Abstract
The clinical management of cervical cancer in HIV-positive patients has challenges mainly due to the concerns on immune status. At present, their mode of management is similar to HIV-seronegative patients involving the use of chemotherapy and radiotherapy concurrently as indicated. HIV infection, cancer, radiotherapy, and chemotherapy lower immunity through reduction in CD4 cell counts. At present there are no treatment guidelines for HIV-positive patients. This study was done to systematically review the literature on cervical cancer management in HIV-positive patients and treatment outcomes. A systematic literature search was done in the major databases to identify studies on the management of HIV-positive patients with cervical cancer. Identified studies were assessed for eligibility and inclusion in the review following the guidelines of The Cochrane Handbook for Systematic Reviews and CRD's (Centre for Reviews and Dissemination) guidance for undertaking reviews in health care. Eight eligible studies were identified from the literature. Three of them were prospective while five were retrospective studies. Notably, the average age at diagnosis of cervical cancer in HIV-positive patients was a decade lower than in seronegative patients. There was no difference in distribution of stages of disease at presentation between HIV-positive and negative patients. Mild acute toxicity (Grades 1 and 2) was higher in HIV-positive patients than in HIV-negative patients in hematopoietic system. In the grades 3 and 4 reactions, anemia was reported in 4% versus 2% while gastrointestinal reactions were reported in 5% versus 2% respectively. In general, patients who were started early on HAART had higher rates of treatment completion. The study supports the suggestion that HAART should be commenced early at cervical cancer diagnosis in HIV-positive patients diagnosed with cervical cancer to ensure less toxicity and better treatment compliance.
Keywords: Cervical cancer, HIV-positive, management
Introduction
Cancer of the uterine cervix is the most common gynecological malignancy and occurs worldwide 1. It has been reported that about eighty percent of cervical cancers occur in the developing countries 2. Furthermore, the mortality due to cervical cancer is higher in the developing countries where screening and treatment modalities are not commonly available or accessible compared with the developed countries 3. According to the GLOBOCAN 2012 estimates, the highest incidence is in sub-Saharan Africa, especially in Eastern African countries 4. The most important risk factor for the development of cervical cancer is the human papilloma virus (HPV) 5.
Cervical cancer is very common in HIV-seropositive patients with an aggressive course and poor treatment outcome 6. Regions of high prevalence of cervical cancer correspond with regions of high prevalence of HIV infection 7. Cervico-vaginal HPV infection has also been reported to be higher in HIV-positive women than in their HIV-negative counterparts. In a study involving 1778 HIV-positive and 500 HIV-negative women, it was found that 63% of the HIV-positive participants tested positive to HPV viral DNA while only 30% of the HIV-negative participants tested positive 8. HIV-positive women have been reported to have seven times more incidence of cervical cancer than their HIV-negative counterparts 9.
HIV infection lowers immunity through the destruction of CD4 lymphocytes. The level of destruction is related to the patient's HIV viral load 10. Progressive reduction in CD4 cell population reduces the ability of the body to ward off infective agents leading to occurrence of opportunistic infections in HIV infected individuals. Dormant infections can also be reactivated under conditions of suppressed immunity. These opportunistic infections add to the deterioration of the clinical states of HIV infected patients leading to poor outcome. Opportunistic infections are common if CD4 cell count is below 200 cells/μL 11.
The three modes of treating cervical cancer are surgery, radiotherapy, and chemotherapy either single or in combination. In areas with high prevalence of cervical cancer, majority of the patients are treated with chemo-radiotherapy which can lower patients' immunity 12,13. Highly active antiretroviral treatment (HAART) has helped to improve the immunological status of HIV-positive patients and control the increase in viral load 14. Patients with compromised immunity usually suffer more treatment toxicities from chemo-radiotherapy used in the treatment of cervical cancer as it affects the immune status of patients 15. The recovery of CD4 cell depends on the state of the thymus gland as they are thymus dependent. The thymus gland undergoes involution in adults and hence recovery of CD4 cell count is usually very slow in those with involute thymus gland. In a study to assess the activity of the thymus gland after chemotherapy, it was reported that in younger patients aged between 18–49 years, the thymus function was evident in 63% of the participants compared with 0% of their counterparts aged 70–91 years 3 months post treatment 16.
Another consideration in HIV-positive patients with cancer is that some chemotherapy and HAART drugs are metabolized by similar cytochrome p450 enzyme pathway. This may affect the clearance of chemotherapy drugs leading to increased toxicity or ineffectiveness 17.
Various treatment modalities and modifications have been used to improve outcome of treatment in HIV-positive patients with cervical cancer. However, the outcome of management is still poor. There is a need to explore ways of improving the effectiveness of treatment and limit potential-associated increased toxicity in these patients. Presently, optimal uniform treatment modalities are yet to be established.
The objectives of this study were to systematically summarize the current available data on mode of treatment and outcome of treatment in HIV-positive patients and compare to HIV-negative ones and to further identify existing gaps in studies regarding the management of patients with cervical cancer and HIV.
Methodology
The method used followed the guidelines of The Cochrane Handbook for Systematic Reviews Version 5.1.0 (http://handbook.cochrane.org/; accessed 17 February 2015) and CRD's (Centre for Reviews and Dissemination) guidance for undertaking reviews in health care (http://www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf; accessed 17 February 2015). The report is presented according to the recommendations of Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) 18. Ethical review of the protocol was not required as this is a systematic review of already published (and therefore anonymized) data.
Sources of Data
A systematic search in electronic data bases of the scientific literature was used. The data bases searched included MEDLINE (PubMed interface), EMBASE (OVID interface), Cochrane Central (OVID interface), Web of Science, Cochrane data base, The NHS Centre for Reviews and Dissemination, Centre for Evidence Based Medicine at Oxford, Scopus, Google Scholar, Chinese BioMed (CBM) and online published doctoral theses from January 1995 until March 2014. The period from 1995 was chosen because HAART was widely introduced into the management of HIV infection at this point in time 19,20. Relevant materials were also obtained through communication with known experts. Clinical Trial.gov database was also searched to identify completed trials that were relevant to the study.
Search Strategy
A systematic search strategy with structured terms of medical subject headings (MeSH) and free keywords were used. The following words were used for this search: ‘treatment of HIV patients with cervical cancer’, ‘cervical cancer in HIV patients’, ‘chemo-radiation in HIV patients with cervical cancer’ and ‘HIV with cervical cancer treatment side effects’. There was no restriction on the study language although the search was only done in English language. Four full articles written in other languages were translated into English for evaluation using google translator. The review was carried out by two people (AN and OC). The study abstracts were first identified from the databases and other sources. They were checked to remove duplicates. Then screening was done according to our eligibility criteria to remove irrelevant studies. Full texts of the screened studies were obtained for further evaluation for eligibility. In addition, retrieved articles were also checked whether any further related articles could be found (cross-references). Eligible studies were selected for the systematic review.
Eligibility Criteria
Studies were included if they consisted of a sample size of more than 20 HIV-positive subjects and met the following criteria.
Population of Interest
Included studies were those with target populations with primary conditions of interest which are patients with cervical cancer and HIV seropositivity. In the case of studies involving HIV-positive and HIV-negative patients with cervical cancer, the results of the HIV subgroup were extracted for the analysis if other study criteria were met. Studies focusing on any of the main objectives namely survival, toxicity, and treatment tolerability were also evaluated.
Interventions of Interest
Studies involving the use of radiotherapy/chemotherapy with or without Highly Active Antiretroviral Treatment (HAART) were considered for the review. Concurrent chemo-radiotherapy is considered a standard of care for cervical cancer and the addition of HAART depends mainly on the CD4 cell count of the patient.
Comparators
The outcome of treatment in HIV-positive patients with cervical cancer was compared with the outcome in cervical cancer patients who were HIV-seronegative and received similar interventions.
Study Outcomes
The primary outcome of interest in this study was survival. This included local control, progression-free survival and loco-regional and distant failure. Secondary endpoints included acute and late toxicity, treatment compliance or adherence and CD4 cell count trend of patients during treatment.
Study Designs
Observational studies, randomized controlled trials (RCTs), and controlled clinical trials (CCTs) for interventions were included. Quasi experimental studies that contained most of the information required were also included. The publication types included were full peer reviewed original papers and published doctoral thesis (if not published otherwise). Reviews, case series, and case reports were excluded since they may not provide needed details for evaluation or do not fulfil our minimal sample size requirements.
Data Extraction
The data extracted included study identification, number of patients and age range, diseases characteristics, treatment modalities, toxicities, treatment compliance, and outcome among others. The data were extracted using a self-designed data extraction form.
Assessment of Methodological Quality of Studies
The quality of the studies was assessed based on whether the information of interest relative to meeting the aims of this study were included. In addition, the quality of interest that the studies to be included in the review should have included information about HIV-positive patients with cervical cancer treated with radiotherapy (external beam + brachytherapy), cisplatin chemotherapy and HAART as indicated. In addition, the studies should also include information on acute and late reactions during treatment assessed in major systems namely skin, hematological (hemoglobin, white blood cells and platelets), neurological, gastrointestinal system, and genito-urinary system using standard toxicity scales. The response rates based on the FIGO stages and treatment compliance were to be assessed as well and all these compared with HIV-negative patients.
Assessment of Quality of the Studies for Risk of Bias
The study quality was assessed according to the guidelines on presentation of assessments of risk of bias as outlined in Cochrane Handbook for Systematic Reviews for Randomized Trials 21. The GRACE (Good ReseArch for Comparative Effectiveness) principle was also employed to assess the quality of observational studies (www.graceprinciples.org; accessed 17 February 2015). The latter is a validated checklist for evaluating the quality of observational cohort studies for decision-making support.
These various domains of the studies were classified into low risk, high risk, and unclear risk (of bias) based on the assessment using the following main domains:
Were the study plans (including research questions, main comparisons, outcomes, etc.) specified before conducting the study?
Was the study conducted and analyzed in a manner consistent with good practice and reported in sufficient detail for evaluation and replication?
How valid is the interpretation of comparative effectiveness (CE) for the population of interest, assuming sound methods and appropriate follow-up?
The check list used for study quality assessment is presented in Table1. In the table, different sets of criteria are used to assess prospective and observational studies because observational studies although do provide valuable information, are relatively weaker in methodology compared to RCT and hence a consensus principle is used to guide the assessment of observational studies 22. The determination of the risk of bias was done by means of personal assessment since the use of scoring quality scales or checklist is discouraged by Cochraine Reviews (http://handbook.cochrane.org/chapter_8/8_3_3_quality_scales_and_Cochrane_reviews.htm; accessed 17 February 2015). Studies were therefore assessed as having ‘low risk’ of bias if the study appears to be free of sources of bias. ‘High risk’ of bias was described if there was at least one important risk of bias for example, the study had a potential source of bias related to the specific study design used or had some other identified issue. Unclear risk of bias was ascribed in cases where there may be a risk of bias, but there is either: insufficient information to assess whether an important risk of bias exists; or insufficient rationale or evidence that an identified problem will introduce bias. A more comprehensive list of criteria for assessing quality of the studies is contained in Table S1.
Table 1.
HIV and Cervical cancer treatment outcome: quality assessment check list for the studies
| Quality assessment domains-Prospective trials RCT/non RCT | ||
| Participation bias | Yes | No |
| Population of interest is adequately described for key characteristics | ||
| Study setting and geographic location is adequately described | ||
| Baseline sample is adequately described for key characteristics | ||
| Inclusion and exclusion criteria are adequately described | ||
| Patients were balanced in all aspects except the intervention | ||
| Attrition bias | Yes | No |
| Follow-up is sufficiently long for outcome to occur (≥6 months) | ||
| Proportion of sample completing the study is adequate (≥80%) | ||
| Description of withdrawal (incomplete outcome data) is provided | ||
| Characteristics of drop-outs versus completers is provided | ||
| Outcome measurement | ||
| Definition of outcome is provided a priori | ||
| Objective definition of outcome is provided | ||
| Data analysis and reporting | Yes | No |
| Alpha error and/or beta error is specified a priori | ||
| Data analysis was based on intention-to-treat analysis principle | ||
| Frequencies of most important data (for example, outcomes) are presented | ||
| Quality assessment domains – Retrospective studies | ||
| Data | Yes | No |
| Were treatment and/or important details of treatment exposure adequately recorded for the study purpose in the data source? | ||
| Were the primary outcomes adequately recorded for the study purpose (e.g., available in sufficient detail through data source | ||
| Was the primary clinical outcome(s) measured objectively rather than subject to clinical judgment (e.g., opinion about whether the patient's condition has improved | ||
| Were primary outcomes validated, adjudicated, or otherwise known to be valid in a similar population? | ||
| Was the primary outcome(s) measured or identified in an equivalent manner between the treatment/intervention group and the comparison group(s)? | ||
| Were important covariates that may be known confounders or effect modifiers available and recorded? | ||
| Methods | Yes | No |
| Was the study (or analysis) population restricted to new initiators of treatment or those starting a new course of treatment? | ||
| If one or more comparison groups were used, were they concurrent comparators? If not, did the authors justify the use of historical comparisons group(s)? | ||
| Were important covariates, confounding and effect modifying variables taken into account in the design and/or analysis? | ||
| Is the classification of exposed and unexposed person-time free of “immortal time bias”? | ||
| Were any meaningful analyses conducted to test key assumptions on which primary results are based? | ||
RCT, randomized controlled trial.
Data Analysis and Presentation
The data extracted from the studies are summarized and presented in tables and figures. Descriptive analyses including proportions/percentages are also used to describe the data. A Summary of Findings (SoF) table is used to summarize the main findings from the studies.
Results
Literature search
A flow diagram showing the process of the literature search with associated data is displayed in Figure1. Initial search identified 1018 potentially relevant citations including four citations in non-English language literature (I French, 1 Italian, I Spanish and 1 Portuguese).
Figure 1.
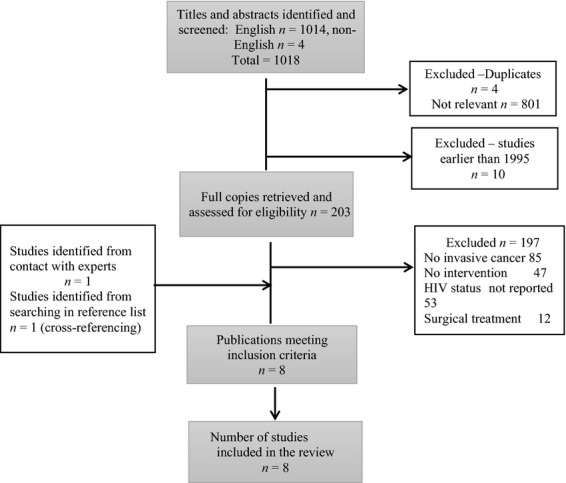
Flow diagram showing the literature search process with associated data.
Four duplicate entries were removed including 801 studies that were not relevant. Ten studies conducted before 1995 were also excluded following initial screening of the titles and abstracts. Further assessment of full texts of the remaining 203 publications led to the exclusion of 197 studies. The reasons for their exclusion are depicted in Figure1. Eight studies met the criteria for inclusion in the studies. There was one RCT, two prospective non-randomized studies, and five retrospective studies. Search on ClinicalTrials.gov (performed May 5, 2014) did not identify any completed study relevant to this topic.
Assessment of methodological quality of the studies
The quality assessment level of the included studies based on GRACE principle and Systematic Reviews principles for retrospective and randomized control trials respectively (Table1) are presented in Table2. In summary, the overall assessment of the included studies was low especially in terms of quality of treatment assessment, treatment allocations, patient's selection, and follow-up.
Table 2.
Results of the methodological quality assessment of studies on HIV patients with cervical cancer. Studies with low levels of bias in at least two domains were assessed as of high overall quality (details see methods section)
| Study type (reference) | Risk of bias | ||
|---|---|---|---|
| Study methods | Data analysis | Overall assessment | |
| Retrospective studies | |||
| Gichangi et al. 43 | High | High | Low |
| Moodley and Mould 44 | High | High | Low |
| Shrivastava et al. 45 | High | Uncertain | Low |
| Simonds et al. 29 | Low | Low | High |
| Al-Noseery 27 | High | Uncertain | Low |
Study characteristics
We did not find any RCT study that randomized HIV-positive patients with cervical cancer on one arm and HIV-negative patients with cervical cancer on the other arm with HIV-positive patients receiving external beam radiotherapy, brachytherapy, chemotherapy with HAART (if indicated based on CD4 cell count). Such studies were also expected to assess the toxicity and response pattern among the two groups. The characteristics of the studies included in the review are summarized in Table3.
Table 3.
HIV-positive patients with cervical cancer: main characteristics of the studies included in the review
| Study, reference | Sample size | Study period | Country | Study type | Characteristics of cervical cancer | Median age (years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage (%) | Histology (%) | |||||||||||||
| I | IIA | IIB | IIIA | IIIB | IVA | Squamous | Adeno | Others | ||||||
| Gichangi 43 | 36 | 1989–1998 | Kenya | Retro | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Moodley 44 | 45 | 2003 | South Africa | Retro | I-IIA (14%) | IIB/IIIA (27%) | IIIB/IVA (49%) | 89 | 4 | 7 | 41 | |||
| Shrivastava 45 | 42 | 1997–2003 | India | Retro | 5 | 7 | 29 | – | 45 | 5 | 98 | 2 | – | 41 |
| Gichangi 15 | 41 | 2000–2002 | Kenya | Prosp (NR) | 2.4 | – | 63.4 | – | 31.7 | 2.4 | 87 | 5.3 | 7.7 | 38.1 |
| Msadabwe 26 | 25 | 2009 | South Africa | Prosp (RCT) | NA | NA | NA | NA | NA | NA | NA | NA | NS | 39.5 |
| Simonds et al. 29 | 59 | 2007–2010 | South Africa | Retro | I/II (16.9%) | III/IV (83.1%) | 94.9 | 1.7 | 3.4 | 41 | ||||
| Munkukpa 23 | 55 | 2012 | Zambia | Prosp (NR) | 13 | 7 | 60 | 2 | 18 | – | 98.2 | 1.8 | – | 40 |
| Al-Noseery 27 | 137 | 2012 | Zambia | Retro | NA | NA | NA | NA | NA | NA | NA | NA | NS | 42 |
Sq, squamous cell carcinoma; Adeno, adenocarcinoma; Retro, retrospective cohort study; Prosp, prospective; NR, nonrandomized; RCT, randomized controlled trial; NA, not available.
The description of the characteristics of patients varied among the different studies. Five studies reported the average age of HIV-positive patients with cervical cancer while only three reported such among HIV-seronegative patients. Similarly, reports on histology and stage of malignancy also varied with no uniformity in the reports. The stages are grouped under early (FIGO stages I to IIB) and late (FIGO stages IIB to IVA) as depicted and summarized in Table4.
Table 4.
Patients characteristics – HIV-positive versus HIV-negative cervical cancer patients from the various studies
| No. of studies that reported item | HIV-positive (%) | No. of studies that reported item | HIV-negative (%) | |
|---|---|---|---|---|
| Median age (years) (Range years) | 5 | 40 years (27–70) | 3 | 52 years (30–80) |
| Histology | 3 | Sum of the 3 studies (N/T) | 2 | Sum of the 2 studies (N/T) |
| Squamous | 130/142 (92) | 448/491 (91) | ||
| Adenocarcinoma | 3/142 (2) | 25/491 (5) | ||
| Others | 9/142 (6) | 18/491 (4) | ||
| Stage | 3 | 2 | ||
| I-IIA (early) | 15/138 (11) | 44/491 (9) | ||
| IIB-IVA (late) | 123/138 (89) | 447/491 (91) |
N = sum of the item within a parameter (histology, stage), T = Total of all items within a parameter.
Toxicity assessment
The pattern of reports on the toxicity and treatment compliance was also very variable among the included studies. Some reports graded toxicity under grades 1 and 2, then 3 and 4 while others separated the various grades. Other studies did not give any information on the toxicity and the proportion of patients that complied with the treatment. The results are summarized in Table5.
Table 5.
Pattern of toxicity and treatment compliance reported by the studies on HIV-positive patients with cervical cancer
| Toxicity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Treatment | Toxicity scale | GIT | GUS | Hem | Skin | Treatment completionon schedule (%) | |
| Gichangi et al. 43 | EBRT NS | G1 | NS | NS | NS | NS | NS | NS |
| Brachy NS | G2 | NS | NS | NS | NS | NS | ||
| CTH NS | G3 | NS | NS | NS | NS | NS | ||
| HAART NS | G4 | NS | NS | NS | NS | NS | ||
| Moodley and Mould 44 | EBRT Yes (dose NS) | G1 | NS | NS | NS | NS | NS | 53% |
| Brachy NS | G2 | NS | NS | NS | NS | NS | ||
| CTH NS | G3 | NS | NS | NS | NS | NS | ||
| HAART NS | G4 | NS | NS | NS | NS | NS | ||
| Shrivastava et al. 45 | EBRT (45–50 Gy) | G1 | 45 | 46 | NS | NS | RTOG | 52% |
| Brachy (25–30 Gy –54% of patients) | G2 | 41 | 18 | NS | NS | |||
| CTH-Nil | G3 | 9 | – | NS | NS | |||
| HAART NS | G4 | 5 | – | NS | NS | |||
| Gichangi et al. 15 | EBRT (40–50 Gy) | G1 | NS | NS | NS | NS | NS | |
| Brachy Nil | G2 | NS | NS | NS | NS | |||
| CTH Nil | G3 | } 34 | 20 | – | 39 | 67% | ||
| HAART Nil | G4 | NS | NS | NS | NS | |||
| Msadabwe 26 | EBRT 45 Gy (Mean) | G1 | 5 | 8 | 15 | NS | RTOG/WHO | 72% |
| Brachy 24 Gy in 3# | G2 | 7 | 1 | 7 | NS | |||
| CTH Cisp 30 mg/m2 weekly | G3 | 2 | – | 4 | NS | |||
| HAART Yes | G4 | – | – | – | NS | |||
| Simonds et al. 29 | EBRT 46–50 Gy | G1 | NS | NS | NS | NS | NS | 45% |
| Brachy 20–26 Gy | G2 | NS | NS | NS | NS | |||
| CTH Cisp 40 mg/m2 | G3 | NS | NS | NS | NS | |||
| HAART Yes | G4 | NS | NS | NS | NS | |||
| Munkukpa 23 | EBRT 46–50 Gy | G1 | 63 | 58 | 34 | 49 | NCI-CTC | 75% |
| Brachy 20-26 Gy | G2 | 33 | 35 | 17 | 47 | |||
| CTH Cisp 80 mg/m2 3 weekly | G3 | – | – | 5 | 5.5 | |||
| HAART Yes | G4 | – | – | 5 | – | |||
| Al-Noseery 27 | EBRT 46–50 Gy | G1 | 84 | 79.6 | 84 | NS | NCI-CTC | 77% |
| Brachy 26 Gy | G2 | |||||||
| CTH Cisp 80 mg/m2 3 weekly | G3 | 6.8 | 6.1 | 12.2 | NS | |||
| HAART Yes | G4 | |||||||
EBRT, external beam radiotherapy; Brachy, brachytherapy; CTH, chemotherapy; NS, not stated; GUS, genitourinary system; GIT, gastro intestinal system; Hem, hematopoietic system; G, grade, #, fractions of brachytherapy; RTOG, Radiation Therapy Oncology Group; NCI-CTC, National Institute-Common Toxicity Criteria.
There was only one study that assessed the acute toxicity in HIV-positive compared with HIV-negative patients treated with radiotherapy, chemotherapy, and HAART 23. In the study, response rate was not reported and late toxicity was also not assessed. This report is summarized in Table6.
Table 6.
Toxicity in HIV-positive versus HIV-negative group-single report (N = 55) (from 23
| Site of toxicity (organ system) | HIV-positive | HIV-negative control | P-value | ||
|---|---|---|---|---|---|
| Treatment (events/patients) | % | Treatment (events/patients) | % | ||
| Acute Gd 1/2 | |||||
| Anemia | 14/55 | 25 | 20/55 | 36 | 0.2 |
| White cell count | 38/55 | 69 | 28/55 | 51 | 0.06 |
| Platelets | 21/55 | 38 | 9/55 | 16 | 0.019 |
| Genitourinary | 52/55 | 95 | 53/55 | 96 | 0.709 |
| Gastrointestinal | 54/55 | 98 | 54/55 | 98 | 1 |
| Neurological | 0/55 | 0 | 0/55 | 0 | 1 |
| Dermatological | 53/55 | 96 | 55/55 | 100 | 0.5 |
| Acute Gd3/4 | |||||
| Haemoglobin | 2/55 | 4 | 1/55 | 2 | not stated |
| White cell count | 5/55 | 9 | 5/55 | 9 | |
| Platelets | 0/55 | 0 | 0/55 | 0 | |
| Genitourinary | 0/55 | 0 | 0/55 | 0 | |
| Gastrointestinal | 3/55 | 5 | 1/55 | 2 | |
| Neurological | 0/55 | 0 | 0/55 | 0 | |
| Dermatological | 1/55 | 2 | 0/55 | 0 | |
CD4 cells count
The CD4 count records were not consistently reported. Some studies used CD4 count of 200 as the minimum level to commence HAART which was the earlier WHO recommendation 24 while only one study among those that reported CD4 count level used CD4 count of ≤350 as minimum level to commence HAART which is the current WHO recommendation for ordinary HIV-positive patients 25.
One study 26 reported the CD4 cells count trend during the treatment and up to 3 months after treatment period. The average initial CD4 cells count was 321.06 cells/mm2 at commencement of treatment. This gradually dropped to 62.56 cells/mm2 at the end of treatment giving a mean difference of 258.5 cells/mm2. There was however, gradual rise after treatment by 3 months which was the end of the follow-up period of the study, but the pretreatment level was not reached. The average count at the end of 3 months was one-third of the pretreatment value.
Summary of findings
The acute toxicity profiles following treatment with complete modalities were available in three studies 23,26,27. They are presented as Grades 1 and 2 and Grades 3 and 4 in Table7. Since there was only one study that reported toxicity in a comparative group (HIV-positive vs. HIV-negative), historical data from studies on HIV-negative group were used to put the numbers in context. The data were from a meta-analysis by Cochrane reviews 28).This work assessed the toxicity of chemo-radiotherapy in cervical cancer patients who were HIV negative.
Table 7.
Summary of findings: study participants (HIV-positive treated with cisplatin and radiotherapy from REFERENCES) versus historical cancer controls (HIV-negative and treated with cisplatin and radiotherapy)
| Toxicity | Cancer patients (HIV (positive) from references | Historical cancer controlsChemoradiotherapy for Cervical Cancer Meta-analysis Collaboration 28) (HIV-negative) | ||||
|---|---|---|---|---|---|---|
| Number of trials | Treatment (events/patients) | % | Number of trials | Treatment (events/patients) | % | |
| Acute Grades 1/2 | ||||||
| Site of toxicity | ||||||
| Hemoglobin | 3 | 110/217 | 51 | 4 | 373/700 | 53 |
| White cell count | 3 | 160/217 | 74 | 8 | 667/1326 | 50 |
| Platelets | 3 | 35/217 | 16 | 7 | 273/1296 | 21 |
| Genitourinary | 3 | 155/217 | 71 | 7 | 228/946 | 24 |
| Gastrointestinal | 3 | 188/217 | 87 | 10 | 585/1150 | 51 |
| Neurological | 3 | 0/217 | 0 | 4 | 64/740 | 9 |
| Dermatological | 3 | 170/217 | 78 | 6 | 180/1011 | 18 |
| Grade 3/4 Toxicity | ||||||
| Site of toxicity | ||||||
| Hemoglobin | 3 | 16/233 | 7 | 4 | 25/429 | 6 |
| White cell count | 3 | 17/233 | 8 | 11 | 265/1479 | 18 |
| Platelets | 3 | 5/233 | 2 | 8 | 26/1356 | 2 |
| Genitourinary | 3 | 8/233 | 4 | 6 | 9/1106 | 0.8 |
| Gastrointestinal | 3 | 33/233 | 9 | 13 | 156/1516 | 10 |
| Neurological | 1 | 0/31 | 0 | 5 | 7/867 | 1 |
| Dermatological | 3 | 23/233 | 10 | 8 | 28/1329 | 2 |
| Response | ||||||
| Complete | 2 | 38/72 | 53 | 10 | 589/1349 | 44 |
| Partial | 2 | 34/72 | 47 | 10 | 220/1374 | 16 |
Information on treatment compliance was not consistently reported in the studies that met the study modalities of treatment. Some patients skipped chemotherapy and continued with radiotherapy due to renal compromise after some doses of cisplatin. Some skipped due to toxicity while others skipped some treatments due to disease progression (malignancy or HIV or both) and development of opportunistic infections which were not primarily linked with treatment toxicity.
The response rate presented were the ones reported by only two studies – (Msadabwe 26 and Simonds 29) whose patients had complete modalities of treatment. The methods of assessing and concluding on complete or partial responds (PAP smear or radiological methods) were not stated in any of the studies.
They are summarized as complete and partial response. These are also compared with data extracted from historical studies reported in Cochrane reviews and are included in Table7.
Discussion
This systematic review summarized available evidence regarding the treatment of cervical cancer patients with HIV infection treated with chemo-radiotherapy which is a mode of treating cervical cancer recommended by many treatment guidelines. Unfortunately, there was no published RCT comparing the treatment outcome in HIV-positive patient with HIV-negative counterparts. The average quality of the included studies was rather low and most of observational nature with suboptimal control for various forms of bias and confounding factors. This reveals a gap in evidence-based management of HIV-positive patients diagnosed with cervical cancer.
All the studies included in the report were carried out in developing countries. This supports the fact that both, cervical cancer and simultaneous HIV burden of disease are high in these regions. This is also reflected in the management modes for these patients. Up to 2005, some centers were not able to treat their patients fully with external beam radiotherapy, brachytherapy, chemotherapy, and HAART. This was probably due to inadequate facilities. An analysis by International Atomic Energy Agency on radiotherapy facilities in 52 African countries noted that, although cancer incidence is increasing in the region only 23 countries had teletherapy facilities while only 20 had brachytherapy treatment units. This situation (which still prevails) cannot guarantee effective treatment of cancer cases 30.
This review shows that median age of HIV-positive cervical cancer patients was at least a decade lower than in their HIV-negative counterparts (40 years vs. 52 years). This has been attributed to the fact that the high virulence and hence progression of HPV infection to cause invasive cervical cancer is faster in HIV-positive patients than their seronegative ones 31. In terms of histological types, the predominant histological cell type is squamous cell carcinoma in both HIV-positive and HIV-negative patients.
The result of this study also shows that there is no major difference in the proportion of patients with early disease (stages I-IIA) from late disease stages IIB-IVA between HIV-positive and HIV-negative (early disease 11% vs. 9%, late disease 89% vs. 91%). This finding is different from the report by Maiman et al. 32 stating that patients who tested HIV positive usually have more advanced cervical cancer than HIV-negative patients 32. This may be because in areas with high incidence of cervical cancer which corresponds with low resource settings, access to screening and early treatment of the disease is low and most patients with cervical cancer present late, irrespective of HIV status 33–36.
The toxicity assessment varied considerably in the included studies and the assessment methods were not uniform. Most are a mixture of toxicities from different chemotherapy and radiotherapy doses in addition to various stages of cervical cancer and HIV. Looking at the proportion of patients that completed the prescribed treatments, those on HAART have higher rates of treatment completion than those without. This confirms earlier findings that patients on HAART are more likely to tolerate chemo-radiotherapy than those without 37.
Still, the coverage of HAART treatment in patients with HIV in some countries is not adequate. A report from Cameroon stated that about 58% coverage was achieved and this was stated to be an improvement. There are logistic issues and lack in facilities, funds and human resources to implement the programs of HIV management in low resource countries 20,38,39. CD4 cells count assays were also lacking in some studies possibly due to unavailability.
The single study that reported toxicity profiles among HIV-positive and negative patients showed that acute toxicity grades 1 and 2 were significantly higher in HIV-positive patients (for platelets 38% vs. 16% P = 0.019). In the acute grades 3 and 4 reactions, Hb was 4% versus 2% while GIT reactions were 5% versus 2%, respectively. These reactions were manageable and 75% of the patients completed their treatment. The reactions in other systems were essentially similar. In that report, all the HIV-positive patients were placed on HAART irrespective of CD4 cell count status according to the institutions' protocol. In the other study by Al-Noseery 27 including patients from the same center, the percentage treatment completion rate was 77%. This observation points to the direction that the commencement of all HIV-positive patients with cervical cancer on HAART irrespective of the CD4 cells count status can ensure a better treatment compliance and hence a better outcome. This is also supported by the fact that in the WHO clinical staging for HIV infections, cervical cancer occurrence in an HIV-positive patient classifies the patient as stage 4 alongside with other AIDS-defining malignancies like Kaposi's sarcoma and lymphoma. In these patients classified as stage 4 infection, it is strongly recommended that HAART should be commenced with CD4 cell count of ≤500 cells/mm3 25.
Furthermore, the trend in CD4 cells count reduction reported in one of the studies 26 is quite informative. An average reduction in CD4 cell count of 258.2 cells/mm2 during treatment shows that patients with normal CD4 cell count can experience very low cell counts during therapy. The recovery of CD4 was also slow as it was noticed that at 3 months post therapy, average CD4 count was one-third of initial level. In addition to this point justifying the commencement of HAART, patients' CD4 count should be monitored closely to know when to commence them on prophylaxes for opportunistic infections like pneumocystis carinii and tuberculosis.
HIV-positive patients on chemotherapy are also known to have increased viral load if they are not on HAART. The viral load decreases as soon as HAART is introduced 40.
The toxicity profile presented in table7 compares acute toxicity from the studies with historical data from systematic reviews and meta-analyses on HIV-negative studies from the literature. Although the sample size difference between the two groups is quite wide and though it may be a comparison with many limitations, certain observations can still be made. In the mild acute reaction groups (grades 1 and 2) reactions are more on the HIV-positive group than HIV-negative in all the systems—WBC, GUS, GIT, and skin. These grades of reactions do not usually lead to treatment interruptions. In the moderate–severe acute reactions (grades 3 and 4) the differences were more in genitourinary and dermatological reactions. The analysis of response rates included in the two studies that reported these differences was not done based on stage of the disease and whether HAART was received or not. The report is presented considering all the patients irrespective of the stage of the disease and whether oncology treatment was completed or not.
An important result in the report by Simonds et al. 29 was that disease stage and completion of radiotherapy (at least 68 Gy) were the only independent factors that predicted good disease control 29. Similarly, the completion of at least three courses of chemotherapy has been reported to ensure benefit from chemotherapy 41.
Factors that usually lead to poor adherence to treatment schedule include toxicity and poor performance status. The use of HAART has been shown to minimize these in HIV-positive patients 23.
Simmonds et al. 29 also suggested that chemotherapy could be withdrawn from HIV-positive patients with stage IIIB and above disease to enable them complete radiotherapy with less toxicity 29. We think that if such patients are on HAART, their tolerance to chemo-radiotherapy will be better and the additional benefit of adding chemotherapy is desirable. This is based on the 2008 report of The Meta-Analysis Group, Medical Research Council Clinical Trials Unit, London, United Kingdom 42 on a systematic reviews and meta-analysis on using radiotherapy and concurrent chemo-radiotherapy in the management of cervical cancer. Fifteen trials were analyzed and it was concluded that there was an absolute benefit of 6% with chemo-radiotherapy over radiotherapy alone in disease-free survival and 8% benefit in disease control over 5 years. The effect was, however, found to be greater in early disease than with advanced disease. The absolute survival benefits were 10% (Stage IA to IIA), 7% (Stage IIB), and 3% (Stage III to IVA) at 5 years. Chemotherapy should, however, be withdrawn in cases with potential renal function impairment.
The level of disease control as reported in Table7 is quite remarkably compared with historical controls though the sample size was comparatively low. It suggests that there are some benefits in treating these patients with chemo-radiotherapy. In HIV-seronegative patients, response rate is highly dependent on stage of the disease with early disease performing better than late disease.
Fortunately, the adverse effects due to drug–drug interaction between cisplatin/carboplatin and most antiretroviral drugs that can be used in the treatment of patients with cervical cancer is mild though some may need minor dose adjustments as illustrated in The University of Liverpool HIV drug interactions website (www.hiv-druginteractions.org; accessed 21 October 2014).
Limitations of the Study
The number of studies identified addressing the management of HIV-positive patients with cervical cancer were very few and the quality of the studies quite low. The mode of reports was also haphazard making it difficult to characterize the studies properly. In addition, outcome measures were difficult to be compared due to nonuniformity of study parameters and data. With regard to patients on HAART, the possible side effects associated with HAART which may influence the side effects of treatment in HIV-positive patients were not taken into consideration.
Conclusion
Currently there is no standard guideline for the management of HIV-positive patients diagnosed with cervical cancer. These patients are managed like their HIV-seronegative counterparts. This is justified based on the results of this review but additional measures should be applied to HIV-positive patients such as commencing all patients on HAART since few studies that commenced all HIV-positive patients with cervical cancer on HAART reported better rates of treatment compliance comparable with their HIV-negative counterparts and with manageable toxicity. The completion of treatment which is an important factor for good disease control is also enhanced by HAART.
Acknowledgments
Uta Schmidt-Strassburger, Medical Faculty, Ulm University, Germany for her advice and support during the study.
Conflict of Interest
None declared.
Supporting Information
Determining low and high risk of bias.
References
- Arbyn M, Castellsague X, de Sanjose S, Bruni L, Saraiya M, Bray F, et al. Worldwide burden of cervical cancer in 2008. Ann. Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- Akhtar-Danesh N, Lytwyn A. Elit L. Five-year trends in mortality indices among gynecological cancer patients in Canada. Gynecol. Oncol. 2012;127:620–624. doi: 10.1016/j.ygyno.2012.08.038. [DOI] [PubMed] [Google Scholar]
- Denny L. Anorlu R. Cervical cancer in Africa. Cancer Epidemiol. Biomarkers Prev. 2012;21:1434–1438. doi: 10.1158/1055-9965.EPI-12-0334. [DOI] [PubMed] [Google Scholar]
- GLOBOCAN. 2014. World cancer statistics 2012 IARC. Available at http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed 5 January 2015)
- Crow JM. HPV: the global burden. Nature. 2012;488:S2–S3. doi: 10.1038/488S2a. [DOI] [PubMed] [Google Scholar]
- Prabha Devi K. Bindhu Priya N. Conventional pap smear screening in HIV seropositive women in South India. J. Obstet. Gynaecol. India. 2013;63:55–58. doi: 10.1007/s13224-012-0204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firnhaber CS. Michelow P. Cervical cancer and the human immunodeficiency virus: a review. South. Afr. J. HIV Med. 2009;10(2):23–27. [Google Scholar]
- Palefsky JM, Minkoff H, Kalish LA, Levine A, Sacks HS, Garcia P, et al. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. J. Natl Cancer Inst. 1999;91:226–236. doi: 10.1093/jnci/91.3.226. [DOI] [PubMed] [Google Scholar]
- Abraham AG, D'Souza G, Jing Y, Gange SJ, Sterling TR, Silverberg MJ, et al. Invasive cervical cancer risk among HIV-infected women: a North American multicohort collaboration prospective study. J. Acquir. Immune Defic. Syndr. 2013;62:405–413. doi: 10.1097/QAI.0b013e31828177d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilen CB, Tilton JC. Doms RW. HIV: cell binding and entry. Cold Spring Harb. Perspect Med. 2012;2:a006866. doi: 10.1101/cshperspect.a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Stem Cells. 2000;18:10–18. doi: 10.1634/stemcells.18-1-10. [DOI] [PubMed] [Google Scholar]
- Kahn S, Jani A, Edelman S, Rossi P, Godette K, Landry J, et al. Matched cohort analysis of outcomes of definitive radiotherapy for prostate cancer in human immunodeficiency virus-positive patients. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:16–21. doi: 10.1016/j.ijrobp.2011.05.047. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Meregalli S, Bonetto E, Mancuso M, Brivio F, Colciago M, et al. Radiotherapy-induced lymphocytopenia: changes in total lymphocyte count and in lymphocyte subpopulations under pelvic irradiation in gynecologic neoplasms. J. Biol. Regul. Homeost. Agents. 2005;19:153–158. [PubMed] [Google Scholar]
- April MD, Wood R, Berkowitz BK, Paltiel AD, Anglaret X, Losina E, et al. The survival benefits of antiretroviral therapy in South Africa. J. Infect. Dis. 2014;209:491–499. doi: 10.1093/infdis/jit584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gichangi P, Bwayo J, Estambale B, Rogo K, Njuguna E, Ojwang S, et al. HIV impact on acute morbidity and pelvic tumor control following radiotherapy for cervical cancer. Gynecol. Oncol. 2006;100:405–411. doi: 10.1016/j.ygyno.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Sfikakis PP, Gourgoulis GM, Moulopoulos LA, Kouvatseas G, Theofilopoulos AN. Dimopoulos MA. Age-related thymic activity in adults following chemotherapy-induced lymphopenia. Eur. J. Clin. Invest. 2005;35:380–387. doi: 10.1111/j.1365-2362.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- Rubinstein P, Aboulsfia D. Zloza A. Malignancies in HIV? AIDS: from epidemiology to therapeutic challenges. AIDS. 2014;28:453–465. doi: 10.1097/QAD.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RD. Chaisson RE. Natural history of HIV infection in the era of combination antiretroviral therapy. AIDS. 1999;13:1933–1942. doi: 10.1097/00002030-199910010-00017. [DOI] [PubMed] [Google Scholar]
- Vella S, Schwartlander B, Sow SP, Eholie SP. Murphy RL. The history of antiretroviral therapy and of its implementation in resource-limited areas of the world. AIDS. 2012;26:1231–1241. doi: 10.1097/QAD.0b013e32835521a3. [DOI] [PubMed] [Google Scholar]
- Higgins J. Green S. 2011. , and Cochrane handbook for systematic reviews of interventions version 5.1.0. The cochrane collaboration. Available at www.cochrane-handbook.org (accessed 17 February 2015)
- National Pharmaceutical Council . Available at http://www.npcnow.org/publication/good-research-comparative-effectiveness-grace-initiative (accessed 7 December 2014)
- Munkukpa H. 2012. Acute toxicity in cervical cancer HIV-positive versus HIV negative patients treated by radical chemo-radiotherapy in Zambia. M.Sc. diss., University of Witswatersrand, Johannesburg, South Africa. Available at https://ujdigispace.uj.ac.za/bitstream/handle/10210/8344/Munkupa.pdf;jsessionid (accessed 18 April 2014)
- WHO. 2006. Guidelines on antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. Available at http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf?ua=1 (accessed 8 February 2015)
- WHO. 2013. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Available at http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf (accessed 21 October 2014)
- Msadabwe S. 2009. A randomized study to compare radical concurrent chemoradiotherapy against radical radiotherapy, as treatment of cancer of the cervix in HIV infected patients. M.Sc. diss., University of Witwatersrand, Johannesburg, South Africa. Available at http://wiredspace.wits.ac.za/bitstream/handle/10539/7468/MMed%20R (accessed 18 November 2014)
- Al-Noseery M. 2012. Effect of pre-treatment CD4 count on acute chemo/radiation reactions and treatment compliance in HIV-positive cancer patients. M.Sc. diss., Ulm University Germany.
- Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration (CCCMAC) Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta-analysis. Cochrane Database Syst. Rev. 2010 doi: 10.1002/14651858.CD008285. Available at http://www.thecochranelibrary.com (accessed 7 December 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds HM, Wright JD, du Toit N, Neugut AI. Jacobson JS. Completion of and early response to chemoradiation among human immunodeficiency virus (HIV)-positive and HIV-negative patients with locally advanced cervical carcinoma in South Africa. Cancer. 2012;118:2971–2979. doi: 10.1002/cncr.26639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Wahab M, Bourque JM, Pynda Y, Izewska J, van der Merwe D, Zubizarreta E, et al. Status of radiotherapy resources in Africa: an International Atomic Energy Agency analysis. Lancet Oncol. 2013;14:e168–e175. doi: 10.1016/S1470-2045(12)70532-6. [DOI] [PubMed] [Google Scholar]
- Joseph B. Chiramana H. Extensive subcutaneous metastasis from squamous cell carcinoma of the cervix in patient with HIV. Int. J. Gynecol. Cancer. 2004;14:176–177. doi: 10.1111/j.1048-891x.2004.014213.x-1. [DOI] [PubMed] [Google Scholar]
- Maiman M, Fruchter RG, Serur E, Remy JC, Feuer G. Boyce J. Human immunodeficiency virus infection and cervical neoplasia. Gynecol. Oncol. 1990;38:377–382. doi: 10.1016/0090-8258(90)90077-x. [DOI] [PubMed] [Google Scholar]
- Chirenje ZM, Rusakaniko S, Akino V. Mlingo M. A review of cervical cancer patients presenting in Harare and Parirenyatwa Hospitals in 1998. Cent. Afr. J. Med. 2000;46:264–267. doi: 10.4314/cajm.v46i10.8566. [DOI] [PubMed] [Google Scholar]
- Eze JN, Emeka-Irem EN. Edegbe FO. A six-year study of the clinical presentation of cervical cancer and the management challenges encountered at a state teaching hospital in southeast Nigeria. Clin. Med. Insights Oncol. 2013;7:151–158. doi: 10.4137/CMO.S12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar A, Anantha N. Venugopal TC. Incidence, mortality and survival in cancer of the cervix in Bangalore, India. Br. J. Cancer. 1995;71:1348–1352. doi: 10.1038/bjc.1995.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndlovu N. Kambarami R. Factors associated with tumour stage at presentation in invasive cervical cancer. Cent. Afr. J. Med. 2003;49:107–111. [PubMed] [Google Scholar]
- Powles T, Imami N, Nelson M, Gazzard BG. Bower M. Effects of combination chemotherapy and highly active antiretroviral therapy on immune parameters in HIV-1 associated lymphoma. AIDS. 2002;16:531–536. doi: 10.1097/00002030-200203080-00003. [DOI] [PubMed] [Google Scholar]
- Barnighausen T, Bloom DE. Humair S. Human resources for treating HIV/AIDS: needs, capacities, and gaps. AIDS Patient Care STDS. 2007;21:799–812. doi: 10.1089/apc.2007.0193. [DOI] [PubMed] [Google Scholar]
- Boyer S, Eboko F, Camara M, Abe C, Nguini ME, Koulla-Shiro S, et al. Scaling up access to antiretroviral treatment for HIV infection: the impact of decentralization of healthcare delivery in Cameroon. AIDS. 2010;24(Suppl 1):S5–S15. doi: 10.1097/01.aids.0000366078.45451.46. [DOI] [PubMed] [Google Scholar]
- Little RF, Pittaluga S, Grant N, Steinberg SM, Kavlick MF, Mitsuya H, et al. Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: impact of antiretroviral therapy suspension and tumor biology. Blood. 2003;101:4653–4659. doi: 10.1182/blood-2002-11-3589. [DOI] [PubMed] [Google Scholar]
- Peters WA, III, Liu PY, Barrett RJ, II, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- Meta-Analysis Group, Medical Research Council Clinical Trials Unit, London, United Kingdom. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J. Clin. Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gichangi P, de Vuyst H, Estambale B, Rogo K, Bwayo J. Temmerman M. HIV and cervical cancer in Kenya. Int. J. Gynaecol. Obstet. 2002;76:55–63. doi: 10.1016/s0020-7292(01)00560-4. [DOI] [PubMed] [Google Scholar]
- Moodley M. Mould S. Invasive cervical cancer and human immunodeficiency virus (HIV) infection in KwaZulu-Natal, South Africa. J. Obstet. Gynaecol. 2005;25:706–710. doi: 10.1080/01443610500294599. [DOI] [PubMed] [Google Scholar]
- Shrivastava SK, Engineer R, Rajadhyaksha S. Dinshaw KA. HIV infection and invasive cervical cancers, treatment with radiation therapy: toxicity and outcome. Radiother. Oncol. 2005;74:31–35. doi: 10.1016/j.radonc.2004.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Determining low and high risk of bias.


