Abstract
Background
Pulmonary metastasectomies are performed for a variety of cancers, though few reports have examined their merit for head and neck cancers. This study examined the relationship between clinical and pathological characteristics and survival after resection of lung metastases of these cancers.
Methods
Between 1986 and 2013, 34 patients presenting with pulmonary metastases of primary head or neck cancers underwent surgical resections at our institution. We retrospectively analyzed their clinical and pathological characteristics and the patients’ survival after metastasectomy in search of adverse prognostic factors.
Results
The primary sites of cancer were: the tongue in eight patients, the pharynx and salivary glands each in seven, the larynx in five, and miscellaneous sites in seven patients. Squamous cell carcinoma (SCC) was present in 19, adenoid cystic carcinomas in 10, and other diagnoses in five patients. The median disease-free interval (DFI) between the initial surgery and the metastasectomies was 40 months. The overall five-year survival rate was 57.9%, and median survival time was 77 months. By single variable analysis, a DFI of ≤26 months, age ≥60 years, and histology of SCC were predictors; by multiple variable analysis, a short DFI (P = 0.018) and older age (P = 0.046) remained independent predictors of poor clinical outcomes.
Conclusions
Young age and a long DFI are factors in favor of pulmonary metastasectomy after surgical treatment of primary head or neck cancers.
Keywords: Cancer survival, head and neck cancer, pulmonary metastasis
Introduction
Resection of pulmonary metastases is often performed after surgical treatment of primary head and neck cancers, as the lungs are the most common site of their distant dissemination.1 The indications and clinical contributions of surgery for metastatic colon and breast cancers have been widely studied. In contrast, only a few reports have described the clinical outcomes and benefits of the resection of pulmonary metastases of head or neck cancers. The number of patients undergoing resection of pulmonary metastases of head or neck cancers is relatively small compared with colon and breast cancers. At present, no consensus has been reached with respect to the adverse postoperative prognosis conferred, in particular, by clinical and pathological factors, after the resection of lung metastases of head or neck cancers. The purpose of this study was to evaluate the clinical outcomes after pulmonary metastasectomy of head and neck cancers, and to identify predictors of adverse clinical outcome among patients treated at our institution.
Patient sample and methods
In this retrospective study, we included 34 patients who presented with lung metastases of primary head or neck cancers, and who underwent surgical resections of the metastatic lesions at our institution, between November 1986 and April 2013. Patients who had histories of skin or thyroid cancers were excluded. Patients with primary head or neck cancers were surgically treated after the detection of lung metastases on chest roentgenograms or computed tomography (CT) scans. We excluded patients with distant metastases other than pulmonary metastases, or those who were suspected to have carcinomatous lymphangiosis detected on CT scans. The disease-free interval (DFI) is defined as the period between initial treatment of a primary tumor and the resection of one or more pulmonary metastases. Overall survival (OS) was measured between the date of pulmonary resection to the date of death from any cause, or the latest date on which the patient was known to be alive.
The operative procedure varied between lobectomies and wedge resections, depending on the numbers and locations of the metastases. Typically, lobectomies were performed through thoracotomy, whereas wedge resections were performed through the video-assisted thoracoscopic approach. We did not perform lymph node dissection during the metastasectomies.
All specimens were submitted to pathologists who were expert in the field of tumors of the head, neck, and lung. In diagnostically challenging cases, particularly when it was unclear whether the tumor was metastatic or primary, a second expert pathologist was consulted and a diagnosis was reached by consensus.
Patients were followed-up via the outpatient department every three or six months after metastasectomy, or received additional treatment, such as chemotherapy, if considered necessary.
This retrospective study, based on an anonymous review of records and pathological specimens, was approved in March 2014 by our institutional review board, which also waived the requirement to obtain written consent from the patients.
Statistical analysis
The data are presented as medians (ranges), or counts and percentages. Survival curves were constructed by the Kaplan–Meier method and compared using the log-rank test. Variables emerging with a P value of <0.05 by single variable analysis were entered in a multiple variable Cox forward stepwise regression model, in search of independent predictors of survival. An analysis of receiver operating characteristic curves was performed in search of cut-off values for the greatest diameter of the metastatic pulmonary tumor, age at the time of diagnosis of metastatic disease, and DFI that predicted a fatal outcome with the highest sensitivity and specificity. All tests were two-sided, and P values <0.05 were considered statistically significant. The data were analyzed using IBM SPSS software, version 21.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient, lesion, and procedural characteristics
The main characteristics of the 34 patients, lesions, and operations are shown in Table 1. The median age of the study sample was 57 years (range 28 to 79), 2/3 of patients were men, the median follow-up was 36 months (range 3 to 321), and the median DFI was 40 months (range 1–120). The sites and histology of the primary head and neck cancers are listed in Table 2. Multiple lung metastases were found in 15 patients, and bilateral lung metastases were present in 11 (Table 1). Wedge resections were performed in 2/3 and lobectomies in 1/3 patients. Lymphadenectomy was not performed systematically in any patients.
Table 1.
Main characteristics of the 34 patients, metastases and operative procedures
| Men | 22 (65) |
| Median age, years | 57 (28–79) |
| Median duration, months | |
| Follow-up | 36 (3–321) |
| Disease-free interval† | 40 (1–120) |
| Metastases per patient | |
| 1 | 19 (56) |
| >1 | 15 (44) |
| Location of metastases | |
| Unilateral | 23 (68) |
| Bilateral | 11 (32) |
| Metastases largest diameter, mm | |
| <25 | 26 (76) |
| ≥25 | 8 (24) |
| Resection type | |
| Partial | 19 (56) |
| Segmentectomy | 5 (15) |
| Lobectomy | 10 (29) |
| Resection extent | |
| Complete | 22 (65) |
| Incomplete | 12 (35) |
Values are medians (range), or numbers (%) of observations. †From the initial treatment of the primary tumor.
Table 2.
Primary sites and histology of primary head and neck cancers
| Carcinomas | |||||
|---|---|---|---|---|---|
| SCC | AC | MC | Others | All | |
| Tongue | 5 | 2 | 1 | 8 | |
| Pharynx | 7 | 7 | |||
| Salivary gland | 4 | 1 | 2 | 7 | |
| Larynx | 4 | 1 | 5 | ||
| Oral cavity | 2 | 1 | 3 | ||
| External ear | 2 | 2 | |||
| Others | 1 | 1 | 2 | ||
| All | 19 | 10 | 2 | 3 | 34 |
Values are numbers of observations. AC, adenocarcinoma; MC, mucoepidermoid carcinoma; SCC, squamous cell carcinoma.
Survival and predictors of death
The OS of the 34 patients was 57.9% at five years, and the median survival duration was 77 months. By receiver operating characteristic curve analysis, the threshold values of the greatest diameter of the metastatic pulmonary tumor, age at the time of diagnosis of metastatic disease, and DFI, which predicted death with the highest sensitivity and specificity were ≥25-mm, ≥60 years, and ≤26 months, respectively. By single variable analysis, histology of squamous cell carcinoma (SCC) (P = 0.005), age ≥60 years (P = 0.003), and DFI ≤26 months (P = 0.001) were associated with a poor prognosis (Table 3). Furthermore, a ≥25-mm maximum tumor diameter tended to be associated with a shorter survival than a <25-mm maximum tumor diameter, though the difference did not reach statistical significance (P = 0.075). The cumulative OS curves constructed by the Kaplan–Meier method, and compared, using the log-rank test, are shown in Figure 1a and b. Using multiple variable analysis, a DFI ≤26 months (hazard ratio [HR] 3.396; 95% confidence interval [CI] 1.133–10.177; P = 0.018), and age ≥60 years (HR 3.256; 95% CI 1.023–10.360; P = 0.046), were independent predictors of adverse outcomes (Table 4). The five-year OS rate of patients ≥60 years of age and whose DFI was ≤26 months was 0%, while in patients in whom a single or neither criterion was met, the five-year survival was 74.5 ± 9.0% (Fig 2).
Table 3.
Clinical and pathological predictors of 5-year survival after resection of pulmonary metastases by single variable analysis
| Variables | P† | |
|---|---|---|
| Men | 22 (51.4) | 0.094 |
| Women | 12 (70.7) | |
| Age, years | 0.003 | |
| ≥60 | 21 (34.2) | |
| <60 | 13 (73.2) | |
| Histology | ||
| Squamous cell carcinoma | 15 (16.4) | 0.001 |
| Other diagnoses | 19 (64.4) | |
| Disease-free interval, months | ||
| ≤26 | 19 (40.2) | 0.005 |
| >26 | 15 (83.3) | |
| Number of metastases | ||
| Solitary | 19 (54.8) | 0.742 |
| Multiple | 15 (60.2) | |
| Tumor size, mm | ||
| ≥25 | 26 (50.0) | 0.075 |
| <25 | 8 (61.5) | |
| Operation | ||
| Partial resection | 19 (65.7) | 0.688 |
| Segmentectomy | 5 (53.3) | |
| Lobectomy | 10 (45.0) | |
| Primary site | ||
| Tongue | 8 (50.0) | 0.642 |
| Others | 26 (60.6) | |
| Resection | ||
| Incomplete | 12 (56.3) | 0.483 |
| Complete | 22 (58.9) |
Values are number of observations (% 5-year survival). †log-rank test.
Figure 1.
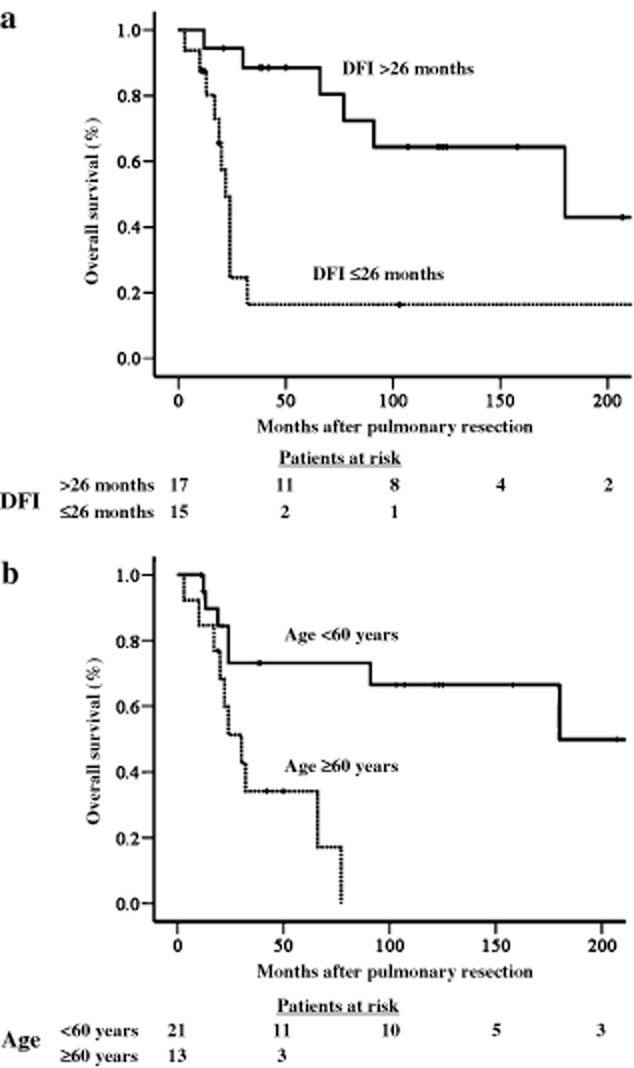
Kaplan-Meier cumulative survival after resection of pulmonary metastases. (a) Comparison according to disease-free interval (DFI). (b) Comparison according to age at the time of diagnosis of metastatic disease.
Table 4.
Outcome of Cox stepwise regression, multiple variable analysis
| Variables | Hazard ratio | 95% confidence interval | P |
|---|---|---|---|
| Age, years | |||
| ≥60 | 3.256 | 1.023–10.360 | 0.046 |
| <60 | |||
| Histology | |||
| Squamous cell carcinoma | 0.359 | 0.092–1.402 | 0.14 |
| Other diagnoses | |||
| Disease-free interval, months | |||
| ≥26 | 3.396 | 1.133–10.177 | 0.018 |
| >26 |
Figure 2.
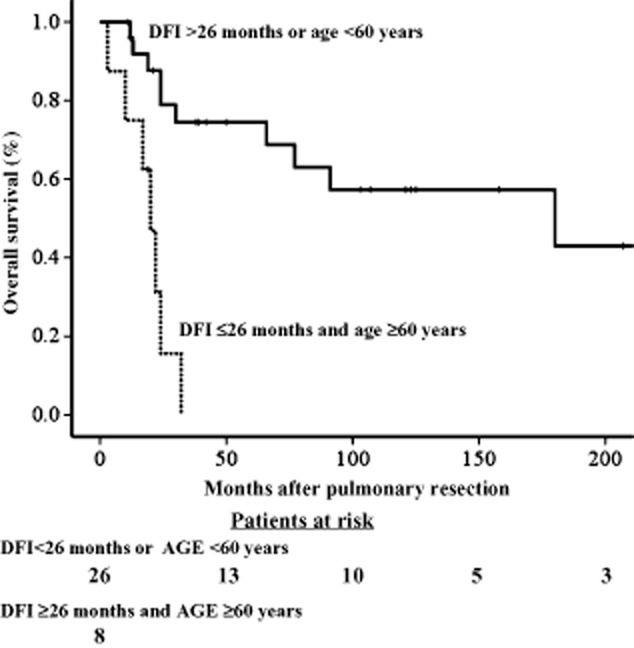
Kaplan-Meier cumulative survival after resection of pulmonary metastases according to disease-free interval (DFI) and age.
Discussion
Head and neck cancers, SCC in particular, often metastasize to the lungs.1,2 Because these metastases respond relatively poorly to chemotherapy, surgery has become an alternate therapeutic option as long as: (i) the primary tumor has been controlled; (ii) the metastases are limited to the lung and can be completely resected; and (iii) the patient can tolerate the procedure. Several studies have described the effectiveness of surgical treatment of pulmonary metastases of various cancers, such as colorectal cancer and osteosarcoma.1,3,4 Although there are few studies of head and neck cancers, the evidence available thus far suggests that pulmonary metastasectomies for these cancers may also significantly improve the prognosis and prolong the median and overall survival compared with other or no therapeutic attempts.3,5 The reported five-year survival after pulmonary metastasectomy of head and neck cancers ranges between 50–60%.6,7 Including our results, this seems to be an acceptable aim of therapy.
In our study, age ≥60 years and DFI ≤26 months were independent predictors of adverse clinical outcomes after resection of pulmonary metastases of primary head and neck cancer. Wedman et al. found that age ≥60 years, histology of SCC, and a DFI ≤1 year were adverse prognostic factors after pulmonary metastasectomy.5 In a study by Winter et al., an incomplete resection of the metastases, complications associated with surgery, and adjuvant chemotherapy were independent predictors of adverse clinical outcomes, while Nibu et al. found cancers of the mouth, metastases to the mediastinal lymph nodes, and pleural invasion to be predictors of poor outcomes.3,8 The variations in prognostic factors after pulmonary metastasectomy detected among these reports, including ours, are attributable to their relatively small sample sizes.1,3–10
In earlier studies focusing on SCC, the survival of surgically treated patients was higher than that of non-surgically treated groups.6,11 In studies limited to SCC by Mazer et al. and Shiono et al., the five-year survival of 44 and 114 patients who underwent pulmonary metastasectomy of the head or neck were 43.0% and 26.5%, respectively.9,12 The biological characteristics of SCC of the head and neck are different from those of other histologic types, and survival of patients presenting with SCC is shorter than that of patients with other cancers as they are most likely to develop recurrences within two years after the resection of metastases.3,7,9,10 In contrast, adenoid cystic carcinoma, the second most common histologic type in our study, is a relatively rare, slow growing, though proliferating tumor. Liu et al. found that 36 patients who had undergone resection of pulmonary metastases of adenoid cystic carcinomas had an estimated five-year survival rate of 84%, though no patient survived beyond 14 years.10 In an attempt to determine whether the prognosis depended on the histologic type of the tumor, we included SCC, adenoid cystic carcinoma, and other types of cancers in our study. However, our multiple variable analyses did not reveal the existence of correlations between specific histologic diagnosis and prognosis.
The prognosis conferred by the presence of multiple pulmonary metastases has been the object of conflicting reports.1,3–10,12 A reason to consider the resection of multiple metastatic lesions might be the poor prognosis known to be associated with conservative treatments. Winter et al. observed no statistically significant difference in the survival of patients presenting with single versus multiple pulmonary metastases.3 Therefore, patients with single or multiple pulmonary metastases of head or neck cancer should undergo surgery if the primary tumor is under control.
The sample sizes of each primary site and histological type included in this study were small. Furthermore, because we only included patients who had undergone surgery, this may have introduced a bias by selecting patients with a priori relatively favorable postoperative prognoses. Finally, the pathological differentiation of a metastasis from a primary cancer may be challenging.
Conclusion
In summary, the results of our multiple variable analysis identified age ≥60 years and DFI ≤26 months as predictors of adverse long-term outcome following the resection of metastases of a variety of head or neck cancers. A long DFI combined with young age suggests that pulmonary metastasectomy is a worthwhile therapeutic endeavor regardless of the primary site or number of metastatic lesions.
Disclosure
The authors of this manuscript have no conflict of interest to disclose.
Acknowledgments
This study was supported by unrestricted institutional funds.
References
- Daiko H, Nagai K, Yoshida J, et al. The role of pulmonary resection in tumors metastatic from head and neck carcinomas. Jpn J Clin Oncol. 2010;40:639–644. doi: 10.1093/jjco/hyq023. [DOI] [PubMed] [Google Scholar]
- Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:202–207. doi: 10.1159/000055740. [DOI] [PubMed] [Google Scholar]
- Winter H, Meimarakis G, Hoffmann G, et al. Does surgical resection of pulmonary metastases of head and neck cancer improve survival? Ann Surg Oncol. 2008;15:2915–2926. doi: 10.1245/s10434-008-0001-4. [DOI] [PubMed] [Google Scholar]
- Finley RK, 3rd, Verazin GT, Driscoll DL, et al. Results of surgical resection of pulmonary metastases of squamous cell carcinoma of the head and neck. Am J Surg. 1992;164:594–598. doi: 10.1016/s0002-9610(05)80714-7. [DOI] [PubMed] [Google Scholar]
- Wedman J, Balm AJ, Hart AA, et al. Value of resection of pulmonary metastases in head and neck cancer patients. Head Neck. 1996;18:311–316. doi: 10.1002/(SICI)1097-0347(199607/08)18:4<311::AID-HED1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Haro A, Yano T, Yoshida T, et al. Results of a surgical resection of pulmonary metastasis from malignant head and neck tumor. Interact Cardiovasc Thorac Surg. 2010;10:700–703. doi: 10.1510/icvts.2009.219766. [DOI] [PubMed] [Google Scholar]
- Chen F, Sonobe M, Sato K, et al. Pulmonary resection for metastatic head and neck cancer. World J Surg. 2008;32:1657–1662. doi: 10.1007/s00268-008-9631-8. [DOI] [PubMed] [Google Scholar]
- Nibu K, Nakagawa K, Kamata S, et al. Surgical treatment for pulmonary metastases of squamous cell carcinoma of the head and neck. Am J Otolaryngol. 1997;18:391–395. doi: 10.1016/s0196-0709(97)90059-4. [DOI] [PubMed] [Google Scholar]
- Shiono S, Kawamura M, Sato T, et al. Pulmonary metastasectomy for pulmonary metastases of head and neck squamous cell carcinomas. Ann Thorac Surg. 2009;88:856–860. doi: 10.1016/j.athoracsur.2009.04.040. [DOI] [PubMed] [Google Scholar]
- Liu D, Labow DM, Dang N, et al. Pulmonary metastasectomy for head and neck cancers. Ann Surg Oncol. 1999;6:572–578. doi: 10.1007/s10434-999-0572-8. [DOI] [PubMed] [Google Scholar]
- Yano T, Shoji F, Maehara Y. Current status of pulmonary metastasectomy from primary epithelial tumors. Surg Today. 2009;39:91–97. doi: 10.1007/s00595-008-3820-9. [DOI] [PubMed] [Google Scholar]
- Mazer TM, Robbins KT, McMurtrey MJ, Byers RM. Resection of pulmonary metastases from squamous carcinoma of the head and neck. Am J Surg. 1988;156:238–242. doi: 10.1016/s0002-9610(88)80282-4. [DOI] [PubMed] [Google Scholar]


