Abstract
Background
To increase the enrollment rate of medication therapy management (MTM) programs in Medicare Part D plans, the US Centers for Medicare & Medicaid Services (CMS) lowered the allowable eligibility thresholds based on the number of chronic diseases and Part D drugs for Medicare Part D plans for 2010 and after. However, an increase in MTM enrollment rates has not been realized.
Objectives
To describe trends in MTM eligibility thresholds used by Medicare Part D plans and to identify patterns that may hinder enrollment in MTM programs.
Methods
This study analyzed data extracted from the Medicare Part D MTM Programs Fact Sheets (2008–2014). The annual percentages of utilizing each threshold value of the number of chronic diseases and Part D drugs, as well as other aspects of MTM enrollment practices, were analyzed among Medicare MTM programs that were established by Medicare Part D plans.
Results
For 2010 and after, increased proportions of Medicare Part D plans set their eligibility thresholds at the maximum numbers allowable. For example, in 2008, 48.7% of Medicare Part D plans (N = 347:712) opened MTM enrollment to Medicare beneficiaries with only 2 chronic disease states (specific diseases varied between plans), whereas the other half restricted enrollment to patients with a minimum of 3 to 5 chronic disease states. After 2010, only approximately 20% of plans opened their MTM enrollment to patients with 2 chronic disease states, with the remaining 80% restricting enrollment to patients with 3 or more chronic diseases.
Conclusion
The policy change by CMS for 2010 and after is associated with increased proportions of plans setting their MTM eligibility thresholds at the maximum numbers allowable. Changes to the eligibility thresholds by Medicare Part D plans might have acted as a barrier for increased MTM enrollment. Thus, CMS may need to identify alternative strategies to increase MTM enrollment in Medicare plans.
Keywords: CMS guidance, medication therapy management (MTM) program, Medicare Part D plans, MTM enrollment, MTM eligibility criteria, MTM thresholds
In a 2013 report, the IMS Institute for Healthcare Informatics estimated that the United States spent more than $213 billion on unnecessary medical expenses as a result of irresponsible medication use.1 Nonadherence, delayed evidence-based practice, the suboptimal use of generic drugs, and mismanaged polypharmacy in the elderly comprised more than 80% of these avoidable costs.1 Poor patient adherence alone is responsible for an estimated 125,000 deaths in the United States annually and 10% of all hospitalizations.2
Collaboration between pharmacists, prescribers, and patients to optimally manage medication therapy is essential for improving clinical outcomes and for reducing overall healthcare costs.3 To this end, starting in 2006, the Centers for Medicare & Medicaid Services (CMS) required that Medicare Part D insurance plans implement medication therapy management (MTM) programs for patients who are at the highest risk for drug therapy problems, which are defined as multiple chronic diseases, multiple covered Part D drugs, and high drug costs.4 Within the minimum standards defined by CMS, Medicare Part D plans (ie, sponsors) are able to determine their own eligibility thresholds.5
KEY POINTS
-
▸
To increase the enrollment of Medicare medication therapy management (MTM) programs in Medicare prescription drug (Part D) plans, CMS lowered the MTM eligibility thresholds for chronic diseases, Part D drugs use, and minimum Part D drug costs for patients starting in 2010.
-
▸
Since 2010, a much greater percentage of MTM programs set their minimum thresholds at or close to the maximum threshold values set by CMS.
-
▸
These higher thresholds might have acted as a barrier for increased patient enrollment in MTM programs.
-
▸
MTM programs can improve clinical outcomes and reduce overall healthcare costs, but enrollment in these programs has not increased even after 2010.
-
▸
Efforts to improve the quality of MTM programs have had more success than efforts to improve access.
-
▸
CMS will need to find alternative strategies to increase future patient enrollment in MTM programs. Reforming the MTM budget allocation for MTM costs may increase enrollment.
-
▸
This study illuminates hidden pitfalls of healthcare policy that may inadvertently negatively impact Medicare beneficiaries.
Initially, regulatory guidance from CMS was permissive: Medicare Part D plans were free to design their own MTM enrollment criteria for Part D enrollees as long as they were based on a minimum number of chronic diseases and Part D drugs, and a minimum annual Part D drug cost of $4000.5 CMS has periodically updated its guidelines to standardize best practices and to boost MTM enrollment.5 Aiming to increase MTM enrollment, CMS set minimum eligibility thresholds for 2010 and after, mandating that plans open enrollment to patients with at least 3 chronic diseases, 8 covered drugs (specific drugs included may differ by patient), and $3000 annual Part D drug costs.5%,6 Plans were also required to automatically enroll eligible patients in the MTM program, which was a change from an opt-in approach (beneficiaries meeting criteria must enroll themselves) to an opt-out method (beneficiaries meeting criteria are automatically enrolled until they withdraw themselves).5%,6 Despite the more expansive guidelines for 2010 and after, the percentage of MTM-eligible beneficiaries has remained relatively constant, and is currently hovering at approximately 8%.5%,7
The goal of this study is to examine the potential causes for continued low beneficiary enrollment, despite the multipronged regulatory effort to expand access to MTM programs in Medicare Part D plans. To do so, this study describes how MTM eligibility and service quality have evolved over time before and after the most recent CMS guidance for 2010 and after.
Methods
This study describes trends in MTM programs utilizing data from the Medicare Part D MTM Programs Fact Sheets for 2008 through 2014, which can be found on the CMS website.8–14 These fact sheets contain annual statistics for targeted patients, as well as the type and manner of service provision. Although MTM programs were initiated in 2006, data for 2006 and 2007 were not available.
The annual percentages of MTM programs utilizing each minimum threshold value of the number of chronic diseases and Part D drugs were analyzed using Microsoft Excel 2013 (IBM; Armonk, NY) charting and graphing tools. These trends were separately examined for the Medicare Advantage prescription drug plans and the Medicare Part D stand-alone prescription drug plans. Other trends of MTM programs were also reviewed, such as Medicare Part D drug specification, chronic disease specification, and the method of enrollment.8–15
Results
Threshold for Chronic Diseases
As shown in Figure 1, approximately half of the plans (48.7%; N = 347:712) originally opened MTM enrollment to patients with only 2 chronic diseases, whereas the other half restricted enrollment to patients with a minimum of 3 to 5 chronic diseases. Subsequent years (2009–2014) showed an approximate 10% annual increase in the percentage of plans restricting MTM eligibility to patients with 3 or more chronic diseases. In 2011, the trend toward restricting MTM access by increasing the required number of chronic diseases as a result of CMS regulations stabilized; however, approximately 80% of the plans (N = 508:641) restricted access to patients with 3 or more chronic diseases, which is the maximum allowable number. This percentage was the highest in 2014, when 84.8% of plans (N = 582:686) required 3 or more chronic disease states.
Figure 1. MTM Programs with Thresholds of 2 versus ≥3 Chronic Diseases.
MTM indicates medication therapy management.
Threshold for Part D Drugs
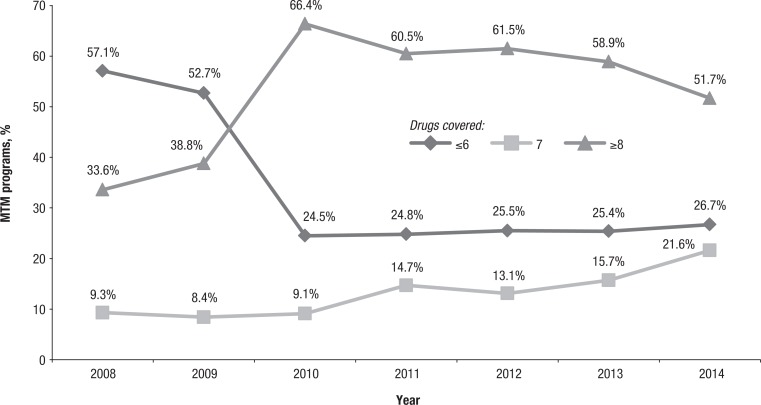
As shown in Figure 2, in 2008, a majority of plans (57.1%; N = 406) opened enrollment in MTM programs to patients taking 6 or fewer Part D drugs, and only 33.6% (N = 240:712) restricted enrollment to patients taking 8 or more Part D drugs. The next year (2009) showed a slight increase in the former percentage, but still only 38.8% of plans (N = 286:736) required a minimum of 8 Part D drugs. The CMS update for 2010 and after was correlated with the majority of plans increasing their minimum threshold to the maximum allowable of 8 Part D drugs. In every year since 2010, the majority of plans have required 8 Part D drugs (eg, 51.7% in 2014; N = 355:686) for participating in MTM programs. The trends for Medicare Advantage prescription drug plans and stand-alone prescription drug plans mirror the trend for all MTM programs, but stand-alone prescription drug plans usually were slightly less restrictive than Medicare Advantage prescription drug plans (results not shown).
Figure 2. MTM Programs with Thresholds of Number of Part D–Covered Drugs.
MTM indicates medication therapy management.
Chronic Disease Specification
Sponsors were required by CMS to target Medicare beneficiaries with any chronic diseases or any specific chronic diseases. However, after CMS changed the thresholds to expand enrollment in MTM programs in 2010, a much greater percentage of Part D plans began to restrict enrollment to patients with chronic diseases that were specified by CMS instead of any chronic diseases. For example, in 2008, 90.0% of plans (N = 641:712) accepted any chronic disease for the purposes of MTM program enrollment; that percentage in 2009 was 85.3% (N = 628:736).
Since the regulation change by CMS in 2010, less than 10% of Part D plans have accepted any chronic disease in any given year (2010: 4.6%, N = 31:678; 2011: 4.6%, N = 29:641; 2012: 6.1%, N = 39:633; 2013: 3.7%, N = 24:645). This percentage of plans decreased to 2.9% (N = 20:686) in 2014. Diabetes, chronic heart failure, dyslipidemia, and hypertension were the most frequently targeted chronic diseases, although the order of frequency with which these diseases were targeted differed slightly over the years.
Part D Drug Specification
In 2008, the majority of all Part D plans (50.7%; N = 361:712) accepted any Part D drug to be considered among the minimum number of Part D drugs, whereas the remaining sponsors required the drugs to be Part D drugs for chronic conditions, disease-specific drugs related to chronic diseases, or specific Part D drug classes. Less than half of the plans (44.6%; N = 328:736) accepted any Part D drug in 2009. After hovering at slightly less than 40% for the 3 years after the CMS update for 2010 and after, the percentage of plans accepting any Part D drug decreased again, to 25.8% (N = 177:686), as of 2014.
Methods of Enrollment
In 2008 and 2009, Part D sponsors were allowed to enroll beneficiaries using methods such as opt-in, opt-out, a combination of opt-in and opt-out, and other options. In 2009, 14.8% of plans (N = 109) used the opt-in method, 52.3% (N = 385) used the opt-out method, 30.2% (N = 222) used a combination method, and 2.7% (N = 20) used other methods. Since 2010, CMS has required all plan sponsors to use the opt-out method of enrollment.
CMS regulations for 2010 and after also required Part D plans to target beneficiaries for enrollment in their MTM program at least on a quarterly basis. From 2010 to 2014, more than 66% of the MTM programs identified the targeted beneficiaries quarterly, and approximately 20% of the programs identified beneficiaries monthly (Table).
Table.
Frequency of Plans Identifying MTM-Eligible Individuals, 2010–2014
| Frequency of eligibility identification | 2010 (N = 678), % | 2011 (N = 641), % | 2012 (N = 633), % | 2013 (N = 645), % | 2014 (N = 686), % |
|---|---|---|---|---|---|
| Quarterly | 68.0 | 71.1 | 70.0 | 69.1 | 66.9 |
| Every other month | 0.6 | 0.6 | 1.0 | 0.6 | — |
| Monthly | 26.5 | 20.0 | 22.0 | 21.7 | 16.0 |
| Weekly | 4.4 | 7.8 | 6.0 | 5.9 | 10.3 |
| Daily | 0.4 | 0.5 | 1.0 | 2.6 | 6.7 |
MTM indicates medication therapy management.
A smaller share of MTM programs identified targeted beneficiaries more frequently than monthly. The number of plans that identified MTM-eligible Part D enrollees weekly and daily increased slightly over the years (Table).
Interventions
For 2008 and 2009, Part D sponsors could include any type or combination of MTM interventions in their programs. As of 2010, sponsors were required to provide a minimum level of MTM services for each enrolled beneficiary, which included interventions for beneficiaries and prescribers, a comprehensive medication review (CMR) by a pharmacist or other qualified provider at least annually, and a performance of quarterly medication reviews with follow-up interventions when necessary. CMRs are interactive person-to-person or telehealth consultations evaluating an enrollee's use of medications, followed by an individualized summary report.
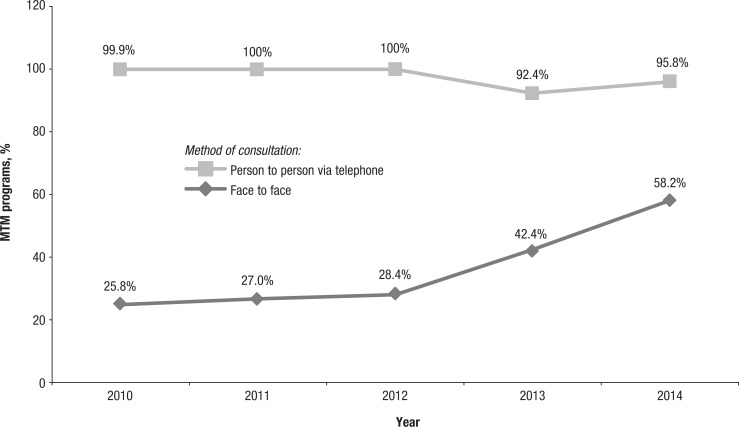
As shown in Figure 3, the proportions of MTM programs that offer face-to-face CMRs have gradually increased over the years. For example, approximately 25% of the MTM programs offered face-to-face consultations in 2010 (25.8%; N = 175:678), 2011 (27.0%; N = 183:641), and 2012 (28.4%; N = 182:633); this percentage increased to 42.4% (N = 273:645) in 2013 and to 58.2% (N = 399:686) in 2014.
Figure 3. MTM Programs Offering Comprehensive Medication Review Consultation Methods.
MTM indicates medication therapy management.
The type of written summaries after CMRs initially varied among the programs. To further standardize MTM and to minimize the differences between the programs, starting in 2013, the Affordable Care Act (ACA) has required programs to offer a CMR to all MTM enrollees, including long-term care enrollees at least annually.16 The ACA also requires plans to develop a uniform format for the CMR action plan and summary that includes 3 components—a beneficiary cover letter, a medication action plan, and a personal medication list.16
Discussion
CMS has made efforts in recent years to expand access to MTM services by adjusting the eligibility threshold criteria; however, there is a dearth of information available in the literature regarding the effects of these efforts. The current study described the trends in MTM eligibility and program requirements related to a CMS rule for 2010 and after that lowered the MTM eligibility thresholds. Using data from the annual Medicare Part D MTM Programs Fact Sheets from 2008 to 2014, this study found that the effects of the CMS rule for 2010 and after may have been offset by the plans' increased restriction in access by whatever means allowable.
It may have been assumed that setting allowable thresholds would not affect the programs that were already within the lower ranges, while ensuring that programs above the lower ranges would decrease their minimum thresholds. Hypothetically, lower threshold ranges would cause sponsors overall to decrease their current thresholds, thereby increasing access to MTM programs. However, the percentage of programs utilizing lower thresholds for minimum chronic diseases and minimum Part D drugs decreased, whereas the percentage of programs utilizing higher thresholds increased.
These findings indicate that since 2010, a much greater percentage of MTM programs have set their minimum thresholds at or close to the maximum threshold values set by CMS (ie, 3 chronic diseases and 8 Part D drugs). This study also shows that the percentage of programs targeting beneficiaries with 2 chronic diseases, and the percentage of programs targeting beneficiaries with fewer than 8 Part D drugs have decreased since 2010. It is possible that many beneficiaries with 2 chronic diseases were enrolled in a program that previously had set the minimum threshold at 2 diseases, which was raised to 3 diseases after the threshold range change. Similarly, it is possible that many beneficiaries using fewer than 8 Part D drugs were previously enrolled in a program that had formerly set its minimum threshold at below 8 drugs, which was raised to 8 drugs after the threshold change. Consequently, beneficiaries who once qualified for lower thresholds might lose coverage to programs and may now qualify for a lesser percentage of programs. The lowering of the annual cost from $4000 to $3000 likely increased the number of beneficiaries who are eligible for the MTM program, but this factor might have been offset by the trend toward restricting eligibility via limits regarding Part D drugs and chronic diseases.
Another factor that might have affected the MTM enrollment rate was the decrease in the percentage of programs accepting any Part D drug. The percentage of sponsors accepting any Part D drug dropped from 2008–2009 to 2010–2014, whereas the percentage of sponsors requiring Part D drugs for chronic conditions increased. In this case, a percentage of beneficiaries may be eligible for fewer programs than before, because their drugs may no longer qualify to be counted within the minimum number of Part D drugs.
The decrease in the percentage of programs accepting any chronic diseases to be classified among the minimum number of diseases might also have affected the enrollment rate. The percentage of plan sponsors accepting any chronic disease decreased by an order of magnitude after the update for 2010 and after. As with Part D drugs, a percentage of beneficiaries may be less likely to be eligible for MTM programs, because their chronic diseases may no longer qualify for MTM enrollment.
Significant changes were made to MTM programs in 2010 and after; the efforts toward improving program quality have been more successful than efforts toward improving program access, as is demonstrated by automatic beneficiary enrollment, the use of CMRs, minimum targeting frequencies, and the increased intensity for interventions and services. CMS established eligibility criteria thresholds with the expectation of expanding eligibility for MTM; however, the shift of the majority of programs to higher thresholds within the allowable range was an unforeseen consequence and requires further investigation.
One possible reason for this shift toward higher thresholds is that MTM programs are paid out of administrative funds as a component of plan bids for a contract with CMS.8–14 Part D sponsors must have their budget allocations for the time and resources associated with their plans approved by CMS to obtain a contract.8–14 To keep the total administrative costs low, sponsors may only have a limited allocation for MTM costs and may be unable to afford a high number of eligible beneficiaries. Therefore, to ensure compliance within allotted budgets, plans may limit their enrollees by raising thresholds to the most restrictive standard allowable.
Unless the budget allocation for MTM costs changes, it will be difficult for programs to increase the number of enrollees. Reforming the current MTM budget process so that plans would not have to depend on administrative funds may enable programs to include more beneficiaries.
Regarding the limited budget and resulting trade-offs between MTM program access and program quality, it might have been more realistic for MTM programs to focus on their existing beneficiaries and to address program quality. Most of CMS's other expanded requirements since 2010 have emphasized program quality and have produced more visible improvements, which have been evidenced by automatic beneficiary enrollment, the use of CMRs, minimum targeting frequencies, and increased intensity for interventions and services.
The shift to automatic enrollment (opt-out option) starting in 2010 might have increased the MTM participation rate, but this effect was likely offset by restricting access to programs by other means. The standardization of these programs reflect the increasing importance of healthcare quality and consistency—2 of the ACA's main objectives—in the US healthcare system.17
However, the subdued approach to improving MTM programs may be giving way to a more aggressive eligibility regulation for the coming years. In the second week of 2014, CMS published a proposal for new rules for 2015 that is intended to dramatically increase the rate of MTM eligibility.5 The proposal suggests reducing the minimum number of disease states to 2 (1 being from the core disease states list published in the annual call letter), reducing the minimum number of Part D drugs to 2, and lowering the minimum cost to the annual average costs of 2 generic prescription drugs (approximately $620.40 in January 2014).5 These proposed changes to the 2015 MTM program have the potential to expand the benefits of MTM to a much wider group of patients. However, CMS later decided not to implement these proposed changes to Part D plans, because various stakeholders opposed other Part D reforms that were proposed along with these MTM changes.18
Although this study is unique in its research topic, the literature has rich examples documenting the unintended consequences of public policies. For example, in the United States, Werner and Asch have warned that publicly reporting quality information may lead to physicians avoiding sick patients.19 Wang and colleagues were the first in the United States to document that non-Hispanic blacks and Hispanics may be less likely to be eligible for Medicare MTM eligibility criteria than non-Hispanic whites, because MTM eligibility criteria were based on utilization, and non-Hispanic blacks and Hispanics historically tend to use fewer healthcare resources.20–22 In China, Yao and colleagues reported that pregnant women may become the victims of smoke-free policies in public places when their husbands shift their smoking location from public places and workplaces to the home.23 Our present research has added another example of hidden pitfalls of the policymaking process and has provided a valuable lesson for policymakers.
Limitations
These findings show that changes in the threshold ranges of eligibility criteria are associated with a substantive impact on MTM program trends, and, consequently, enrollment rates for Medicare beneficiaries; however, there are some limitations to this study.
This study does not include longitudinal information for specific plans or specific data on patient characteristics for MTM programs.
There is also insufficient information to determine the exact causes of the low enrollment rate and the shift in programs toward higher thresholds.
In addition, trends that are not included in the CMS Fact Sheets, such as data on beneficiaries' race, ethnicity, and socioeconomic characteristics, should be investigated to explore the possible socioeconomic effects on MTM enrollment rates.
Conclusion
This study illuminates hidden pitfalls of healthcare policy that may inadvertently negatively impact Medicare beneficiaries. Changes to CMS regulation on threshold ranges for the MTM eligibility criteria, including to the minimum number of chronic diseases, the minimum number of Part D drugs, and the minimum cost likely to be incurred from Part D drugs, are associated with increasing proportions of plans using the maximum levels of eligibility thresholds allowable. In addition, plans have become more restrictive in terms of which chronic diseases and Part D drugs they allow. Alternative strategies may be warranted to increase MTM beneficiary enrollment. Furthermore, future studies should evaluate these changes to MTM services and should examine their impact on quality of care and the resulting outcomes.
Acknowledgments
The authors would like to acknowledge the research assistance of Degan Lu, Yanru Qiao, and Satya Subhi.
Funding Source
This project was funded by grant R01AG040146 from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Author Disclosure Statement
Dr Wang, Dr Shih, Ms Qin, and Mr Young reported no conflicts of interest. Dr Thomas is an employee of Walgreens. Dr Spivey, Dr Solomon, and Dr Chisholm-Burns reported no conflicts of interest.
Contributor Information
Junling Wang, Professor, Health Outcomes and Policy Research, Department of Clinical Pharmacy, University of Tennessee College of Pharmacy, Memphis.
Ya-Chen Tina Shih, Professor of Health Economics, and Chief, Section of Cancer Economics and Policy, Department of Health Services Research, University of Texas M.D. Anderson Cancer Center, Houston.
Yolanda Qin, Student, Duke University, Durham, NC, and was a summer intern at the University of Tennessee, College of Pharmacy, Memphis, at the time of this study.
Theo Young, Student, University of Mississippi School of Pharmacy, Jackson, and was a summer intern at the University of Tennessee College of Pharmacy, Memphis, at the time of this study.
Zachary Thomas, Student, University of Tennessee College of Pharmacy, Memphis.
Christina A. Spivey, Assistant Professor, Department of Clinical Pharmacy, University of Tennessee College of Pharmacy, Memphis.
David K. Solomon, Professor, Department of Clinical Pharmacy, University of Tennessee College of Pharmacy, Memphis.
Marie Chisholm-Burns, Dean and Professor, University of Tennessee College of Pharmacy, Memphis..
References
- 1.IMS Institute for Healthcare Informatics. Avoidable costs in U.S. healthcare: the $200 billion opportunity from using medicines more responsibly. June 2013. www.imshealth.com/deployedfiles/imshealth/Global/Content/Corporate/IMS%20Institute/RUOM-2013/IHII_Responsible_Use_Medicines_2013.pdf. Accessed February 18, 2015.
- 2.Pellegrino AN, Martin MT, Tilton JJ, Touchette DR. Medication therapy management services: definitions and outcomes. Drugs. 2009; 69:393–406. [DOI] [PubMed] [Google Scholar]
- 3.Bluml BM. Definition of medication therapy management: development of professionwide consensus. J Am Pharm Assoc (2003). 2005; 45:566–572. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; Medicare prescription drug benefit. Final rule. Fed Regist. 2005; 70:4193–4585. [PubMed] [Google Scholar]
- 5.Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; contract year 2015 policy and technical changes to the Medicare Advantage and the Medicare Prescription Drug Benefit programs. Final rule. Fed Regist. 2014; 79:29843–29968. [PubMed] [Google Scholar]
- 6.Blum J; for the Centers for Medicare & Medicaid Services. Issuance of the 2010 Call Letter. March 30, 2009. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/downloads/2010CallLetter.pdf. Accessed February 18, 2014.
- 7.Stuart B, Loh FE, Roberto P, Miller LM. Increasing Medicare Part D enrollment in medication therapy management could improve health and lower costs. Health Aff (Millwood). 2013; 32:1212–1220. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services. 2008 Medicare Part D Medication Therapy Management (MTM) programs. 2008 fact sheet.
- 9.Centers for Medicare & Medicaid Services. Medicare Part D Medication Therapy Management (MTM) programs. 2009 fact sheet. Updated July 21, 2009. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/downloads/MTMFactSheet_2009_06-2009_fnl.pdf. Accessed February 18, 2015.
- 10.Centers for Medicare & Medicaid Services. 2010 Medicare Part D Medication Therapy Management (MTM) programs. Fact sheet. June 8, 2010. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/downloads/MTMFactSheet_2010_06-2010_final.pdf. Accessed February 18, 2015.
- 11.Centers for Medicare & Medicaid Services. 2011 Medicare Part D Medication Therapy Management (MTM) programs. Fact sheet. June 30, 2011. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/downloads/MTMFactSheet2011063011Final.pdf. Accessed February 18, 2015.
- 12.Centers for Medicare & Medicaid Services. 2012 Medicare Part D Medication Therapy Management (MTM) programs. Fact sheet. November 12, 2012. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/CY2012-MTM-Fact-Sheet.pdf. Accessed February 18, 2015.
- 13.Centers for Medicare & Medicaid Services. 2013 Medicare Part D Medication Therapy Management (MTM) programs. Fact sheet: summary of 2013 MTM programs. September 12, 2013. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/CY2013-MTM-Fact-Sheet.pdf. Accessed February 18, 2015.
- 14.Centers for Medicare & Medicaid Services. 2014 Medicare Part D Medication Therapy Management (MTM) programs. Fact sheet: summary of 2014 MTM programs. August 21, 2014. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/CY2014-MTM-Fact-Sheet.pdf. Accessed February 18, 2015.
- 15.Tudor CG; for the Centers for Medicare & Medicaid Services. CY 2013 Medication Therapy Management program guidance and submission instructions. April 10, 2012. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovGenIn/Downloads/Memo-Contract-Year-2013-Medication-Therapy-Management-MTM-Program-Submission-v041012.pdf. Accessed February 18, 2015.
- 16.The Office of the Legislative Counsel. Compilation of Patient Protection and Affordable Care Act. May 1, 2010. http://housedocs.house.gov/energycommerce/ppacacon.pdf. Accessed July 15, 2015.
- 17.Parekh AK, Goodman RA, Gordon C, Koh HK; for the HHS Interagency Workgroup on Multiple Chronic Conditions. Managing multiple chronic conditions: a strategic framework for improving health outcomes and quality of life. Public Health Rep. 2011; 126:460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barlas S. Medicare backs off on MTM changes: congressional and patient-group opposition to other, more controversial Part D reforms sank the plan. P T. 2014; 39:463–519, vi. [PMC free article] [PubMed] [Google Scholar]
- 19.Werner RM, Asch DA. The unintended consequences of publicly reporting quality information. JAMA. 2005; 293:1239–1244. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Mullins CD, Brown LM, et al. , Disparity implications of Medicare eligibility criteria for medication therapy management services. Health Serv Res. 2010; 45:1061–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Qiao Y. Historical trend of disparity implications of Medicare MTM eligibility criteria. Res Social Adm Pharm. 2013; 9:758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Brown LM, Hong SH. Racial and ethnic disparities in meeting Part D MTM eligibility criteria among the non-Medicare population. J Am Pharm Assoc (2003). 2012; 52:e87–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao T, Lee AH, Mao Z. Potential unintended consequences of smoke-free policies in public places on pregnant women in China. Am J Prev Med. 2009; 37 (2 suppl):S159–S164. [DOI] [PMC free article] [PubMed] [Google Scholar]