Abstract
Background
Little is known about the clinical use of intensity-modulated radiotherapy (IMRT) in postoperative radiotherapy (PORT) of esophageal cancer; therefore, we retrospectively investigated the clinical value of postoperative IMRT among resected thoracic esophageal squamous cell carcinoma (TESCC) patients.
Methods
We enrolled a total of 228 patients with resected TESCC who underwent IMRT between January 2004 and June 2009 in the study. PORT was applied via IMRT with a median total dose of 60 Gy. The Kaplan–Meier method was used to calculate survival rates, and a log-rank test was used for univariate analysis. The Cox proportional model was used for multivariate analysis.
Results
The one, three, and five-year overall survival rates of all patients were 89.9%, 56.7%, and 45.1%, respectively. Univariate analysis showed that significant prognostic factors included Union for International Cancer Control 2002 stage, lymphatic metastasis, number of metastatic lymph nodes, the degree of metastatic lymph nodes, the degree of differentiation, and vascular tumor thrombus (P < 0.05). Treatment failure occurred in 98 (45.2%) patients because of recurrence or metastases. Early reactions were observed at rates of 18.0% for radiation esophagitis and 5.7% for radiation pneumonitis more than grade 2. Late side effects included anastomotic stenosis (1.3%) and gastrointestinal bleeding (3.1%).
Conclusions
The postoperative prophylactic IMRT of TESCC provided a favorable local control rate and acceptable toxicity.
Keywords: Esophageal neoplasms/surgery, intensity-modulated radiotherapy, postoperative, prognosis
Introduction
Surgery is the primary treatment for esophageal cancer as it provides a chance of cure; however, the survival rate after surgery remains poor. Even after a radical surgery, a large number of patients experience recurrence. Five-year overall survival (OS) rates of patients with postoperative thoracic esophageal squamous cell carcinoma (TESCC) at stage II and III are only 36% and 10%, respectively.1 The main reason for postoperative failure is local-regional recurrence, which has an incidence of 20.5–43%.2–5 Therefore, extended lymphadenectomy is combined with more aggressive resection or postoperative radiotherapy (PORT), which is one of the important strategies that decreases regional recurrence.6–9
The outcomes of PORT for the improvement of OS are debatable, and previous studies have acknowledged that two-dimensional conventional radiation therapy (2D-RT) possesses some limitations, such as uneven dose distribution and inability to converge to target volumes.8–14 Our postoperative 2D-RT for esophageal cancer improved the survival of patients with positive lymph nodes and stage III disease, while the three-year survival rate of patients with stage IIA and negative lymph nodes only increased by 7–10%. Note, however, that the five-year survival rate did not increase.9 Furthermore, the use of extensive portals in postoperative 2D-RT caused late toxicity.
Intensity-modulated radiotherapy (IMRT), based on computer planning studies, possesses improved target volume coverage with less off-target delivery, potentially reducing toxicity and dose escalation, even compared with three-dimensional conformal radiotherapy (3DCRT) against non-small cell lung cancer and esophageal cancer.15,16
However, little is known about the clinical use of IMRT in PORT of esophageal cancer, and whether postoperative IMRT may improve survival rate. We hypothesized that postoperative IMRT may improve the survival rate of resected TESCC patients. Therefore, we retrospectively investigated the clinical value of postoperative IMRT among the resected patients in our hospital.
Methods
Patients
Between January 2003 and June 2009, 551 patients with TESCC were treated with surgery plus radiotherapy at the Cancer Hospital & Institute, Chinese Academy of Medical Sciences (CAMS), Peking Union Medical College, China. The ethics committee of the CAMS Cancer Hospital approved the study. Informed consent was obtained from all patients. Patients meeting the following criteria were included in the study: (i) had undergone radical resection (R0); (ii) had a diagnosed squamous cell carcinoma confirmed by pathological studies; (iii) had Karnofsky performance status scale ≥ 70; (iv) did not receive pre-operative neoadjuvant therapy; and (v) underwent postoperative IMRT. Of the 551 patients, 323 were excluded according to the criteria above for postoperative radiation because of recurrence (144 cases), postoperative 2D-RT (66 cases), R1 or R2 resection (92 cases), postoperative 3DCRT (20 cases), postoperative IMRT, and one case was lost to follow-up. The remaining 228 patients were eligible for further analysis. Of the 228 patients, 198 underwent PORT without chemotherapy and 30 in stage III PORT with chemotherapy, with 18 and 12 receiving chemotherapy before and after radiotherapy, respectively.
Surgery
Surgical details have been previously reported.9 Surgeons determined patient selection, approach, and operative procedures. A three-phase abdominothoracic McKeown resection through a right thoracotomy using the stomach for esophageal replacement was performed for lesions in the upper third of the thoracic segment. For lesions in the mid and lower third, esophagectomy was performed on the left side using the stomach to establish digestive continuation. In each case, lymph nodes were removed as completely as possible. Juxtatumoral, paraesophageal, superior gastric, left gastric, and paracardial lymph nodes were individually analyzed to determine a final stage classification according to the 2002 (6th) edition of the International Union Against Cancer (UICC) tumor node metastasis (TNM) classification system. A total of 5298 lymph nodes (median 22, range 3 to 89) were dissected for pathologic staining and stage examination using a hematoxylin and eosin method. Metastases were found in 504 lymph nodes.
Postoperative radiotherapy
Radiotherapy was initiated four to six weeks following the surgery. All patients received a planning computed tomography (CT) scan and were immobilized in a supine position with their arms raised in a customized alpha-cradle mold for treatment. The clinical target volume (CTV) of the upper thoracic (including with or without lymph node metastasis) and middle thoracic (included stage IIA or without lymph node metastasis) encompassed the bilateral supraclavicular areas with the tip of the cricoid cartilage serving as the upper border (upper thoracic) or chest vertebrae 1 on the edge (middle thoracic) and 3.0 cm below the lower margin of the tumor as the lower border or 2.0∼3.0 cm below the carina. This CTV included levels 1, 2, 4, and 7, the site of anastomosis (upper thoracic), and tumor bed. The CTV of the middle thoracic (including with lymph node metastasis) and lower thoracic included levels 2, 4, 7, and 8, the left gastric artery, celiac artery, and tumor bed. The planning target volume (PTV) was defined by the CTV plus a 5 mm margin based on the observation of the average setup. The contoured image was transferred to our treatment planning system (TPS; Pinnacle, version 9.0, Philips, Amsterdam, The Netherlands). All patients were treated by IMRT using five coplanar beams. The prescription doses of PTV were 60 Gy in 30 fractions over six weeks. The dose levels for the PTV were prescribed at the maximum isodose surface that encompassed at least 95% of the volume. The maximum tolerance doses to the critical normal structures were as follows: the maximum spinal cord dose was kept below 45 Gy; the total lung was limited V20 to ≤28%; the stomach was limited V40 to ≤50%.
Follow-up of patients
Patients were instructed to return for follow-up that included clinical examination, barium swallow, chest radiography, abdominal ultrasonography, and thoracic CT at three to six month intervals. If ultrasonography results of the abdomen were suspicious, an abdominal CT was performed. Local failure was determined by positive pathologic diagnosis or roentgenographic evidence of mediastinal lesions revealed by CT scan. Signs or symptoms of vocal cord paralysis or tracheal compression combined with mediastinal lesions shown on CT were also considered local failures. Follow-up concluded on 1 February 2012.
Statistical analysis
Disease-free survival (DFS) and overall survival (OS) endpoints were evaluated. OS was measured from the date of operation of TESCC to the date of death, or censored at the last follow-up date. For the other endpoints, the time duration was computed from day one of surgery to the date of event occurrence. The Kaplan–Meier method was used to estimate survival, and the log-rank test to determine significant difference at a P value < 0.05. A Cox proportional-hazards model was used to estimate the prognostic factors for survival. Statistical analysis was performed using SPSS 17.0 software (IBM Inc., Armonk, NY, USA).
Results
Clinical characteristics of patient population
The 228 esophageal cancer patients were 35 to 78 years old (median, 56 years), with 16.7% (38) at stage IIa, 20.6% (47) at stage IIb, and 62.7% (143) at stage III. Detailed patient characteristics are shown in Table 1.
Table 1.
Clinical characteristics of the patient population
| Characteristics | No. | % |
|---|---|---|
| Gender | ||
| Male | 195 | 85.5 |
| Female | 33 | 14.5 |
| Age (years) | ||
| Median age | 56 | |
| Range | 35∼78 | |
| Location | ||
| Upper | 24 | 10.5 |
| Middle | 119 | 52.2 |
| Lower | 85 | 37.3 |
| Tumor differentiation | ||
| High-moderate | 160 | 70.2 |
| Low- undifferentiated | 68 | 29.8 |
| T-stage (UICC02) | ||
| T1 | 18 | 7.9 |
| T2 | 30 | 13.2 |
| T3 | 172 | 75.4 |
| T4 | 8 | 3.5 |
| N-stage (UICC02) | ||
| N0 | 42 | 18.4 |
| N1 | 186 | 81.6 |
| P-stage (UICC02) | ||
| Stage IIA | 38 | 16.7 |
| Stage IIB | 47 | 20.6 |
| Stage III | 143 | 62.7 |
UICC, International Union Against Cancer.
Survival
The patients were followed for 31 to 92 months (median, 45 months). Overall one, three, and five-year survival rates were 89.9%, 56.7%, and 45.1%, respectively (Fig 1a). The disease-free one, three, and five-year survival rates were 78.9%, 47.9%, and 40.3%, respectively (Fig 1b).
Figure 1.
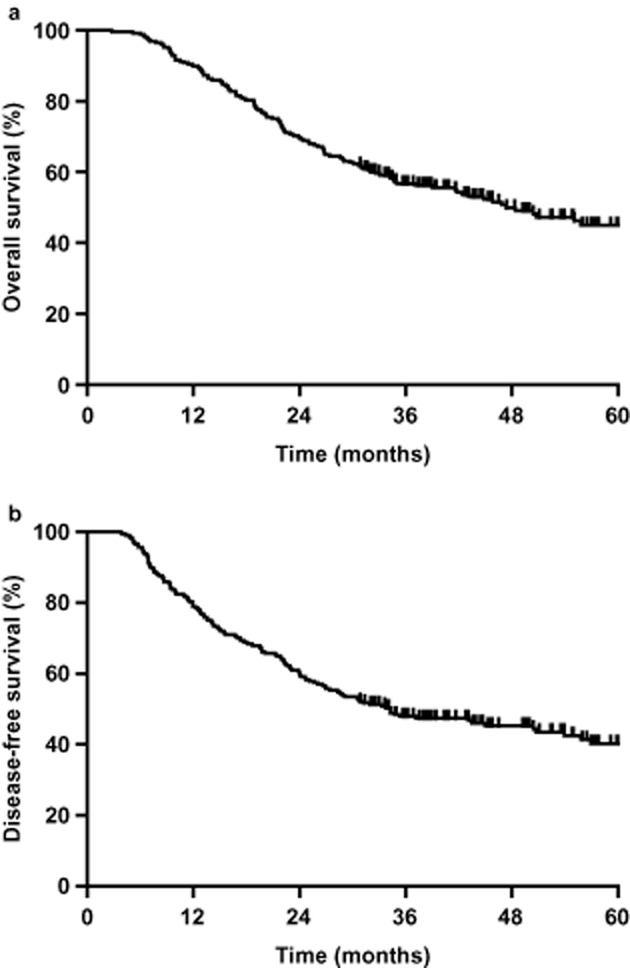
(a) Overall survival (OS) and (b) disease-free survival (DFS) for all patients. The one, three, and five-year OS rates were 89.9%, 56.7%, and 45.1%, respectively (Fig 1a). The one, three, and five-year DFS rates were 78.9%, 47.9%, and 40.3%, respectively (Fig 1b).
Five-year overall survival rates of stage IIa, IIb, and III patients were 72.3%, 42.7%, and 38.0%, respectively (P < 0.01, Fig 2a). The median OS of stage IIb and III was 55.0 and 34.8 months, respectively. Five-year DFS rates of stage IIa, IIb, and III patients were 65.3%, 43.4%, and 32.7%, respectively (P < 0.001, Fig 2b).
Figure 2.
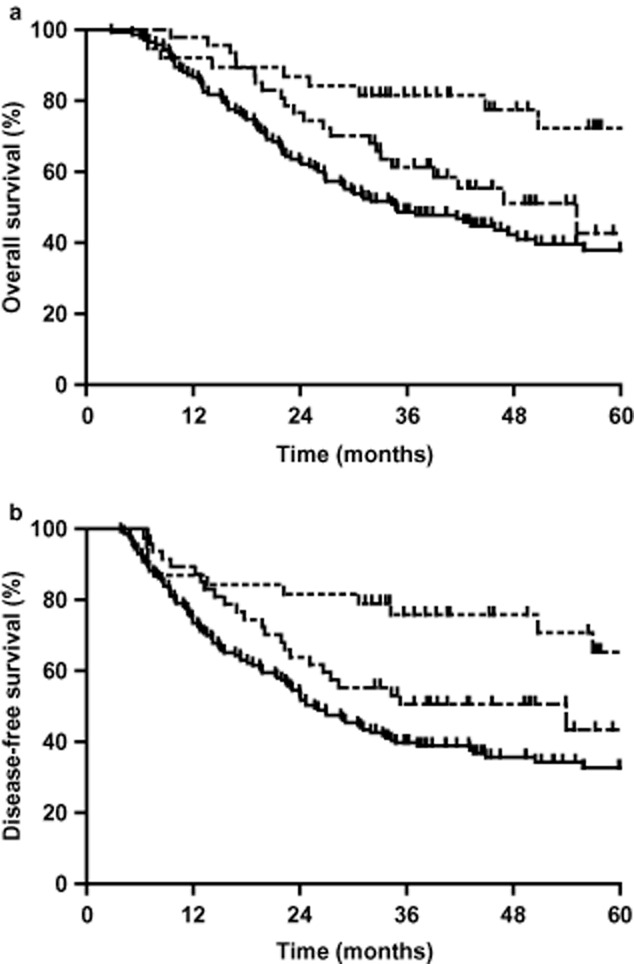
Overall survival (OS) and disease-free survival (DFS) rates of the stage IIa, IIb, and III patients. (a) The five-year OS rates were 65.6%, 42.7%, and 38.0%, respectively, (P < 0.01). (b) The one, three, and five-year DFS rates were 82.1%, 55.5%, and 49.3%, respectively (P < 0.01).  , IIa stage;
, IIa stage;  , IIb stage;
, IIb stage;  , III stage.
, III stage.
Influence of pathologic lymph node status on survival
A total of 5298 lymph nodes were removed from the 228 patients (mean, 22 nodes per patient; range, 3–89 nodes). The cases with and without nodal metastases were 186 (81.6%) and 42 (18.4%), respectively. The one, three, and five-year survival rates of patients with negative nodes were 92.9%, 80.6%, and 71.9%, respectively. The one, three, and five-year survival rates of patients with positive nodes were 89.2%, 51.3%, and 38.9%, respectively. Median survival in patients with nodal metastases was 39 months, and less than half of the patients without nodal metastases died during follow-up (P < 0.001, Fig 3a). The disease-free one, three, and five-year survival rates of patients with negative nodes were 88.1%, 75.4%, and 65.6%, respectively. The disease-free one, three, and five-year survival rates of patients with positive nodes was 76.9%, 41.7%, and 34.7%, respectively (P < 0.001, Fig 3b).
Figure 3.
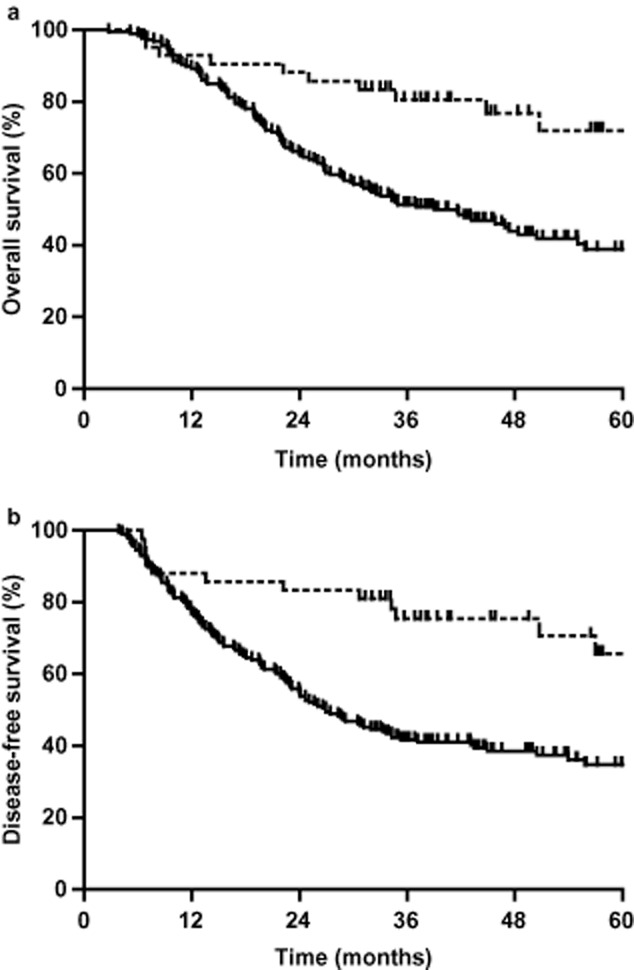
(a) One, three, and five-year overall survival (OS) rates and (b) disease-free survival (DFS) rates of patients with negative or positive lymph nodes. The one, three, and five-year OS rates of patients with negative lymph nodes were 93.6%, 76.0%, and 66.0%, respectively; the one, three, and five-year OS rates of patients with positive lymph nodes were 89.2%, 51.3%, and 38.9%, respectively (Fig 3a, P < 0.01). The one, three, and five-year DFS rates of patients with negative lymph nodes were 91.2%, 81.8%, and 73.6%, respectively; the one, three, and five-year DFS rates of patients with positive lymph nodes were 79.7%, 48.3%, and 42.9%, respectively (P < 0.001, Fig 3b).  , NO;
, NO;  , N+.
, N+.
Univariate and multivariate analyses of prognostic factors
Univariate analysis revealed the following significant prognostic factors, including UICC 2002 stage, lymphatic metastasis, the number of metastatic lymph nodes, the degree of metastatic lymph nodes, the degree of differentiation, and vascular tumor thrombus (χ2 = 13.270, 12.150, 15.138, 6.836, 6.333, 13.616; P = 0.001, 0.000, 0.002, 0.009, 0.042, 0.000; Table 2). Multivariate analysis revealed that UICC 2002 stage and vascular tumor thrombus were independent prognostic factors (χ2 = 7.655, 6.434; P = 0.0.006, 0.011; Table 3).
Table 2.
Univariate analysis of prognostic factors and survival
| Variable | No. | Survival rate (%) | Median survival time (months) | χ2 value | P value | ||
|---|---|---|---|---|---|---|---|
| 1 year | 3 year | 5 year | |||||
| Gender | 1.433 | 0.231 | |||||
| Male | 195 | 90.3 | 55.0 | 43.2 | 45.8 | ||
| Female | 33 | 87.9 | 66.7 | 55.6 | 75.0 | ||
| Age (years) | 1.734 | 0.629 | |||||
| ≤50 | 49 | 89.8 | 55.0 | 46.1 | 44.8 | ||
| 51–60 | 98 | 88.8 | 63.9 | 43.9 | 50.5 | ||
| 61–70 | 65 | 90.8 | 50.3 | 46.0 | 41.8 | ||
| >70 | 16 | 93.8 | 43.8 | 43.8 | 30.1 | ||
| Location | 0.683 | 0.711 | |||||
| Upper | 24 | 87.5 | 58.3 | 30.2 | 42.4 | ||
| Middle | 119 | 90.8 | 57.7 | 49.0 | 50.8 | ||
| Lower | 85 | 89.4 | 54.8 | 40.5 | 48.4 | ||
| Differentiation | 6.333 | 0.042 | |||||
| High | 39 | 92.3 | 71.8 | 45.0 | 55.8 | ||
| Middle | 121 | 92.6 | 57.4 | 48.3 | 47.3 | ||
| Low | 68 | 83.8 | 46.7 | 40.2 | 30.1 | ||
| Degree of metastatic lymph nodes (%) | 6.836 | 0.009 | |||||
| ≤20% | 195 | 90.8 | 60.2 | 48.2 | 55.1 | ||
| >20% | 33 | 84.8 | 36.4 | 27.3 | 29.0 | ||
| Vascular tumor thrombus | 13.616 | 0.000 | |||||
| No | 186 | 90.3 | 62.0 | 50.4 | 64.5 | ||
| Yes | 42 | 88.1 | 33.3 | 21.4 | 24.4 | ||
| UICC02 T-stage | 0.422 | 0.516 | |||||
| T1+T2 | 48 | 97.9 | 60.1 | 41.8 | 55.1 | ||
| T3+T4 | 180 | 87.8 | 55.8 | 45.4 | 47.3 | ||
| UICC02 N-stage | 12.150 | 0.000 | |||||
| N0 | 42 | 92.9 | 80.6 | 71.9 | — | ||
| N1 | 186 | 89.2 | 51.3 | 38.9 | 39.0 | ||
| Number of metastatic lymph nodes | 15.138 | 0.002 | |||||
| 0 | 42 | 92.9 | 80.6 | 71.9 | — | ||
| 1–2 | 110 | 90.9 | 56.9 | 40.1 | 45.9 | ||
| 3–6 | 64 | 85.9 | 42.2 | 40.1 | 26.9 | ||
| 7- | 12 | 91.7 | 50.0 | 37.5 | 29.8 | ||
| UICC02 stage | 13.270 | 0.001 | |||||
| IIA | 38 | 92.1 | 81.6 | 72.3 | — | ||
| IIB | 47 | 97.9 | 61.3 | 42.7 | 55.1 | ||
| III | 143 | 86.7 | 48.7 | 38.8 | 34.8 | ||
UICC, International Union Against Cancer.
Table 3.
Multivariate analyses of prognostic factors
| Prognostic factors (group) | P value | HR value | 95% CI |
|---|---|---|---|
| Differentiation (H/M/L) | 0.078 | 1.294 | 0.971–1.724 |
| Vascular tumor thrombus (No/Yes) | 0.011 | 1.717 | 1.131–2.607 |
| UICC02 stage (IIA/IIB/III) | 0.006 | 1.502 | 1.126–2.005 |
HR, hazard ratio; UICC, International Union Against Cancer.
Site of failure
Among the 228 patients, 217 patients were included for a study of site and cause of failure because 11 patients died from unspecified death. Treatment failure occurred in 98 (45.2%) patients because of recurrence or metastases (Table 4). Fifty-eight patients (26.7%) developed a hematogenous recurrence, including nine patients (4.1%) with simultaneous locoregional and hematogenous recurrence; 26 (12.0%) with recurrence in the supraclavicular (12, 5.5%) and celiac (14, 6.5%) lymph nodes; and 27 patients (12.4%) developed intrathoracic recurrence.
Table 4.
Analysis of failure site (98/217)
| Type of failure | No. (%) |
|---|---|
| Intrathoracic recurrence | 27 (12.4%) |
| Supraclavicular and celiac recurrence | 26 (12.0%), 7 patients with simultaneous hematogenous recurrence |
| Supraclavicular | 12 (5.5%) |
| Celiac | 14 (6.5%) |
| Hematogenous recurrence | 58 (26.7%), 49 patients with hematogenous recurrence alone |
Toxicity
Early reactions related to radiation treatment were observed at rates of 18.0% (41/228) for radiation esophagitis, including 33 for grade 2, eight for grade 3; 13 (5.7%) for radiation pneumonitis more than grade 2; and 63 (27.6%) for leucopenia, including nine for grade 3. Late side effects included anastomotic stenosis and gastrointestinal bleeding. The frequency of anastomotic stenosis higher than grade 2 in all patients in this study was 1.3% (3/228). Seven patients died from gastrointestinal bleeding (3.1%, 7/228).
Discussions
We have reported previously that postoperative conventional radiotherapy reduced local-regional recurrence and increased the survival rate in lymph node positive and stage III patients with esophageal cancer; however, the frequency of local-regional recurrence was still high. To investigate the clinical therapeutic outcomes of IMRT to resected stage II/III TESCC, we retrospectively studied resected TESCC patients treated with IMRT. To our knowledge, this is the first report detailing the treatment outcome of postoperative prophylactic IMRT of TESCC patients. We found that postoperative prophylactic IMRT of resected TESCC provided a favorable local control rate and acceptable toxicity.
The effect of PORT on esophageal cancer is constantly debated. While several prospective randomized studies using 2D–RT indicated that PORT could decrease local recurrence, discrepancy exists as to whether five-year OS is improved.8–11 Chen et al. retrospectively analyzed 1715 patients who had undergone extended esophagectomies with three field lymph node dissection with (n = 438) or without (n = 1277) PORT and found that PORT can improve OS for patients with poor disease-related prognostic factors: positive nodal disease, three or more positive lymph nodes, stage III/IV, and largely or deeply invading tumors. For patients with stage III/IV disease, five-year OS rates were 27.9% for surgery only versus 37.2% for surgery plus radiation subgroups (P < 0.001).13 Schreiber et al. used the Surveillance, Epidemiology, and End Results database to retrospectively evaluate the benefit of adjuvant RT in T3–4N0M0 or T1–4N1M0 esophageal cancer patients definitively treated with esophagectomy. The study involved 1046 patients: 683 (65.3%) had surgery alone, and 363 (34.7%) received PORT. For American Joint Committee on Cancer (AJCC) stage III esophageal carcinoma (T3N1M0 or T4N0–1M0), in which 346 patients had surgery alone and 231 patients received PORT, PORT significantly improved the median OS from 15 to 19 months and the three-year OS rate from 18.2% to 28.9% (P < 0.001). This benefit was evident in both squamous cell carcinoma and adenocarcinoma.14 Xiao et al. reported that PORT improved OS in lymph node positive or stage III patients.9
In all of the previous studies mentioned, 2D-RT was applied. In the current study, IMRT was used. Mechanistically, IMRT applies multiple beams (typically 5–7), which are modulated further, and computer-controlled multileaf collimation to dynamically block the path of the radiation when the beam is on. This effectively allows the dose to be “painted” with various intensities, thus producing the greatest treatment conformality to the tumor and avoiding normal structures. It has been reported that IMRT is superior to 3DCRT in dosiology and can better protect normal tissue;17 therefore, IMRT protects normal tissues, leading to a low incidence of adverse events.18 Lin et al. analyzed 676 nonrandomized patients (3DCRT, n = 413; IMRT, n = 263) with esophageal cancers who were treated with chemoradiotherapy and found that 3DCRT treated patients had a significantly greater risk of mortality (72.6% vs. 52.9%, P < 0.0001) and local-regional recurrence (P = 0.0038) compared to IMRT treated patients, and that the cumulative incidence of cardiac death increased in patients receiving 3DCRT.16
We assumed that extensive portal in postoperative 2D-RT devoted to late toxicity. As such, in this study, we diminished the radiation portal, including only the bilateral supraclavicular areas and 3.0 cm below the lower margin of the tumor as the lower border or 2.0∼3.0 cm below the carina for patients with stage IIA and negative lymph nodes. This area, which included the mediastinum superius and bilateral supraclavicular, is difficult to dissect extensively and has a high risk for recurrence. In this study, IMRT was applied to replace the conventional RT. As reported previously, we found that the three and five-year survival rates of patients with stage IIA TESCC were 81.6% and 72.3%, respectively, which were higher than the rates associated with 2D-RT (64.0% and 50.3%) or surgery alone (56.0% and 51.3%).9 However, another study demonstrated that PORT cannot improve the five-year OS of thoracic esophageal squamous cell carcinoma at stages I-II.13 Therefore, further study is required to determine whether patients with stage IIA squamous cell carcinoma could benefit from PORT.
Several retrospective studies have reported that PORT can improve survival in patients in stage III, such as the five-year OS reported by Chen et al., Schreiber et al., and Xiao et al.9,13,14 In our study, the five-year OS rates of resected stage III esophageal cancer patients following postoperative prophylactic IMRT were 38.0%, which was slightly higher than in patients treated with 2D-PORT, suggesting that postoperative IMRT might be beneficial to the improvement of OS in patients with esophageal cancer in stage III.
The 7th edition of 2009 UICC/AJCC esophageal cancer staging system defined N as N1, N2, and N3, according to the number of positive lymph nodes.19 The same stage III cancer may lead to different prognoses as a result of the different rate and numbers of lymph node metastases.5,20,21 The five-year OS rates of patients with lymph nodes equal or greater than three receiving IMRT was 38.5%, which was higher than the five-year OS (20.6%) of patients receiving 2D-RT in previous studies,13,21 suggesting that IMRT for PORT in esophageal cancer with lymph nodes equal or greater than three is superior to 2D-RT.
In this study, the intrathoracic recurrence rate was 12.4% after postoperative IMRT and 13.7% in patients with positive lymph nodes. In our previous study using 2D-RT, the intrathoracic recurrence rate of patients with stage N0 and N1 were 13.3% and 21.5%, respectively,9 and 25–40%2,3,22 after surgery alone without any postoperative RT. These results indicate that postoperative IMRT tends to decrease intrathoracic recurrence, thus improving OS rates. Our results showed that 26.7% of patients underwent hematogenous recurrence, which was the main contributing cause for failure in the postoperative IMRT group. This result was in accordance with findings in a study in which postoperative CRT appears to prolong survival in patients with lymph node positive and resected esophageal carcinoma.23 Other studies have shown that postoperative adjuvant chemoradiotherapy, in combination with esophagectomy, prolonged survival duration and recurrence-free survival in patients with locoregionally advanced or node-positive esophageal carcinoma.24,25 Future studies are warranted to evaluate the effect of postoperative adjuvant chemoradiotherapy on patients with high-risk hematogenous metastasis.
IMRT was found to protect the tissue from radiation-induced toxicity more efficiently than routine radiotherapy. The frequency of anastomotic stenosis higher than grade 2 in all patients in this study was 1.3%, which was lower than 4.0% found in a previous study.9 Higher toxicity rates of 25.9% (92/355) in radiation esophagitis and 22.8% (81/355) in radiation pneumonitis were also found in a previous study.9,13 Higher toxicity may result from the use of routine radiotherapy. However, in the present study, we found that IMRT caused 3.1% gastrointestinal bleeding, which was higher than gastrointestinal bleeding rates of 1.1%9 and 1.0%26 reported previously. This may have resulted from a high dose of radiation to the stomach because we used a median dose of 60 Gy of RT, while previous studies used 50 Gy.26 Therefore, IMRT as PORT should be used with caution.
Conclusions
We have demonstrated that postoperative IMRT may be a promising technology with acceptable toxicity in the treatment of postoperative TESCC. Our findings in this study suggest that postoperative IMRT may be used as a standard treatment strategy for patients with stage III esophageal cancer or positive lymph nodes after surgery. However, the advantages of postoperative IMRT as treatment for patients at stage II or with negative lymph nodes requires further confirmation by a larger patient population.
Acknowledgments
This work is supported by grants from the Chinese National Natural Sciences Foundation (Grant no. 81021061, 39925020) and the Science and Technology Project of Beijing (No. Z121107001012004).
Disclosure
No authors report any conflict of interest.
References
- Visbal AL, Allen MS, Miller DL, et al. Ivor Lewis esophagogastrectomy for esophageal cancer. Ann Thorac Surg. 2001;71:1803–1808. doi: 10.1016/s0003-4975(01)02601-7. [DOI] [PubMed] [Google Scholar]
- Clark GW, Peters JH, Ireland AP, et al. Nodal metastasis and sites of recurrence after en bloc esophagectomy for adenocarcinoma. Ann Thorac Surg. 1994;58:646–653. doi: 10.1016/0003-4975(94)90722-6. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kanda T, Kosugi S, Ohashi M, Suzuki T, Hatakeyama K. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg. 2004;198:205–211. doi: 10.1016/j.jamcollsurg.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Doki Y, Ishikawa O, Takachi K, et al. Association of the primary tumor location with the site of tumor recurrence after curative resection of thoracic esophageal carcinoma. World J Surg. 2005;29:700–707. doi: 10.1007/s00268-005-7596-4. [DOI] [PubMed] [Google Scholar]
- Smit JK, Pultrum BB, van Dullemen HM, Van Dam GM, Groen H, Plukker JT. Prognostic factors and patterns of recurrence in esophageal cancer assert arguments for extended two-field transthoracic esophagectomy. Am J Surg. 2010;200:446–453. doi: 10.1016/j.amjsurg.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: Five-year survival of a randomized clinical trial. Ann Surg. 2007;246:992–1000. doi: 10.1097/SLA.0b013e31815c4037. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Yoshimura H, Kinugasa S, et al. Postoperative chemotherapy vs chemoradiotherapy for thoracic esophageal cancer: A prospective randomized clinical trial. Eur J Surg Oncol. 2003;29:580–587. doi: 10.1016/s0748-7983(03)00111-2. [DOI] [PubMed] [Google Scholar]
- Xiao ZF, Yang ZY, Liang J, et al. Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. Ann Thorac Surg. 2003;75:331–336. doi: 10.1016/s0003-4975(02)04401-6. [DOI] [PubMed] [Google Scholar]
- Ténière P, Hay JM, Fingerhut A, Fagniez PL. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet. 1991;173:123–130. [PubMed] [Google Scholar]
- Fok M, Sham JS, Choy D, Cheng SW, Wong J. Postoperative radiotherapy for carcinoma of the esophagus: A prospective, randomized controlled study. Surgery. 1993;113:138–147. [PubMed] [Google Scholar]
- Zieren HU, Müller JM, Jacobi CA, Pichlmaier H, Müller RP, Staar S. Adjuvant postoperative radiation therapy after curative resection of squamous cell carcinoma of the thoracic esophagus: A prospective randomized study. World J Surg. 1995;19:444–449. doi: 10.1007/BF00299187. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhu J, Pan J, et al. Postoperative radiotherapy improved survival of poor prognostic squamous cell carcinoma esophagus. Ann Thorac Surg. 2010;90:435–442. doi: 10.1016/j.athoracsur.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Schreiber D, Rineer J, Vongtama D, et al. Impact of postoperative radiation after esophagectomy for esophageal cancer. J Thorac Oncol. 2010;5:244–250. doi: 10.1097/JTO.0b013e3181c5e34f. [DOI] [PubMed] [Google Scholar]
- Sura S, Gupta V, Yorke E, Jackson A, Amols H, Rosenzweig KE. Intensity-modulated radiation therapy (IMRT) for inoperable non-small cell lung cancer: the Memorial Sloan-Kettering Cancer Center (MSKCC) experience. Radiother Oncol. 2008;87:17–23. doi: 10.1016/j.radonc.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84:1078–1085. doi: 10.1016/j.ijrobp.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole TP, Aghayere O, Kwah J, Yorke ED, Goodman KA. Comparison of heart and coronary artery doses associated with intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83:1580–1586. doi: 10.1016/j.ijrobp.2011.10.053. [DOI] [PubMed] [Google Scholar]
- Veldeman L, Madani I, Hulstaert F, De Meerleer G, Mareel M, De Neve W. Evidence behind use of intensity-modulated radiotherapy: A systematic review of comparative clinical studies. Lancet Oncol. 2008;9:367–375. doi: 10.1016/S1470-2045(08)70098-6. [DOI] [PubMed] [Google Scholar]
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, editors. AJCC Cancer Staging Manual (7th ed) New York: Springer; 2010. [Google Scholar]
- Mariette C, Balon JM, Piessen G, Fabre S, Van Seuningen I, Triboulet JP. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer. 2003;97:1616–1623. doi: 10.1002/cncr.11228. [DOI] [PubMed] [Google Scholar]
- Xiao ZF, Yang ZY, Miao YJ, et al. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: Report of 549 cases. Int J Radiat Oncol Biol Phys. 2005;62:82–90. doi: 10.1016/j.ijrobp.2004.08.046. [DOI] [PubMed] [Google Scholar]
- Chen G, Wang Z, Liu XY, Liu FY. Recurrence pattern of squamous cell carcinoma in the middle thoracic esophagus after modified Ivor-Lewis esophagectomy. World J Surg. 2007;31:1107–1114. doi: 10.1007/s00268-006-0551-1. [DOI] [PubMed] [Google Scholar]
- Bedard EL, Inculet RI, Malthaner RA, Brecevic E, Vincent M, Dar R. The role of surgery and postoperative chemoradiation therapy in patients with lymph node positive esophageal carcinoma. Cancer. 2001;91:2423–2430. [PubMed] [Google Scholar]
- Rice TW, Adelstein DJ, Chidel MA, et al. Benefit of postoperative adjuvant chemoradiotherapy in locoregionally advanced esophageal carcinoma. J Thorac Cardiovasc Surg. 2003;126:1590–1596. doi: 10.1016/s0022-5223(03)01025-0. [DOI] [PubMed] [Google Scholar]
- Chen J, Pan J, Liu J, et al. Postoperative radiation therapy with or without concurrent chemotherapy for node-positive thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2013;86:671–677. doi: 10.1016/j.ijrobp.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Chen J, Pan J, Zheng X, et al. Number and location of positive nodes, postoperative radiotherapy, and survival after esophagectomy with three-field lymph node dissection for thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:475–482. doi: 10.1016/j.ijrobp.2010.08.037. [DOI] [PubMed] [Google Scholar]


