Abstract
The acetylenic tricyclic bis(cyanoenone) TBE-31 is a highly potent cysteine targeting compound with a reversible covalent mode of action; its best-characterized target being Kelch-like ECH-associated protein-1 (Keap1), the cellular sensor for oxidants and electrophiles. TBE-31 reacts with cysteines of Keap1, impairing its ability to target nuclear factor-erythroid 2 p45-related factor 2 (Nrf2) for degradation. Consequently, Nrf2 accumulates and orchestrates cytoprotective gene expression. In this study we investigated the pharmacokinetic and pharmacodynamic properties of TBE-31 in C57BL/6 mice. After a single oral dose of 10 μmol/kg (∼200 nmol/animal), the concentration of TBE-31 in blood exhibited two peaks, at 22.3 nM and at 15.5 nM, 40 min and 4 h after dosing, respectively, as determined by a quantitative stable isotope dilution LC-MS/MS method. The AUC0–24h was 195.5 h/nmol/l, the terminal elimination half-life was 10.2 h, and the kel was 0.068 h−1. To assess the pharmacodynamics of Nrf2 activation by TBE-31, we determined the enzyme activity of its prototypic target, NAD(P)H:quinone oxidoreductase 1 (NQO1) and found it elevated by 2.4- and 1.5-fold in liver and heart, respectively. Continuous feeding for 18 days with diet delivering the same daily doses of TBE-31 under conditions of concurrent treatment with the immunosuppressive agent azathioprine had a similar effect on Nrf2 activation without any indications of toxicity. Together with previous reports showing the cytoprotective effects of TBE-31 in animal models of carcinogenesis, our results demonstrate the high potency, efficacy and suitability for chronic administration of cysteine targeting reversible covalent drugs.
Keywords: Cysteine targeting, Keap1, NQO1, Nrf2, Reversible covalent drug
Highlights
-
•
TBE-31 is a cysteine targeting compound with a reversible covalent mode of action.
-
•
After a single oral dose, the blood concentration of TBE-31 exhibits two peaks.
-
•
Oral TBE-31 is a potent activator of Nrf2-dependent enzymes in multiple organs.
-
•
Chronic dietary administration of TBE-31 at doses that show efficacy is not toxic.
1. Introduction
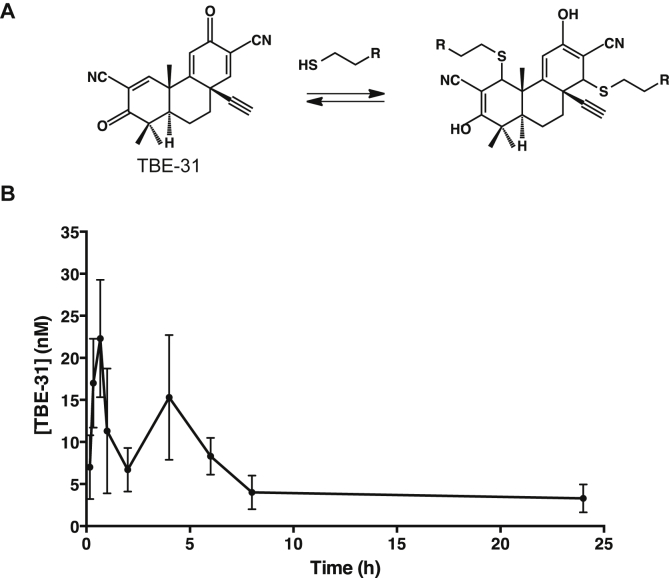
Electrophilic olefins react readily with nucleophilic cysteine sulfhydryl groups in a reversible manner. The presence of an electron withdrawing nitrile group adjacent to an olefin increases the reactivity, and furthermore, enhances the reversibility of the reaction [1]. The ability of small molecules to bind their protein targets covalently but reversibly is gaining an increasing interest in drug development as such compounds provide sustained covalent inhibition without accumulating permanently modified (and potentially cytotoxic) proteins. The concept of designing reversible covalent inhibitors has been recently employed to obtain noncatalytic cysteine targeting drug candidates with high ligand efficiency and selectivity for the MSK/RSK-family protein kinases [2]. A reversible covalent mode of action combines the desirable features of both irreversible covalent agents (such as high potency and long-lasting activity) as well as reversible non-covalent drugs (such as lack of permanent target modification). One example of a cysteine targeting compound with a reversible covalent mode of action is the acetylenic tricyclic bis(cyanoenone) (±)-(4bS,8aR,10aS)-10a-ethynyl-4b,8,8-trimethyl-3,7-dioxo-3.4b,7,8,8a,9,10, 10a-octahydrophenanthrene-2,6-dicarbonitrile (TBE-31, Fig. 1A).
Fig. 1.
(A) TBE-31 binds to sulfhydryl groups in a Michael addition reaction forming covalent, but reversible adducts. (B) Pharmacokinetics of TBE-31 in female C57/BL6 mice (n = 3). TBE-31 (10 μmol/kg) was administered by oral gavage. Blood (10 μl) was drawn from the tail vein at 10, 20, 40, 60 min post-dosing, and subsequently at 2, 4, 6, 8, and 24 h. The blood levels of TBE-31 were determined at each time point using a stable isotope dilution LC-MS method on nano-flow LC-Orbitrap. Values are means ± 1 S.E.M.
TBE-31 has two doubly activated Michael acceptors that render this compound highly reactive with sulfhydryl groups (Fig. 1A). The best-characterized intracellular target of TBE-31 is Kelch-like ECH-associated protein-1 (Keap1), the protein sensor for oxidants and electrophiles [3]. Under homeostatic conditions, Keap1 binds transcription factor nuclear factor-erythroid 2 p45-related factor 2 (Nrf2) [4,5] and functions as a substrate adapter for the Cul3-mediated ubiquitination and proteasomal degradation of Nrf2 [6–8] by use of a highly efficient cyclic mechanism [9]. Consequently, the half-live of Nrf2 is very short (∼10–20 min) [10]. Pharmacological activators of Nrf2 react with specific cysteine residues of Keap1 [3,11–13], disrupting the cycle of Nrf2 degradation and leading to Nrf2 accumulation, nuclear translocation and enhanced target gene expression. The battery of Nrf2 transcriptional targets comprises genes encoding proteins with versatile cytoprotective functions, including antioxidant, anti-inflammatory and metabolic enzymes [14].
TBE-31 is one of the most potent Nrf2 activators known to date. In vitro, this acetylenic tricyclic bis(cyanoenone) binds reversibly to sulfhydryl groups such as those present in the reduced form of Cleland's reagent (dithiothreitol, DTT) [15,16] and to cysteine sensors of recombinant Keap1 [16,17]. In cells, at sub-to low nanomolar concentrations, TBE-31 induces Nrf2 transcriptional targets, and inhibits pro-inflammatory responses, such as the transcriptional upregulation of inducible nitric oxide synthase (iNOS) mediated by lipopolysaccharide or interferon-γ (IFN-γ). In vivo, small (nmol) doses of TBE-31 robustly induce Nrf2-dependent cytoprotective responses [16,18] and protect animals against hepatocarcinogenesis induced by aflatoxin [15], as well as against cutaneous carcinogenesis mediated by solar-simulated ultraviolet radiation [19]. The aims of the present study were to: (i) define the pharmacokinetics of a single dose of orally administered TBE-31 in mice, (ii) evaluate the pharmacodynamics of Nrf2 activation by TBE-31 when administered either at a single dose or chronically, and (iii) to evaluate the safety of chronic administration of TBE-31 as exemplar of a highly potent Nrf2 activator with a reversible covalent mode of action.
2. Materials and methods
2.1. Materials
All reagents were of the highest purity available, purchased from common commercial suppliers. (±)-TBE-31 and a stable isotope labeled (±)-[13C215N2]-TBE-31 were synthesized as described [17,20].
2.2. Animals and treatments
The animal experiments were performed in accordance with the regulations described in the UK Animals (Scientific Procedures) Act 1986, and were in strict compliance with institutional guidelines. C57BL/6 mice were bred in our facility and maintained on a 12-h light/12-h dark cycle, 35% humidity with free access to water and pelleted RM1 diet (SDS Ltd., Witham, Essex, UK). All experimental animals were female, 6–12 weeks of age, uniformly distributed between the treated and the control groups.
For single dose experiments, TBE-31 [10 μmol/kg, or ∼200 nmol/mouse (n = 3), dissolved in 2% DMSO (v/v) in corn oil] was administered by oral gavage. Control mice received an equivalent volume of 2% DMSO (v/v) in corn oil. Blood (10 μl) was drawn from the tail vein at 10, 20, 40, 60 min post-dosing, and subsequently at 2, 4, 6, 8, and 24 h. The blood was collected in heparin-containing tubes and stored at – 80 °C until analysis.
For continuous dietary administration of TBE-31, the compound was mixed with powdered RM1 diet (27.6 mg of TBE-31 per kg of food). The estimated dose of TBE-31 delivered by this diet was ∼10 μmol/kg, or ∼200 nmol/mouse (n = 6) per day. The TBE-31 diet was stored at 4 °C, and the feeders were refilled daily over the course of the experiment (18 days). Control mice (n = 6) were fed RM1 diet that was stored and delivered under identical conditions. The animals in both groups were receiving azathioprine (10 mg/kg) in the drinking water. Body weights were recorded twice a week. At termination of the experiments, the animals were euthanized by CO2 asphyxiation, and immediately exsanguinated by cardiac puncture. Blood was drawn in EDTA-containing tubes, plasma was isolated by centrifugation, and stored at – 80 °C until analysis. Livers, hearts, skin, kidneys, and stomachs were harvested, immediately frozen in liquid N2, and stored at – 80 °C.
2.3. Determination of blood levels of TBE-31
The blood levels of TBE-31 were determined using a stable isotope dilution LC-MS method. Briefly, 10 μl whole blood was extracted in 0.5 ml acetonitrile using 500 pmol [13C215N2]-TBE-31 (10 μl of a 50 μM solution) as an internal standard. The sample was sonicated in a water bath for 10 min, and subjected to centrifugation at 13,000 × g for 20 min at 4 °C. The supernatant was transferred to a new tube, diluted to 10% acetonitrile (v/v), and subjected to solid phase extraction on an HLB Oasis Cartridge (30 mg, Waters, Manchester). Following a wash with 40% acetonitrile/0.1% formic acid (v/v) in water, the analytes were eluted with 70% acetonitrile (v/v) in water, dried under vacuum, and resuspended in 50 μl of 2% acetonitrile/0.1% formic acid (v/v) in water. LC-MS/MS was performed on nano-flow LC-Orbitrap as described in detail [20]. To determine pharmacokinetic parameters, logarithmic linear regression was used to calculate the slope of the terminal disposition phase, based on which the terminal phase elimination half-life (t1/2) and the elimination constant (Kel) were determined using a single compartment model with curve stripping.
2.4. Enzyme assays
Frozen tissue was pulverized into powder under liquid N2. Approximately 30 mg of powder were homogenized in an ice bath in ice-cold buffer [100 mM potassium phosphate, pH 7.4; 100 mM KCl; 0.1 mM ethylenediaminetetraacetic acid (EDTA)], and subjected to centrifugation at 4 °C (15,000 × g for 10 min). Supernatant fractions were used to determine the enzyme activities of NAD(P)H:quinone oxidoreductase 1 (NQO1) with menadione as a substrate [21], glutathione S-transferase (GST) with CDNB as a substrate [22], and the protein concentration using the bicinchoninic acid (BCA) assay (Thermo Scientific).
2.5. Blood tests
Blood tests were performed at the Mary Lyon Centre (MRC, Harwell, UK) on plasma samples free of hemolysis that had been isolated from mice fed continuously with TBE-31 (27.6 mg of TBE-31 per kg of food) for 18 days.
2.6. Statistical analysis
Values are means ± 1 S.D. or 1 S.E.M., as indicated in the figure legends. The differences between groups were determined by Students t-test using Excel (Microsoft Corp.).
3. Results and discussion
3.1. Pharmacokinetics and pharmacodynamics of a single dose of orally administered TBE-31
To determine the blood levels of TBE-31, we employed a quantitative liquid chromatography/mass spectrometry-based approach coupled with the use of a stable isotope-labeled internal standard, in which both nitrile groups of TBE-31 are labeled with 13C and 15N atoms, i.e. (±)-[13C215N2]-TBE-31 [20]. The high sensitivity of this method (which has an estimated on-column limit of detection of 80 fmol) enabled us to examine the pharmacokinetic profile of orally administered TBE-31 by drawing small amounts (10 μl) of blood from the mouse tail vein. This allowed us to determine the blood levels of the compound at 9 different time-points using three animals per group. Following a single oral dose of 10 μmol/kg, the concentration of TBE-31 in whole blood exhibited two peaks (Fig. 1B). The first peak was at 22.3 nM 40 min after dosing. After a rapid decline, a second broader peak was observed at 15.5 nM 4 h after dosing. The reason for the presence of two peaks is unclear; however it is noteworthy that sulforaphane, another potent Nrf2 activator, which reacts reversibly with sulfhydryl groups, such as the cysteine sensors of Keap1 [3,23–25], or reduced glutathione [26–28] shows a similar pharmacokinetic behavior in Sprague–Dawley rats [29], and could be due to accumulation of glutathione conjugates, and their subsequent deconjugation to give the parent compound.
The area under the concentration–time curve (AUC) from time zero to 24 h after dosing was 195.5 h/nmol/l, the terminal phase elimination half-life (t1/2) was 10.2 h, and the kel was 0.068 h−1. As expected, there was no detectable TBE-31 in blood from vehicle-treated animals, confirming the absence of any endogenous sources and the specificity of the detection method. Importantly, the TBE-31 concentrations that were detected in the blood of the TBE-31-treated animals are entirely consistent with the concentrations of the compound which are typically used in cell culture experiments and have been shown to activate robustly Nrf2 and to protect against the toxicities of damaging agents, such as peroxynitrite or the combination of 6-thioguanine and ultraviolet radiation [15,16,18,30].
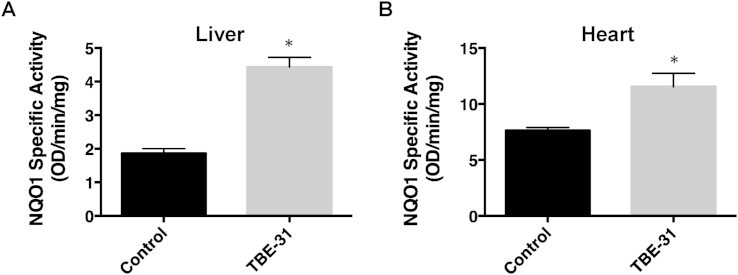
To assess the pharmacodynamics of Nrf2 activation by TBE-31, we determined the activity of NQO1, an enzyme encoded by a prototypic Nrf2-dependent gene, in liver and heart of the animals. In comparison with vehicle-treated animals, the specific enzyme activity of hepatic NQO1 was higher by 2.4-fold (n = 3, p = 0.00003) 24 h after a single orally administered dose (10 μmol/kg, or ∼200 nmol/mouse) of TBE-31 (Fig. 2A). Although to a lower degree, perhaps reflecting the much higher (by ∼4-fold) levels of the enzyme in heart compared to liver, cardiac NQO1 was also induced and was 1.5-fold higher in TBE-31-treated than in vehicle-treated animals (n = 3, p = 0.003) (Fig. 2B).
Fig. 2.
Pharmacodynamics of Nrf2 activation by a single oral dose of TBE-31 in female C57/BL6 mice (n = 3). Specific enzyme activity of NQO1 in liver (A) and heart (B) 24 h after a single oral dose (10 μmol/kg) of TBE-31. Values are means ± 1 S.D.
3.2. Pharmacodynamics of chronically administered TBE-31
Due to the reversibility of the reaction with their protein targets, reversible covalent drugs do not permanently modify their protein targets and are suitable for chronic administration. To assess the pharmacodynamic effects of chronic TBE-31 administration, the compound was mixed with the diet and the animals were fed with this diet for 18 days, receiving continuously a daily dose of ∼200 nmol TBE-31. In relation to our interest in developing preclinical models of cutaneous carcinogenesis under immunosuppressed conditions, during the last two weeks of the experiment, the mice were also receiving the immunosuppressive agent azathioprine (10 mg/kg) in the drinking water.
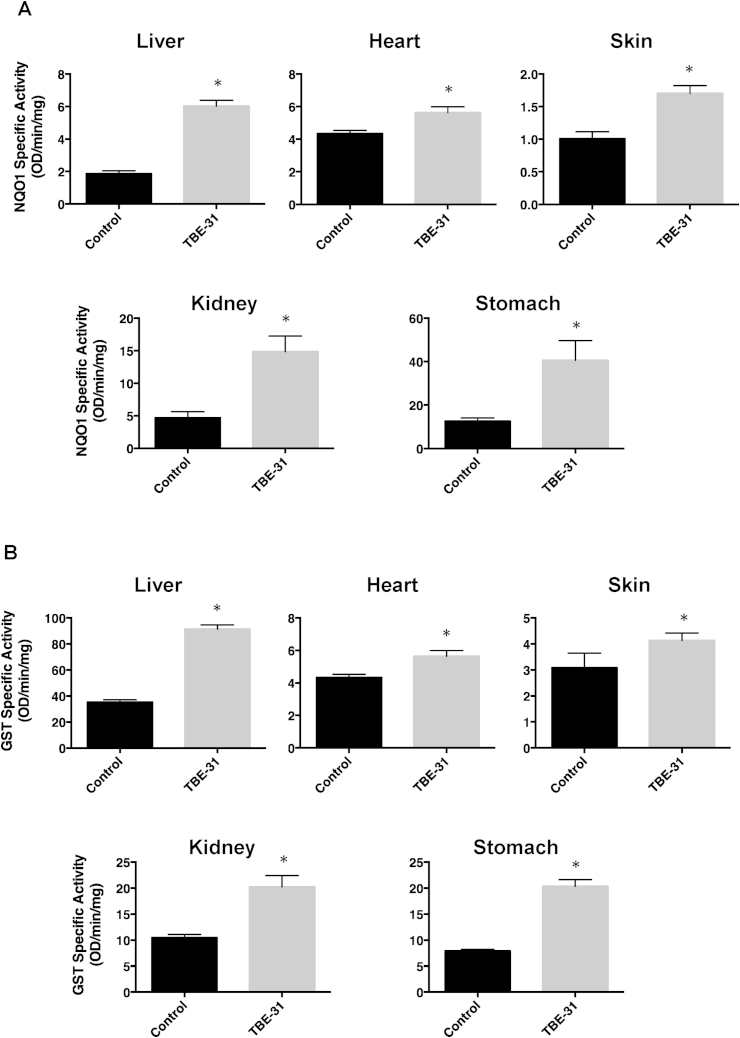
The TBE-31 treatment led to upregulation of the activity of NQO1 by 3.2-, 1.3-, 1.7-, 3.2- and 3.2-fold in liver, heart, skin, kidney, and stomach, respectively (n = 6, p < 0.00001) (Fig. 3A). We also measured the specific activity of glutathione S-transferase (GST), another Nrf2-dependent enzyme. Similar to the effect on NQO1, chronic dietary administration of TBE-31 induced the activity of GST by 2.6-, 1.4-, 1.4-, 1.9-, and 2.6-fold in liver, heart, skin, kidney, and stomach, respectively (n = 6, p < 0.00001) (Fig. 3B). The highly statistically significant induction of both enzymes in all organs examined demonstrated the pharmacodynamic effects of chronic TBE-31 administration and further confirmed that TBE-31 is a potent Nrf2 activator in vivo.
Fig. 3.
Continuous feeding with TBE-31 (27.6 mg of TBE-31 per kg of food) for 18 days increases the specific enzyme activity of NQO1 (menadione as a substrate) (A) and GST (CDNB as a substrate) (B) in female C57/BL6 mice (n = 6). Values are means ± 1 S.D.
3.3. Toxicity assessment of chronically administered TBE-31
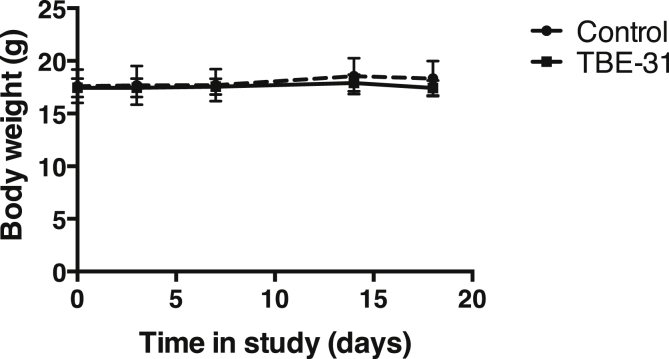
There were no obvious signs of toxicity, as judged by the similarity in body weight (Fig. 4) and behavior between the animals on the control diet and those receiving continuously a daily dose of ∼200 nmol TBE-31 in the diet for 18 days. To further strengthen this conclusion, we performed a detailed analysis of the plasma levels of creatinine, alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol, low-density lipoproteins (LDL), high-density lipoproteins (HDL), triglycerides, free fatty acids, glucose, bilirubin, and ketone bodies (Table 1). The activities of ALP, ALT and AST were not significantly different between the two groups, indicating unaltered liver function and confirming the absence of toxicity by the continuous TBE-31 treatment. Most of the other evaluated parameters were also very similar between the groups. There were two exceptions: the levels of LDL were higher (by 1.3-fold, p < 0.05) in the TBE-31-treated group compared to the control group, whereas the levels of bilirubin were lower (by 35%, p < 0.05).
Fig. 4.
Continuous feeding with TBE-31 (27.6 mg of TBE-31 per kg of food) for 18 days does not alter body weight in female C57/BL6 mice (n = 6). Values are means ± 1 S.D.
Table 1.
Blood plasma tests of samples from female C57BL/6 mice (n = 4–6) administered continuously TBE-31 in the diet (27.6 mg of TBE-31 per kg of food) for 18 days.
| Blood test | Control diet | TBE-31 diet | p value |
|---|---|---|---|
| Creatinine (μmol/L) | 8.58 ± 0.995 | 8.75 ± 0.704 | 0.7445 |
| Alkaline phosphatase (IU/L) | 44.0 ± 32.5 | 16.7 ± 9.27 | 0.0810 |
| Alanine aminotransferase (IU/L) | 49.4 ± 26.7 | 38.3 ± 22.5 | 0.4739 |
| Aspartate aminotransferase (IU/L) | 150.8 ± 17.1 | 93.8 ± 60.8 | 0.2968 |
| Total cholesterol (mmol/L) | 1.82 ± 0.457 | 2.19 ± 0.178 | 0.2994 |
| HDL (mmol/L) | 1.23 ± 0.360 | 1.42 ± 0.156 | 0.6572 |
| LDL (mmol/L) | 0.480 ± 0.092 | 0.643 ± 0.052 | 0.0150 |
| Glucose (mmol/L) | 11.6 ± 3.05 | 15.3 ± 1.67 | 0.2211 |
| Triglycerides (mmol/L) | 1.05 ± 0.317 | 1.18 ± 0.271 | 0.3568 |
| Free fatty acids (mmol/L) | 0.395 ± 0.142 | 0.472 ± 0.294 | 0.4519 |
| Total bilirubin (μmol/L) | 3.38 ± 0.577 | 2.18 ± 0.519 | 0.0478 |
| Ketone bodies (mmol/L) | 0.073 ± 0.015 | 0.123 ± 0.078 | 0.2899 |
The numbers shown in bold are those that are statistically significantly different between the groups.
The increase in LDL by the TBE-31 treatment is consistent with the pro-atherogenic effects of Nrf2 that have been previously described in apolipoporotein E (ApoE)-deficient mice [31–33]. However, it is not clear at present whether Nrf2 is pro-atherogenic in man, and perhaps more importantly, whether the pro-atherogenic role of Nrf2 requires its persistent (rather than transient) activation. Nonetheless, this result warrants caution when designing dosing regimens with Nrf2 activators in humans.
The decrease in the levels of bilirubin in the plasma of the TBE-31-treated animals is in agreement with the role of Nrf2 in regulating the gene expression of microsomal UDP-glucuronosyltransferases, such as UGT1A1 [34,35], the enzymes that are principally responsible for the glucuronidation and elimination of bilirubin [36]. This result suggests that Nrf2 activation by agents such as TBE-31 could be potentially developed as a therapeutic approach for conditions of impaired bilirubin glucuronidation, such as Crigler–Najjar and Gilbert's syndromes.
Notably, although TBE-31 is largely eliminated by 24 h after dosing, its cytoprotective effects are evident for much longer periods of time. This is because the ultimate cytoprotective agents are not TBE-31, or even Nrf2, but the Nrf2-dependent transcriptional targets, which are proteins with long half-lives. We have recently demonstrated that NQO1 is induced to essentially the same extent at 24- or 72-h after dosing with TBE-31 in the murine skin, where the half-life of the compound is ∼10 h [19]. This long-lasting pharmacodynamic effect makes the maintenance of steady-state plasma levels of the inducer unnecessary, allowing for chronic dosing at a low frequency. In addition, because reversible covalent binding does not lead to formation of stable adducts with the target protein, repeated dosing regimens are possible without immunological consequences. Indeed, chronic (∼30 weeks) topical application of small quantities (40 nmol per animal) of TBE-31 twice a week resulted in dramatically reduced tumor multiplicity and burden in a model of ultraviolet radiation-mediated skin carcinogenesis in SKH-1 hairless mice receiving azathioprine treatment [19]. In a rat model of aflatoxin-mediated hepatocarcinogenesis, oral administration of TBE-31, three times per week for three weeks, essentially abolished the formation of pre-neoplastic foci in liver [15]. Taken together, these findings illustrate the advantages of reversible covalent drugs and especially those whose protein targets subsequently affect the activity of transcription factors that, in turn orchestrate the expression of networks of genes, and encourage future drug development of compounds of this class.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We thank Sheila Sharp at the Biomarker and Drug Analysis Core Facility for providing services in pharmacokinetics analysis, the Mary Lyon Centre's Clinical Pathology Service Laboratory (MRC, Harwell, UK) for performing the plasma clinical chemistry tests, and Cancer Research UK (C20953/A18644), Reata Pharmaceuticals, and Stony Brook Foundation for financial support.
References
- 1.Zheng S., Laxmi Y.R.S., David E., Dinkova-Kostova A.T., Shiavoni K.H., Ren Y., Zheng Y., Trevino I., Bumeister R., Ojima I., Wigley W.C., Bliska J.B., Mierke D.F., Honda T. Synthesis, chemical reactivity as Michael acceptors, and biological potency of monocyclic cyanoenones, novel and highly potent anti-inflammatory and cytoprotective agents. J. Med. Chem. 2012;55:4837–4846. doi: 10.1021/jm3003922. [DOI] [PubMed] [Google Scholar]
- 2.Miller R.M., Paavilainen V.O., Krishnan S., Serafimova I.M., Taunton J. Electrophilic fragment-based design of reversible covalent kinase inhibitors. J. Am. Chem. Soc. 2013;135:5298–5301. doi: 10.1021/ja401221b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinkova-Kostova A.T., Holtzclaw W.D., Cole R.N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi A., Kang M.I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cullinan S.B., Gordan J.D., Jin J., Harper J.W., Diehl J.A. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell. Biol. 2004;24:8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang D.D., Lo S.C., Cross J.V., Templeton D.J., Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird L., Lleres D., Swift S., Dinkova-Kostova A.T. Regulatory flexibility in the Nrf2-mediated stress response is conferred by conformational cycling of the Keap1-Nrf2 protein complex. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15259–15264. doi: 10.1073/pnas.1305687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahon M., Thomas N., Itoh K., Yamamoto M., Hayes J.D. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J. Biol. Chem. 2006;281:24756–24768. doi: 10.1074/jbc.M601119200. [DOI] [PubMed] [Google Scholar]
- 11.McMahon M., Lamont D.J., Beattie K.A., Hayes J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. U. S. A. 2010;107:18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi M., Li L., Iwamoto N., Nakajima-Takagi Y., Kaneko H., Nakayama Y., Eguchi M., Wada Y., Kumagai Y., Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell. Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang D.D., Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Liby K., Yore M.M., Roebuck B.D., Baumgartner K.J., Honda T., Sundararajan C., Yoshizawa H., Gribble G.W., Williams C.R., Risingsong R., Royce D.B., Dinkova-Kostova A.T., Stephenson K.K., Egner P.A., Yates M.S., Groopman J.D., Kensler T.W., Sporn M.B. A novel acetylenic tricyclic bis-(cyano enone) potently induces phase 2 cytoprotective pathways and blocks liver carcinogenesis induced by aflatoxin. Cancer Res. 2008;68:6727–6733. doi: 10.1158/0008-5472.CAN-08-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinkova-Kostova A.T., Talalay P., Sharkey J., Zhang Y., Holtzclaw W.D., Wang X.J., David E., Schiavoni K.H., Finlayson S., Mierke D.F., Honda T. An exceptionally potent inducer of cytoprotective enzymes: elucidation of the structural features that determine inducer potency and reactivity with Keap1. J. Biol. Chem. 2010;285:33747–33755. doi: 10.1074/jbc.M110.163485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honda T., Yoshizawa H., Sundararajan C., David E., Lajoie M.J., Favaloro F.G., Jr., Janosik T., Su X., Honda Y., Roebuck B.D., Gribble G.W. Tricyclic compounds containing nonenolizable cyano enones. A novel class of highly potent anti-inflammatory and cytoprotective agents. J. Med. Chem. 2011;54:1762–1778. doi: 10.1021/jm101445p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalra S., Knatko E.V., Zhang Y., Honda T., Yamamoto M., Dinkova-Kostova A.T. Highly potent activation of Nrf2 by topical tricyclic bis(cyano enone): implications for protection against UV radiation during thiopurine therapy. Cancer Prev. Res. (Phila.) 2012;5:973–981. doi: 10.1158/1940-6207.CAPR-12-0041. [DOI] [PubMed] [Google Scholar]
- 19.Knatko E.V., Ibbotson S.H., Zhang Y., Higgins M., Fahey J.W., Talalay P., Dawe R.S., Ferguson J., Huang J.T., Clarke R., Zheng S., Saito A., Kalra S., Benedict A.L., Honda T., Proby C.M., Dinkova-Kostova A.T. Nrf2 Activation Protects against Solar-Simulated Ultraviolet Radiation in Mice and Humans. Cancer Prev. Res. (Phila.) 2015;8:475–486. doi: 10.1158/1940-6207.CAPR-14-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng S., Huang J.T., Knatko E.V., Sharp S., Higgins M., Ojima I., Dinkova-Kostova A.T., Honda T. Synthesis of (13) C2 (15) N2 -labeled anti-inflammatory and cytoprotective tricyclic bis(cyanoenone) ([(13) C2 (15) N2 ]-TBE-31) as an internal standard for quantification by stable isotope dilution LC-MS method. J. Label. Compd. Radiopharm. 2014;57:606–610. doi: 10.1002/jlcr.3230. [DOI] [PubMed] [Google Scholar]
- 21.Prochaska H.J., Santamaria A.B. Direct measurement of NAD(P)H:quinone reductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers. Anal. Biochem. 1988;169:328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- 22.Habig W.H., Jakoby W.B. Glutathione S-transferases (rat and human) Methods Enzymol. 1981;77:218–231. doi: 10.1016/s0076-6879(81)77029-0. [DOI] [PubMed] [Google Scholar]
- 23.Hong F., Freeman M.L., Liebler D.C. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem. Res. Toxicol. 2005;18:1917–1926. doi: 10.1021/tx0502138. [DOI] [PubMed] [Google Scholar]
- 24.Hu C., Eggler A.L., Mesecar A.D., van Breemen R.B. Modification of keap1 cysteine residues by sulforaphane. Chem. Res. Toxicol. 2011;24:515–521. doi: 10.1021/tx100389r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eggler A.L., Luo Y., van Breemen R.B., Mesecar A.D. Identification of the highly reactive cysteine 151 in the chemopreventive agent-sensor Keap1 protein is method-dependent. Chem. Res. Toxicol. 2007;20:1878–1884. doi: 10.1021/tx700217c. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y. Molecular mechanism of rapid cellular accumulation of anticarcinogenic isothiocyanates. Carcinogenesis. 2001;22:425–431. doi: 10.1093/carcin/22.3.425. [DOI] [PubMed] [Google Scholar]
- 27.Ye L., Zhang Y. Total intracellular accumulation levels of dietary isothiocyanates determine their activity in elevation of cellular glutathione and induction of Phase 2 detoxification enzymes. Carcinogenesis. 2001;22:1987–1992. doi: 10.1093/carcin/22.12.1987. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis. 2000;21:1175–1182. [PubMed] [Google Scholar]
- 29.Cornblatt B.S., Ye L., Dinkova-Kostova A.T., Erb M., Fahey J.W., Singh N.K., Chen M.S., Stierer T., Garrett-Mayer E., Argani P., Davidson N.E., Talalay P., Kensler T.W., Visvanathan K. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Ahn Y.H., Benjamin I.J., Honda T., Hicks R.J., Calabrese V., Cole P.A., Dinkova-Kostova A.T. HSF1-dependent upregulation of Hsp70 by sulfhydryl-reactive inducers of the KEAP1/NRF2/ARE pathway. Chem. Biol. 2011;18:1355–1361. doi: 10.1016/j.chembiol.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sussan T.E., Jun J., Thimmulappa R., Bedja D., Antero M., Gabrielson K.L., Polotsky V.Y., Biswal S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PloS One. 2008;3:e3791. doi: 10.1371/journal.pone.0003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barajas B., Che N., Yin F., Rowshanrad A., Orozco L.D., Gong K.W., Wang X., Castellani L.W., Reue K., Lusis A.J., Araujo J.A. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler. Thromb. Vasc. Biol. 2011;31:58–66. doi: 10.1161/ATVBAHA.110.210906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harada N., Ito K., Hosoya T., Mimura J., Maruyama A., Noguchi N., Yagami K., Morito N., Takahashi S., Maher J.M., Yamamoto M., Itoh K. Nrf2 in bone marrow-derived cells positively contributes to the advanced stage of atherosclerotic plaque formation. Free Radic. Biol. Med. 2012;53:2256–2262. doi: 10.1016/j.freeradbiomed.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Kundu R., Dasgupta S., Biswas A., Bhattacharya S., Pal B.C., Rao P.G., Barua N.C., Bordoloi M. Carlinoside reduces hepatic bilirubin accumulation by stimulating bilirubin-UGT activity through Nrf2 gene expression. Biochem. Pharmacol. 2011;82:1186–1197. doi: 10.1016/j.bcp.2011.07.069. [DOI] [PubMed] [Google Scholar]
- 35.Wang M., Chen S., Wang S., Sun D., Chen J., Li Y., Han W., Yang X., Gao H.Q. Effects of phytochemicals sulforaphane on uridine diphosphate-glucuronosyltransferase expression as well as cell-cycle arrest and apoptosis in human colon cancer Caco-2 cells. Chin. J. Physiol. 2012;55:134–144. doi: 10.4077/CJP.2012.BAA085. [DOI] [PubMed] [Google Scholar]
- 36.Kadakol A., Ghosh S.S., Sappal B.S., Sharma G., Chowdhury J.R., Chowdhury N.R. Genetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler–Najjar and Gilbert syndromes: correlation of genotype to phenotype. Hum. Mutat. 2000;16:297–306. doi: 10.1002/1098-1004(200010)16:4<297::AID-HUMU2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]