Abstract
Background
To compare the overall survival (OS) of patients with advanced lung adenocarcinoma in China before and after the approved use of gefitinib, and analyze clinical factors that may affect OS.
Methods
Clinical data of 558 patients with advanced lung adenocarcinoma who received chemotherapy from January 2002 to December 2010 were retrospectively analyzed. According to the matched-pair case-control study design, 255 patients who only received chemotherapy and 255 patients who received gefitinib treatment after its approval were stringently matched by age, gender, and smoking history and enrolled in the study. Clinical factors including age, gender, smoking history, Eastern Cooperative Oncology Group (ECOG) performance status (PS), tumor stage, organ metastasis, and the number of prior chemotherapies were analyzed to determine their correlations with OS.
Results
The median survival time (MST) of the 510 enrolled patients with advanced lung adenocarcinoma was 22.8 months. The MST of the patients who received gefitinib treatment was significantly longer than that of patients who did not receive gefitinib treatment (33.5 vs. 14.1 months, P < 0.001). The OS in patients who received gefitinib treatment was significantly longer than in patients who did not receive gefitinib treatment in almost all clinical factor-based subgroups, including age, gender, smoking history, ECOG PS 0–1, tumor stage, the presence or absence of lung, pleural, bone, brain, adrenal gland and liver metastasis, and the number of prior chemotherapies (all P < 0.001), except in the ECOG PS ≥2 subgroup.
Conclusions
Gefitinib treatment significantly improved the survival of patients with advanced lung adenocarcinoma in China.
Keywords: Adenocarcinoma, gefitinib, non-small cell lung cancer, chemotherapy
Introduction
Lung cancer is one of the most common malignant tumors worldwide; non-small cell lung cancer (NSCLC) accounts for 80–85%, and more than 50% of patients with advanced NSCLC are older than 65 years at the time of diagnosis.1 According to an American epidemiologic investigation, the median age at initial visit was 70 years.2 Distant metastasis had occurred in most NSCLC patients at the time of confirmed diagnosis, and their five-year survival rate was only about 15%.3 A platinum-based third generation chemotherapy regimen is the standard first-line therapy recommended for the treatment of advanced NSCLC, offering a median survival time (MST) of 8–10 months. The standard second-line chemotherapy as represented by docetaxel and pemetrexed has further improved the survival of advanced NSCLC patients from 4.6 months in those who received best supportive care to 6.3–9.2 months.4–6 With further research on the molecular biology of lung cancer, targeted therapy has been applied to clinical study and treatment. Tremendous progress has been made in the clinical use of some epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKI) for the treatment of NSCLC. In March 2005, in China, gefitinib (Iressa, AstraZeneca, London,) was the first EGFR-TKI approved for clinical use as a result of relatively mild adverse reactions (mainly rash and diarrhea) and good tolerance.7
Since its approval in China, more patients with lung cancer, especially those with lung adenocarcinoma, have been treated with gefitinib. However, the benefits of gefitinib in Chinese lung cancer patients have not been reported. Therefore, it is of clinical significance to compare the survival of advanced lung adenocarcinoma patients who received gefitinib treatment after March 2005 with those who did not in order to provide clinical clues for the selection of gefitinib in lung adenocarcinoma patients. In the present study, we collected data of patients with stage IIIB or IV lung adenocarcinoma treated at the Cancer Hospital and Institute of the Chinese Academy of Medical Sciences and Peking Union Medical College between January 2002 and December 2010, who only received chemotherapy before the approved use of gefitinib and those who received gefitinib treatment after its approval. According to gender, age (<60 vs. ≥60 years) and smoking history, they were 1:1 matched for the control study to explore the difference in survival between patients before and after the approved use of gefitinib. At the same time, clinical factors were analyzed as subgroups to determine whether they were correlated with patient survival in an attempt to provide clinical clues for the choice of individualized chemotherapy for advanced lung adenocarcinoma patients.
Materials and methods
Subjects and groups
From January 2002 to December 2010, a total of 14 668 NSCLC patients were admitted to the Cancer Hospital and Institute of the Chinese Academy of Medical Sciences and Peking Union Medical College, including 3191 stage IIIB and IV patients in the Department of Medical Oncology. There were 1286 patients whose pathological types were adenocarcinoma. The inclusion criteria were: cytologically or pathologically confirmed diagnosis of stage IIIB or IV lung adenocarcinoma according to the criteria of the American Joint Committee on Cancer Staging (AJCC); patients who had received at least one chemotherapy regimen; and patients who had been assessed for therapeutic outcome according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.0. The exclusion criteria were: patients who lacked cytological or pathological diagnosis, or patients whose pathological type was not adenocarcinoma; and who had not received systematic treatment specific for advanced lung cancer. Finally, 558 patients met the criteria and their complete clinical and follow-up data were included in this study.
Gefitinib was used as the demarcation of group classification for simple chemotherapy and gefitinib groups. The former included 269 stage IIIB or IV lung adenocarcinoma patients who were admitted between 2002 and 2004, received at least one systemic chemotherapy without any gefitinib treatment, and died before 31 December 2004. The latter included 289 stage IIIB or IV lung adenocarcinoma patients who had received at least one systemic chemotherapy before the use of gefitinib administered orally at a daily dose of 250 mg until the presence of progress for objective evaluation or intolerable toxicity.
To minimize differences between the two groups, the patients in the two groups were 1:1 matched by age (<60 vs. ≥60 years), gender, and smoking history. The random number table determined patient screening. Finally, 255 patients in each group were enrolled in the study.
Tumor responses
Objective tumor responses were evaluated by RECIST 1.0. Overall survival (OS) was defined as the time from the first systemic chemotherapy after objectively confirmed diagnosis of stage IIIB or IV lung adenocarcinoma to death from any reason.
Statistical analysis
The baseline characteristics of the patients included age, gender, smoking history, Eastern Cooperative Oncology Group (ECOG) performance status (PS), clinical stage, organ metastasis, and the number of prior chemotherapy regimens. Clinical stage and organ metastasis are defined as the stage and metastatic site at the time of starting the first systemic chemotherapy after objectively confirmed diagnosis of stage IIIB or IV lung adenocarcinoma, and the number of prior chemotherapy regimens is defined as the number of systemic chemotherapy regimens that patients in the gefitinib group received before approved used of the drug.
All data were analyzed by SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The baseline characteristics of the patients were used for retrospective single and multivariate analyses of OS. Comparisons of the tumor responses between the subgroups were performed by χ2 test. Survival time was analyzed by the Kaplan–Meier method. The log-rank test was used to statistically assess significance between survival curves. Cox regression was used for multivariate analysis. Bilateral P < 0.05 was considered statistically significant. To clarify factors that may affect the benefits of gefitinib, other factors including age, gender, smoking history, ECOG PS, clinical stage, organ metastasis, and the number of prior chemotherapies were analyzed by Cox regression tests in both groups.
Results
This retrospective control study included 255 strictly matched patients in a simple chemotherapy group and 255 patients in a gefitinib group, diagnosed with stage IIIB or IV lung adenocarcinoma according to the criteria described previously. The baseline characteristics of these patients are shown in Table 1.
Table 1.
Baseline characteristics of the patients
| Characteristics | Simple chemotherapy group n = 255 (%) | Gefitinib group n = 255 (%) | P value | |
|---|---|---|---|---|
| Age (year) | Median | 55.9 | 54.6 | |
| Range | 27∼76 | 28∼81 | ||
| <60 | 164 (64.3) | 164 (64.3) | NA | |
| ≥60 | 91 (35.7) | 91 (35.7) | ||
| Gender | Male | 105 (41.2) | 105 (41.2) | NA |
| Female | 150 (58.8) | 150 (58.8) | ||
| Smoking history | No | 197 (77.2) | 197 (77.2) | NA |
| Yes | 58 (22.8) | 58 (22.8) | ||
| ECOG | 0–1 | 243 (95.3) | 238 (93.3) | 0.449 |
| ≥2 | 12 (4.7) | 17 (6.7) | ||
| Stage | IIIB | 16 (6.3) | 21 (8.2) | 0.502 |
| IV | 239 (93.7) | 234 (91.8) | ||
| Organ metastasis | Lung | 98 (38.4) | 135 (52.9) | 0.004 |
| Pleura | 111 (43.5) | 119 (46.7) | 0.543 | |
| Bone | 96 (37.6) | 81 (31.8) | 0.610 | |
| Brain | 66 (25.9) | 60 (23.5) | 0.165 | |
| Adrenal gland | 13 (5.1) | 10 (3.9) | 0.655 | |
| Liver | 27 (10.6) | 17 (6.7) | 0.153 | |
| Prior chemo | 1–2 | 187 (73.3) | 227 (89.0) | <0.001 |
| ≥3 | 68 (26.7) | 28 (11.0) |
NA, Not applicable. ECOG, Eastern Cooperative Oncology Group.
The median age was 55.1 (range 27–81): 55.9 (range 27–76) in the simple chemotherapy group and 54.6 (range 28–81) years in the gefitinib group. Over both groups, there were 164 (64.3%) patients aged under 60 years. The female/male ratio was 58.8% versus 42.2%; 77.2% patients had no smoking history; and 95.3% patients in the simple chemotherapy group and 93.3% patients in the gefitinib group had an ECOG PS of 0–1. Over both groups, 92.7% of patients were clinically classified as stage IV.
Although only three factors (age, gender, and smoking status) were used for matched screening, there was no significant difference in ECOG PS (P = 0.449), clinical stage (P = 0.502), pleural (P = 0.543), bone (P = 0.610), brain (P = 0.165), adrenal gland (P = 0.655), and liver metastases (P = 0.153) between the subgroups. However, there was a significant difference in lung metastasis (P = 0.004). There was also a significant difference in the number of prior chemotherapies between the two groups (P < 0.001). Significantly more patients in the simple chemotherapy group received three or more chemotherapies compared with the gefitinib group (26.7% vs. 11.0%). The most common metastatic sites were the lung, pleura, bone, brain, liver, and adrenal gland in sequence in both groups.
The median survival time (MST) was 22.8 months in all 510 patients (95% confidence interval [CI], 20.7–24.9 months): 14.1 months (95% CI, 12.4–15.9 months) in the simple chemotherapy group versus 33.5 months (95% CI, 31.0–36.0 months) in the gefitinib group (P < 0.001) (Fig 1).
Figure 1.
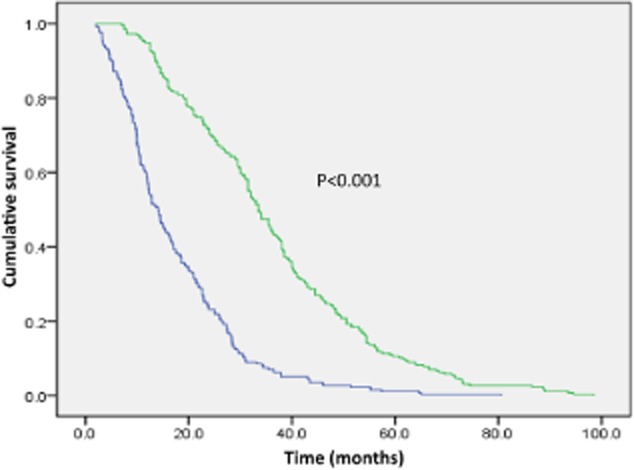
Kaplan–Meier survival curves for overall survival OS comparison between the simple chemotherapy and gefitinib groups.  , Simple chemotherapy group;
, Simple chemotherapy group;  , Gefitinib group.
, Gefitinib group.
Single factor analysis on the survival of the 510 patients revealed that gefitinib treatment was a factor that significantly improved survival. In addition, survival was significantly longer in patients with no smoking history (P = 0.005), associated lung metastasis (P = 0.001), no adrenal gland metastasis (P = 0.014), no liver metastasis (P = 0.009), and the number of prior chemotherapies ≥3 (P < 0.001). Age, gender, ECOG PS, clinical stage, and associated pleural, bone, and brain metastases were not significant factors affecting OS.
Multivariate analysis on the survival of the 510 patients revealed the factors that significantly improved OS included gefitinib treatment (hazard ratio [HR] 0.175, 95% CI 0.140–0.219, P < 0.001), age <60 years (HR 0.787, 95% CI 0.649–0.955, P = 0.015), no smoking history (HR 2.103, 95% CI 1.608–2.750, P < 0.001), no liver metastasis (HR 1.501, 95% CI 1.085–2.077, P = 0.014), and the number of prior chemotherapies ≥3 (HR 0.298, 95% CI 0.230–0.386, P < 0.001) (Table 2).
Table 2.
Multivariate analysis of OS of the 510 patients
| Patients n = (%) | HR (95% CI) | P value | ||
|---|---|---|---|---|
| Total number | 510 (100) | |||
| Group | Simple chemotherapy | 255 (50.0) | 0.175 (0.140–0.219) | <0.001 |
| Gefitinib | 255 (50.0) | |||
| Age (year) | <60 | 328 (64.3) | 0.787 (0.649–0.955) | 0.015 |
| ≥60 | 182 (35.7) | |||
| Gender | Male | 210 (41.2) | 1.243 (0.993–1.555) | 0.058 |
| Female | 300 (58.8) | |||
| Smoking history | No | 394 (77.3) | 2.103 (1.608–2.750) | <0.001 |
| Yes | 116 (22.7) | |||
| ECOG PS | 0–1 | 481 (94.3) | 1.119 (0.761–1.645) | 0.568 |
| ≥2 | 29 (5.7) | |||
| Stage | IIIB | 37 (7.3) | 0.871 (0.589–1.290) | 0.491 |
| IV | 473 (92.7) | |||
| Organ metastasis | No | 277 (54.3) | 0.934 (0.773–1.130) | 0.483 |
| Yes | 233 (45.7) | |||
| Pleural | No | 280 (54.9) | 1.115 (0.913–1.361) | 0.286 |
| Yes | 230 (45.1) | |||
| Bone | No | 333 (65.3) | 1.100 (0.901–1.343) | 0.351 |
| Yes | 177 (34.7) | |||
| Brain | No | 384 (75.3) | 1.099 (0.883–1.368) | 0.399 |
| Yes | 126 (24.7) | |||
| Adrenal gland | No | 487 (95.5) | 1.193 (0.765–1.861) | 0.436 |
| Yes | 23 (4.5) | |||
| Liver | No | 466 (91.4) | 1.501 (1.085–2.077) | 0.014 |
| Yes | 44 (8.6) | |||
| Prior chemo | 1–2 | 414 (81.2) | 0.298 (0.230–0.386) | <0.001 |
| ≥3 | 96 (18.8) |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; OS, overall survival; PS, performance status.
Analyses of OS of the two groups by subgroups of age, gender, smoking history, ECOG PS, clinical stage, organ metastasis, and the number of prior chemotherapies showed that the survival of the patients in the gefitinib group was significantly longer than the patients in the simple chemotherapy group regardless of age (<60 years or ≥60 years), gender, smoking history, clinical stage IIIB or IV, the presence or absence of lung, pleural, bone, brain, adrenal gland, and liver metastasis, or the number of prior chemotherapies 1–2 or ≥3 (P < 0.001). In the ECOG 0–1 subgroup, the survival of patients in the gefitinib group was significantly longer than that of patients in the simple chemotherapy group (P < 0.001). In the ECOG ≥2 subgroup, there was no significant difference in OS between the 12 patients in the simple chemotherapy group and the 17 patients in the gefitinib group (P = 0.090) (Table 3).
Table 3.
Comparison of OS between the simple chemotherapy and gefitinib groups
| Simple chemotherapy MST ( month) 95% CI | Gefitinib MST (mon) 95% CI | HR (95% CI) | P value | ||
|---|---|---|---|---|---|
| Age (year) | <60 | 14.1 (11.4–16.8) | 33.5 (30.9–36.1) | 0.290 (0.229–0.367) | <0.001 |
| ≥60 | 13.0 (10.5–15.4) | 33.5 (28.0–39.0) | 0.348 (0.256–0.475) | <0.001 | |
| Gender | Male | 14.2 (10.2–18.2) | 33.0 (30.2–35.8) | 0.363 (0.273–0.481) | <0.001 |
| Female | 14.1 (12.2–16.0) | 33.9 (30.5–37.3) | 0.273 (0.212–0.352) | <0.001 | |
| Smoking history | No | 14.6 (12.5–16.7) | 35.5 (32.0–39.0) | 0.332 (0.269–0.411) | <0.001 |
| Yes | 9.9 (8.4–11.3) | 30.0 (26.3–33.7) | 0.228 (0.151–0.346) | <0.001 | |
| ECOG PS | 0–1 | 14.2 (12.4–16.0) | 34.0 (31.4–36.6) | 0.310 (0.256–0.376) | <0.001 |
| ≥2 | 9.2 (7.2–11.2) | 19.5 (6.0–32.9) | 0.490 (0.215–1.117) | 0.090 | |
| Stage | IIIB | 8.3 (6.6–9.9) | 35.7 (31.8–39.6) | 0.093 (0.035–0.248) | <0.001 |
| IV | 14.5 (12.4–16.6) | 33.5 (31.1–35.9) | 0.331 (0.273–0.402) | <0.001 | |
| Organ metastasis | No | 12.8 (10.9–14.7) | 32.0 (27.4–36.6) | 0.323 (0.249–0.418) | <0.001 |
| Yes | 16.2 (13.4–18.9) | 34.0 (29.7–38.2) | 0.327 (0.248–0.430) | <0.001 | |
| Pleural | No | 14.1 (12.2–16.1) | 33.5 (30.5–36.6) | 0.263 (0.202–0.342) | <0.001 |
| Yes | 14.4 (11.6–17.3) | 33.0 (29.5–36.4) | 0.374 (0.285–0.490) | <0.001 | |
| Bone | No | 15.5 (12.0–18.9) | 33.9 (31.1–36.7) | 0.332 (0.264–0.418) | <0.001 |
| Yes | 12.3 (10.7–13.9) | 32.4 (25.5–39.2) | 0.304 (0.220–0.420) | <0.001 | |
| Brain | No | 12.7 (10.9–14.6) | 34.0 (31.1–36.9) | 0.282 (0.227–0.352) | <0.001 |
| Yes | 16.4 (11.8–21.0) | 31.5 (28.8–34.2) | 0.416 (0.288–0.600) | <0.001 | |
| Adrenal gland | No | 14.4 (12.2–16.7) | 34.0 (31.5–36.5) | 0.312 (0.258–0.378) | <0.001 |
| Yes | 12.0 (7.7–16.3) | 21.0 (11.7–30.3) | 0.397 (0.164–0.960) | 0.040 | |
| Liver | No | 14.5 (12.3–16.6) | 33.5 (31.0–36.0) | 0.325 (0.268–0.395) | <0.001 |
| Yes | 12.2 (9.8–14.5) | 30.8 (3.2–58.4) | 0.234 (0.107–0.514) | <0.001 | |
| Prior chemo | 1–2 | 10.6 (9.5–11.8) | 33.0 (31.3–34.8) | 0.190 (0.152–0.238) | <0.001 |
| ≥3 | 27.3 (25.0–29.6) | 40.5 (27.6–53.3) | 0.406 (0.252–0.653) | <0.001 |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; OS, overall survival; PS, performance status.
Discussion
Gefitinib is a small molecule orally administered quinazoline compound and a reversible EGFR-TKI.8 It blocks cell proliferation and angiogenesis and promotes the apoptosis of tumor cells by specifically inhibiting the activity of the protein tyrosine kinase of EGFR by competing with adenosine triphosphate for binding to the structural domain of the extracellular ligand.9
The phase III Iressa Survival Evaluation in Lung Cancer clinical trial (ISEL) using gefitinib and a placebo in 1692 patients with recurrent or refractory advanced NSCLC showed that there was no significant difference in MST between the two groups (5.6 vs. 5.1 months, P = 0.087).10 Their further observation of 342 Asian patients revealed that patients who were pathologically confirmed as the adenocarcinoma type benefited more from gefitinib treatment (P = 0.0028).11 Satouchi et al. retrospectively analyzed 221 Japanese patients with advanced NSCLC and found that the patients with lung adenocarcinoma, no smoking history, female gender, good PS, and EGFR mutation were optimal populations for gefitinib treatment.12 In their INTEREST study, Kim et al. reported that the survival of patients with lung adenocarcinoma, no smoking history, and female gender was higher than that of patients with non adenocarcinoma, smoking history, non Asian origin, and male gender.13 These results indicate that patients with pathological adenocarcinoma can clinically benefit more from gefitinib treatment.
Korean researchers analyzed the survival of patients with advanced NSCLC before and after the approved use of gefitinib and found that there was significant difference in survival between the two groups (11.5 vs. 19.3 months, P < 0.001). Multivariate analysis showed the factors that significantly improved the OS of all patients enrolled included gefitinib treatment (HR 0.58, P < 0.001), no smoking history, pathological adenocarcinoma type, good ECOG PS, stage IIIB, more than three chemotherapeutic regimens, prior use of platinum-based chemotherapy, and prior use of chemotherapy regimens containing docetaxel or pemetrexed. When the two groups were sub-classified as subgroups according to age (<60 vs. ≥60 years), gender, smoking history, clinical stage (IIIB vs. IV), pathological type (adenocarcinoma vs. non adenocarcinoma), ECOG PS (0–1 vs. ≥2), the number of prior chemotherapies (1–2 vs. ≥3), prior chemotherapy with versus without platinum, prior chemotherapy with versus without docetaxel or pemetrexed, the OS of the gefitinib group was still significantly longer than that of the simple chemotherapy group (P < 0.001), except in the subgroups of non adenocarcinoma (P = 0.109), ECOG PS ≥2 (P = 0.245), and prior chemotherapy without platinum (P = 0.073). A multivariate interaction test showed that the survival benefit in advanced NSCLC patients in the gefitinib group was mainly in patients with lung adenocarcinoma.14
To determine whether there was any difference in survival in patients with lung adenocarcinoma before and after the approved use of gefitinib in China, we analyzed the survival of 255 patients who did not receive gefitinib treatment and 255 patients who received gefitinib treatment, and found that the MST of the latter group was significantly longer (33.5 vs. 14.1 months, 95% CI 31.0–36.0 months, P < 0.001). This result is similar to that reported by Korean researchers (19.3 vs. 11.5 months, P < 0.001).14 However, it is worthwhile to point out that the Korean study included both adenocarcinoma and non-adenocarcinoma patients; their final conclusion was that patients who benefited from gefitinib treatment were mainly those with adenocarcinoma, with a MST of 24.2 months (95% CI, 20.8–27.6 months). Since gefitinib was approved for use in China in March 2005, some studies have suggested that patients with certain characteristics benefit more from gefitinib treatment, including women, patients without a smoking history, of Asian origin, and with adenocarcinoma.11–13 Therefore, patients with these characteristics have been termed “optimal patients” and are often chosen for gefitinib treatment in clinical practice in China. Further comparison revealed that there were some differences in the characteristics of the enrolled patients between the present study and the Korean study. For example, there were more female patients (58.5% vs. 30.5%), patients with no smoking history (77.2% vs. 39.4%), and ECOG PS 0–1 (93.3% vs. 83.2%) in the gefitinib group of our study compared with the Korean group. Single factor analysis of OS in the Korean study showed that age, gender, and smoking history were all factors that significantly improved the survival of their patients. Therefore, the difference in the baseline characteristics of the patients enrolled in the two studies may be an important reason why OS in our study was longer than in the Korean study (33.5 vs. 24.2 months).
Multivariate analysis showed that gefitinib treatment, age <60 years, no smoking history, no liver metastasis, and more than three prior chemotherapies were factors that significantly improved the OS of the 510 patients in our study. Our results indicate that gefitinib treatment is one of the independent factors that can improve survival of Chinese patients with advanced lung adenocarcinoma. Another significant similarity between our study and the Korean study is the impact of the number of prior chemotherapies on survival. Both single factor and multivariate analyses of the Korean study revealed that the survival of patients who received more than three prior chemotherapy regimens was significantly longer than that of patients who received only one to two prior chemotherapies (22.8 vs. 13.6 months, P < 0.001). Other prior studies on gefitinib also suggested that patients who continued with further chemotherapy during tumor progression lived longer than those who received relatively fewer chemotherapy regimens.11,14
There was no significant difference in OS in the ECOG ≥2 subgroup between the simple chemotherapy group before March 2005 and the gefitinib group after March 2005 (P = 0.090). The reason for this result may be because the number of patients in this subgroup was relatively small (n = 12 [4.7%] in the simple chemotherapy group and n = 17 [6.7%] in the gefitinib group). The Korean study similarly found no significant difference in OS the in ECOG ≥2 subgroup (P = 0.245). The author analyzed that ECOG PS was not included as a matching factor for the control study. ECOG PS was not equally balanced between the two groups, despite the fact of statistically significant difference. Therefore, the difference in distribution of patients with an ECOG PS 0 and 1 may be the reason for improved survival in post-gefitinib patients.
There were some limitations to this retrospective study. We only selected age, gender, and smoking history as factors for 1:1 group matching. Although most other factors were balanced between the two groups, there was a significant difference in the number of patients between the two groups in two subgroups: lung metastasis and one to two prior chemotherapies. In addition, there may be some differences in best supportive care and treatment modalities between the two groups because of the differences in time periods. These factors need to be further explored in future prospective studies.
Conclusion
In summary, this is the first retrospective study to compare differences in the survival of patients with advanced lung adenocarcinoma before and after gefitinib was officially approved for clinical use in China in March 2005. The results suggest that gefitinib significantly improved the survival of patients with advanced lung adenocarcinoma. Our findings serve as a useful reference for further clinical study of gefitinib.
Acknowledgments
This study was financially supported by the Chinese National Major Project for New Drug Innovation (2008ZX09312, 2012ZX09303012); the Chinese Central Health Authority Special Fund (B2009B124); the Chinese National High Technology Research and Development Program (863 Program) (2011AA02A110); the Beijing Municipal Science and Technology Commission Major Project for New Drug Innovation (Z111102071011001); and the Special Foundations of Wu Jieping Medical Foundation of China (320.6799.1109).
Disclosure
No authors report any conflict of interest.
References
- Gridelli C, Perrone F, Monfardini S. Lung cancer in the elderly. Eur J Cancer. 1997;33:2313–2314. doi: 10.1016/s0959-8049(97)10050-8. [DOI] [PubMed] [Google Scholar]
- Havlik RJ, Yancik R, Long S, Ries L, Edwards B. The National Cancer Institute on aging and the National Cancer Institute SEER collaborative study on comorbidity and early diagnosis of cancer in the elderly. Cancer. 1994;74(Suppl. 7):2101–2106. doi: 10.1002/1097-0142(19941001)74:7+<2101::aid-cncr2820741718>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens: The Tax 320 Non-small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- Tiseo M, Rossi G, Capelletti M, et al. Predictors of gefitinib outcomes in advanced non-small cell lung cancer (NSCLC): Study of a comprehensive panel of molecular markers. Lung Cancer. 2010;67:355–360. doi: 10.1016/j.lungcan.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Baselga J, Averbuch SD. ZD1839 (‘Iressa’) as an anticancer agent. Drugs. 2000;60(Suppl. 1):33–40. doi: 10.2165/00003495-200060001-00004. [DOI] [PubMed] [Google Scholar]
- Wakeling AE, Guy SP, Woodburn JR, et al. ZD1839 (Iressa): An orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–5754. [PubMed] [Google Scholar]
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- Chang A, Parikh P, Thongprasert S, et al. Gefitinib (IRESSA) in patients of Asian origin with refractory advanced non-small cell lung cancer: Subset analysis from the ISEL study. J Thorac Oncol. 2006;1:847–855. [PubMed] [Google Scholar]
- Satouchi M, Negoro S, Funada Y, et al. Predictive factors associated with prolonged survival in patients with advanced non-small-cell lung cancer (NSCLC) treated with gefitinib. Br J Cancer. 2007;96:1191–1196. doi: 10.1038/sj.bjc.6603710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): A randomised phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- Kim HS, Park K, Jun HJ, et al. Comparison of survival in advanced non-small cell lung cancer patients in the pre- and post-gefitinib eras. Oncology. 2009;76:239–246. doi: 10.1159/000205386. [DOI] [PubMed] [Google Scholar]


