Abstract
Microtubule-binding proteins (MBPs) are structurally and functionally diverse regulators of microtubule-mediated cellular processes. Alteration of MBPs has been implicated in the pathogenesis of human diseases, including cancer. MBPs can stabilize or destabilize microtubules or move along microtubules to transport various cargoes. In addition, MBPs can control microtubule dynamics through direct interaction with microtubules or coordination with other proteins. To better understand microtubule structure and function, it is necessary to identify additional MBPs. In this study, we isolated microtubules and MBPs from mammalian cells by a taxol-based method and then profiled a panel of MBPs by mass spectrometry. We discovered a number of previously uncharacterized MBPs, including several membrane-associated proteins and proteins involved in post-translational modifications, in addition to several structural components. These results support the notion that microtubules have a wide range of functions and may undergo more exquisite regulation than previously recognized.
Keywords: Mass spectrometry, microtubule, microtubule-binding protein, taxol, tubulin
Introduction
Microtubules are long hollow polymers composed of α- and β-tubulin heterodimers. As one of the major components of the cytoskeleton present in nearly all eukaryotic cells, microtubules play important roles in many cellular processes, such as intracellular transport, cell motility, and cell division. Microtubules are highly dynamic, and the dynamic property is crucial for microtubules to carry out most of their cellular functions.1,2 Microtubule dynamics also render microtubules having different behaviors in different cell types or different cell cycle phases. In some tissue-specific cells, microtubules are stable and form highly specialized structures, such as cilia and axon. Microtubules are known to undergo post-translational modifications (PTMs), such as acetylation and detyrosination, which play a critical role in the modulation of microtubule dynamics and functions.3–5
In addition to PTMs, microtubule-binding proteins (MBPs) are essential for the regulation of microtubule dynamics.6–8 Defects in MBPs can result in disorganized assembly or deregulated dynamics of microtubules, leading to cell dysfunction and various diseases.9–13 Microtubule-dependent motors, such as kinesin and dynein, are highly conserved MBPs that generate force and movement on microtubules by adenosine triphosphate hydrolysis.2,14 Microtubule plus end-tracking proteins (+TIPs), such as end-binding protein 1 (EB1) and cytoplasmic linker protein of 170 kDa (CLIP-170), are another group of MBPs that are critically involved in the regulation of microtubule dynamics.15 In addition, a number of enzymes, such as the deubiquitinase cylindromatosis (CYLD), histone deacetylase 6 (HDAC6), and the E3 ubiquitin ligase parkin, can associate with microtubules and regulate microtubule dynamics.16–22 To better understand microtubule structure and function, we sought to discover novel MBPs by taxol-based microtubule separation followed by mass spectrometry.
Materials and methods
Materials
Taxol was purchased from Sigma-Aldrich (St Louis, MO, USA), and guanosine-5′-triphosphate from Millipore (Bedford, MA, USA). Antibodies against α-tubulin were obtained from Sigma-Aldrich, EB1 from BD Transduction Laboratories (San José, CA, USA), and CYLD, CLIP-170, and HDAC6 from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Centrosomal protein of 70 kDa (Cep70) antibody was generated as described previously.23 Horseradish peroxidase-conjugated secondary antibodies were obtained from Amersham Biosciences (Chandler, AZ, USA). Microtubule-associated protein (MAP)-free tubulin was obtained from the Cytoskeleton Inc. (Denver, CO, USA).
Cell culture
HeLa cells were obtained from the American Type Cell Collection (Manassas, VA, USA) and grown at 37°C in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum in humidified air with 5% CO2.
Isolation of microtubules and mass spectrometry
Microtubules and MBPs were purified based on taxol-induced microtubule stabilization and cold-induced microtubule depolymerization. Proteins were subjected to standard in-gel tryptic digestion and analyzed by mass spectrometry, as previously described.24
Western blot analysis
Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride membranes (Millipore). The membranes were blocked with 5% fat-free milk in Tris-buffered saline containing 0.1% Tween 20, and probed with primary antibodies, followed by horseradish peroxidase-conjugated secondary antibodies, as previously described.25 The target proteins were visualized with enhanced chemiluminescence detection reagent following the manufacturer’s instructions (Pierce Biotechnology, Rockford, IL, USA).
Results
To obtain MBP-containing fractions, we lysed HeLa cells and then separated cytosolic components from membranes and nuclei by centrifugation. Microtubules and MBPs were then purified based on taxol-induced microtubule stabilization and cold-induced microtubule depolymerization (Fig 1a). After two cycles of polymerization and depolymerization followed by ultracentrifugation, we obtained the PIII and SIII fractions, containing microtubules and MBPs, respectively (Fig 1a). Coomassie blue and silver staining showed the high purity of isolated microtubules (Fig 1b). In addition, silver staining revealed that the SIII fraction obtained from a salt wash of microtubule pellets contained a much lower amount of proteins compared with the SII fraction (Fig 1c).
Figure 1.
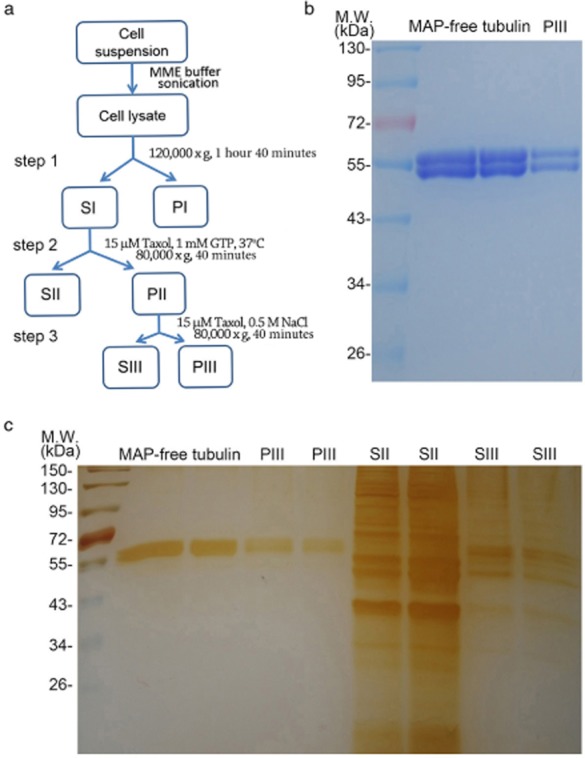
Isolation of microtubules and microtubule-binding proteins (MBPs) from HeLa cells. (a) Schematic diagram of taxol-based microtubule purification from HeLa cells. (b) Coomassie blue staining of microtubule-associated protein (MAP)-free tubulin and PIII purified from HeLa cells. (c) Silver staining of MAP-free tubulin, PIII, SII, and SIII. GTP, guanosine-5′-triphosphate; S, supernatant; P, pellet.
To verify the specificity of the isolated MBPs, we examined several known MBPs in the SII and SIII fractions. In agreement with previous findings that CLIP-170 and EB1 bound tightly to microtubules, Western blot analysis showed that the +TIPs proteins CLIP-170 and EB1 were present in both SII and SIII fractions (Fig 2).15 By contrast, Cep70, CYLD, and HDAC6 were entirely or primarily present in the SII fraction (Fig 2), consistent with their relatively low binding affinity to microtubules.23,26,27 Thus, the MBPs in the SIII fraction of HeLa cells were highly specific.
Figure 2.
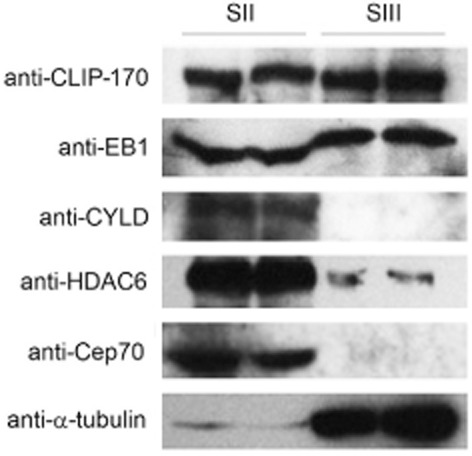
Western blot analysis of SII and SIII fractions with antibodies against several known microtubule-binding proteins (MBPs). Note that cytoplasmic linker protein of 170 kDa (CLIP-170) and end-binding protein 1 (EB1) exist in large amounts in both SII and SIII fractions, whereas deubiquitinase cylindromatosis (CYLD), histone deacetylase 6 (HDAC6), and centrosomal protein of 70 kDa (Cep70) are entirely or primarily in the SII fraction.
To profile individual MBPs in the SIII fraction, we loaded the acetone-precipitated proteins to the SDS-PAGE gel and concentrated them into a single band. This band was subjected to in-gel digestion and resulting peptides were analyzed by nano-liquid chromatography-mass spectrometry/liquid chromatography-tandem mass spectrometry for protein identification (Fig 3a). A representative total ion chromatogram for the MBP-derived peptide analysis shown in Figure 3b indicates the complexity of protein composition in the SIII fraction. A number of novel MBPs identified in our study are listed in Table 1. Interestingly, these candidate MBPs include several membrane-associated proteins, such as importin 5, clathrin, and vinculin. We also identified a few proteins involved in PTM regulation, such as ubiquitin-like modifier activating enzyme 1 and isoform 1 of protein diaphanous homolog 1. These results support the notion that microtubules have a wide range of functions and may undergo more exquisite regulation than previously recognized.
Figure 3.
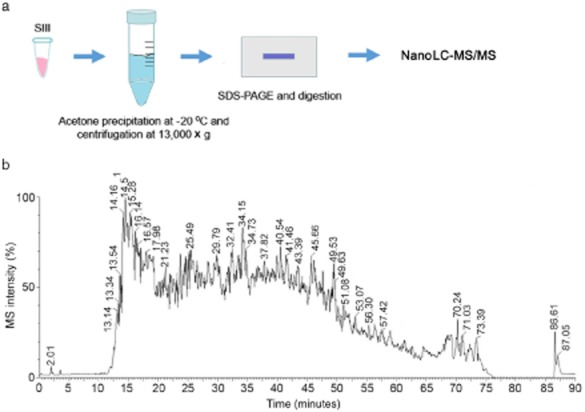
Identification of novel microtubule-binding proteins (MBPs) through mass spectrometry analysis. (a) Schematic diagram for the preparation of MBP peptides by acetone precipitation and in-gel digestion. (b) The total ion chromatogram for nano liquid chromatography-mass spectrometry/liquid chromatography-tandem mass spectrometry (nanoLC-MS/MS) analysis of MBP peptides. MS, mass spectrum; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Table 1.
List of MBPs identified by high-resolution mass spectrometry
| Accession | Description | M.W. (kDa) | Pi (pH) | Biological process |
|---|---|---|---|---|
| IPI00792677.1 | cDNA FLJ60097 highly similar to tubulin alpha ubiquitous chain | 46 | 4.78 | Microtubule-based process |
| IPI00009342.1 | Ras GTPase activating like protein IQGAP1 | 189 | 6.04 | Cell signaling |
| IPI00021439.1 | Actin cytoplasmic 1 | 42 | 5.14 | Cytoskeleton organization |
| IPI00645452.1 | Tubulin beta | 48 | 4.50 | Microtubule-based process |
| IPI00179330.6 | Ubiquitin 40S ribosomal protein S27a | 18 | 10.02 | Cell signaling |
| IPI00553169.5 | Uncharacterized protein | 246 | 5.56 | Unknown |
| IPI00645078.1 | Ubiquitin like modifier activating enzyme 1 | 118 | 5.37 | Protein modification |
| IPI00414676.6 | HSP 90 beta | 83 | 4.77 | Stress and immune response |
| IPI00382470.3 | Isoform 2 of HSP 90 alpha | 98 | 4.88 | Stress and immune response |
| IPI00003865.1 | Isoform 1 of heat shock cognate 71 kDa protein | 71 | 5.20 | Stress response |
| IPI00000877.1 | Hypoxia up-regulated protein 1 | 111 | 4.97 | Stress response |
| IPI00002966.2 | Heat shock 70 kDa protein 4 | 94 | 4.91 | Stress response |
| IPI00291175.7 | Isoform 1 of vinculin | 117 | 5.72 | Cell junction/cell adhesion |
| IPI00793443.2 | Isoform 1 of importin 5 | 124 | 4.64 | Protein transport |
| IPI00914026.1 | Dynactin subunit 1 isoform 4 | 127 | 5.16 | Nuclear migration |
| IPI00022434.4 | Uncharacterized protein | 72 | 6.30 | Unknown |
| IPI00024067.4 | Isoform 1 of clathrin heavy chain 1 | 191 | 5.35 | Protein transport |
| IPI00028275.2 | Isoform 1 of cytoskeleton associated protein 5 | 225 | 7.67 | Microtubule-based process |
| IPI00037283.3 | Isoform 5 of dynamin 1 like protein | 79 | 6.48 | Membrane fission, endocytosis |
| IPI00643920.3 | cDNA FLJ54957 highly similar to Transketolase | 68 | 7.47 | Unknown |
| IPI00020035.4 | Protein NipSnap homolog 3B | 28 | 9.57 | Unknown |
| IPI00100160.3 | Isoform 1 of cullin associated NEDD8 dissociated protein 1 | 136 | 5.41 | Cell differentiation |
| IPI00852685.1 | Isoform 1 of protein diaphanous homolog 1 | 141 | 5.14 | Microtubule-based process |
| IPI00979442.1 | cDNA FLJ46846 fis clone UTERU3004635 | 181 | 5.71 | Unknown |
| IPI00880007.2 | Microtubule associated protein | 245 | 5.83 | Microtubule-based process |
| IPI00022058.3 | Isoform 1 of ArfGAP with SH3 domain ANK repeat and PH domain containing protein 2 | 112 | 6.22 | Cilium morphogenesis |
| IPI00216694.3 | Isoform 3 of plastin | 71 | 5.26 | Bone development |
| IPI00465128.4 | Isoform 1 of large proline rich protein BAG6 | 119 | 5.28 | Cell differentiation and tissue development |
ANK, ankylin; BAG6, BCL2-associated athanogene 6; cDNA, complementary DNA; HSP, heat shock protein; MBPs, microtubule-binding proteins.
Discussion
Diverse microtubule-based cellular processes not only rely on microtubules per se but also MBPs. MBPs have also been demonstrated to regulate the binding of microtubule-targeting drugs to microtubules, thereby modulating cancer cell sensitivity to these drugs.21,28–31 Therefore, the identification of MBPs is of great significance in order to understand microtubule structure and function, as well as the mechanisms underlying cancer chemotherapeutic sensitivity. For example, CYLD is a recently identified MBP that regulates microtubule dynamics and participates in cell migration, cell division, angiogenesis, and ciliogenesis.16,17,19,27,32 CYLD has also been shown to stimulate noscapine activity in acute lymphoblastic leukemia via a microtubule-dependent mechanism.31 In addition, parkin, CLIP-170, and EB1 have been reported to bind microtubules and promote the sensitivity of breast cancer cells to taxol.21,29,30
MBPs are known to interact with microtubule via specific domains or motifs. For example, the cytoskeleton-associated protein-glycine-rich domain mediates the interaction of CYLD with microtubules, whereas calponin homology domain mediates the interaction of EB1 with microtubules.16,33,34 In this study, using a combination of taxol-based microtubule isolation and mass spectrometry, we have identified a list of proteins that may potentially associate with microtubules. It is important to validate these MBPs through biochemical analysis and immunofluorescence microscopy. In the future, examination of the profiles of these MBPs in different cell types and cell cycle stages and the mechanistic details for how these proteins interact with microtubules is necessary. Investigation of these proteins using their known structural and functional information may provide useful knowledge for the diverse roles of microtubules. In addition, MBPs are known to act in concert to regulate microtubule structure and function.18,35 Therefore, it will be important to analyze whether the novel MBPs coordinate with known MBPs to carry out their functions.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31271437, 31401150, and 31371382).
Disclosure
No authors report any conflict of interest.
References
- Kaverina I, Straube A. Regulation of cell migration by dynamic microtubules. Semin Cell Dev Biol. 2011;22:968–974. doi: 10.1016/j.semcdb.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente JJ, Wordeman L. Mitosis, microtubule dynamics and the evolution of kinesins. Exp Cell Res. 2015;334:61–69. doi: 10.1016/j.yexcr.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiera MM, Janke C. Post-translational modifications of tubulin. Curr Biol. 2014;24:R351–354. doi: 10.1016/j.cub.2014.03.032. [DOI] [PubMed] [Google Scholar]
- Song Y, Brady ST. Post-translational modifications of tubulin: Pathways to functional diversity of microtubules. Trends Cell Biol. 2015;25:125–136. doi: 10.1016/j.tcb.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: Mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- Amos LA, Schlieper D. Microtubules and maps. Adv Protein Chem. 2005;71:257–298. doi: 10.1016/S0065-3233(04)71007-4. [DOI] [PubMed] [Google Scholar]
- Maiato H, Sampaio P, Sunkel CE. Microtubule-associated proteins and their essential roles during mitosis. Int Rev Cytol. 2004;241:53–153. doi: 10.1016/S0074-7696(04)41002-X. [DOI] [PubMed] [Google Scholar]
- Sun X, Shi X, Liu M, et al. Mdp3 is a novel microtubule-binding protein that regulates microtubule assembly and stability. Cell Cycle. 2011;10:3929–3937. doi: 10.4161/cc.10.22.18106. [DOI] [PubMed] [Google Scholar]
- Bhat KM, Setaluri V. Microtubule-associated proteins as targets in cancer chemotherapy. Clin Cancer Res. 2007;13:2849–2854. doi: 10.1158/1078-0432.CCR-06-3040. [DOI] [PubMed] [Google Scholar]
- Dong X, Liu F, Sun L, et al. Oncogenic function of microtubule end-binding protein 1 in breast cancer. J Pathol. 2010;220:361–369. doi: 10.1002/path.2662. [DOI] [PubMed] [Google Scholar]
- Liu M, Wang X, Yang Y, et al. Ectopic expression of the microtubule-dependent motor protein Eg5 promotes pancreatic tumourigenesis. J Pathol. 2010;221:221–228. doi: 10.1002/path.2706. [DOI] [PubMed] [Google Scholar]
- Liu M, Li D, Sun L, et al. Modulation of Eg5 activity contributes to mitotic spindle checkpoint activation and Tat-mediated apoptosis in CD4-positive T-lymphocytes. J Pathol. 2014;233:138–147. doi: 10.1002/path.4333. [DOI] [PubMed] [Google Scholar]
- Tala, Xie S, Sun X, et al. Microtubule-associated protein Mdp3 promotes breast cancer growth and metastasis. Theranostics. 2014;4:1052–1061. doi: 10.7150/thno.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennerich A, Vale RD. Walking the walk: How kinesin and dynein coordinate their steps. Curr Opin Cell Biol. 2009;21:59–67. doi: 10.1016/j.ceb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia SM, Akhmanova A. Cell and molecular biology of microtubule plus end tracking proteins: End binding proteins and their partners. Int Rev Cell Mol Biol. 2010;285:1–74. doi: 10.1016/B978-0-12-381047-2.00001-3. [DOI] [PubMed] [Google Scholar]
- Gao J, Huo L, Sun X, et al. The tumor suppressor CYLD regulates microtubule dynamics and plays a role in cell migration. J Biol Chem. 2008;283:8802–8809. doi: 10.1074/jbc.M708470200. [DOI] [PubMed] [Google Scholar]
- Gao J, Sun L, Huo L, et al. CYLD regulates angiogenesis by mediating vascular endothelial cell migration. Blood. 2010;115:4130–4137. doi: 10.1182/blood-2009-10-248526. [DOI] [PubMed] [Google Scholar]
- Li D, Gao J, Yang Y, et al. CYLD coordinates with EB1 to regulate microtubule dynamics and cell migration. Cell Cycle. 2014;13:974–983. doi: 10.4161/cc.27838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu M, Li D, et al. CYLD regulates spindle orientation by stabilizing astral microtubules and promoting dishevelled-NuMA-dynein/dynactin complex formation. Proc Natl Acad Sci U S A. 2014;111:2158–2163. doi: 10.1073/pnas.1319341111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela-Fernández A, Cabrero JR, Serrador JM, Sánchez-Madrid F. HDAC6: A key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 2008;18:291–297. doi: 10.1016/j.tcb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu B, Zhang C, et al. Parkin regulates paclitaxel sensitivity in breast cancer via a microtubule-dependent mechanism. J Pathol. 2009;218:76–85. doi: 10.1002/path.2512. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zhao J, Feng J. Parkin binds to alpha/beta tubulin and increases their ubiquitination and degradation. J Neurosci. 2003;23:3316–3324. doi: 10.1523/JNEUROSCI.23-08-03316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Sun X, Liu M, Li D, Aneja R, Zhou J. CEP70 protein interacts with gamma-tubulin to localize at the centrosome and is critical for mitotic spindle assembly. J Biol Chem. 2011;286:33401–33408. doi: 10.1074/jbc.M111.252262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- Sun L, Gao J, Dong X, et al. EB1 promotes Aurora-B kinase activity through blocking its inactivation by protein phosphatase 2A. Proc Natl Acad Sci U S A. 2008;105:7153–7158. doi: 10.1073/pnas.0710018105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Liu M, Li D, Wang J, Aneja R, Zhou J. Cep70 contributes to angiogenesis by modulating microtubule rearrangement and stimulating cell polarization and migration. Cell Cycle. 2012;11:1554–1563. doi: 10.4161/cc.19954. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ran J, Liu M, et al. CYLD mediates ciliogenesis in multiple organs by deubiquitinating Cep70 and inactivating HDAC6. Cell Res. 2014;24:1342–1353. doi: 10.1038/cr.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzier R, Rajan R, Wagner P, et al. Microtubule-associated protein tau: A marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci U S A. 2005;102:8315–8320. doi: 10.1073/pnas.0408974102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Li D, Yang Y, et al. Microtubule-binding protein CLIP-170 is a mediator of paclitaxel sensitivity. J Pathol. 2012;226:666–673. doi: 10.1002/path.3026. [DOI] [PubMed] [Google Scholar]
- Luo Y, Li D, Ran J, et al. End-binding protein 1 stimulates paclitaxel sensitivity in breast cancer by promoting its actions toward microtubule assembly and stability. Protein Cell. 2014;5:469–479. doi: 10.1007/s13238-014-0053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ran J, Sun L, et al. CYLD regulates noscapine activity in acute lymphoblastic leukemia via a microtubule-dependent mechanism. Theranostics. 2015;5:656–666. doi: 10.7150/thno.10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Gao J, Huo L, et al. Tumour suppressor CYLD is a negative regulator of the mitotic kinase Aurora-B. J Pathol. 2010;221:425–432. doi: 10.1002/path.2723. [DOI] [PubMed] [Google Scholar]
- Hayashi I, Ikura M. Crystal structure of the amino-terminal microtubule-binding domain of end-binding protein 1 (EB1) J Biol Chem. 2003;278:36430–36434. doi: 10.1074/jbc.M305773200. [DOI] [PubMed] [Google Scholar]
- Bu W, Su LK. Characterization of functional domains of human EB1 family proteins. J Biol Chem. 2003;278:49721–49731. doi: 10.1074/jbc.M306194200. [DOI] [PubMed] [Google Scholar]
- Li D, Sun X, Zhang L, et al. Histone deacetylase 6 and cytoplasmic linker protein 170 function together to regulate the motility of pancreatic cancer cells. Protein Cell. 2014;5:214–223. doi: 10.1007/s13238-013-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


