Abstract
Background
We have occasionally encountered advanced lung cancer patients with disseminated carcinomatosis throughout the body and/or within the lung. This study investigated the clinical characteristics and outcomes of advanced lung adenocarcinoma patients with miliary disseminated carcinomatosis.
Methods
Patients with adenocarcinomas harboring epidermal growth factor receptor (EGFR) mutations who presented with miliary disseminated carcinomatosis (either intrapulmonary or distant site) were enrolled in the study. Clinical characteristics, treatment responses, and survival outcomes were collected from medical records.
Results
The most frequent EGFR mutation was an in-frame deletion in exon 19 (n = 44, 68.8%). Arginine substitution of leucine 858 in exon 21 and alanine substitution of glycine 719 in exon 18 were detected in 19 patients (29.7%) and one patient (1.6%), respectively. Patients with miliary disseminated carcinomatosis tended to be female and non-smokers. They expressed the E19 deletion more frequently than patients without miliary dissemination and had shorter progression-free survival times in response to EGFR tyrosine kinase inhibitors (9.7 vs. 12.8 months, P = 0.003) and poorer overall survival (15.9 vs. 29.0 months, P = 0.077). Multivariate analyses revealed that metabolic tumor volume correlated with shorter overall survival time.
Conclusions
Our data indicate that lung adenocarcinoma patients with miliary dissemination have relatively shorter survival times than those without miliary dissemination. The poor prognosis of patients with miliary dissemination may reflect a high tumor burden, as represented by metabolic tumor volume.
Keywords: Carcinomatosis, epidermal growth factor, lung adenocarcinoma, miliary, receptor
Introduction
Lung cancer is the most common cause of cancer death among South Koreans. According to the Korea National Statistical Office, in 2010, 20 711 individuals were diagnosed with lung cancer, and 15 867 died from lung cancer.1 Since 2010, the mortality rate has increased steadily, while the death rate has dropped only slightly. Approximately two-thirds of non-small cell lung cancer (NSCLC) patients present with advanced disease at initial diagnosis. These patients have a median survival time of four to five months and a one-year survival rate of 10% when managed with best supportive care alone.2
Chest radiographs of pulmonary metastases of lung cancer show multiple pulmonary nodules, interlobular septal thickening, pleural effusions, and enlarged lymph nodes.3,4 Metastatic pulmonary nodules are usually multiple, 3 mm to >10 cm in size, and randomly distributed via hematogenous spread. In rare cases, numerous tiny discrete nodules appear in a pattern analogous to that of miliary tuberculosis. Some investigators have termed this pattern “miliary intrapulmonary carcinomatosis” (MIPC). The micronodules are generally uniform in size (<5 mm) and diffusely distributed throughout the lungs on contrast-enhanced chest computed tomography (CT) scans.5 They appear to result from a type of hematogenous spread, but may be associated with adenocarcinoma and epidermal growth factor receptor (EGFR) mutations in NSCLC patients.6–10 Although the prognosis of NSCLC patients with MIPC is extremely poor, some studies report good responses to EGFR-tyrosine kinase inhibitor (TKI) therapy.8,11,12
We have occasionally encountered advanced lung cancer patients with multiple miliary metastatic lesions on distant organs.13–15 We collectively refer to miliary intrapulmonary and distant site metastases as “milary dissemination carcinomatosis” and describe its clinical phenotype in lung adenocarcinomas with EGFR mutations in this study. We also compare the response of miliary and non-miliary disseminated carcinomatosis to EGFR-TKIs.
Materials and methods
Patients
Patients with pathologically confirmed lung adenocarcinoma with activating EGFR gene mutations treated at Konkuk University Hospital between September 2005 and June 2012 were enrolled in the study. Patients at stages I–III were excluded. Clinical data collected included patient demographics, smoking status, comorbidities, outcome, EGFR mutation subtype, and tumor response to EGFR-TKIs evaluated according to Response Evaluation Criteria in Solid Tumors, version 1.1.16 For comparative purposes, the medical records of advanced lung adenocarcinoma patients without miliary dissemination treated between September 2005 and June 2012 were also reviewed. The institutional ethics committee at Konkuk University Hospital approved this study.
Image-based criteria for miliary disseminated carcinomatosis of lung adenocarcinoma
A pulmonologist and a radiologist who were blinded to the results of EGFR mutation analysis and clinical course reviewed all images. MIPC was defined as: (i) tiny discrete pulmonary micronodules generally uniform in size (≤5 mm) and diffusely distributed throughout the lungs on contrast-enhanced chest CT scans5 (Fig 1); (ii) numerous nodules not easily countable on CT scans; and (iii) absence of unilateral intrapulmonary carcinomatosis, multifocal ground-glass opacities, and lymphangitic carcinomatosis.5,7–9 The same criteria defined multiple miliary metastatic lesions in other organs; however, different imaging modalities were used. Bone was analyzed via a whole body bone scan and positron emission tomography (PET)-CT, the liver and adrenal glands via contrast-enhanced abdominal CT, and the brain via gadolinium-enhanced brain magnetic resonance imaging.
Figure 1.
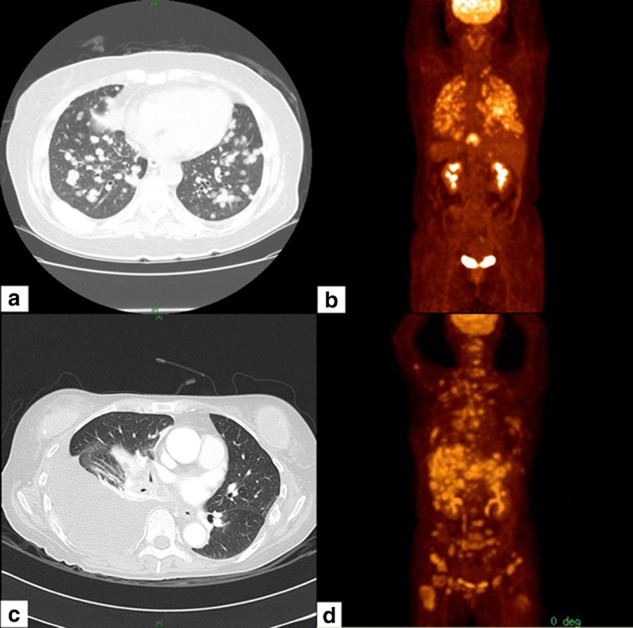
Miliary disseminated carcinomatosis of a lung adenocarcinoma. (a) A computed tomography (CT) scan, and (b) positron emission tomography (PET)-CT showing lung cancer with miliary intrapulmonary carcinomatosis. (c) A CT scan, and (d) PET-CT showing miliary disseminated metastasis in the liver and bone of a lung cancer patient without intrapulmonary metastasis.
Clinical definitions
We defined patients who had smoked fewer than 100 cigarettes in their lifetime as non-smokers, patients who had smoked cigarettes within a year of diagnosis as current smokers, and the remaining patients as former smokers. Disease stages were determined according to the 7th Lung Cancer Tumor Node Metastasis Classification and Staging System.
Epidermal growth factor receptor (EGFR) mutation analysis
After diagnosis of lung adenocarcinoma via lung mass biopsy, metastatic sites or pleural effusion cytology, DNA was extracted from tissue samples or cytology slides containing tumor cells. The polymerase chain reaction primer sequences used for amplification of EGFR mutation sites were as follows: exon 18, 5′-biotin-GCTCCCAACCAAG-CTCTCTT-3′ (forward) and 5′-TATACACCGTGCCGAACGC-3′ (reverse); exon 19, 5′-GCATGTGGC-ACCATCTCA-3′ (forward) and 5′-biotin-AAAAGGTGGGCCTGAGGTT-3′ (reverse); exon 20, 5′- biotin-ATGGCCAGCGTGGACAAC-3′ (forward) and 5′-TTTGTGTTCCCGGACATAGTC-3′ (reverse); and exon 21, 5′- ACCGCAGCATGTCAAGATCAC-3′ (forward) and 5′-biotin-TCCGCACCCAGCA-GTTTG-3′ (reverse). The samples were analyzed using the PyroMark ID system (Biotage, Uppsala, Sweden) and a single nucleotide polymorphism reagent kit (Biotage). Procedures were performed according to the manufacturer’s instructions.
Statistical analyses
Associations between miliary disseminated carcinomatosis and EGFR mutation, clinical characteristics (gender, smoking status, tumor stage), and pathologic subtype were assessed using the Chi-square test. Associations between miliary disseminated carcinomatosis and clinical responses to EGFR-TKIs were assessed using Fisher’s exact test. The Kaplan–Meier method and log-rank test were used to determine overall survival (OS). Potential prognostic factors were identified by univariate and multivariate Cox regression analysis. All analyses were performed using SAS version 9.3 software (SAS Institute Inc., Cary, NC, USA).
Results
Clinical characteristics of patients
From September 2005 to June 2012, 490 patients were diagnosed with lung adenocarcinoma at Konkuk University Hospital. Of these, 357 underwent EGFR mutation testing that required both informed consent and adequate tissue samples; 94 (26.3%) harbored activating EGFR mutations. After excluding 30 patients with stage I–III disease, the study group consisted of 64 patients with stage IV disease (Table 1) and included 39 women (60.9%) and 38 non-smokers (59.4%). Median age was 65 years (range 39–84 years). Fifteen patients (24.4%) presented with miliary disseminated carcinomatosis of lung adenocarcinoma at initial diagnosis; 12 (80%) were women and 12 (80%) were non-smokers. There were no statistically significant differences in gender, age or smoking status of patients with miliary or non-miliary carcinomatosis, although the former were more likely to be female (80.0% vs. 55.1%, P = 0.084) and non-smokers (80.0% vs. 53.1%, P = 0.063).
Table 1.
Demographic factors of patients with disseminated carcinomatosis
| Variables | Miliary type | Non-miliary type | Total | P-value |
|---|---|---|---|---|
| Age (year) | ||||
| Mean ± SD | 60.1 ± 8.6 | 65.6 ± 12.0 | 62.7 ± 9.8 | 0.108 |
| Median (Range) | 63 (47–76) | 67 (39–84) | 65 (39–84) | |
| Gender | ||||
| Male | 3 (20.0%) | 22 (44.9%) | 25 (39.1) | 0.084 |
| Female | 12 (80.0%) | 27 (55.1%) | 39 (60.9%) | |
| Smoking status | ||||
| Never smokers | 12 (80.0%) | 26 (53.1%) | 38 (59.4%) | 0.063 |
| Former or current smokers | 3 (20.0%) | 23 (46.9%) | 26 (40.6%) | |
| Total | 15 (23.4%) | 49 (76.6%) | 64 |
SD, standard deviation.
Sites of disseminated carcinomatosis
The most common sites of miliary dissemination were the lung (n = 11, 73.3%), followed by bone (n = 6, 40.0%) (Table 2). Interestingly, four patients had miliary disseminated carcinomatosis in the brain, bone or liver without lung involvement. In addition to miliary dissemination, 10 patients had distant metastases comprised of single or multiple nodules; the most frequent sites were the brain (n = 6, 40.0%) and bone (n = 5, 33.3%).
Table 2.
Patterns of miliary disseminated carcinomatosis
| No. | Gender/Age | Miliary disseminated organ | Single/multiple metastasis |
|---|---|---|---|
| 1 | Male/47 | Lung, Bone | |
| 2 | Male/63 | Bone | |
| 3 | Female/66 | Brain | |
| 4 | Female/76 | Brain | Adrenal gland (S) |
| 5 | Female/64 | Lung | Brain (M), Bone (M) |
| 6 | Female/55 | Lung, Peritoneum | |
| 7 | Female/71 | Lung, Liver | Bone (M) |
| 8 | Female/64 | Lung | Brain (M) |
| 9 | Female/55 | Lung, Bone, Brain | |
| 10 | Female/50 | Lung, Bone | Brain (M), Abdominal LN (M) |
| 11 | Female/65 | Bone, Liver | Brain (M) |
| 12 | Male/50 | Lung | Bone (M), Brain (M) |
| 13 | Female/50 | Lung | Brain (S) |
| 14 | Female/66 | Lung | Bone (M) |
| 15 | Female/60 | Lung | Bone (M) |
LN, lymph nodes; M, multiple metastasis (at least two metastatic nodules); S, single metastasis.
EGFR mutations and clinical responses to EGFR-tyrosine kinase inhibitors
Epidermal growth factor receptor mutations in the 64 patients in our study group were as follows: in-frame deletions in exon 19 (E19 deletion; 42 patients, 65.7%); arginine substitution of leucine 858 (L858R) in exon 21 (19 patients, 29.7%); and alanine substitution of glycine 719 (G719A) in exon 18 (1 patient, 1.6%) (Table 3). The E19 deletion was more frequent in patients with compared to without miliary disseminated carcinomatosis without statistical significance (86.7% vs. 63.3%, P = 0.07).
Table 3.
EGFR mutations, treatment patterns, and clinical responses to EGFR-TKIs
| Variables | Miliary type | Non-miliary type | Total | P-value |
|---|---|---|---|---|
| EGFR mutation | ||||
| Exon 19 deletions | 13 (86.7%) | 31 (63.3%) | 44 (68.8%) | 0.070 |
| Others | 2 (13.3%) | 18 (36.7%) | 20 (31.2%) | |
| Exon 21 L858R | 2 (13.3%) | 17 (34.7%) | 19 (29.7%) | |
| Exon 18 G719A | 0 (0.00%) | 1 (2.0%) | 1 (1.6%) | |
| Drug selection | ||||
| TKIs alone | 2 (13.3%) | 9 (18.4%) | 11 (17.2%) | 0.205 |
| TKIs→chemotherapy | 1 (6.7%) | 9 (18.4%) | 10 (15.6%) | |
| Chemotherapy→TKIs | 9 (60.0%) | 26 (53.1%) | 35 (54.7%) | |
| Chemotherapy alone | 3 (20.0%) | 2 (4.1%) | 5 (7.8%) | |
| None | 0 (0.0%) | 3 (6.1%) | 3 (4.7%) | |
| Types of EGFR-TKIs | ||||
| Gefitinib | 9 (60.0%) | 37 (75.5%) | 46 (71.9%) | 0.531 |
| Erlotinib | 2 (13.3%) | 5 (10.2%) | 7 (10.9%) | |
| Afatinib | 1 (6.7%) | 2 (4.1%) | 3 (4.7%) | |
| None | 3 (20.0%) | 5 (10.2%) | 8 (12.5%) | |
| Treatment order | ||||
| First line | 3 (25.0%) | 18 (40.9%) | 21 (37.5%) | 0.313 |
| Second line | 9 (75.0%) | 26 (59.1%) | 35 (62.5%) | |
| Maximal response to EGFR-TKIs | ||||
| CR | 0 (0.0%) | 1 (2.0%) | 1 (1.6%) | 0.645 |
| PR | 6 (40.0%) | 23 (46.9%) | 29 (45.3%) | |
| SD | 5 (33.3%) | 13 (26.5%) | 18 (28.1%) | |
| PD | 0 (0.0%) | 4 (8.2%) | 4 (6.3%) | |
| NE | 4 (26.7%) | 8 (16.3%) | 12 (18.8%) | |
| Total | 15 (23.4%) | 49 (76.6%) | 64 |
CR, complete response; EGFR, epidermal growth factor receptor; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease; TKIs, tyrosine kinase inhibitors.
Fifty-six of the 64 patients received EGFR-TKIs during the course of their treatment. Thirty-five patients (54.7%) were treated with EGFR-TKIs as second-line after chemotherapy, 21 patients with EGFR-TKIs in first-line, and cytotoxic chemotherapy was followed in 10 patients. Sixteen patients were re-treated with the same or other EGFR-TKIs in their clinical course after disease progression occurred (5 and 11 in patients with and without miliary dissemination, respectively). The most-prescribed EGFR-TKI was gefitinib (n = 46, 71.9%), followed by erlotinib (n = 7, 10.9%), and afatinib (n = 3, 4.7%). One (1.6%) patient had a complete response, 29 (45.3%) had a partial response, and 18 (28.1%) achieved stable disease. The overall disease control rate was 75.0%; the rate increased to 92.3% when 12 patients whose responses to EGFR-TKIs could not be assessed, were excluded. There was no significant difference between patients with miliary or non-miliary disseminated carcinomatosis.
Overall survival and prognostic factors
The OS of lung adenocarcinoma patients without miliary dissemination was slightly longer than that of the patients with miliary dissemination (Fig 2). Objective response rates (ORR), progression-free survival (PFS) after treatment with EGFR-TKIs, and metabolic tumor volume (MTV) were identified as prognostic factors for OS via univariate and multivariate survival analyses (Table 4). Patients with miliary disseminated carcinomatosis had larger tumor burdens (as represented by MTVs) and shorter OS time despite the presence of activating EGFR mutations, compared with patients without miliary dissemination.
Figure 2.
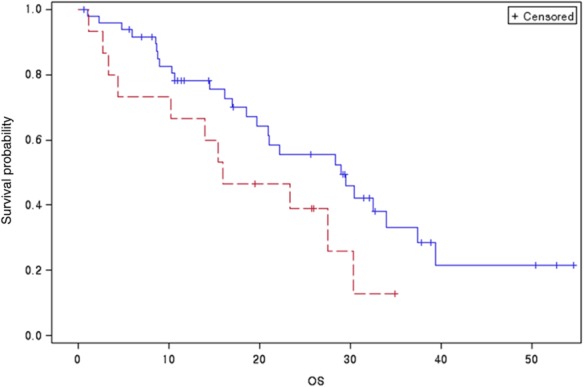
Overall survival curves of patients with miliary or non-miliary dissemination carcinomatosis.  , miliary metastasis;
, miliary metastasis;  , non-miliary metastasis. Log-rank test P-value: 0.077. OS: overall survival.
, non-miliary metastasis. Log-rank test P-value: 0.077. OS: overall survival.
Table 4.
Univariate and multivariate Cox regression analyses for overall survival in lung adenocarcinoma patients with disseminated carcinomatosis
| Factor | Univariate model | Multivariate model | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | P overall | Hazard ratio (95% CI) | P-value | |
| Age | 1.015 (0.986–1.045) | 0.3166 | |||
| Smoking | 0.7231 | ||||
| Never smoker | 0.887 (0.455–1.726) | ||||
| Former or current smoker | |||||
| T stage | |||||
| 1 | 1.487 (0.439–5.036) | 0.5238 | 0.9014 | ||
| 2 | 1.234 (0.565–2.697) | 0.5984 | |||
| 3 | 1.049 (0.312–3.523) | 0.9386 | |||
| 4 | |||||
| N stage | |||||
| 1 | 0.664 (0.306–1.443) | 0.3014 | 0.6402 | ||
| 2 | 0.0000 (0.0000) | 0.9918 | |||
| 3 | 0.639 (0.285–1.428) | 0.2748 | |||
| M stage | |||||
| M1a | 0.726 (0.361–1.457) | 0.3676 | |||
| M1b | |||||
| Miliary dissemination | 1.900 (0.923–3.912) | 0.0816 | |||
| EGFR mutation | |||||
| Exon 18 | 0.397 (0.049–3.198) | 0.3857 | 0.2643 | ||
| Exon 19 | 0.581 (0.292–1.156) | 0.1218 | |||
| Exon 21 | |||||
| 19 Deletion | 0.614 (0.319–1.180) | 0.1432 | |||
| EGFR TKIs | |||||
| None | 2.007(0.221–18.192) | 0.5356 | 0.7245 | ||
| Gefitinib | 1.700(0.230–12.585) | 0.6036 | |||
| Erlotinib | 1.050 (0.118–9.329) | 09650 | |||
| Afatinib | |||||
| Responses to EGFR-TKIs | |||||
| CR + PR | 2.403 (1.131–5.106) | 0.0226 | 2.810 (1.131–6.980) | 0.0261 | |
| SD + PD | |||||
| Progression-free survival | 0.869 (0.813–0.929) | <0.0001 | 0.856 (0.783–0.936) | 0.0007 | |
| Metabolic tumor volume | 1.001 (1.000–1.001) | 0.0470 | 1.001 (1.000–1.002) | 0.0267 | |
After 10% selection via univariate regression, treatment responses to epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR–TKIs), progression-free survival (PFS), metabolic tumor volume (MTV), and miliary dissemination were included in the multivariate model. CI, confidence interval; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Discussion
Several researchers have reported that NSCLC patients with miliary dissemination have a higher EGFR mutation rate, especially those with E19 deletions.8–10 Previous studies have reported that women, non-smokers, patients with adenocarcinoma pathology, and East Asians exhibit the best responses to EGFR-TKI treatment.17–21 In this report, we focused on lung adenocarcinomas with EGFR mutations and miliary disseminated presentations in patients selected from a population of NSCLC lung cancer patients at our institution. Although not statistically significant, miliary disseminated carcinomatosis was more frequent in women and non-smokers than men and smokers, respectively. We suggest that the lack of significance may reflect the small number of patients in our study. Miliary dissemination is an end result of disease progression; however, it may also be a distinct characteristic of lung adenocarcinomas with activating EGFR mutations.
The E19 deletion was present in the lung adenocarcinomas of 86.7% of patients with miliary dissemination, but in only 63.3% of patients without miliary dissemination (P = 0.07, Table 3). Wu et al. reported that this mutation was more frequent in patients with MIPC than in those without MIPC (35% vs. 25%, respectively).8 Laack et al. examined five non-smoking adenocarcinoma patients with MIPC and the E19 deletion.9 Their results agree with ours, and we suggest that this mutation contributes to miliary dissemination carcinomatosis in lung adenocarcinoma.
As expected, patients with classic activating EGFR mutations had favorable responses to EGFR-TKIs; the disease control rate was 92.3%. Although the E19 deletion, a more favorable subtype than the L858R mutation, was more frequent in patients with miliary dissemination than without, there was no significant difference in ORR to EGFR-TKIs between the two groups.22,23 OS was shorter in patients with miliary dissemination than in patients without miliary dissemination, although not significantly so (P = 0.077, Fig 2). This result is inconsistent with previous studies showing better EGFR-TKI responses in NSCLC patients with intrapulmonary metastases harboring EGFR mutations.8,12 Despite the prevalence of the E19 deletion, the poor prognosis of lung adenocarcinoma patients with miliary disseminating carcinomatosis was likely a result of the tumor burden in our study. Lee et al. reported that high tumor burden (assessed on the basis of MTV determined via PET-CT) was an independent predictor of poor prognosis in lung cancer, regardless of stage or cell type.24 Park et al. measured tumor burden by the number of metastatic sites and found that tumor burden is predictive of inferior survival in NSCLC patients with activating EGFR mutations who received gefitinib.25 We measured tumor burden on the basis of MTV or total lesion glycolysis (TLG) via PET-CT and observed an inverse relationship between MTV and OS (P = 0.0267, Table 4). TLG exhibited the same pattern as MTV. In summary, miliary disseminated carcinomatosis indicates a high disease burden and could be a prognostic factor for poor survival, despite the presence of activating EGFR mutations and initial favorable responses to EGFR-TKIs.
Although lung adenocarcinoma patients with miliary dissemination have relatively poor survival, EGFR-TKIs are the first-line treatment of choice for patients with activating EGFR mutations. In our study, patients showed promising initial responses to EGFR-TKIs despite their disease burdens, and the better the response to EGFR-TKIs (PFS and ORR), the longer overall survival expected (Table 4).
Our study is from a single center, included only a small number of patients, and had a retrospective observational design, which are substantial limitations.
Conclusion
Our data indicate that PFS after EGFR-TKI treatment is shorter and OS is poorer in lung adenocarcinoma patients with miliary dissemination than in those without miliary dissemination. The poor clinical course of patients with miliary disseminating carcinomatosis may be associated with a high tumor burden, as represented by MTV.
Acknowledgments
This work was supported by Konkuk University. The authors would like to thank Hyo-Jin Min for advice on the statistical analyses.
Disclosure
No authors report any conflict of interest.
References
- Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: Incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn L, Pao W, Johnson DH. Neoplasms of the lung. In: Longo DL, Fauci AS, Kasper DL, Hause SL, Jameson L, Loscalzo J, editors. Harrison’s Principles of Internal Medicine. 18th Ed. New York: McGraw-Hill; 2012. pp. 737–753. In: (eds) [Google Scholar]
- Marom EM, Patz EF, Jr, Swensen SJ. Radiologic findings of bronchogenic carcinoma with pulmonary metastases at presentation. Clin Radiol. 1999;54:665–668. doi: 10.1016/s0009-9260(99)91088-7. [DOI] [PubMed] [Google Scholar]
- Quinn D, Gianlupi A, Broste S. The changing radiographic presentation of bronchogenic carcinoma with reference to cell types. Chest. 1996;110:1474–1479. doi: 10.1378/chest.110.6.1474. [DOI] [PubMed] [Google Scholar]
- Andreu J, Mauleón S, Pallisa E, Majó J, Martinez-Rodriguez M, Cáceres J. Miliary lung disease revisited. Curr Probl Diagn Radiol. 2002;31:189–197. [PubMed] [Google Scholar]
- Zompatori M, Bná C, Poletti V, et al. Diagnostic imaging of diffuse infiltrative disease of the lung. Respiration. 2004;71:4–19. doi: 10.1159/000075642. [DOI] [PubMed] [Google Scholar]
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- Wu SG, Hu FC, Chang YL, et al. Frequent EGFR mutations in nonsmall cell lung cancer presenting with miliary intrapulmonary carcinomatosis. Eur Respir J. 2013;41:417–424. doi: 10.1183/09031936.00006912. [DOI] [PubMed] [Google Scholar]
- Laack E, Simon R, Regier M, et al. Miliary never-smoking adenocarcinoma of the lung: Strong association with epidermal growth factor receptor exon 19 deletion. J Thorac Oncol. 2011;6:199–202. doi: 10.1097/JTO.0b013e3181fb7cf1. [DOI] [PubMed] [Google Scholar]
- Togashi Y, Masago K, Kubo T, et al. Association of diffuse, random pulmonary metastases, including miliary metastases, with epidermal growth factor receptor mutations in lung adenocarcinoma. Cancer. 2011;117:819–825. doi: 10.1002/cncr.25618. [DOI] [PubMed] [Google Scholar]
- Umeki S. Association of miliary lung metastases and bone metastases in bronchogenic carcinoma. Chest. 1993;104:948–950. doi: 10.1378/chest.104.3.948. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Takeuchi T, Bandobashi K, et al. Diffuse micronodular pulmonary metastasis of lung adenocarcinoma predicts gefitinib response in association with epidermal growth factor receptor mutations. Anticancer Res. 2006;26:1621–1626. [PubMed] [Google Scholar]
- Kahveci R, Gürer B, Kaygusuz G, Sekerci Z. Miliary brain metastases from occult lung adenocarcinoma: Radiologic and histopathologic confirmation. J Neurosci Rural Pract. 2012;3:386–389. doi: 10.4103/0976-3147.102638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro HB, Paiva TF, Jr, Mamprin GP, Gorzoni ML, Rocha AJ, Lancellotti CL. Carcinomatous encephalitis as clinical presentation of occult lung adenocarcinoma: Case report. Arq Neuropsiquiatr. 2007;65:841–844. doi: 10.1590/s0004-282x2007000500022. [DOI] [PubMed] [Google Scholar]
- Inomata M, Hayashi R, Kambara K, et al. Miliary brain metastasis presenting with calcification in a patient with lung cancer: A case report. J Med Case Rep. 2012;6:279. doi: 10.1186/1752-1947-6-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Armour A. Gefitinib in advanced non-small cell lung cancer: Clinical experience in patients of Asian origin. Asia Pac J Clin Oncol. 2007;3:66–78. [Google Scholar]
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: Biological and clinical implications. Cancer Res. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- Park K, Goto K. A review of the benefit-risk profile of gefitinib in Asian patients with advanced non-small-cell lung cancer. Curr Med Res Opin. 2006;22:561–573. doi: 10.1185/030079906X89847. [DOI] [PubMed] [Google Scholar]
- Bell DW, Lynch TJ, Haserlat SM, et al. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: Molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol. 2005;23:8081–8092. doi: 10.1200/JCO.2005.02.7078. [DOI] [PubMed] [Google Scholar]
- Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:3908–3914. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- Zhu JQ, Zhong WZ, Zhang GC, et al. Better survival with EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small cell lung cancer patients is due to differential inhibition of downstream signals. Cancer Lett. 2008;265:307–317. doi: 10.1016/j.canlet.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Lee P, Weerasuriya DK, Lavori PW, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys. 2007;69:328–333. doi: 10.1016/j.ijrobp.2007.04.036. [DOI] [PubMed] [Google Scholar]
- Park JH, Kim TM, Keam B, et al. Tumor burden is predictive of survival in patients with non-small-cell lung cancer and with activating epidermal growth factor receptor mutations who receive gefitinib. Clin Lung Cancer. 2013;14:383–389. doi: 10.1016/j.cllc.2012.10.007. [DOI] [PubMed] [Google Scholar]


