Abstract
The antibody-dependent respiratory burst and opsonic phagocytosis assays have been associated with protection against malaria; however, other mechanisms may also be involved. The antibody-dependent cellular inhibition (ADCI) assay is yet to be correlated with protection in longitudinal cohort studies (LCS). We investigated the relationship between ADCI activity of immunoglobulin G before malaria season and risk of malaria in a LCS involving Ghanaian children. High ADCI activity was significantly associated with reduced risk against malaria. Findings here suggest a potential usefulness of the ADCI assay as a correlate of protection to guide malaria vaccine studies.
Keywords: antibody-dependent cellular inhibition, children, longitudinal cohort study, malaria, monocytes, Plasmodium falciparum
The importance of immunoglobulin (Ig) G antibodies in immunity against Plasmodium falciparum malaria remains undisputed; however, the possible mechanisms by which these antibodies function have not been adequately explained. The antibody-dependent cellular inhibition (ADCI) assay measures the overall functional effect of antibodies and monocyte (MN) collaboration on in vitro parasite growth and has aided the discovery and development of malaria vaccine candidates such as merozoite surface protein 3 and the glutamate rich protein [1, 2]. Several studies have assessed ADCI activity in relation to malaria immunity [1–5]; however, the importance of this mechanism in protection against malaria in longitudinal cohort studies (LCS) is yet to be evaluated. In this study, we successfully assessed the relationship between ADCI activity of IgG before malaria season and P. falciparum infection outcome for the first time in well characterized LCS of Ghanaian children.
MATERIALS AND METHODS
Ethics Statement
The LCS was approved by the Institutional Review Board of Noguchi Memorial Institute for Medical Research of University of Ghana, Accra, Ghana. Written informed consent was obtained from parents or guardians of children before enrollment into the study. Use of Danish blood donor samples was approved by the Scientific Ethics Committee of Copenhagen and Frederiksberg, Denmark.
Study Design
From May 2008 to February 2009, a 42-week malaria LCS, detailed elsewhere [6], was conducted in Asutsuare, Ghana. In brief, 797 children (aged 1–12 years) were enrolled and observed both actively and passively for malaria case detection. Baseline venous blood plasma was stored at −80°C until use, and thick and thin film blood slides (TTBS) were obtained. Sickle cell status and ABO blood group were determined by the sodium metabisulphite test and a commercial blood grouping kit (Biotec Laboratories Limited, UK), respectively. Once every month, TTBS was obtained from each child for asymptomatic parasitemia assessment. Febrile malaria was defined as fever (axillary temperature ≥37.5°C, measured or reported) with slide positive for any asexual P. falciparum parasitemia and at least 1 other sign of malaria such as vomiting, diarrhoea, or malaise. At the end of the study, children in whom parasitemia was associated with febrile malaria were considered susceptible, whereas those who did not experience any febrile malaria despite parasitemia were considered protected. Others had no detectable parasitemia by microscopy and no malaria symptoms [6].
Antibodies
Test IgG was purified from approximately 300–500 µL of plasma from Ghanaian children and Danish controls using protein G coupled to Sepharose (GE Healthcare) as described previously [7]. Purity and concentration were checked with sodium dodecyl sulfate polyacrylamide gel electrophoresis and NanoDrop, respectively, and IgG was stored at −20°C until use. Pooled IgG from hyperimmune African adults (PHIG) or malaria-naive Danes (PNIG) were the positive and negative controls, respectively [8].
Antibody-Dependent Cellular Inhibition Assay
The NF54 strain of P. falciparum was cultured and ADCI assay performed as described [8, 9]. In brief, Danish blood donor peripheral blood mononuclear cells were isolated by LymphoPrep (Lonza), and approximately 2 × 105 MNs/well were selected by adherence in a flat-bottom 96-well culture plate (Nunc, Denmark). Highly synchronized schizont stage P. falciparum at 0.5% parasitemia and 2.5% hematocrit were added at 100 µL/well followed by test and control IgG at 0.5 mg/mL and 1.0 mg/mL final concentrations, respectively, to designated duplicate wells. Volume was adjusted to 200 µL with parasite growth medium (PGM) (RPMI 1640 [Lonza] + 0.5% Albumax) [9]. An additional 50 μL PGM was added per well at 48 hours and 72 hours, and the assay was stopped after 96 hours. Final parasitemia was determined as described previously [8], and specific growth inhibitory index (SGI) was calculated: SGI = 100 × (1 – [%parasitemia with MN and test antibodies/% parasitemia test antibodies]/[%parasitemia with MN and PNIG/% parasitemia PNIG]).
Schizont Extract Enzyme-Linked Immunosorbent Assay
Flat-bottom 96-well microtiter plates (Nunc) were coated with 100 µL/well of 5 μg/mL crude schizont antigen, obtained as described previously [10], in 0.05 M carbonate buffer and incubated overnight at 4°C. The remaining enzyme-linked immunosorbent assay procedure was as described previously [11], with modifications. Samples were tested at 65 μg/mL and 130 μg/mL for IgG and subclasses quantification, respectively. The detection antibodies were as follows: horseradish peroxidase (HRP)-conjugated polyclonal rabbit anti-human IgG (Dako, Denmark) at 1:5000 dilution; HRP-conjugated sheep anti-human IgG1 (AP006), IgG2 (AP007), or IgG3 (AP008) (The Binding Site, UK) at 1:4000, 1:2000, 1:3000 dilutions, respectively.
Statistical Analysis
Data analyses were performed with R, version 3.1.2 (http://www.R-project.org/). Age was categorized (1–5 and 6–12 years), and associations between febrile malaria and covariates (age group, sex, sickle cell status, and ABO blood group) were assessed by multivariate logistic regression models and likelihood ratio tests (LRTs). The parasitemia after the 96-hour ADCI assay were mean = 3.67%, 95% confidence interval [CI], 3.12–4.22 and mean = 5.89%, 95% CI, 5.21–6.57, for wells with and without MNs, respectively.
To facilitate interplate comparisons, sample ADCI activity (ie, SGI) values were normalized to the PHIG as follows: (Sample-SGI/Plate PHIG-SGI) × Mean PHIG-SGI all plates. The PHIG-SGI coefficient of variation was 29.85%. The association between SGI and age (continuous scale) was assessed by multivariate linear regression analysis adjusting for covariates. To evaluate whether the ADCI activity influences time-to-first malaria episode, SGI was categorized into tertiles. Kaplan–Meier survival curves and log-rank test were used to compare the tertile categories, and hazard ratios were calculated by Cox proportional hazards regression analysis. Children were either high ADCI responders (with SGI values in the top tertile) or low ADCI responders (with SGI values in the bottom and middle tertile). Receiver-operating characteristic (ROC) analysis was used to determine sensitivity and specificity associated with the SGI top tertile as a cutoff for classifying protected and susceptible children. Antibody titers were compared between high and low ADCI responders (Welch's t test). Raw P values and confidence limits with Bonferroni-corrected P values are shown for antibody data analyses. Significance level was at 5%.
RESULTS
Study Demographics and Malaria Incidence
Febrile malaria incidence was low (15.1%), and 168 children were considered “definitively” exposed by recording at least 1 febrile malaria episode (susceptible group) or 1 monthly blood slide positive for asexual stage P. falciparum without any clinical symptoms (protected group) throughout the study period [12]. Data presented here are for 98 (protected = 35; susceptible = 63) children with definitive exposure who had sufficient volumes of baseline plasma available for IgG purification. These 98 children were similar in age (P = .27, Welch's t test) to the 168 exposed children [12] but older (P = .025, Welch's t test) than the entire 669 children who successfully completed follow-up [6], because exposed but younger children were excluded from the current study due to insufficient plasma for IgG purification. Older children (6–12 year old) were protected from febrile malaria (odds ratio [OR] = 0.34; 95% CI, .13–.84; P = .021) compared with younger (1–5 year old) children. Similarly to previous reports on the larger cohort [12], sex, sickle cell status, and ABO blood group also had no influence on the outcome of P. falciparum infection in this subpopulation (PLRT > .05) (Supplementary Table 1). Baseline parasitemia levels showed no association (P = .29) with febrile malaria in a multivariate logistic regression adjusting for covariates.
Antibody-Dependent Cellular Inhibition Activity Increases With Age
The distribution of ADCI activity (SGI) ranged from −30.39% to 103.10% with a median of 46.18%. Multivariate linear regression found a significant increase (OR = 5.21; 95% CI, 1.22–22.27; P = .026) in SGI with age of children but not with the other covariates (P > .05).
Association Between Antibody-Dependent Cellular Inhibition and Febrile Malaria
Multivariate logistic regression analysis adjusting for the covariates showed increasing SGI was significantly (OR = 0.94; 95% CI, .90–.97; P = .00056) associated with reduced risk against febrile malaria. Redefining febrile malaria with parasitemia ≥2500 did not change (OR = 0.93; 95% CI, .88–.97; P = .0026; n = 64) the association between SGI and malaria. There was a significant difference (log-rank test P = .00085) in the time-to-first malaria episode for children in the different SGI tertiles (Figure 1). Using children in the SGI bottom tertile as reference in a Cox regression model adjusted for age group, we noted a significantly reduced risk of febrile malaria for children in the top tertile (hazard ratio [HR] = 0.30; 95% CI, .15–.59; P = .00044) but not those in the middle tertile (HR = 0.64; 95% CI, .37–1.14; P = .13). Thus, it appears a rather high threshold of ADCI activity was necessary to achieve a significant reduction in the risk of febrile malaria. The ROC analysis showed that a cutoff at the top SGI tertile (SGI ≥ 52.5%) had a sensitivity and specificity of 79.4% and 57.1%, respectively, in classifying protected and susceptible children in this cohort.
Figure 1.
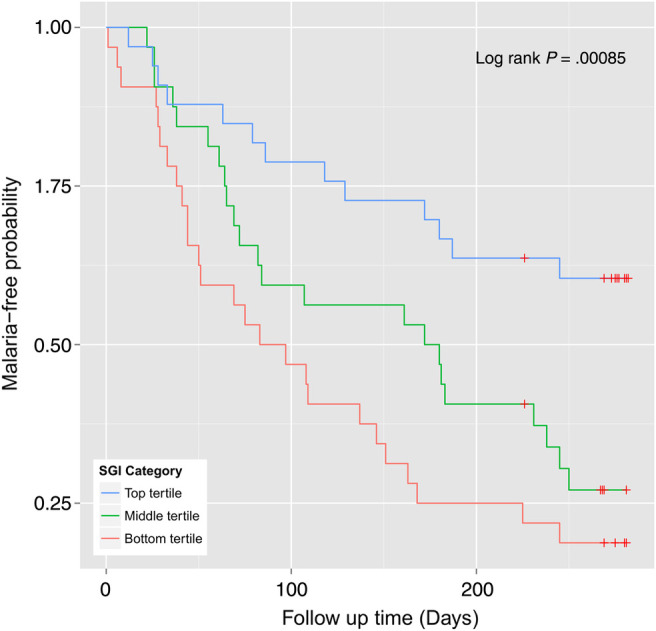
High threshold of antibody-dependent cellular inhibition (ADCI) activity is associated with reduced risk of febrile malaria. Children were categorized based on ADCI activity (ie, specific growth inhibition index [SGI]) into tertiles: top tertile (blue line), middle tertile (green line), and bottom tertile (red line). There was a statistically significant difference in the risk of malaria (log-rank test P = .00085) among children in the different SGI categories. The Kaplan–Meier estimates of malaria-free probability showed that children in the top SGI tertile category seemed to have a much reduced risk compared with those in the middle and bottom tertiles. Red crosses denote censored observations.
Relationship Between Anti-schizont Extract Immunoglobulin G Levels, Risk of Malaria, and Specific Growth Inhibitory Index
Total IgG (P = .0035), IgG1 (P = .031), and IgG3 (P = .001) but not IgG2 levels against crude schizont antigens were significantly associated with reduced risk against febrile malaria in a multivariate logistic regression adjusting for age groups (Supplementary Table 2). To determine whether ADCI was associated with IgG responses, children were categorized as either high (SGI ≥ 52.5%) or low (SGI < 52.5%) ADCI responders. Only IgG3 levels was significantly different (t = 2.66; 95% CI, .59–4.06; P = .037) between high and low responders (Table 1).
Table 1.
Association Between Antibody-Dependent Cellular Inhibition and Antibody Responses Against Crude Schizont Antigensa
| Anti-schizont Extract | High Responders (n = 33) Mean | Low Responders (n = 65) Mean | t Statistic (95% CI) | P Value | Adjusted P Value |
|---|---|---|---|---|---|
| IgG | −0.08 | −1.93 | 2.19 (.16–3.55) | .032 | .12 |
| IgG1 | 1.33 | 0.067 | 1.23 (−.68–3.20) | .20 | .80 |
| IgG2 | −0.68 | −1.80 | 1.40 (−.48–2.72) | .17 | .68 |
| IgG3 | −0.90 | −3.23 | 2.66 (.59–4.06) | .0093 | .037 |
Abbreviations: CI, confidence interval; IgG, immunoglobulin G; SGI, specific growth inhibition index.
a Children were divided on the basis of SGI into high responder (ie, SGI values in the top tertile, SGI ≥ 52.5%) and low responder (ie, SGI values in the bottom and middle tertile, SGI < 52.5%) groups, and antibody levels were compared between the groups by the Welch's t test. Antibody titers were log to base 2 transformed, and P values are shown without adjustment and after Bonferroni adjustment for multiple testing. Some antibody titers were negative after log base 2 transformation, which ultimately resulted in some negative mean values. Bold text indicate significant P value after multiple testing correction.
DISCUSSION
Using a well characterized LCS, we show for the first time that ADCI activity of IgG is significantly associated with reduced risk against febrile malaria. Although ADCI activity increased with age, its association with malaria remained significant in multivariate models that were adjusted for age. Children with ADCI activity within the top tertile of the distribution had a significantly delayed time-to-first malaria episode in the cohort, and this threshold classified protected and susceptible children with sensitivity and specificity of 79.4% and 57.1%, respectively. The lower specificity was due to the overlapping distribution of ADCI activity among protected and susceptible children, suggesting that other mechanisms may contribute to protection in this cohort.
Several studies have evaluated the ADCI assay as a potential correlate of malaria immunity [3, 4], but until now its importance in assessing immunity in LCS had not been evaluated. The ADCI mechanism is thought to be triggered by synergistic activation of FcγRIIA and FcγRIIIA on MNs when cross-linked with cytophilic antibodies bound to target antigens [4, 5]. This interaction signals the release of largely uncharacterized soluble factors, including tumor necrosis factor (TNF)-α, which act in concert to block the division of surrounding intraerythrocytic uninucleate stage parasites [4]. The unspecific nature of the active antiparasite mediators makes the ADCI mechanism parasite strain independent [3], which suggests that it may play a vital role in premunition, a nonsterile form of immunity in which low levels of parasites are tolerated in the absence of clinical symptoms [13]. In malaria-endemic populations, protective immunity develops gradually in relation to exposure to the parasite and age. This is consistent with the significant association of age with both protection against febrile malaria and increasing ADCI activity here. The apparent lack of protection with sickle cell may be due to its low prevalence in the cohort. The rather high ADCI activity necessary for protection against malaria may reflect the time-dependent nature of a mechanism that mirrors cumulative exposure and premunition.
Malaria immunity may be a composite of several mechanisms. In the current cohort, we previously identified polymorphisms in FcγRIIIB that modify antibody functionality to be associated with risk of malaria [12], thus implicating neutrophil-mediated mechanisms. In this study, cytophilic IgG antibodies were associated with reduced risk [14], and the importance of IgG3 in the ADCI mechanism was substantiated [5]. Although the ADCI assay used here and the opsonic phagocytosis assay recently associated with protection against malaria [15] were different, they share fundamental similarities. In essence, both assays are mediated by cytophilic IgG and involve merozoites opsonization and MN activation to release soluble factors, including TNF-α [4, 15], which facilitate parasite killing [4]. Thus, it is plausible that these 2 mechanisms are not mutually exclusive; however, the extent of any such potential overlap is not clear [4].
A limitation of this study is the small sample size involved, potentially restricting a wider generalization of our conclusions. Nonetheless, we have clearly shown that the ADCI mechanism is associated with reduced risk against malaria in this LCS. Further studies in larger cohorts would be crucial in improving our understanding of ADCI in malaria immunity.
CONCLUSIONS
In conclusion, we have shown for the first time in a malaria LCS that the ADCI assay is associated with reduced risk against malaria, suggesting its potential usefulness as a correlate of protection in malaria vaccine studies.
Supplementary Material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank the children and their parents and guardians from Asutsuare and its environs who volunteered to participate in the study, and without whose cooperation this study would have been impossible. The Afro Immuno Assay 2 Field and laboratory personnel are duly acknowledged for their support.
Financial support. This study was supported by grants from the Danish Council for Strategic Research (grant 13127), the European and Developing Countries Clinical Trials Partnership (grants IP.2007.31100.001 and TA.2007.40200.012), and the African Malaria Network Trust (grant 008/2008AIA).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Oeuvray C, Bouharoun-Tayoun H, Gras-Masse H et al. . Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 1994; 84:1594–602. [PubMed] [Google Scholar]
- 2.Theisen M, Soe S, Oeuvray C et al. . The glutamate-rich protein (GLURP) of Plasmodium falciparum is a target for antibody-dependent monocyte-mediated inhibition of parasite growth in vitro. Infect Immun 1998; 66:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouharoun-Tayoun H, Attanath P, Sabchareon A et al. . Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med 1990; 172:1633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouharoun-Tayoun H, Oeuvray C, Lunel F et al. . Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med 1995; 182:409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jafarshad A, Dziegiel MH, Lundquist R et al. . A novel antibody-dependent cellular cytotoxicity mechanism involved in defense against malaria requires costimulation of monocytes FcgammaRII and FcgammaRIII. J Immunol 2007; 178:3099–106. [DOI] [PubMed] [Google Scholar]
- 6.Adu B, Dodoo D, Adukpo S et al. . Fc gamma receptor IIIB (FcgammaRIIIB) polymorphisms are associated with clinical malaria in Ghanaian children. PLoS One 2012; 7:e46197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlback M, Jorgensen LM, Nielsen MA et al. . The chondroitin sulfate A-binding site of the VAR2CSA protein involves multiple N-terminal domains. J Biol Chem 2011; 286:15908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiendrebeogo RW, Adu B, Singh SK et al. . High-throughput tri-colour flow cytometry technique to assess Plasmodium falciparum parasitaemia in bioassays. Malar J 2014; 13:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jogdand PS, Singh SK, Christiansen M et al. . Flow cytometric readout based on Mitotracker Red CMXRos staining of live asexual blood stage malarial parasites reliably assesses antibody dependent cellular inhibition. Malar J 2012; 11:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wahlgren M, Berzins K, Perlmann P et al. . Characterization of the humoral immune response in Plasmodium falciparum malaria. I. Estimation of antibodies to P. falciparum or human erythrocytes by means of microELISA. Clin Exp Immunol 1983; 54:127–34. [PMC free article] [PubMed] [Google Scholar]
- 11.Nebie I, Diarra A, Ouedraogo A et al. . Humoral and cell-mediated immunity to MSP3 peptides in adults immunized with MSP3 in malaria endemic area, Burkina Faso. Parasite Immunol 2009; 31:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adu B, Jepsen MP, Gerds TA et al. . Fc gamma receptor 3B (FCGR3B-c.233C>A-rs5030738) polymorphism modifies the protective effect of malaria specific antibodies in Ghanaian children. J Infect Dis 2014; 209:285–9. [DOI] [PubMed] [Google Scholar]
- 13.Sergent E. [Definition of immunity & premunition]. Ann Inst Pasteur (Paris) 1950; 79:786–97. [PubMed] [Google Scholar]
- 14.Perraut R, Marrama L, Diouf B et al. . Distinct surrogate markers for protection against Plasmodium falciparum infection and clinical malaria identified in a Senegalese community after radical drug cure. J Infect Dis 2003; 188:1940–50. [DOI] [PubMed] [Google Scholar]
- 15.Osier FH, Feng G, Boyle MJ et al. . Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med 2014; 12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


