Abstract
Third and fourth line chemotherapy agents are not helpful in the setting of extensive stage small cell lung cancer (SCLC). We describe the case of a 43-year-old Korean patient with T4N3M1b extensive stage SCLC who responded remarkably well to treatment and experienced a prolonged progression-free survival (PFS) period following treatment with fourth line ifosfamide and carboplatin after experiencing disease progression with three previous regimens. Additionally, third line cyclophosphamide, doxorubicin (Adriamycin), and vincristine demonstrated long-term PFS periods.
Keywords: Cyclophosphamide, ifosfamide, refractory, small cell lung carcinoma
Introduction
Small cell lung cancer (SCLC) is a particularly aggressive form of lung cancer characterized by rapid tumor growth and early dissemination.1 Although initial tumor response rates to cytotoxic chemotherapy are very high, most patients with SCLC relapse within a few months of commencing treatment. Most are subsequently treated with second line chemotherapy.2,3 Generally, second line chemotherapy is the final chemotherapy regimen utilized.
There are two types of relapse based on version 1.2015 of the National Comprehensive Cancer Network (NCCN) Guidelines. A patient’s response to the next regimen is highly dependent on the time between the patient’s initial therapy and relapse. If this interval is less than three months (refractory or resistant disease), the patient’s response to most agents or regimens is poor (≤10%). If more than three months have elapsed (sensitive disease), the response rate is approximately 25%.4 The NCCN guidelines recommend a therapeutic challenge in the setting of a patient’s second relapse. Paclitaxel, docetaxel, topotecan, irinotecan, vinorelbine, gemcitabine, ifosfamide, temozolomide, and oral etoposide may be used in subsequent treatments for SCLC.4 The overall survival (OS) and progression-free survival (PFS) rates of each regimen are poor, regardless of the numbers or types of therapeutic challenges utilized.
We present a rare and interesting case of a 43-year-old Korean patient with T4N3M1b extensive stage SCLC, who responded remarkably well to third line cyclophosphamide, doxorubicin (Adriamycin, Pfizer, New York, USA), and vincristine (CAV) and fourth line ifosfamide and carboplatin. Although she was believed to have refractory relapsed disease, third and fourth line chemotherapy elicited a dramatic response and prolonged her survival.
Case
A 43 year-old non-smoking woman came to the Chungnam National University Hospital with a six-month history of worsening dyspnea on exertion. She had no past medical history. Her Eastern Cooperative Oncology Group (ECOG) performance status was grade 1.
The patient underwent testing; a chest X-ray revealed mass-like consolidation in the left upper lung field, adjacent to aortic arch. Chest computed tomography (CT) revealed a large central mass and mediastinal and hilar lymph node enlargement in the left upper lobe. Bronchoscopic washing and biopsies revealed the presence of small cell carcinoma based on immunostaining for CD56, synaptophysin, chromogranin, and leucocyte common antigen (LCA). There was no evidence of metastatic brain lesions on magnetic resonance imaging (MRI). Additionally, positron emission tomography (PET)-CT) demonstrated hot areas of uptake in the para-aortic lymph nodes and the right retrocrural lymph nodes, as well as pleural metastasis and a malignant pleural effusion. She was diagnosed with extensive stage SCLC (Fig 1).
Figure 1.
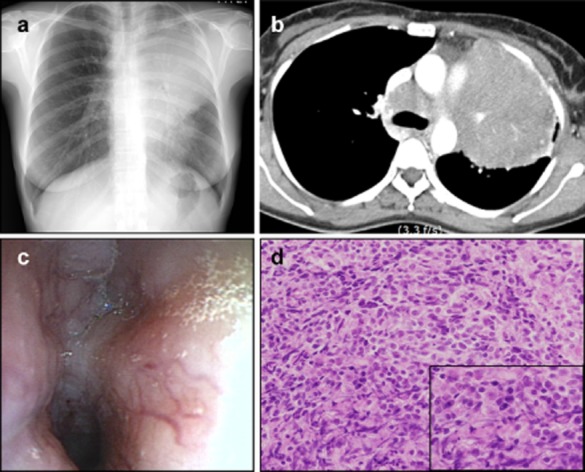
(a) Initial chest X-ray revealed a left upper lobe (LUL) mass-like consolidation. (b) Initial chest computed tomography revealed a large central mass and mediastinal and hilar lymph node enlargement in the LUL. (c) Bronchoscopy revealed endobronchial infiltration along the left main bronchus and LUL bronchus. (d) Bronchoscopic biopsy specimen showed small-cell lung cancer (hematoxylin-eosin, original magnification ×200).
Chemotherapy with cisplatin (60 mg/m2) and etoposide (100 mg/m2) was initiated and maintained for six cycles. A chest CT following the third cycle demonstrated dramatic improvement, as the tumor remained only as a peribronchial nodular mass along the upper division of the left upper lobe. Following the sixth cycle, however, the tumor increased in size to a long diameter of 81.71 mm indicating progressive disease (PD) according to Response Evaluation Criteria In Solid Tumors. A change took place over a period of four months. Therefore, second line chemotherapy with carboplatin and irinotecan was initiated. After two cycles, the patient developed obstructive pneumonia; the tumor was noted to be 106.85 mm in size via X-ray and her regimen was changed. The patient was placed in the refractory relapse group; we carefully undertook third line chemotherapy using CAV (Adriamycin, [40 mg/m2], vincristine [1.4 mg/m2] and cyclophosphamide [1000 mg/m2]). Third line chemotherapy was maintained for 11 cycles, with the last cycle at a reduced dose (75%) because the patient developed neutropenia. The size of the tumor decreased immediately following the infusion of CAV, but subsequently increased after approximately 10 days, as noted via a follow up chest X-ray (follow up chest CT images were obtained every 3 to 4 cycles, Fig 2e–g). This pattern was repeated for 10 cycles of CAV. After 11 cycles, the patient developed PD; the tumor was noted to be 134 mm in long diameter via chest CT and her regimen was unable to be continued. No additional regimens were recommended following 11 cycles of treatment with third line CAV. However, the patient was relatively young and had a good performance status score (ECOG 1); she had not experienced significant toxicity (only neutropenia) or unbearable symptoms. A carboplatin, ifosfamide and etoposide (ICE) regimen had proven effective, but had shown horrible toxicity; therefore an ICE regimen without etoposide: twice-weekly therapy with carboplatin (AUC 5) and ifosfamide (5 g/m2), as well as mesna for prophylaxis against ifosfamide induced hemorrhagic cystitis, was commenced as a fourth line therapy. Contrary to what was originally scheduled, the patient’s chemotherapy was performed every three weeks because of intermittent bone marrow depression characterized by absolute neutrophil counts of less than 1000/uL. A CT obtained following the third cycle of carboplatin and ifosfamide demonstrated a partial response (PR) to therapy, as the mass decreased in size from a long diameter of 13.4 cm to a long diameter of 7.2 cm. This regimen was maintained until completion of the fifth cycle. A PET-CT following the fifth cycle demonstrated mild decreases in both the size and uptake of the tumor (Figs 2, 3). As the patient’s pain, which had been unbearable, gradually dissipated during the course of her regimen, she refused further treatment.
Figure 2.
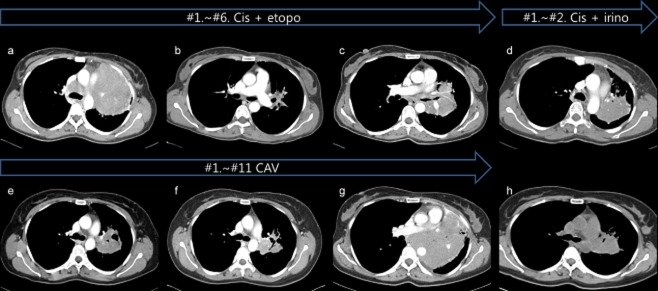
The series of computed tomography (CT) scans are listed with the regimen used. (a) Initial chest CT. (b) After thee cycles of first line chemotherapy with cisplatin and etoposide, chest CT demonstrated dramatic improvement with partial response. (c) However, the tumor increased to 81.71 mm following the sixth cycle, resulting in progressive disease (PD). Therefore, second line chemotherapy with carboplatin and irinotecan was performed. After two cycles, obstructive pneumonia developed. (g) Third line chemotherapy with cyclophosphamide, Adriamycin, and vincristine was maintained until the patient developed PD; the tumor was noted to be 134 mm. (h) Fourth line regimen with carboplatin and ifosfamide demonstrated a partial response, as the mass decreased in size from 134 mm to 72 mm.
Figure 3.
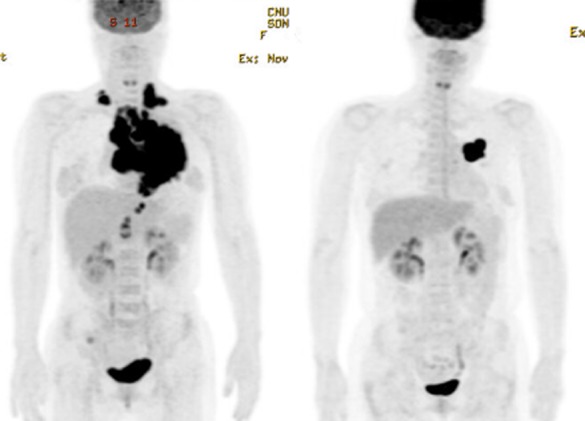
Initial positron emission tomography-computed tomography (PET-CT) (left) and follow up PET-CT (right) after 19 months. Even though the patient went through four different regimens, a dramatic decrease in tumor size can be seen.
The patient presented to the emergency department for dyspnea and rib pain eight months following the completion of her fifth cycle of carboplatin and ifosfamide. A chest CT demonstrated that her mass had increased in size from 7.2 cm to 18 cm. The day following her admission, a sixth cycle of carboplatin and ifosfamide was initiated for palliation of her symptoms. Her dyspnea and chest pain improved, as did her oxygen requirements, and she was able to sleep lying down. However, she expired due to aspiration pneumonia 11 days later. She survived for approximately 27 months after beginning chemotherapy.
Discussion
In this case, a patient diagnosed with refractory relapsed SCLC responded well to therapy and experienced long-term PFS following treatment with a third line CAV regimen and fourth line carboplatin-ifosfamide therapy. During third line CAV therapy, the tumor size decreased and increased several times between each cycle, as viewed via X-ray. Following fourth line carboplatin-ifosfamide therapy, the size of the tumor was smaller than the mass’ initial size, and its growth rate had also decreased. Possible reasons for this dramatic response may have been the tumor’s distinct characteristics and the effects of the modified carboplatin and ifosfamide regimen.
Decades ago, several studies described the effects of CAV as a first line therapy.5,6 A CAV regimen is often used following first line treatment with cisplatin and etoposide. In two studies involving sensitive and refractory patients, CAV therapy yielded second line response rates of 13% and 28%.7,8 Topotecan, a camptothecin analog, replaced CAV as a second line therapy based on clinical trials comparing CAV and topotecan. The response rates were 18.3% (CAV) and 24.3% (topotecan), with median survival times of 24.7 and 25.0 weeks; differences that were not statistically significant.9 We had only limited information regarding third line treatment with CAV based on clinical trials; therefore, further treatment was not proposed.
Regarding third line chemotherapy, a previous study regarding the use of amrubicin for refractory relapsed SCLC was undertaken. The results of this study included a PFS period of 3.0 months and an OS period of 5.1 months.10 Additionally, another study regarding amrubicin therapy in the setting of refractory SCLC was performed. Eighty-two patients with chemotherapy-refractory SCLC received 40 mg/m2 of amrubicin for three consecutive days, every 21 days. The median PFS and OS periods were 3.5 months (95% confidence interval [CI], 3.0–4.3 months) and 8.9 months (95% CI, 7.6–11.3 months), respectively.11
Long term PFS following third line treatment with CAV was one of the unusual results observed in this study. Similar unusual results were observed with fourth line therapy. We assumed that the patient’s tumor was highly sensitive to both cyclophosphamide and a cyclophosphamide analog (ifosfamide).
A notable finding of our study was the intermittent growth of the patient’s tumor between each round of chemotherapy. It is difficult to find references explaining rapid tumor shrinkage and tumor regrowth. The best explanation for this phenomenon is that the tumor’s multiplication rate was faster than the tumor suppression rate of the chemotherapy regimen.
The mechanism of action of ifosfamide is very similar to that of cyclophosphamide, but slower. Cyclophosphamide is metabolized extensively following cellular uptake. The drug is first transformed into hydroxylated intermediate via the cytochrome P-450 system. The hydroxylated intermediate is subsequently broken down to form active compounds, including phosphoramide mustard and acrolein. The subsequent reaction between the phosphoramide mustard and DNA is the cytotoxic step in this process.12
Significant cross-resistance between ifosfamide and cyclophosphamide was not reported. A study comparing the activity of ifosfamide with cyclophosphamide has been performed previously. The antitumor activity of ifosfamide (increases in lifespan and cure rate) was greater when the drug was used to treat several experimental tumors, some of which were primarily resistant to cyclophosphamide. Although it is difficult to prove that there was no cross resistance between ifosfamide and cyclophosphamide, ifosfamide has demonstrated activity against malignant lymphomas refractory to multiple drugs and treatment regimens, as well as activity against SCLC that was unresponsive to adriamycin, vincristine, and etoposide, and activity against soft tissue and bone sarcomas.
Cardiac toxicity and acute cardiac failure (hemorrhagic necrosis) may occur when cyclophosphamide is used, particularly if the drug is administered at high doses, as is the case when preparing patients for bone marrow transplantation (>120 mg/kg), or if the drug is given along with doxorubicin or daunorubicin, or given to patients receiving concomitant radiation therapy to either the cardiac vessels or the heart itself.13 The patient in our study did not suffer any cardiac toxicity because the dose she received was relatively low (1000 mg/m2 cyclophosphamide).
Carboplatin and ifosfamide are not commonly used to treat SCLC. During the 90’s and early 2000’s, a limited number of articles were written, and a limited number of trials were undertaken. Trials using paclitaxel (175 mg/m2 over 1 hour), ifosfamide (2.5 gm/m2 over 1 hour) and carboplatin (AUC = 6 over 0.5 hours) as a first line regimen in patients with extensive stage SCLC have been completed. The overall response rate of these trials was 71% (15% complete response, 56% partial response). Quality of life appeared to be stable over time. The median survival time was 9.5 months (95% CI, 6.7–13.2 months), with one and two-year survival rates of 43% (95% CI, 26–59%) and 16% (95% CI, 2–30%), respectively.14 Trials using a modified ICE chemotherapy regimen (carboplatin 6x [glomerular filtration rate +25]mg, ifosfamide 3 g/m2 + mesna 3 g/m2, mesna 1.8 g/m2 bolus, and 50 mg of oral etoposide twice daily for 7 days) as first line chemotherapy, given every four weeks, were also completed. Twenty-five patients (83%, 95% CI, 70–97%) experienced either a partial or a complete response to therapy during treatment.15 However, these trials had limitations because they used more than ifosfamide and carboplatin alone; some regimens included etoposide, and others included paclitaxel. Therefore, we cannot determine whether remission from SCLC was a result of ifosfamide or other chemo-compounds. In this study, we noted the obvious effects of ifosfamide in the setting of refractory SCLC. Carboplatin was not the primary reason for the regimen’s effects because it was previously used as a second line therapy.
We observed the obvious effects of fourth line treatment with ifosfamide and carboplatin in the setting of refractory SCLC; however, we have only limited information regarding the effectiveness of ifosfamide alone or the effectiveness of ifosfamide and carboplatin in the setting of refractory SCLC. We hope that additional studies investigating the effects of ifosfamide will be performed.
Disclosure
No authors report any conflict of interest.
References
- Watson WL, Berg JW. Oat cell lung cancer. Cancer. 1962;15:759–768. doi: 10.1002/1097-0142(196207/08)15:4<759::aid-cncr2820150410>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hurwitz JL, McCoy F, Scullin P, Fennell DA. New advances in the second-line treatment of small cell lung cancer. Oncologist. 2009;14:986–994. doi: 10.1634/theoncologist.2009-0026. [DOI] [PubMed] [Google Scholar]
- Schneider BJ. Management of recurrent small cell lung cancer. J Natl Compr Canc Netw. 2008;6:323–331. doi: 10.6004/jnccn.2008.0027. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. 2015. Small Cell Lung Cancer, Version 1. NCCN clinical practice guidelines in Oncology (NCCN guidelines); 2014. [Cited 18 Jun 2014.] Available from URL: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- Kawahara M, Tsuruta M, Furuse K, et al. [Cyclophosphamide, adriamycin and vincristine (CAV) in the treatment of small cell lung cancer] Gan to Kagaku Ryoho. 1988;15:457–462. (In Japanese.) [PubMed] [Google Scholar]
- Lee SM. Treatment of small cell lung cancer with cyclophosphamide, adriamycin and vincristine (CAV) combination therapy: experience at University Hospital, Kuala Lumpur, Malaysia. Singapore Med J. 1990;31:317–320. [PubMed] [Google Scholar]
- Sculier JP, Klastersky J, Libert P, et al. Cyclophosphamide, doxorubicin and vincristine with amphotericin B in sonicated liposomes as salvage therapy for small cell lung cancer. Eur J Cancer. 1990;26:919–921. doi: 10.1016/0277-5379(90)90203-6. [DOI] [PubMed] [Google Scholar]
- Shepherd FA, Evans WK, MacCormick R, Feld R, Yau JC. Cyclophosphamide, doxorubicin, and vincristine in etoposide- and cisplatin-resistant small cell lung cancer. Cancer Treat Rep. 1987;71:941–944. [PubMed] [Google Scholar]
- von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- Asai N, Ohkuni Y, Matsunuma R, Nakashima K, Iwasaki T, Kaneko N. Efficacy and safety of amurubicin for the elderly patients with refractory relapsed small cell lung cancer as third-line chemotherapy. J Cancer Res Ther. 2012;8:266–271. doi: 10.4103/0973-1482.98983. [DOI] [PubMed] [Google Scholar]
- Murakami H, Yamamoto N, Shibata T, et al. A single-arm confirmatory study of amrubicin therapy in patients with refractory small-cell lung cancer: Japan Clinical Oncology Group Study (JCOG0901) Lung Cancer. 2014;84:67–72. doi: 10.1016/j.lungcan.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Harvey RA. Pharmacology. 5th edn. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2012. [Google Scholar]
- Cancer Care Ontario. 2014. Cyclophosphamide: CCO Formulary. [Cited 29 May 2014.] Available from URL: http://www.cancercare.on.ca.
- Socinski MA, Neubauer MA, Olivares J, et al. Phase II trial of paclitaxel, ifosfamide, and carboplatin in extensive-stage small cell lung cancer. Lung Cancer. 2003;40:91–97. doi: 10.1016/s0169-5002(02)00527-5. [DOI] [PubMed] [Google Scholar]
- Shevlin PM, Muers MF, Peake MD, et al. Modified ice study: a phase II study of an intensive, modified ICE regimen (ifosfamide, carboplatin and etoposide) in patients with better prognosis, small cell lung cancer. Lung Cancer. 1998;21:115–126. doi: 10.1016/s0169-5002(98)00035-x. [DOI] [PubMed] [Google Scholar]


