon cytochrome c-specific T cell hybridomas. Evidence that a single peptide binds major histocompatibility complex in different conformations. J Exp Med. 1989 Nov 1;170(5):1609–1625. doi: 10.1084/jem.170.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Braciale T. J., Morrison L. A., Sweetser M. T., Sambrook J., Gething M. J., Braciale V. L. Antigen presentation pathways to class I and class II MHC-restricted T lymphocytes. Immunol Rev. 1987 Aug;98:95–114. doi: 10.1111/j.1600-065x.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Ceppellini R., Frumento G., Ferrara G. B., Tosi R., Chersi A., Pernis B. Binding of labelled influenza matrix peptide to HLA DR in living B lymphoid cells. Nature. 1989 Jun 1;339(6223):392–394. doi: 10.1038/339392a0. [DOI] [PubMed] [Google Scholar]
- Demotz S., Sette A., Sakaguchi K., Buchner R., Appella E., Grey H. M. Self peptide requirement for class II major histocompatibility complex allorecognition. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8730–8734. doi: 10.1073/pnas.88.19.8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornmair K., Rothenhäusler B., McConnell H. M. Structural intermediates in the reactions of antigenic peptides with MHC molecules. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):409–416. doi: 10.1101/sqb.1989.054.01.050. [DOI] [PubMed] [Google Scholar]
- Gorga J. C., Horejsí V., Johnson D. R., Raghupathy R., Strominger J. L. Purification and characterization of class II histocompatibility antigens from a homozygous human B cell line. J Biol Chem. 1987 Nov 25;262(33):16087–16094. [PubMed] [Google Scholar]
- Gorga J. C. Structural analysis of class II major histocompatibility complex proteins. Crit Rev Immunol. 1992;11(5):305–335. [PubMed] [Google Scholar]
- Harris P. E., Liu Z., Suciu-Foca N. MHC class II binding of peptides derived from HLA-DR 1. J Immunol. 1992 Apr 1;148(7):2169–2174. [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kropshofer H., Bohlinger I., Max H., Kalbacher H. Self and foreign peptides interact with intact and disassembled MHC class II antigen HLA-DR via tryptophan pockets. Biochemistry. 1991 Sep 24;30(38):9177–9187. doi: 10.1021/bi00102a008. [DOI] [PubMed] [Google Scholar]
- Kropshofer H., Max H., Müller C. A., Hesse F., Stevanovic S., Jung G., Kalbacher H. Self-peptide released from class II HLA-DR1 exhibits a hydrophobic two-residue contact motif. J Exp Med. 1992 Jun 1;175(6):1799–1803. doi: 10.1084/jem.175.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan D., Arrhenius T., Sidney J., Del Guercio M. F., Albertson M., Wall M., Oseroff C., Southwood S., Colón S. M., Gaeta F. C. On the interaction of promiscuous antigenic peptides with different DR alleles. Identification of common structural motifs. J Immunol. 1991 Oct 15;147(8):2663–2669. [PubMed] [Google Scholar]
- Olson R. R., De Magistris M. T., Di Tommaso A., Karr R. W. Mutations in the third, but not the first or second, hypervariable regions of DR(beta 1*0101) eliminate DR1-restricted recognition of a pertussis toxin peptide. J Immunol. 1992 May 1;148(9):2703–2708. [PubMed] [Google Scholar]
- Rees A. D., Lombardi G., Scoging A., Barber L., Mitchell D., Lamb J., Lechler R. Functional evidence for the recognition of endogenous peptides by autoreactive T cell clones. Int Immunol. 1989;1(6):624–630. doi: 10.1093/intimm/1.6.624. [DOI] [PubMed] [Google Scholar]
- Rosloniec E. F., Vitez L. J., Buus S., Freed J. H. MHC class II-derived peptides can bind to class II molecules, including self molecules, and prevent antigen presentation. J Exp Med. 1990 May 1;171(5):1419–1430. doi: 10.1084/jem.171.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenhäusler B., Dornmair K., McConnell H. M. Specific binding of antigenic peptides to separate alpha and beta chains of class II molecules of the major histocompatibility complex. Proc Natl Acad Sci U S A. 1990 Jan;87(1):352–354. doi: 10.1073/pnas.87.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. Acquisition of immunologic self-tolerance. Cell. 1989 Jun 30;57(7):1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Srinivasan M., Marsh E. W., Pierce S. K. Characterization of naturally processed antigen bound to major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7928–7932. doi: 10.1073/pnas.88.18.7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampé R., Clark B. R., McConnell H. M. Energy transfer between two peptides bound to one MHC class II molecule. Science. 1991 Oct 4;254(5028):87–89. doi: 10.1126/science.1656526. [DOI] [PubMed] [Google Scholar]
- Tampé R., McConnell H. M. Kinetics of antigenic peptide binding to the class II major histocompatibility molecule I-Ad. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4661–4665. doi: 10.1073/pnas.88.11.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt S. N., McConnell H. M. A first-order reaction controls the binding of antigenic peptides to major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8164–8168. doi: 10.1073/pnas.88.18.8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koster H. S., Anderson D. C., Termijtelen A. T cells sensitized to synthetic HLA-DR3 peptide give evidence of continuous presentation of denatured HLA-DR3 molecules by HLA-DP. J Exp Med. 1989 Mar 1;169(3):1191–1196. doi: 10.1084/jem.169.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]