Abstract
Beta glucans are cell wall constituents of yeast, fungi and bacteria, as well as mushrooms and barley. Glucans are not expressed on mammalian cells and are recognized as pathogen-associated molecular patterns (PAMPS) by pattern recognition receptors (PRR). Beta glucans have potential activity as biological response modifiers for hematopoiesis and enhancement of bone marrow recovery after injury. We have reported that Maitake beta glucan (MBG) enhanced mouse bone marrow (BMC) and human umbilical cord blood (CB) cell granulocyte-monocyte colony forming unit (GM-CFU) activity in vitro and protected GM-CFU forming stem cells from doxorubicin (DOX) toxicity. The objective of this study was to determine the effects of MBG on expansion of phenotypically distinct subpopulations of progenitor and stem cells in CB from full-term infants cultured ex vivo and on homing and engraftment in vivo in the nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse. MBG promoted a greater expansion of CD34+CD33+CD38− human committed hematopoietic progenitor (HPC) cells compared to the conventional stem cell culture medium (P = 0.002 by ANOVA). CD34+CXCR4+CD38− early, uncommitted human hematopoietic stem cell (HSC) numbers showed a trend towards increase in response to MBG. The fate of CD34+ enriched CB cells after injection into the sublethally irradiated NOS/SCID mouse was evaluated after retrieval of xenografted human CB from marrow and spleen by flow cytometric analysis. Oral administration of MBG to recipient NOS/SCID mice led to enhanced homing at 3 days and engraftment at 6 days in mouse bone marrow (P = 0.002 and P = 0.0005, respectively) compared to control mice. More CD34+ human CB cells were also retrieved from mouse spleen in MBG treated mice at 6 days after transplantation. The studies suggest that MBG promotes hematopoiesis through effects on CD34+ progenitor cell expansion ex vivo and when given to the transplant recipient could enhance CD34+ precursor cell homing and support engraftment.
Keywords: eta-glucan, stem cell, transplantation, cord blood, hematopoiesis, progenitor cell
Introduction
Beta glucans are cell wall constituents of yeast, fungi and bacteria, as well as edible mushrooms and barley. Beta glucans are not expressed on mammalian cells and are recognized as pathogen-associated molecular patterns (PAMPS) by pattern recognition receptors (PRR), primarily the C-type lectin receptor dectin-1, and also interact via the complement receptor 3 (CR3) (1–4). Dectin-1 is a small type II transmembrane receptor with a lectin-like carbohydrate recognition domain, which recognizes beta1, 3- and beta1, 6-linked and intact yeast, while CR3 is a widely expressed beta 2-integrin containing a lectin domain, which mediates carbohydrate recognition (1, 5). Both CR3 and dectin-1 expression are affected by bone marrow injury and have a role in the restorative effects of soluble beta glucan on hematopoiesis after radiation or chemotherapy (6, 7).
Studies in the mouse have shown that specific beta glucans, such as PGG-glucan derived from yeast (Saccharomyces cerevisiae) or from mushrooms such as Grifola frondosa, Sclerotinia sclerotiorum and Sparassis crispa, can enhance hematopoiesis and protect bone marrow cells from radiation and chemotherapeutic injury (8–12). PGG-glucan (poly-1-6 beta-D-glucopyranosyl 1,3-beta-glucopyranose) has been shown to synergize with colony-stimulating growth factors leading to increased colony forming activity and to have direct effects on committed hematopoietic progenitor cells (10, 13, 14). Increase in colony growth factor production after intraperitoneal injection of SSG, a 1,3-beta-D-glucan obtained from the culture filtrate of S. sclerotiorum led to increases in both splenic hematopoiesis and peripheral leukocyte numbers (9). Administration of SCG a 1,3-beta-D-glucan from Sparassis crispa to mice after cyclophosphamide treatment restored hematopoiesis and the effect was mediated by beta glucan binding to dectin-1 (7, 9, 15).
We have previously reported that Maitake beta glucan (MBG), a purified endotoxin-free extract from the fruit body of mushroom Grifola frondosa characterized by a 1,6 main chain with 1,3 branches, enhanced mouse bone marrow (BMC) granulocyte-monocyte colony forming unit (GM-CFU) activity in vitro and protected GM-CFU forming stem cells from doxorubicin (DOX) toxicity in a dose-dependent manner (8). In subsequent studies we found that MBG enhanced human umbilical cord blood (CB) cell GM-CFU activity in vitro and protected human GM-CFU forming stem cells from DOX toxicity. In these studies we discovered that MBG directly induced G-CSF production in CD33+ CB monocytes but not in adult monocytes, suggesting that lineage specific CB monocytes had an enhanced response to MBG and performed as important accessory cells in the CFU-GM assay (16).
Human umbilical cord blood contains a rich population of primitive hematopoietic cells including lineage-restricted committed progenitors (HPC), and primitive uncommitted hematopoietic stem cells (HSC) that sustain multilineage hematopoiesis (17). HSC develop into all of the blood forming cells of the hematopoietic system, while the myeloid restricted HPC are critical for the initial phase of clinical transplantation (18, 19). Although there are no surface markers that singly or in combination can identify functionally active stem cells as discrete and homogenous populations, the CD34 cell dose is the one factor consistently associated with rate of engraftment, reduced morbidity and survival (20). Functional assays are required to assess the biological activity of progenitor and stem cells (21). Committed myeloid progenitors (HPC) form discrete colonies of mature cells in response to hematopoietic cytokines in semi-solid medium and these cells are measured as colony-forming units (CFU) in validated CFU assays (22). Human CD34+ cells with HSC function are identified by in vivo functional assay in the NOD/SCID mouse by xenotransplantation assay (23). After brief exposure to irradiation the NOD/SCID mouse models can be repopulated with human cells over days to weeks and offer a validated approach to assess HSC homing and engraftment (23, 24). The severe combined immune deficient mouse repopulating cell (SRC) assay, which measures relative SRC activity in the NOD/SCID mouse, provides a clinically useful correlate for graft function (22). CXCR4, the G− protein coupled receptor that binds to stromal cell-derived factor-1 alpha (SDF-1) is a critical determinant for CD34+ human precursor cell migration leading to homing and engraftment in the nonobese diabetic/ severe combined immunodeficient (NOD/SCID) mouse assay for transplantation (25–27). CXCR4 expression on the surface of CD34+ precursor cells denotes very early-uncommitted HSC proliferation and homing and correlates with long-term culture-initiating activity (28).
Cord blood is emerging as an important source of progenitor cells for hematopoietic reconstitution in the treatment of both malignant and non-malignant blood diseases. Compared to bone marrow, cord blood stem cells cause less graft-versus-host disease (29, 30). The limitation in precursor cell number, especially in smaller volume cord blood samples has led to the development of methods to expand cord blood precursor cells including HPC and HSC ex vivo (31, 32) and the investigation of approaches to maximize homing and engraftment potential in vivo (33). The objective of the present study was to determine the effects of MBG on the proliferation and differentiation of phenotypically distinct subpopulations of CB progenitor and stem cells during expansion of freshly obtained CB from healthy full-term infants cultured ex vivo and to evaluate the potential role of oral administration of MBG on the fate of CB CD34+ precursor cells in vivo in the NOD/SCID mouse model for homing and engraftment.
Material and Methods
Chemicals and Reagents
Maitake mushroom beta-glucan (MBG) is an extract from fruit body of Maitake mushroom (Grifola frondosa), which was made under patented methods (Japan Pat. No. 2859843/US Pat. No. 5,854,404) and provided by Yuikiguni Maitake Corp. through the Tradeworks group. The extract was stored in a refrigerator at 4°C under dark conditions until use. The lot of MBG used in this study was sent to NAMSA to test for endotoxin contamination using limulus amebocyte lysate (LAL) assay. The result showed that there was no detectable endotoxin activity (maximum level = 0.012 EU/mg). MBG powder dissolved readily in RPMI 1640 with 25 mM HEPES buffer and was initially prepared at a concentration of 20 mg/mL and sterilized by filtration through 0.2 µm cellulose acetate low protein binding membrane, and stored at −20°C. The stock solution was diluted to the required concentration in RPMI 1640 medium freshly at the time of use.
Mice
NOD.CB17-Prikdc scid/J mice were purchased from Jackson Laboratory and maintained under a restricted barrier facility at Memorial Sloan-Kettering Cancer Center (MSKCC, New York, NY). All animal experiments were approved by the Animal Care Committee of MSKCC. Mice were maintained on regular food, Certified Rodent Diet # 5053 (LabDiet) throughout the study. Mice, 8–10 weeks old, were given a sublethal dose (350 cGy) of whole body irradiation at a rate of 65 cGy/min from a Gammacell 40 Exactor containing 137Cs (MDS Nordion; Kanata, Ontario Canada). Within 24 hrs, mice were injected through the tail vein with CD34+ enriched human umbilical cord blood cells (2–5 × 105 cells/mouse). Mice were sacrificed using the CO2 technique at different time points after transplantation as indicated. Mouse peripheral blood was obtained by cardiac puncture bleeding at the time of sacrifice, and mouse bone marrow and spleen cells were collected and resuspended as single-cell suspensions.
Human Cord Blood Samples
Human umbilical cord blood (CB) samples from healthy full-term infants were obtained under an approved IRB protocol at Weill Medical College of Cornell University. All CB units used in this study were released by the New York Blood Bank Cord Blood Banking program at New York Presbyterian Hospital—Weill Cornell due to low volume or for logistical reasons. All samples were collected at the time of delivery into blood bags containing anticoagulant Citrate-Phosphate Dextrose Adenine and processed freshly in the Weill Cornell Cellular Immunology Laboratory.
Cord Blood Cell Dectin-1 Expression
Freshly collected CB samples were stained with mouse anti-human dectin-1/CLEC7A antibody (R&D Systems, Minneapolis, MN) using a modified indirect staining protocol. Briefly, blood samples were lysed with BD Pharm Lyse TM for 10 mins in room temperature (RT) to remove red blood cells. After washing with staining buffer (PBS/0.5% BSA) twice, cells were incubated with 400 µL blocking buffer I containing 0.5% human IgG, 5% BSA, 2 mM NaN3 in PBS at 4°C for 20 mins to block against human Ig Fc receptor (FcR). Cells were then washed with 2 mL staining buffer once, followed by staining with mouse anti-human dectin-1/CLEC7A antibodies or matching isotype antibodies for 30 mins at 4°C. After washing with 2 mL staining buffer once, cells were incubated with secondary antibodies, FITC conjugated goat anti-mouse IgG (R&D Systems, Minneapolis, MN) at 4°C for 30 mins, then washed with 2 mL staining buffer. Afterwards, cells were incubated with 0.5 mL of blocking buffer II (5% mouse serum in PBS) for 20 mins at 4°C, and washed once with 2 mL staining buffer. Directly conjugated fluorescent antibodies of interest were then added (e.g., CD14 PE for detecting monocytes, CD45 PerCP and CD19 PE for detecting lymphocytes), and samples were incubated at RT for 15 mins, then washed with 2 mL staining buffer. Cells were resuspended in 400 µL fixative solution containing 1% paraformaldehyde, 0.25% BSA, 1 mM NaN3 in PBS. The cells were then acquired and analyzed in a FACSCalibur flow cytometer (BD) using Cell Quest and FlowJo software. Gating was initially performed on monocytes by light scatter properties and on lymphocytes using anti CD45. We also used the anti-dectin-1 antibody GE2 (IgG1) provided by J. A. Willament as a reference.
Cord Blood Stem Cell Enrichment
Freshly collected CB samples were enriched for CD34+ stem cells using the RosetteSep cord blood progenitor enrichment system (StemCell Technologies Inc., Vancouver, Canada) according to the manufacturer’s instructions. Briefly, RosetteSep human progenitor enrichment cocktail was added into CB at 50 µL/mL blood, incubated at room temperature (RT) for 20 mins. After incubation, the CB was diluted with PBS/2% FBS at 1:4 (v/v) and mixed well. The diluted blood was layered on top of Ficoll-Paque. After centrifugation for 25 mins at 2000 rpm at RT, the enriched cells were collected from the Ficoll-Paque plasma interface. Cells were washed with PBS/2% FBS twice, and then resuspended in PBS/2% FBS. Cell aliquots were diluted and mixed using Turk’s stain and counted by light microscope using a hemocytometer. For each CB sample, a small aliquot of enriched cells was assessed by flow cytometric technique to detect the percentage of enriched CD34+CD33+CD38− cells as well as CD34+CXCR4 +CD38− cells; the mean percentages for the CB samples used in ex vivo expansion studies were 8.2 ± 10.0 and 2.8 ± 1.9, respectively.
Ex Vivo Expansion Assay
Cord blood was enriched for CD34+ progenitor cells using RosetteSep cord blood progenitor enrichment system (StemCell Technologies Inc., Vancouver, Canada), after separation of mononuclear cells by density gradient centrifugation as described above. CD34− enriched cord blood cells were then cultured in expansion culture medium, which was StemSpan® H3000 medium (StemCell Technologies Inc.) with StemSpan™ CC100 cytokine cocktail containing 100 ng/mL rh Flt-3 ligand, 100 ng/mL rh Stem Cell Factor (SCF), 20 ng/mL rh interleukin-3 (IL-3) and 20 ng/mL rh interleukin-6 (IL-6). Briefly, after washing with PBS/2% FBS, CD34-enriched cord blood cells were resuspended in expansion medium at 6.69 ± 1.74 × 104 cells/mL. After adding MBG (final concentrations were 0, 50, 100, 200 µg/mL), CD34+ enriched cells were cultured in expansion culture medium at a total volume of 2 mL in T25 tissue culture flasks. The cells were cultured at 37°C, in a 5% CO2 humidified incubator. Harvesting and evaluation of cell populations in cell cultures was performed at specific time points: 0, 4, 7, and 14 days. Effects of MBG on expansion of cell populations with either CD34+CD33+CD38− HPC or CD34+CD38− CXCR4+ HSC phenotypes were assessed by flow cytometry. Flow cytometric analysis was performed on cells stained with directly conjugated moAb using a FACSCalibur (BD Biosciences) instrument. Stepwise gating was performed first to gate on CD38− mononuclear cells expressing CD34, then to determine percentage of populations co-expressing CD33 (HPC) or co-expressing CXCR4 by three-color flow cytometry. Data acquisition and analysis were performed with CellQuest and FlowJo software.
Labeling of CD34+ Enriched Cord Blood Cells with CFSE
For the homing studies, CD34+ enriched cord blood cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) 18 hrs before injection into NOD/SCID mice. Briefly the CFSE solution was added to enriched CB cell suspensions, and samples were incubated at 37°C for 15 mins. Then pre-chilled (4°C) PBS/0.1% BSA was added to wash the cells. Aspiration was performed and cells were washed twice more with pre-chilled PBS/0.1% BSA. After the final aspiration, 5 mL of pre-warmed (37°C) RPMI-1640/5% FBS was added and cells were put into a 37°C, 5% CO2 incubator overnight.
Injection of CD34+ Enriched Cord Blood Cells into NOD/SCID Mice
Before injection, the cells were washed with PBS once and resuspended in PBS at 1~2.5 × 106 cells/mL. Injection of 200 µL /mouse of the enriched CB cells was performed through the tail vein into NOD/ SCID mouse which had been given sublethal irradiation on the previous day.
MBG Oral Administration
In the MBG treatment group, mice were orally given 4 mg/kg/day of MBG by gavage at the same time as the transplantation and then were given MBG daily in the subsequent experimental days in the same way. The mice were weighed each day before gavage.
Collection of Mouse Peripheral Blood, Bone Marrow, and Spleen
Peripheral Blood
After experiment period, mice were sacrificed with the CO2 technique. Mouse peripheral blood was obtained by cardiac puncture allowing free flow bleeding into small, heparinized sterile tubes.
Bone Marrow
Mouse bone marrow cells were collected by standard procedure as previously described (8). Briefly, mouse bone marrow cells were collected from femoral shafts by flushing with 3 mL of cold RPMI-1640. The cell suspensions were passed up and down six times through an 18-gauge needle in RPMI-1640 to disperse cell clumps. After washing once with RPMI-1640, bone marrow cells were incubated with 15% FBS/RPMI-1640 at RT for 30 mins. Washing was performed on cells with serum-free RPMI-1640 twice, then cells were washed once with PBS, and resuspended in PBS for staining with fluorescent conjugated monoclonal antibodies.
Spleen
Mouse spleen cells were collected with smearing between two sterile glass-slides a few times in ~3 mL RPMI-1604 medium. The cell suspensions were passed up and down through an 18-gauge needle in RPMI-1640 to disperse cell clumps. After washing with RPMI-1640 once, mouse spleen cells were incubated with 15% FBS/RPMI-1640 at RT for 30 mins. Spleen cells were washed twice with serum-free RPMI-1640, then washed once with PBS, and then resuspended in PBS for staining with fluorescent-conjugated monoclonal antibodies.
Staining with Anti-Human CD45 and CD34 Antibodies to Detect Engrafted Human Cells
After washing, bone marrow, or spleen cells with PBS, peripheral whole mouse blood, bone marrow or spleen cells were pre-incubated with Mouse BD Fc Block (purified anti-mouse CD16/CD32 mAB, 2.4 G2, BD Biosciences) at ≤1 µg/ million cells in 100 µL, at 4°C for 5–10 mins. Then monoclonal antibodies of interest were added: mouse IgG R-PE and mouse IgG-PerCP for isotype detection tubes, anti-human CD45-PerCP and anti-human CD34-PE for the human cell detection tubes. After incubating at RT for 15 mins in the dark in the presence of Mouse BD Fc Block, fixative-free lysing solution was added at 2 mL/tube (High-Yield Lyse, CALTAG, Carlsbad, CA), followed by vortexing and incubation at RT in dark conditions for 10 mins. Then tubes were centrifuged at 1500 rpm for 5 mins and vacuum aspirated to remove supernatants. After washing the cells once with PBS, followed by aspiration to remove supernatants, 7-AAD was added and tubes were incubated at 4°C for 15 mins. Then cells were treated with 0.5 mL of fixative solution (7.5 g paraformaldehyde + 2.5 mL FBS in 500 mL of PBS). The cells were then acquired and analyzed in a FACSCalibur flow cytometer (BD) using Cell Quest and FlowJo software.
Flow Cytometric Analysis
Flow cytometric analysis was used to determine the percentage and number of human CD45 and CD34 cells retrieved from the NOD/SCID mouse bone marrow, spleen and peripheral blood. For homing studies, human CB cells were identified first with CFSE labeling, then CD34 R-PE or CD45 PerCP positive cells were gated. For engraftment studies, the gating strategy was performed as described (34). Dead cells were excluded using 7-AAD by plotting 7-AAD against forward light scatter. Living CD34 R-PE and/or CD45-FITC positive cells were then gated. To determine the number of CD34+ CB cells retrieved from the NOD/SCID mouse 6 days after transplantation, dead cells were excluded first using 7-AAD. Living CD45dim cells possessing large forward light scatter properties were then gated and then plotted using CD45 FITC versus CD34 R-PE. The number of these cells represented the CD34+ CB cells retrieved from the NOD/ SCID mouse bone marrow.
Statistical Analysis
Data are presented as mean percentage ± SD or mean ± SD. To study the effects of MBG on the ex vivo expansion of CD34+ cells, one-way ANOVA was used to examine the difference in average cell counts across different treatment groups. Dunnett’s test was then used to compare the average cell counts between each of the MBG treated group and the control while properly adjusting for multiple comparisons. To further examine the differential treatment effects on HPCs and HSCs, two-way ANOVA with an interaction term of cell type and treatment was used. For the homing and engraftment studies, two-way ANOVA was used to examine the association between CD34+ cell homing and engraftment and MBG treatment while controlling for different cord blood samples transplanted. These analyses were carried out using statistical programming and software package R (35).
Results
Effects of MBG on Expansion of CD34+ Cells Ex Vivo
Umbilical cord blood samples were collected at delivery from healthy infants and processed within 12 hrs. CD34+ progenitor cells were enriched using the RosetteSep human progenitor enrichment cocktail. Mononuclear cells were isolated by density gradient centrifugation, washed and evaluated for CD34+ cells by flow cytometry and then expanded ex vivo in StemSpan H3000 defined medium supplemented with growth factors and cytokines: rhFlt-3, rhSCF, rhIL-3, rhIL-6, in the presence or absence of MBG at various doses as indicated. The objective of these experiments was to assess the effect of MBG on expansion of the committed CD34+ progenitor cell expressing CD33+ an early marker of myeloid maturation as a correlate of potential HPC progenitor cell activity and on CD34+CXCR4+CD38− cells, putative HSC stem cells, as a correlate of uncommitted hematopoietic potential (36, 37). Absence of CD38 on CD34+ precursor cells was used to define the initial gate (37–39).
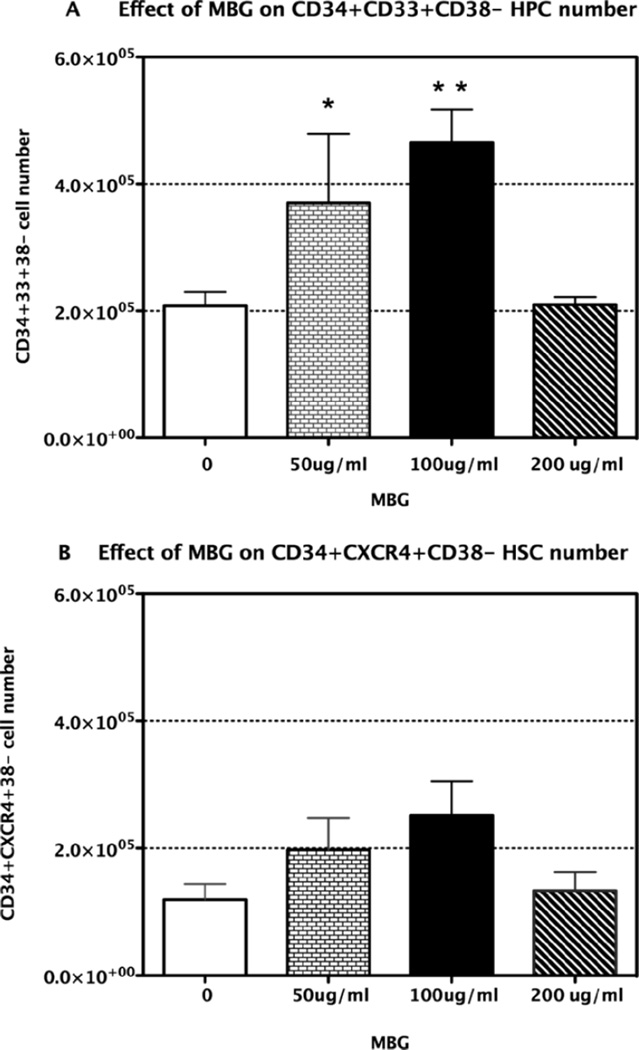
In the absence of cytokines and growth factors, CB cells did not proliferate and cultures were poorly viable (data not shown). After 4 days’ culture in conventional expansion media with and without added MBG, expression of CD34, CD33, CD38, and CXCR4 was assessed by flow cytometry. After gating on CD34+CD38− cells, expression of CD33+ was assessed. Mean data from 4 experiments with CB from 4 different infants are shown in Figure 1 panel A and panel B. As shown in panel A, MBG elicited a dose-related enhancement of CD34+CD33+CD38− cells. Significant differences were observed using one-way ANOVA to analyze changes in HPC across all doses of MBG compared to conventional expansion medium for this population (P = 0.002). Dunnett’s test was then applied to evaluate pair-wise differences for specific doses of MBG. Significant increases in HCP were observed when MBG was added at 50 µg/mL and 100 µg/mL (P = 0.022 and 0.003, respectively); expansion was maximal at 100 µg/mL. At 200 µg/mL the MBG response declined to the level of control cultures. The fall off was not due to any cytostatic or cytotoxic effects as determined in separate experiments adding MBG at 200 µg/ mL to CB cells in the 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) cytotoxicity assay. This could be due to cross-linking of the 1.3 branches at high concentration and failure to trigger monocyte activation.
Figure 1.
Effect of MBG on expansion of HPC and HSC in cord blood ex vivo. Panel A. Mononuclear cells were separated from cord blood from healthy infants (n = 4) enriched for CD34+ cells and cultured ex vivo in the presence or absence of MBG at the indicated doses. The effects of MBG on expansion of cell populations were determined after 4 days of culture followed by harvesting, staining with anti-human CD34, CD38, CD33 antibodies, and assessment by three-color flow cytometry. Data in Panel A show the CD34+CD33+D38- (HPC) cell population. Data in Panel B show the CD34+CD38-CXCR4+ (HSC) cell population from the same cultures as in Panel A. Data present mean cell numbers ± SD, * P< 0.05 vs. control with no MBG.
The same samples from the same cultures were also assessed for expansion of precursor cells defined by expression of the CD34+CD38− CXCR4+ phenotype, which correlates with early, uncommitted hematopoietic stem cells (HSC) capable of repopulating the NOD/SCID mouse (37). Compared to conventional expansion medium alone, MBG treatment led to an increase in cells with HSC phenotype but these differences were not statistically significant by one-way ANOVA. Data are shown in Figure 1 panel B. As noted in Methods, mean concentration of the inoculum was 6.69 ± 1.74 × 104 cells/mL allowing comparison of all MBG doses and time points across all samples. There was no discernible impact of this difference on the results of the expansion studies.
In all samples there were more HPC cells than HSC cells. After enrichment the percentage of HPC cells was 8.2% ± 10.0 and that of HSC cells was 2.8% ± 1.9 as mentioned in the Methods section. The starting population of HSC cells was lower than that of HPC cells and could have influenced the overall expansion. To further examine whether there was a difference between the effect of MBG on HPCs and HSCs on ex vivo expansion in responding to MBG, a two-way ANOVA with an interactive term of cell type and treatment was used. The analysis showed that the HPCs showed a trend towards greater expansion compared to cells with HSC phenotype in response to MBG at 100 µg/ mL.
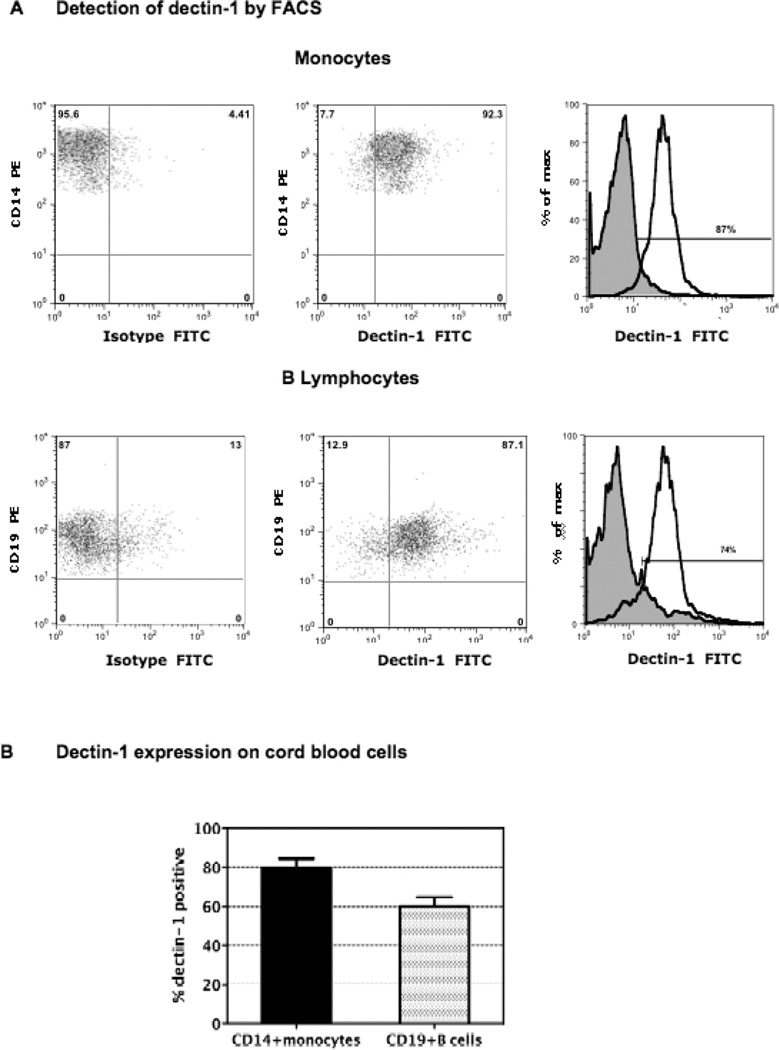
Studies of other beta glucans have shown that monocytes, macrophages, and neutrophils are the principal responding cell type and that this is associated with expression of the dectin-1 receptor on these cells (2, 15, 57, 58, 62). Therefore we evaluated freshly obtained CB from 12 full-term infants for expression of dectin-1 by flow cytometry. As shown in Figure 2, we report that dectin-1 is expressed on both monocytes and B lymphocytes. Panel A shows the flow cytometry of a single representative infant, and Panel B shows the group results.
Figure 2.
Expression of dectin-1 on cord blood cells. CB samples were stained with dectin-1 antibody, using a modified indirect staining protocol to detect dectin-1, and directly conjugated fluorescent antibodies to CD45, CD19, and anti-CD14 were used for assessment of B cells and monocytes; all analyzed by three-color flow cytometry. Data in Panel A show flow cytometric histogram overlays for one-term infant’s monocyte and B cell populations. Data show the percentage of each respective gated population that expresses dectin-1 compared to the isotype control. Data in Panel B represent the mean percentage ± SD of monocytes and B cells in the CB group that express dectin-1. Samples were from 12 healthy full-term infants.
Effects of MBG on CB CD34+ Cells Homing and Engraftment to NOD/SCID Mouse
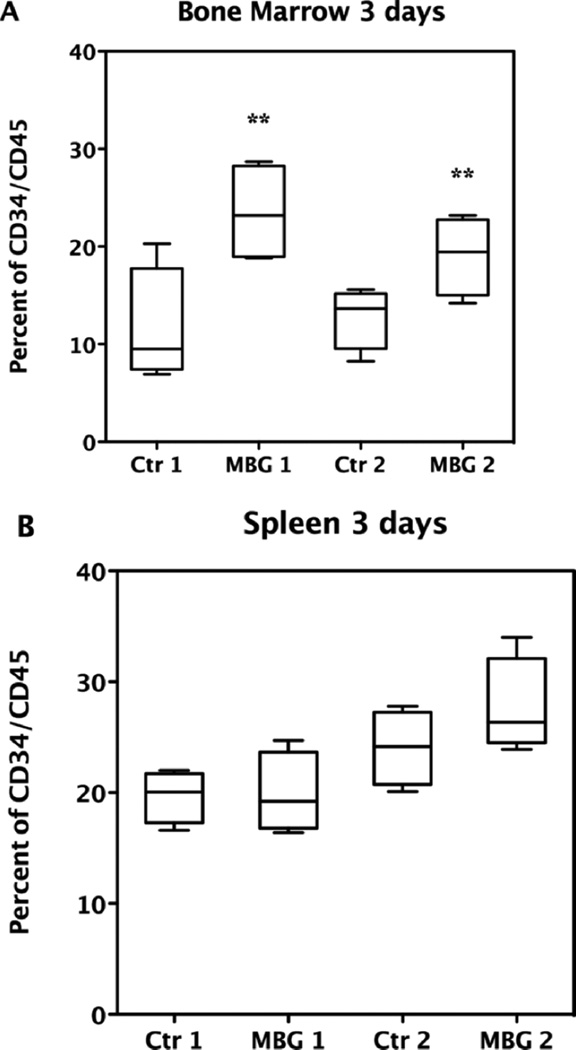
To evaluate the effects of MBG on homing and engraftment, we then transplanted freshly obtained CB enriched for CD34+ precursor cells without expansion into MBG treated compared to untreated NOD/SCID mice (24, 40). Beta glucan given orally is taken up by intestinal macrophages, which then migrate to the bone marrow where further degradation of the beta glucan occurs (41). The purpose of these experiments was to determine if giving MBG by oral supplementation to the recipient mouse would affect homing and engraftment. Three independent experiments were carried out using 3 to 8 mice in each defined group (n = 36 mice); 16 mice were treated with MBG and compared to 16 controls. The other 4 mice were used as irradiation controls without transplantation. For each experiment, one unit CB was transplanted to 8 mice. Mice were then randomly divided such that 4 mice were in both the control and MBG groups. Multiple units of CB samples were used for both homing and engraftment studies. Two-way ANOVA was applied to examine the association between CD34+ cell homing and engraftment and MBG treatment while controlling for different cord blood samples transplanted. For the homing studies, enriched human CD34+ cells prepared from CB samples were labeled with CFSE and transplanted into NOD/SCID mice that had been sublethally irradiated on the previous day. At 3 days the results showed that daily oral administration of MBG led to significantly increased numbers of CFSE-labeled CB CD34+ cells when retrieved from NOD/SCID mouse bone marrow after sacrifice regardless of the cord blood sample used (P value = 0.002) as shown in Figure 3 panel A. In contrast, augmentation of human precursor cell recovery in the spleen compared to conventional transplantation was not observed. As shown in Figure 3 panel B, although CB CD34+ cells retrieved from MBG treated NOD/SCID mouse spleen (SP) were slightly higher on average than the control group using the second unit cord blood sample, the overall percentage of CD34+ cells recovered remained at the same level as in control mice (P value = 0.30). MBG also did not affect recovery of CD34+CB stem cells in peripheral blood at 3 days compared to conventional transplantation (data not shown). Therefore the studies indicated that MBG augmented CB CD34+ cells homing to bone marrow but not to spleen in this early stage of transplantation.
Figure 3.
Effect of MBG on homing of CB CD34+ cells in NOD/SCID mice. Data show effect of daily oral MBG treatment at 4 mg/kg/day at 3 days after CB transplant compared to control group mice. Control 1 (Ctr1) group mice (n = 4) were transplanted with same CB as the MBG1 group of mice (n = 4), while control 2 (Ctr2) mice (n = 4) and MBG 2 mice (n = 4) received the same other unit of CB. CD34+CD45+ human CB cells were retrieved from bone marrow (A) and spleen (B) and analyzed by flow cytometry (** P < 0.01 vs. control).
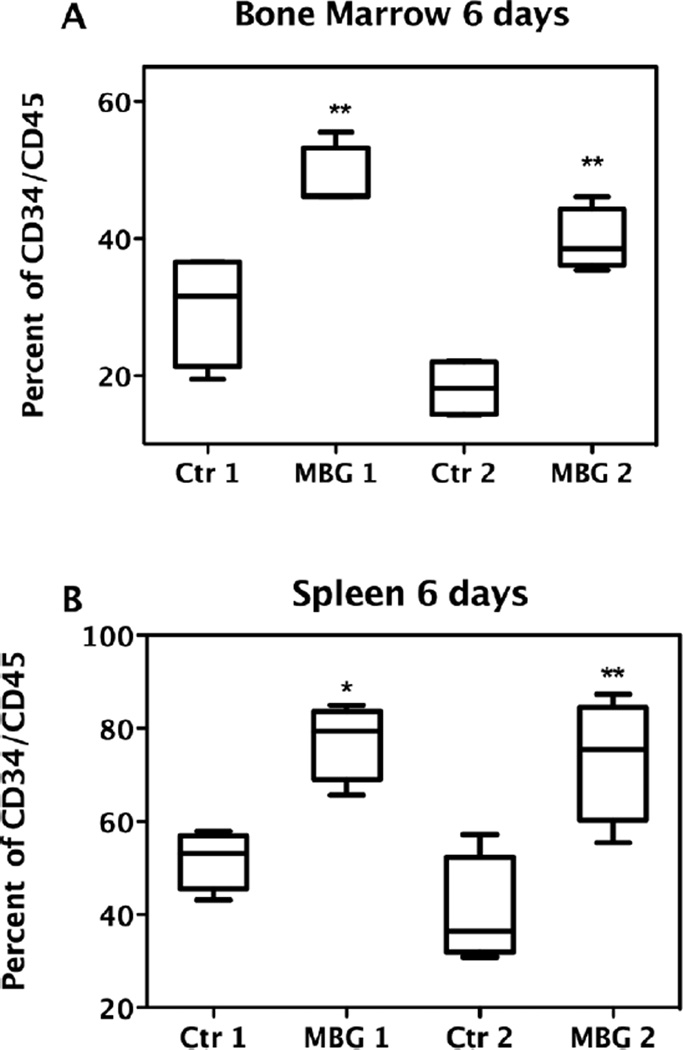
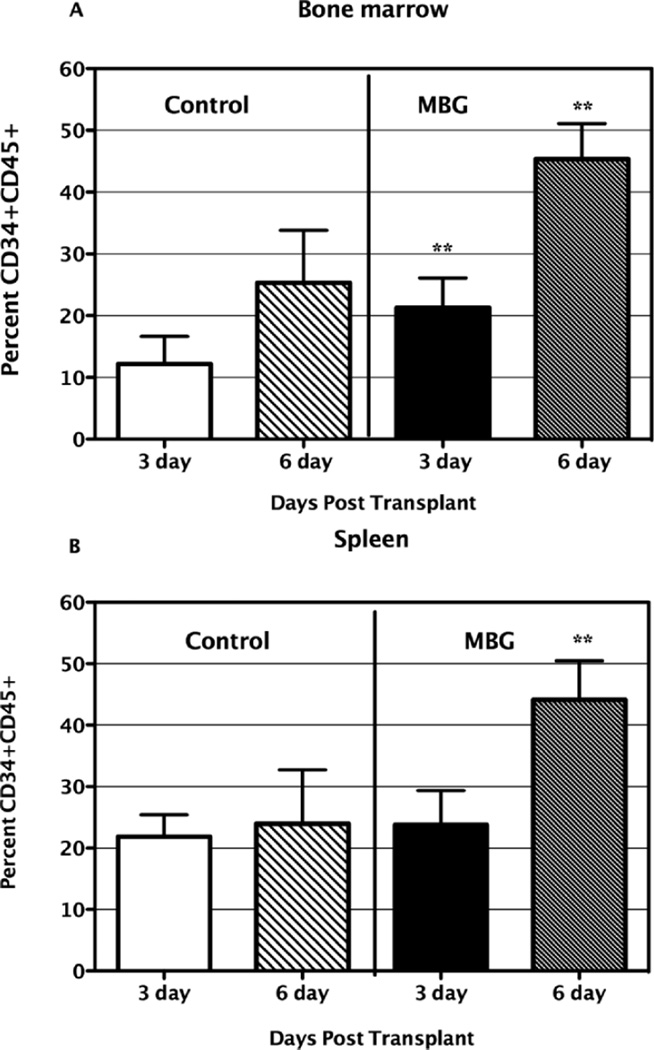
For the engraftment study, at 6 days after transplantation with enriched human CB CD34+ precursor cells, cellular populations were collected from NOD/SCID mice bone marrow and spleen. Cells were prepared as described and analyzed with flow cytometry. Dead cells were excluded using 7-AAD and living CD34+CD45+ cells were selectively gated. As clearly shown in Figure 4 panel A, after 6 days, the percentages of human cord blood identified by coexpression of CD34+CD45+ cells, retrieved from MBG treated NOD/SCID mice (n = 8) bone marrow, were very significantly higher than those from control groups (n= 8), regardless of the different units of CB used (P value < 0.001). Similarly, as shown in Figure 4 panel B, the percentages of human CD34+CD45+ cells retrieved from MBG treated NOD/SCID mouse spleens were very significantly higher than those from the control groups of mice not treated with MBG (P < 0.001).
Figure 4.
Effects of MBG on engraftment of CB CD34+ cells in NOD/ SCID mice. The MBG group mice were given 4 mg/kg/day of MBG beginning on the day of CB transplantation and during the subsequent 6 days. Control 1 (Ctr1) group of mice and MBG1 group mice were transplanted with the same unit of CB, while control 2 (Ctr2) group of mice and the MBG2 group of mice received the other same unit of CB. Human CD34+CD45+ cells retrieved from NOD/ SCID mice bone marrow (A) or spleen (B) were analyzed by flow cytometry (** P < 0.01 vs. control).
When the effects of transplantation were combined for different cord bloods and compared as groups, the results clearly showed that MBG enhanced homing of human CD34+CD45+ cells to bone marrow compared to conventional transplantation as shown in Figure 5 panel A. Overall the percentage of human CD34+CD45+ cells increased 1fold by 6 days in mouse bone marrow and spleen compared to 3 days, for both the control group and the MBG group treated group. In contrast as shown in panel B, there was no effect of MBG on the level of CD34+CD45+ CB cells in spleen at 3 days while at 6 days this population showed a much greater increase in the MBG group compared to the untreated group. This could have reflected an effect of MBG on enhancement of human stem cell proliferation in the spleen.
Figure 5.
Comparison of response to MBG after transplantation in NOD/SCID mice. The MBG group mice were given 4 mg/kg/day of MBG on the day of transplantation and over the subsequent 3 or 6 days (n = 8, n = 8, respectively). Human CD34+CD45+cells retrieved from NOD/SCID mice bone marrow (Panel A) or spleen (Panel B) were analyzed by flow cytometry. Data show mean percentage ± SD, ** P < 0.01 vs. control.
Discussion
These studies are the first to show that a beta glucan, as described here for MBG, promotes the expansion of human umbilical cord blood CD34+ precursor cells ex vivo and enhances human CD34+ precursor cell homing and engraftment in the NOD/SCID mouse. Compared to conventional expansion media, the dose-dependent effect of MBG on expansion of the CD34+ cell population containing myeloid committed HPC progenitor cell was highly significant. The potential relevance for engraftment was evaluated in the xenograft NOD/SCID mouse model assay for human transplantation. Mice were given MBG by oral administration with the intent of influencing the bone marrow microenvironment in the recipient. As shown by recovery of more human cells from mouse bone marrow and spleen of treated mice compared to untreated mice, MBG enhanced human CB CD34+ cell homing and engraftment.
Despite clear evidence that the rates of acute graft-versus-host disease are much lower with CB transplants, the use of CB for adult transplantation has been limited (42). The main reasons are the lower number of CD34+ cells or total nucleated cells and the longer average time of three weeks for neutrophil reconstitution after CB transplant compared to bone marrow (43). However CD34+ CB cells do have a higher level of engraftment than do CD34+ adult bone marrow cells in the severe combined immune deficient mouse repopulating cell (SRC) assay (44), suggesting greater intrinsic potential. Ex vivo expansion methods have shown promise for increasing the numbers of functionally active progenitors and are currently in clinical trials for efficacy (42, 45, 46). In this study we found that MBG showed dose-dependent expansion of the CD34+CD33+ CD38− progenitor cell population, which includes the myeloid committed HPC. The maximum effect was observed at MBG 100 µg/mL and is consistent with our previous studies of MBG enhancement of CFU-GM response in vitro for mouse bone marrow and human cord blood stem cells (8, 16). Mazurier et al. defined the term rapid SCID repopulating cells (rSRC) to describe the stem cell population that rapidly repopulates the NOD/SCID mice (47). Several groups have shown that among fresh stem cells CD34+CD38+ (low) cells are responsible for rapid repopulation and appearance of a myeloerythroid graft at 2 weeks (40, 48). Van Heusden et al. have shown that after ex vivo expansion of cord blood precursor cells under conditions similar to those we used here, the CD34+CD33+CD38− cell population is responsible for early repopulation in the NOD/SCID mouse (37). After culture the rSRC lacks expression of CD38 while expression of CD33, an early myeloid marker, is not affected by culture (36). Since CD33 is also expressed in the thymus on early lymphoid precursors, some CD34+CD33+ precursor cells may also develop into lymphoid long-term culture initiating cells (37, 49). As reported here, we found that MBG treatment led to expansion of the uncommitted HSC stem cell identified as CD34+CXCR4+CD38− but these differences were not significant and two-way ANOVA analysis suggested an interactive effect. In part this may have been reflected by the generally lower percentage of HSC cells after CD34+ cell enrichment compared to the HPC population. However when we attempted to correlate differences in starting cell numbers across the cord blood samples for either HPC or HSC to numbers of matched populations after expansion, we could not find a correlation. One limitation of our study was that we did not measure intracellular expression of CXCR4, which could have led to lower estimation of CXCR4 expressing cells (25). For clinical transplantation, both HPC and HSC are important since heterogeneous progenitors provide cells that transiently sustain hematopoietic function until a permanent hematopoietic stem cell-derived graft is established (50, 51).
The mechanism of action in the CD34+ precursor cell expansion studies shown here may involve MBG activation of G-CSF production by CD33+ cord blood monocytes as we previously reported (16) and could require dectin-1. The effects of MBG on HPC are likely to be indirect and mediated by cells that express dectin-1 or other beta glucan receptors such as CR3. Dectin-1 is not known to be expressed on CD34+ precursor cells. CR3 involvement in the actions of beta glucans appears to vary according to physical characteristics of the specific beta glucan. Particulate beta glucan treatment led to increased CR3 expression on HPC in mouse bone marrow, while soluble PGG-glucan, which was shown to enhance HPC mobilization, did not affect CR3 expression (14, 52). The metabolites of MBG also mediate biological effects. Oral activity of a barley beta glucan was shown to involve macrophage uptake and subsequent degradation of glucan into smaller fragments in bone marrow (41). After phagocytosis by neutrophil effector cells, killing of tumor target cells was enhanced (53). Particulate beta glucans are phagocytosed while the larger forms of beta glucan appear to activate by receptor binding and signal transduction (52).
The restorative effects of other beta glucans after bone marrow injury after radiation or chemotherapeutic treatment and in the repair process have been investigated previously and support our study findings (54–56). Beta-glucan PGG (Betafectin) increased the short-term colony forming potential in vitro of human CD34+ bone marrow mononuclear cells treated with suboptimal levels of GM-CSF or G-CSF but did not stimulate cytokine or growth factor production (13). In contrast MBG does activate CD33+ CB cells to produce G-CSF (16). Betafectin PGG was shown to accelerate the recovery of peripheral blood leukocytes, after myelosuppressive or myeloablative doses of cyclophosphamide (10). Cramer et al. showed that whole glucan particles purified from baker’s yeast would prime CR3+ HPCs tethered through iC3b on injured bone marrow stroma leading to HPC proliferation and leukocyte recovery in the mouse (14). Harada et al. have shown that the restorative effects of the major 6-branched 1,3-beta-D-glucan, SCG, from the edible cultivable mushroom S. crispa on hematopoietic response of cyclophosphamide-induced leukopenic mice are regulated by the level of endogenous GM-CSF production and/or dectin-1 expression (15).
Studies of other beta glucans have shown that monocytes, macrophages, and neutrophils are the principal target cell types and that response is associated with expression of the dectin-1 receptor on these cells (2, 57, 58). Dectin-1 is a germline-encoded pattern recognition receptor that is analogous to members of the TLR family, and can mediate phagocytosis, production of reactive oxygen intermediates and also interacts with TLR signals to induce inflammatory response. Human dectin-1 is widely expressed on myeloid cells, dendritic cells, B cells and a subpopulation of T cells. Data shown here confirm that dectin-1 is strongly expressed on both CB monocytes and B cells. Whether dectin-1 is expressed on CD34+ precursor cells or early hematopoietic progenitor cells after expansion is unknown. Since major fungal pathogens such as Candida albicans, Aspergillus niger, Pneumocystis carinii and also Cryptococcus neoforms, and Histoplasma capsulatum express beta glucans which directly elicit immune response, botanical beta glucans appear to have potential as natural agonists for the host defense system (59–66).
Beta glucan binding to dectin-1 was recently discovered to drive production of IL-17 (67). The IL-17A member of the IL-17 family of cytokines causes neutrophilia when over expressed in the mouse, is present in inflammatory conditions, and drives G-CSF response (68). IL-17 receptor knockout mice have enhanced myelotoxicity and impaired hematopoietic recovery following gamma irradiation (69). Increased hematopoiesis occurs in response to infection and other stresses such as bone marrow injury due to radiation or chemotherapy. Stress hematopoiesis such as after sublethal irradiation of immunodeficient mice (e.g., NOD/SCID mouse) leads to increased expression of stromal cell-derived factor (SDF)-1 alpha and CXCR4. In these mice human CXCR4 responds to murine SDF-1, which mediates homing and migration of human transplanted cells to mouse bone marrow (70). Cramer et al. have shown that injecting normal mice with a beta glucan mobilized HPC from bone marrow and increased the plasma levels of SDF-1. While our studies did not address the mechanism of action that led to enhanced homing and engraftment, it seems plausible that MBG supports bone marrow recovery in the NOD/SCID mouse and that recruitment of human cord blood cells to the mouse bone marrow and spleen is part of this process.
Overall our studies show that MBG treatment increased the number of CD34+ precursor cells ex vivo in expansion culture and promoted homing and engraftment of CD34+ enriched cord blood cells in the NOD/SCID mouse in vivo. The effects of MBG on the expansion of hematopoietic progenitor cells ex vivo could have been mediated by the CD33+ monocyte population, which we have shown in other studies produces G-CSF in response to beta glucan (16). How MBG affects human precursor cell engraftment in the mouse is unclear but could involve parallel effects on mouse bone stromal macrophages and/or other indirect mechanisms such as support of mouse bone marrow recovery. Further studies addressing the potential for long-term engraftment and the mechanism(s) of action, including the requirement for beta-glucan receptors such as dectin-1, CR3, and TLRs are warranted. The discovery that MBG enhances CD34+ precursor cell expansion ex vivo and homing and engraftment in vivo when given to the cord blood recipient has potential significance for the development of cord blood as a resource for clinical transplantation.
Acknowledgments
The study was supported by NIH NCI 29502: CNRU Pilot Study: (PI H. Lin) Effect of MBG (MBG) on hematopoietic expansion & engraftment of cord blood cells in NOD/ SCID mouse; NIH NCI R25 105012 Collaborative Program in Nutrition and Cancer Prevention; H. Lin, Training and Career Development Fellowship Award; NIH NCCAM and ODS: 1-P50-AT02779 Botanical Research Center for Botanical Immunomodulators. We thank J. A. Willament for provision of the anti-dectin-1 antibody GE2 (IgG1).
References
- 1.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 2.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willment JA, Gordon S, Brown GD. Characterization of the human beta-glucan receptor and its alternatively spliced isoforms. J Biol Chem. 2001;276:43818–43823. doi: 10.1074/jbc.M107715200. [DOI] [PubMed] [Google Scholar]
- 4.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 5.Thornton BP, Vetvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18) J Immunol. 1996;156:1235–1246. [PubMed] [Google Scholar]
- 6.Cramer DE, Allendorf DJ, Baran JT, Hansen R, Marroquin J, Li B, Ratajczak J, Ratajczak MZ, Yan J. Beta-glucan enhances complement-mediated hematopoietic recovery after bone marrow injury. Blood. 2006;107:835–840. doi: 10.1182/blood-2005-07-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harada T, Kawaminami H, Miura NN, Adachi Y, Nakajima M, Yadomae T, Ohno N. Mechanism of enhanced hematopoietic response by soluble beta-glucan SCG in cyclophosphamide-treated mice. Microbiol Immunol. 2006;50:687–700. doi: 10.1111/j.1348-0421.2006.tb03841.x. [DOI] [PubMed] [Google Scholar]
- 8.Lin H, She YH, Cassileth BR, Sirotnak F, Cunningham-Rundles S. Maitake beta-glucan MD-fraction enhances bone marrow colony formation and reduces doxorubicin toxicity in vitro. Int Immunopharmacol. 2004;4:91–99. doi: 10.1016/j.intimp.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto K, Suzuki I, Ohsawa M, Oikawa S, Yadomae T. Enhancement of hematopoietic response of mice by intraperitoneal administration of a beta-glucan, SSG, obtained from Sclerotinia sclerotiorum. J Pharmacobiodyn. 1990;13:512–517. doi: 10.1248/bpb1978.13.512. [DOI] [PubMed] [Google Scholar]
- 10.Patchen ML, Vaudrain T, Correira H, Martin T, Reese D. In vitro and in vivo hematopoietic activities of Betafectin PGG-glucan. Exp Hematol. 1998;26:1247–1254. [PubMed] [Google Scholar]
- 11.Patchen ML, MacVittie TJ, Weiss JF. Combined modality radio-protection: the use of glucan and selenium with WR-2721. Int J Radiat Oncol Biol Phys. 1990;18:1069–1075. doi: 10.1016/0360-3016(90)90442-m. [DOI] [PubMed] [Google Scholar]
- 12.Harada T, Miura N, Adachi Y, Nakajima M, Yadomae T, Ohn N. Effect of SCG, 1,3-beta-D-glucan from Sparassis crispa on the hematopoietic response in cyclophosphamide induced leukopenic mice. Biol Pharm Bull. 2002;25:931–939. doi: 10.1248/bpb.25.931. [DOI] [PubMed] [Google Scholar]
- 13.Turnbull JL, Patchen ML, Scadden DT. The polysaccharide, PGG-glucan, enhances human myelopoiesis by direct action independent of and additive to early-acting cytokines. Acta Haematol. 1999;102:66–71. doi: 10.1159/000040972. [DOI] [PubMed] [Google Scholar]
- 14.Patchen ML, Liang J, Vaudrain T, Martin T, Melican D, Zhong S, Stewart M, Quesenberry PJ. Mobilization of peripheral blood progenitor cells by Betafectin PGG-Glucan alone and in combination with granulocyte colony-stimulating factor. Stem Cells. 1998;16:208–217. doi: 10.1002/stem.160208. [DOI] [PubMed] [Google Scholar]
- 15.Harada T, Ohno N. Contribution of dectin-1 and granulocyte macro-phage-colony stimulating factor (GM-CSF) to immunomodulating actions of beta-glucan. Int Immunopharmacol. 2008;8:556–566. doi: 10.1016/j.intimp.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Lin H, Cheung SW, Nesin M, Cassileth BR, Cunningham-Rundles S. Enhancement of umbilical cord blood cell hematopoiesis by maitake beta-glucan is mediated by granulocyte colony-stimulating factor production. Clin Vaccine Immunol. 2007;14:21–27. doi: 10.1128/CVI.00284-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gan OI, Murdoch B, Larochelle A, Dick JE. Differential maintenance of primitive human SCID-repopulating cells, clonogenic progenitors, and long-term culture-initiating cells after incubation on human bone marrow stromal cells. Blood. 1997;90:641–650. [PubMed] [Google Scholar]
- 18.Ballen KK. New trends in umbilical cord blood transplantation. Blood. 2005;105:3786–3792. doi: 10.1182/blood-2004-10-4125. [DOI] [PubMed] [Google Scholar]
- 19.Barker JN, Wagner JE. Umbilical-cord blood transplantation for the treatment of cancer. Nat Rev Cancer. 2003;3:526–532. doi: 10.1038/nrc1125. [DOI] [PubMed] [Google Scholar]
- 20.Wagner JE. Umbilical cord transplantation. Leukemia 12 Suppl. 1998;1:S30–S32. [PubMed] [Google Scholar]
- 21.Coulombel L. Identification of hematopoietic stem/progenitor cells: strength and drawbacks of functional assays. Oncogene. 2004;23:7210–7222. doi: 10.1038/sj.onc.1207941. [DOI] [PubMed] [Google Scholar]
- 22.Broxmeyer HE, Srour EF, Hangoc G, Cooper S, Anderson SA, Bodine DM. High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proc Natl Acad Sci U S A. 2003;100:645–650. doi: 10.1073/pnas.0237086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice AM, Wood JA, Milross CG, Collins CJ, McCarthy NF, Vowels MR. Conditions that enable human hematopoietic stem cell engraftment in all NOD-SCID mice. Transplantation. 2000;69:927–935. doi: 10.1097/00007890-200003150-00044. [DOI] [PubMed] [Google Scholar]
- 24.Lowry PA, Shultz LD, Greiner DL, Hesselton RM, Kittler EL, Tiarks CY, Rao SS, Reilly J, Leif JH, Ramshaw H, Stewart FM, Quesenberry PJ. Improved engraftment of human cord blood stem cells in NOD/LtSz-scid/scid mice after irradiation or multiple-day injections into unirradiated recipients. Biol Blood Marrow Transplant. 1996;2:15–23. [PubMed] [Google Scholar]
- 25.Kollet O, Petit I, Kahn J, Samira S, Dar A, Peled A, Deutsch V, Gunetti M, Piacibello W, Nagler A, Lapidot T. Human CD34(+)CXCR4(−) sorted cells harbor intracellular CXCR4, which can be functionally expressed and provide NOD/SCID repopulation. Blood. 2002;100:2778–2786. doi: 10.1182/blood-2002-02-0564. [DOI] [PubMed] [Google Scholar]
- 26.Voermans C, Kooi ML, Rodenhuis S, van der Lelie H, van der Schoot CE, Gerritsen WR. In vitro migratory capacity of CD34+cells is related to hematopoietic recovery after autologous stem cell transplantation. Blood. 2001;97:799–804. doi: 10.1182/blood.v97.3.799. [DOI] [PubMed] [Google Scholar]
- 27.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 28.Yao CL, Feng YH, Lin XZ, Chu IM, Hsieh TB, Hwang SM. Characterization of serum-free ex vivo-expanded hematopoietic stem cells derived from human umbilical cord blood CD133(+) cells. Stem Cells Dev. 2006;15:70–78. doi: 10.1089/scd.2006.15.70. [DOI] [PubMed] [Google Scholar]
- 29.Gluckman E, Rocha V, Chevret S. Results of unrelated umbilical cord blood hematopoietic stem cell transplant. Transfus Clin Biol. 2001;8:146–154. doi: 10.1016/s1246-7820(01)00132-x. [DOI] [PubMed] [Google Scholar]
- 30.Hwang WY, Samuel M, Tan D, Koh LP, Lim W, Linn YC. A meta-analysis of unrelated donor umbilical cord blood transplantation versus unrelated donor bone marrow transplantation in adult and pediatric patients. Biol Blood Marrow Transplant. 2007;13:444–453. doi: 10.1016/j.bbmt.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Tanavde VM, Malehorn MT, Lumkul R, Gao Z, Wingard J, Garrett ES, Civin CI. Human stem-progenitor cells from neonatal cord blood have greater hematopoietic expansion capacity than those from mobilized adult blood. Exp Hematol. 2002;30:816–823. doi: 10.1016/s0301-472x(02)00818-4. [DOI] [PubMed] [Google Scholar]
- 32.Levac K, Karanu F, Bhatia M. Identification of growth factor conditions that reduce ex vivo cord blood progenitor expansion but do not alter human repopulating cell function in vivo. Haematologica. 2005;90:166–172. [PubMed] [Google Scholar]
- 33.Li K, Chuen CK, Lee SM, Law P, Fok TF, Ng PC, Li CK, Wong D, Merzouk A, Salari H, Gu GJ, Yuen PM. Small peptide analogue of SDF-1alpha supports survival of cord blood CD34+ cells in synergy with other cytokines and enhances their ex vivo expansion and engraftment into nonobese diabetic/severe combined immunodeficient mice. Stem Cells. 2006;24:55–64. doi: 10.1634/stemcells.2005-0082. [DOI] [PubMed] [Google Scholar]
- 34.van Hennik PB, de Koning AE, Ploemacher RE. Seeding efficiency of primitive human hematopoietic cells in nonobese diabetic/severe combined immune deficiency mice: implications for stem cell frequency assessment. Blood. 1999;94:3055–3061. [PubMed] [Google Scholar]
- 35.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Mahwah, New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 36.Dorrell C, Gan OI, Pereira DS, Hawley RG, Dick JE. Expansion of human cord blood CD34(+)CD38(−) cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood. 2000;95:102–110. [PubMed] [Google Scholar]
- 37.Vanheusden K, Van Coppernolle S, De Smedt M, Plum J, Vandekerckhove B. In vitro expanded cells contributing to rapid severe combined immunodeficient repopulation activity are CD34+38- 33+90+45RA. Stem Cells. 2007;25:107–114. doi: 10.1634/stemcells.2006-0256. [DOI] [PubMed] [Google Scholar]
- 38.Forraz N, Pettengell R, McGuckin CP. Characterization of a lineage-negative stem-progenitor cell population optimized for ex vivo expansion and enriched for LTC-IC. Stem Cells. 2004;22:100–108. doi: 10.1634/stemcells.22-1-100. [DOI] [PubMed] [Google Scholar]
- 39.Peled T, Mandel J, Goudsmid RN, Landor C, Hasson N, Harati D, Austin M, Hasson A, Fibach E, Shpall EJ, Nagler A. Pre-clinical development of cord blood-derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamine. Cytotherapy. 2004;6:344–355. doi: 10.1080/14653240410004916. [DOI] [PubMed] [Google Scholar]
- 40.Kerre TC, De Smet G, De Smedt M, Offner F, De Bosscher J, Plum J, Vandekerckhove B. Both CD34+38+ and CD34+38- cells home specifically to the bone marrow of NOD/LtSZ scid/scid mice but show different kinetics in expansion. J Immunol. 2001;167:3692–3698. doi: 10.4049/jimmunol.167.7.3692. [DOI] [PubMed] [Google Scholar]
- 41.Hong F, Yan J, Baran JT, Allendorf DJ, Hansen RD, Ostroff GR, Xing PX, Cheung NK, Ross GD. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol. 2004;173:797–806. doi: 10.4049/jimmunol.173.2.797. [DOI] [PubMed] [Google Scholar]
- 42.Brunstein CG, Setubal DC, Wagner JE. Expanding the role of umbilical cord blood transplantation. Br J Haematol. 2007;137:20–35. doi: 10.1111/j.1365-2141.2007.06521.x. [DOI] [PubMed] [Google Scholar]
- 43.Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, Goldman A, Kersey J, Krivit W, MacMillan ML, Orchard PJ, Peters C, Weisdorf DJ, Ramsay NK, Davies SM. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 44.Ueda T, Yoshida M, Yoshino H, Kobayashi K, Kawahata M, Ebihara Y, Ito M, Asano S, Nakahata T, Tsuji K. Hematopoietic capability of CD34+ cord blood cells: a comparison with CD34+ adult bone marrow cells. Int J Hematol. 2001;73:457–462. doi: 10.1007/BF02994007. [DOI] [PubMed] [Google Scholar]
- 45.Shpall EJ, Quinones R, Giller R, Zeng C, Baron AE, Jones RB, Bearman SI, Nieto Y, Freed B, Madinger N, Hogan CJ, Slat-Vasquez V, Russell P, Blunk B, Schissel D, Hild E, Malcolm J, Ward W, McNiece IK. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant. 2002;8:368–376. doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- 46.Kim SK, Koh SK, Song SU, Shin SH, Choi GS, Kim WC, Lee MH, Seoh JY, Park SK, Fraser JK. Ex vivo expansion and clonality of CD34+ selected cells from bone marrow and cord blood in a serum-free media. Mol Cells. 2002;14:367–373. [PubMed] [Google Scholar]
- 47.Mazurier F, Doedens M, Gan OI, Dick JE. Rapid myeloerythroid repopulation after intrafemoral transplantation of NOD-SCID mice reveals a new class of human stem cells. Nat Med. 2003;9:959–963. doi: 10.1038/nm886. [DOI] [PubMed] [Google Scholar]
- 48.McKenzie JL, Gan OI, Doedens M, Dick JE. Human short-term repopulating stem cells are efficiently detected following intrafemoral transplantation into NOD/SCID recipients depleted of CD122+ cells. Blood. 2005;106:1259–1261. doi: 10.1182/blood-2005-03-1081. [DOI] [PubMed] [Google Scholar]
- 49.Blom B, Spits H. Development of human lymphoid cells. Annu Rev Immunol. 2006;24:287–320. doi: 10.1146/annurev.immunol.24.021605.090612. [DOI] [PubMed] [Google Scholar]
- 50.Zhao P, Liu W, Cui Y. Rapid immune reconstitution and dendritic cell engraftment post-bone marrow transplantation with heterogeneous progenitors and GM-CSF treatment. Exp Hematol. 2006;34:951–964. doi: 10.1016/j.exphem.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 51.Hogan CJ, Shpall EJ, Keller G. Differential long-term and multilineage engraftment potential from subfractions of human CD34+ cord blood cells transplanted into NOD/SCID mice. Proc Natl Acad Sci U S A. 2002;99:413–418. doi: 10.1073/pnas.012336799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cramer DE, Wagner S, Li B, Liu J, Hansen R, Reca R, Wu W, Surma EZ, Laber DA, Ratajczak MZ, Yan J. Mobilization of hematopoietic progenitor cells by yeast-derived beta-glucan requires activation of matrix metalloproteinase-9. Stem Cells. 2008;26:1231–1240. doi: 10.1634/stemcells.2007-0712. [DOI] [PubMed] [Google Scholar]
- 53.Cheung NK, Modak S. Oral (1->3),(1->4)-beta-D-glucan synergizes with antiganglioside GD2 monoclonal antibody 3F8 in the therapy of neuroblastoma. Clin Cancer Res. 2002;8:1217–1223. [PubMed] [Google Scholar]
- 54.Patchen ML, MacVittie TJ, Solberg BD, Souza LM. Survival enhancement and hemopoietic regeneration following radiation exposure: therapeutic approach using glucan and granulocyte colony-stimulating factor. Exp Hematol. 1990;18:1042–1048. [PubMed] [Google Scholar]
- 55.Vos AP, M’Rabet L, Stahl B, Boehm G, Garssen J. Immune-modulatory effects and potential working mechanisms of orally applied nondigestible carbohydrates. Crit Rev Immunol. 2007;27:97–140. doi: 10.1615/critrevimmunol.v27.i2.10. [DOI] [PubMed] [Google Scholar]
- 56.Vetvicka V, Dvorak B, Vetvickova J, Richter J, Krizan J, Sima P, Yvin JC. Orally administered marine (1->3)-beta-D-glucan Phycarine stimulates both humoral and cellular immunity. Int J Biol Macromol. 2007;40:291–298. doi: 10.1016/j.ijbiomac.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, Wong SY. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002;169:3876–3882. doi: 10.4049/jimmunol.169.7.3876. [DOI] [PubMed] [Google Scholar]
- 58.Willment JA, Marshall AS, Reid DM, Williams DL, Wong SY, Gordon S, Brown GD. The human beta-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol. 2005 doi: 10.1002/eji.200425725. [DOI] [PubMed] [Google Scholar]
- 59.Borchers AT, Keen CL, Gershwin ME. Mushrooms, tumors, and immunity: an update. Exp Biol Med (Maywood) 2004;229:393–406. doi: 10.1177/153537020422900507. [DOI] [PubMed] [Google Scholar]
- 60.Weck MM, Appel S, Werth D, Sinzger C, Bringmann A, Grunebach F, Brossart P. hDectin-1 is involved in uptake and cross-presentation of cellular antigens. Blood. 2008;111:4264–4272. doi: 10.1182/blood-2006-10-051375. [DOI] [PubMed] [Google Scholar]
- 61.Gow NA, Netea MG, Munro CA, Ferwerda G, Bates S, Mora-Montes HM, Walker L, Jansen T, Jacobs L, Tsoni V, Brown GD, Odds FC, Van der Meer JW, Brown AJ, Kullberg BJ. Immune recognition of Candida albicans beta-glucan by Dectin-1. J Infect Dis. 2007;196:1565–1571. doi: 10.1086/523110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dennehy KM, Brown GD. The role of the {beta}-glucan receptor Dectin-1 in control of fungal infection. J Leukoc Biol. 2007;82:253–258. doi: 10.1189/jlb.1206753. [DOI] [PubMed] [Google Scholar]
- 63.Ishibashi K, Yoshida M, Nakabayashi I, Shinohara H, Miura NN, Adachi Y, Ohno N. Role of anti-beta-glucan antibody in host defense against fungi. FEMS Immunol Med Microbiol. 2005;44:99–109. doi: 10.1016/j.femsim.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 64.Krajicek BJ, Limper AH, Thomas CF., Jr Advances in the biology, pathogenesis and identification of Pneumocystis pneumonia. Curr Opin Pulm Med. 2008;14:228–234. doi: 10.1097/MCP.0b013e3282f94abc. [DOI] [PubMed] [Google Scholar]
- 65.Akamatsu N, Sugawara Y, Kaneko J, Tamura S, Makuuchi M. Preemptive treatment of fungal infection based on plasma (1 -> 3)beta-D-glucan levels after liver transplantation. Infection. 2007;35:346–351. doi: 10.1007/s15010-007-6240-7. [DOI] [PubMed] [Google Scholar]
- 66.Rappleye CA, Eissenberg LG, Goldman WE. Histoplasma capsulatum alpha-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proc Natl Acad Sci U S A. 2007;104:1366–1370. doi: 10.1073/pnas.0609848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 68.Schwarzenberger P, Huang W, Oliver P, Byrne P, La Russa V, Zhang Z, Kolls JK. Il-17 mobilizes peripheral blood stem cells with short- and long-term repopulating ability in mice. J Immunol. 2001;167:2081–2086. doi: 10.4049/jimmunol.167.4.2081. [DOI] [PubMed] [Google Scholar]
- 69.Tan W, Huang W, Gu X, Zhong Q, Liu B, Schwarzenberger P. IL-17F/IL-17R interaction stimulates granulopoiesis in mice. Exp Hematol. 2008 doi: 10.1016/j.exphem.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Lapidot T. Mechanism of human stem cell migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice The role of SDF-1/CXCR4 interactions. Ann N Y Acad Sci. 2001;938:83–95. doi: 10.1111/j.1749-6632.2001.tb03577.x. [DOI] [PubMed] [Google Scholar]