We have assessed for the first time the influence of iron levels on malaria risk in a longitudinal prospective cohort during pregnancy. Elevated iron levels were significantly associated with increased risk of malarial episodes and P.falciparum density throughout the pregnancy in the context of IPTp and ITN use.
Keywords: iron levels, pregnancy-associated malaria
Abstract
Background. Pregnancy-associated malaria (PAM) remains a significant health concern in sub-Saharan Africa. Cross-sectional studies report that iron might be associated with increased malaria morbidity, raising fears that current iron supplementation policies will cause harm in the present context of increasing resistance against intermittent preventive treatment in pregnancy (IPTp). Therefore, it is necessary to assess the relation of iron levels with malaria risk during the entire pregnancy.
Methods. To investigate the association of maternal iron levels on malaria risk in the context of an IPTp clinical trial, 1005 human immunodeficiency virus-negative, pregnant Beninese women were monitored throughout their pregnancy between January 2010 and May 2011. Multilevel models with random intercept at the individual levels and random slope for gestational age were used to analyze the factors associated with increased risk of a positive blood smear and increased Plasmodium falciparum density.
Results. During the follow-up, 29% of the women had at least 1 episode of malaria. On average, women had 0.52 positive smears (95% confidence interval [CI], 0.44–0.60). High iron levels (measured by the log10 of ferritin corrected on inflammation) were significantly associated with increased risk of a positive blood smear (adjusted odds ratio = 1.75; 95% CI, 1.46–2.11; P < .001) and high P falciparum density (beta estimate = 0.22; 95% CI, 0.18–0.27; P < .001) during the follow-up period adjusted on pregnancy parameters, comorbidities, environmental and socioeconomic indicators, and IPTp regime. Furthermore, iron-deficient women were significantly less likely to have a positive blood smear and high P falciparum density (P < .001 in both cases).
Conclusions. Iron levels were positively associated with increased PAM during pregnancy in the context of IPTp. Supplementary interventional studies are needed to determine the benefits and risks of differently dosed iron and folate supplements in malaria-endemic regions.
Pregnancy-associated malaria (PAM) remains a public health concern in sub-Saharan Africa with over 35 million pregnant women at risk [1]. Pregnancy-associated malaria is defined as a peripheral or placental infection by Plasmodium, and it is correlated with increased maternal morbidity and mortality [2, 3] and severe anemia (defined as hemoglobin [Hb] <70 g/L or <80 g/L) [3]. Furthermore, PAM is associated with an increased risk for placental malaria (PM), prematurity and low birth weight (LBW) [3, 4]. Preventive strategies such as intermittent preventive treatment in pregnancy (IPTp) or insecticide-treated mosquito nets (ITNs) have shown their efficacy in reducing PAM and its subsequent morbidity [5, 6]. Indeed the World Health Organization (WHO) recommends IPTp with sulphadoxine-pyrimethamine (SP) for all pregnant women as early as possible in the second trimester and at each scheduled antenatal visit (ANV) at least 1 month apart [7].
However, IPTp does not always completely clear Plasmodium falciparum parasitemia, and residual parasitemia increases as a consequence of the growing resistance [8]. In addition, the effect of residual parasitemia is not harmless [9, 10]. For these reasons, it is necessary to further investigate additional factors influencing P falciparum parasitemia during pregnancy among women receiving IPTp.
Environmental, obstetric, and hematologic genetic risk factors for PAM have been extensively assessed. The association of gravidity with parasitemia increases with transmission [11], and a young maternal age (≤20 years) is also associated with increased malarial risk especially in high-transmission settings [12–14]. Pregnancy-associated malaria seems to vary depending on gestational age with the period before the first IPTp intake seemingly at particular risk [15]. Nevertheless, important knowledge gaps need to be filled with regard to the influence of nutritional indicators on PAM. This aspect is of special concern, because iron has been repeatedly linked to increased infectious morbidity, and it is simultaneously involved in the hematological outcomes of P falciparum infection. A recent Cochrane review on iron supplementation during pregnancy found only 2 studies (of the 23 studies of malaria-endemic countries) that reported results concerning malarial infection. It concluded that there was no evidence that iron supplements were associated to PM [16]. However, an important cohort in Tanzania indicated that iron deficiency (ID) was significantly protective for PM in terms of both prevalence and severity [17]. Therefore, it is necessary to further investigate the association of iron and folate with malarial risk in a prospective longitudinal cohort during pregnancy. More precisely, the study of the influence of maternal iron and folate levels on P falciparum parasitemia in the context of IPTp will help to better understand PAM and provide important knowledge on supplementary factors influencing malarial risk during pregnancy among women receiving IPTp.
The aim of our study was to investigate the relationship of maternal iron and folate levels with malarial risk and P falciparum parasite density during pregnancy in the context of IPTp in Benin, taking into account environmental and obstetric risk factors and simultaneous comorbidities. In addition, we aimed to explore the association of iron and folate with PAM outcomes such as LBW and PM.
MATERIALS AND METHODS
Study Design
One thousand five human immunodeficiency virus (HIV)-negative pregnant women under 28 weeks of gestational age were observed until delivery in the context of the Anemia in Pregnancy: Etiology and Consequences (APEC) study, an observational study nested in the Malaria in Pregnancy Preventive Alternative Drugs (MiPPAD) clinical trial (http://clinicaltrials.gov/ct2/show/NCT00811421). Further details are given in González et al [18].
Study Site and Population
The APEC study was conducted in 3 maternity clinics in the district of Allada, between January 2010 and May 2012. Allada is a semirural area of 91 778 inhabitants located 50 km North of Cotonou (Benin). Malaria has a perennial transmission pattern with 2 transmission peaks corresponding to the rainy seasons in April–July and October–November. Plasmodium falciparum is the species responsible for the majority of infections.
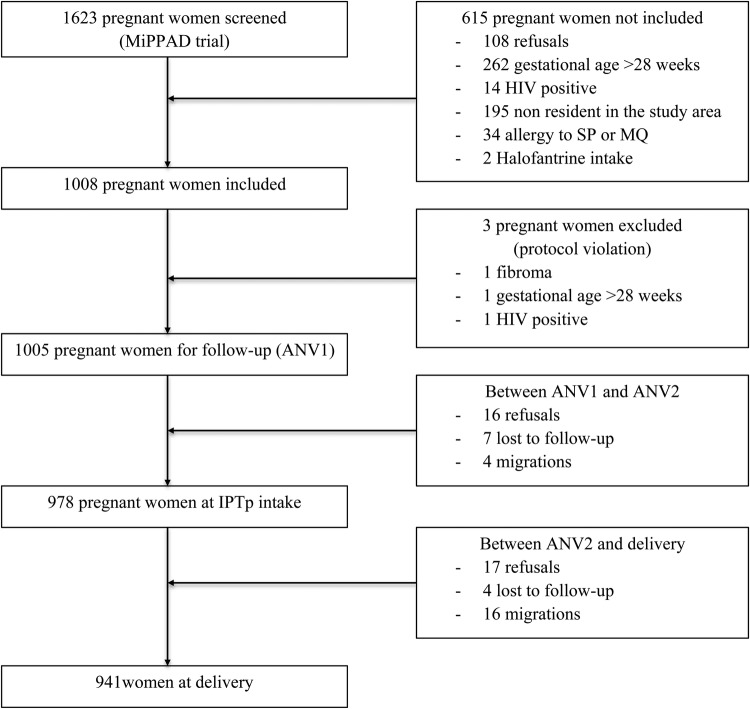
Further details of the study are described elsewhere [19], but, briefly, the eligibility criteria included no intake of IPTp, iron, folic acid, vitamin B12, or antihelminthic treatment. All women were offered confidential pretest HIV counseling and thereafter informed consent was obtained. The study was approved by the Ethics Committee of the Faculty of Medicine of Cotonou. Precise details of the follow-up are presented in Figure 1.
Figure 1.
Study profile. Abbreviations: ANV, antenatal visit; HIV, human immunodeficiency virus; IPTp, intermittent preventive treatment; MiPPAD, Malaria in Pregnancy Preventive Alternative Drugs; MQ, mefloquine; SP, sulphadoxine-pyrimethamine.
Study Procedures
Clinical Data Collection
The pregnant women were observed through 2 systematic ANV, the first taking place at inclusion, and through unscheduled visits in case of disease. The observations were completed after the women gave birth. At the first ANV, each woman was given an ITN, she was examined, and her clinical and gynecological histories were recorded. At each systematic ANV, 2-dose IPTp (1500/75 mg of SP per dose or 15 mg/kg mefloquine [MQ], either single or split intake) was administered 1 month apart, the first given to pregnant women after 15 weeks of gestation. Women were also systematically given 600 mg of albendazole as well as supplements of oral ferrous sulfate (200 mg per day) and folic acid (5 mg/day) for home treatment. In case of Hb concentration <110 g/L, women were treated as follows: ie, they received 200 mg of oral ferrous sulfate twice a day for mild or moderate anemia (Hb between 70 and 110 g/L, according to the national recommendations in Benin); and they were referred to the tertiary hospital in case of severe anemia (Hb < 70 g/L, according to the national recommendations in Benin). In case of sickness, women were examined and, if necessary, treated in unscheduled visits. Clinical data were collected at each ANV, unscheduled visit, and at delivery.
Blood and Stools Samples Collection
At ANV1, ANV2, and at delivery, 8 mL venous blood were collected. A container was also given to the woman to collect stools to examine the presence of intestinal helminths. At delivery, a placental blood smear was performed to investigate the existence of PM. The study sample examination techniques have been described elsewhere [20]. Microbiological exams were realized as follows: the Lambaréné technique [21] was used to assess malaria infection on thick smears; and helminthic infestations were assessed using the Kato-Katz concentration method.
Environmental Data
Because no entomological data were available, we used rain quantity instead as a surrogate for the anopheline presence. Because of the anopheline timeliness, rain was calculated as the mean rainfall of the 7 days before the 2 weeks before the consultation.
Definitions
Pregnancy-associated malaria was defined as peripheral or placental infection by Plasmodium, whereas PM was defined as presence of Plasmodium in the placenta. Low birth weight corresponds to newborn weights <2500 g, and prematurity refers to offspring born before 37 weeks of gestation. Severe, moderate, and mild anemia were defined as Hb concentrations <80 g/L, 80–99 g/L, and 100–109 g/L, respectively, following WHO criteria [22]. Inflammation was determined by C-reactive protein (CRP) levels ≥5 mg/mL. We corrected serum ferritin in the context of inflammation following the procedure inspired by the meta-analysis by Thurnham et al [23] before conducting the analyses, so we multiplied serum ferritin by 0.76 in the presence of Plasmodia without inflammation, and we multiplied serum ferritin by 0.53 in case of concurrent Plasmodia infection and inflammation. Iron deficiency was then defined as corrected serum ferritin <15 µg/L. Iron deficiency anemia (IDA) was defined as Hb < 110 g/L with ID. Folic acid deficiency was defined as a serum concentration <6 ng/mL. Vitamin B12 deficiency was defined as a serum concentration <150 pg/mL. Intestinal helminth infestations were diagnosed by the presence of intestinal helminth eggs in the stool sample.
Socioeconomic items (home possession of latrines, electricity, a refrigerator, a television, a vehicle with at least 2 wheels, being married, and working outside the home) were plotted into a multiple correspondence analysis. Then, a predictor was created to synthesize the information, and it was kept as the final socioeconomic index.
Statistical Analysis
Data were double entered and analyzed with ACCESS2003 and STATA12.0 (StataCorp, College Station, TX). The Kruskal-Wallis test was also used to analyze continuous variables. The χ2 test was used for comparing categorical variables by gravidity status. Univariate analysis was conducted to assess the association of all variables with positive smear and maternal peripheral parasitemia using multilevel models with a random intercept at the individual level. Thereafter, 2 different multilevel models regressions were built: the first on the risk of having a positive blood smear during the follow-up period and the second on P falciparum parasite density. Both models included the smears and blood films of both systematic and unscheduled visits. The variables with P < .2 in univariate analysis were included in the multilevel models. Maternal age squared was used due to the quadratic relationship of age with the malarial risk. For both the analysis of the possibility of a positive blood smear and for the analysis of parasite density, random coefficient models were used because they were statistically better than fixed effects according to Akaike information criterion (AIC) and Bayesian information criterion (BIC). The AIC and BIC compare maximal likelihood models. More precisely, random intercept was applied in both cases at the individual level and random slope was applied to gestational age, because the effect of the variables might differ among women and the effect of gestational age might also vary differently according to the timing of the measure. Multivariable linear regression was used in the analysis of birth weight, and logistic regression was used for PM and LBW assessment. Certain variables were forced into the model because of their meaning in the analyses according to the literature: socioeconomic status and rainfall in the case of malarial indicators, and body mass index (BMI) in the case of LBW. The statistical significance in the final multivariable models was set to P < .05. The presented P values and the significance threshold were 2-sided.
RESULTS
Study Population
Between January 2010 and May 2011, 1005 pregnant women were included in the cohort, 978 continued until the second ANV (second IPTp dose), and 941 (93.63%) completed the follow-up until delivery. During the follow-up period, 29% of the women had at least 1 malarial episode. On average, women had 0.52 positive smears (standard deviation [SD] = 1.23, with a median of 0 [25th percentile = 0, 75th percentile = 1], and range of 0–6 positive smears). Demographic and clinical characteristics were statistically different in univariate analyses between primigravid, secundigravid, and multigravid women with regard to age, BMI, socioeconomic status, number of positive blood smears, PM, and LBW (Table 1). Sixty-nine of the 751 placentas analyzed had placental malaria (9.2%). The mean of positive blood smears during pregnancy was significantly higher for primi- and secundigravidae than for multigravidae (0.84, 0.86, and 0.32, respectively; P < .01). The percentage of women with placental malaria decreased as gravidity increased: placental malaria was found in 15.3% of primigravid, 13.4% of secundigravid, and 6% of multigravid women (P < .01). The proportion of LBW was also inversely correlated with gravidity: 18%, 10.7%, and 7.5% for primi-, secundi-, and multigravid women, respectively (P < .01). However, gravidity was not significant in the multivariable analysis of positive blood smears and parasite density after the inclusion of maternal age in the model (P value for gravidity in the multivariable model = .16 and .08, respectively; data not shown).
Table 1.
Characteristics of the Study Population, by Gravidity Statusa
| Characteristic | Primigravidae (n = 172, 18.45%) | Secundigravidae (n = 187, 20.06%) | Multigravidae (n = 573, 61.48%) | P Value |
|---|---|---|---|---|
| Age, years | 20.10 (19.74; 20.46) | 22.29 (21.80; 22.79) | 28.77 (28.38; 29.16) | <.001 |
| BMI before pregnancy (kg/m2) | 20.41 (19.98; 20.84) | 20.66 (20.18; 21.13) | 21.35 (21.02; 21.68) | .01 |
| IPTp regime | ||||
| SP | 56 (32.56%) | 64 (34.22%) | 198 (34.55%) | .89 |
| MQ | 116 (67.44%) | 123 (65.78%) | 375 (65.45%) | .89 |
| Gestational age at ANV1 (weeks) | 22.06 (21.52; 22.61) | 22.11 (21.50; 22.71) | 22.20 (21.87; 22.52) | .77 |
| Gestational age at ANV2 | 28.41 (27.82; 29.01) | 28.88 (28.33; 29.42) | 28.97 (28.66; 29.28) | .21 |
| Gestational age at delivery | 38.37 (37.85; 38.89) | 37.86 (37.38; 38.34) | 38.20 (37.92; 38.48) | .42 |
| Number of positive smears during pregnancy | 0.84 (0.63; 1.05) | 0.86 (0.63; 1.09) | 0.32 (0.24; 0.40) | .42 |
| Placental malaria | 20 (15.27%) | 20 (13.42%) | 28 (5.97%) | .001 |
| Low birth weight | 31 (18.02%) | 20 (10.70%) | 43 (7.50%) | <.001 |
Abbreviations: ANV, antenatal visit; BMI, body mass index; IPTp, intermittent preventive treatment; MQ, mefloquine; SP, sulphadoxine-pyrimethamine.
a For continuous variables, the mean is provided followed by the 95% confidence interval in brackets. For categorical variables, n is presented followed by the % in brackets.
Follow-Up
Indicators of nutritional status such as folate, vitamin B12, and ferritin changed significantly during pregnancy (Table 2). The mean ferritin levels decreased after the first iron supplements were given at ANV1 from 37 mg/L (SD = 42.7) to 25.1 mg/L (SD = 31.3) at the second ANV, and then it increased up to 60.2 mg/L (SD = 83.1) at delivery. In parallel, the proportion of women with a positive smear decreased after IPTp (from 15.3% at ANV1 to 3.9% at ANV2), and then it increased again up to 9.6% at delivery. Nevertheless, the trend was slightly different concerning parasite density. Plasmodium falciparum parasite density was higher at ANV1 than at ANV2 (382.4, SD = 3709.2 and 214.1, SD = 2728.5 parasites/µL, respectively) but then rose up to 3098.8, SD = 31120.7 parasites/µL at delivery. There were no significant differences between SP and MQ IPTp with regard to the women malarial risk or parasite density within the whole follow-up period. There were no significant differences in ferritin levels or ID rates depending on the IPTp regime.
Table 2.
Indicators of Malaria, Folate, and Iron Indicators During Pregnancya
| Parameters | ANV 1 (n = 932) | ANV 2(n = 906) | Delivery (n = 858) |
|---|---|---|---|
| Gestational age (weeks) | 22.15 (21.90; 22.41) | 28.85 (28.60; 29.09) | 39.51 (39.34; 39.68) |
| Folate (ng/mL) | 9.52 (9.12; 9.91) | 10.47 (9.91; 11.02) | 11.25 (10.09; 12.40) |
| Folate deficiency (serum folate <6 ng/mL) | 294 (31.55%) | 155 (17.09%) | 330 (39.01%) |
| Vitamin B12 (pg/mL) | 397.55 (385.34; 409.77) | 370.36 (356.65; 384.06) | 337.09 (322.20; 351.98) |
| Vitamin B12 deficiency (vitamin B12 <150 pg/mL) | 32 (3.43%) | 33 (3.64%) | 62 (7.32%) |
| Ferritin (mg/L) | 36.99 (34.24; 39.73) | 25.10 (23.05; 27.14) | 60.19 (54.58; 65.80) |
| Inflammation (CRP >5 mg/mL) | 195 (20.92%) | 110 (12.13%) | 292 (34.11%) |
| Iron deficiency (corrected SF <15 µg/L) | 277 (33.09%) | 359 (44.16%) | 183 (23.11%) |
| Hemoglobin (g/L) | 10.30 (10.22; 10.38) | 10.50 (10.43; 10.57) | 11.16 (11.07; 11.26) |
| Anemia (Hb <110 g/L) | 636 (68.24%) | 589 (65.01%) | 346 (40.37%) |
| Severe anemia (Hb <80 g/L) | 32 (3.43%) | 15 (1.66%) | 20 (2.33%) |
| Positive blood smear | 143 (15.34%) | 35 (3.86%) | 82 (9.56%) |
| Plasmodium falciparum parasitemia (parasites/µL) | 382.40 (143.96; 620.84) | 214.09 (36.19; 392.00) | 3098.82 (1013.53; 5184.12) |
| Kato-Katz test positivity | 104 (11.33%) | 65 (7.30%) | 28 (3.75%) |
Abbreviations: ANV, antenatal visit; CRP, C-reactive protein; Hb, hemoglobin; SF, serum ferritin.
aFor continuous variables, the mean is provided followed by the 95% confidence interval in brackets. For categorical variables, n is presented followed by the % in brackets.
Malarial Outcomes
High iron levels (log10 of ferritin corrected on inflammation) were significantly associated with increased risk of a positive blood smear (adjusted odds ratio [aOR] = 1.75; 95% CI, 1.46–2.11; P < .001) and P falciparum parasite density (coefficient = 0.22; 95% CI, 0.18–0.27; P < .001) during the follow-up in logistic and linear-mixed multivariable models, respectively (Tables 3 and 4). More precisely, high corrected ferritin levels were associated with malaria risk at each visit unless the one following iron supplements (P value in univariate analysis = .07; data not shown). However, corrected ferritin was statistically associated with parasite density at each visit. Women with ID were significantly less likely to have a positive blood smear and a high P falciparum density (P < .001; data not shown). In parallel, high folate levels were statistically associated with decreased odds of a positive blood smear (aOR = 0.36; 95% CI, 0.19–0.70; P < .001) and to a lower P falciparum parasite density (beta coefficient = −0.2; 95% CI, −0.37 to −0.08; P < .001). When adjusted on maternal age, gravidity was not significantly correlated with malaria risk or parasite density. Young maternal age, early gestational age, and inflammatory status were significantly positively correlated to increased malarial risk with regard to both having a positive smear and to higher parasite density. High socioeconomic status was associated with reduced malaria risk and P falciparum parasite density (aOR = 0.82; 95% CI, 0.69–0.96; P = .02 and beta coefficient = −0.05; 95% CI, −0.09 to −0.01; P = .01, respectively).
Table 3.
Multilevel Model on Factors Associated With Having Positive Blood Smears During Pregnancy
| Factor | AOR (95% CI) | P Value |
|---|---|---|
| Ferritin corrected on inflammation (logarithm of µg/L) | 1.75 (1.46; 2.11) | <.001 |
| Folate (logarithm of ng/mL) | 0.37 (0.19; 0.70) | .002 |
| IPTp with MQ (SP = reference) | 1.06 (0.76; 1.48) | .74 |
| Gestational Age (weeks) | 0.95 (0.93; 0.98) | .001 |
| Maternal age (years) | 0.64 (0.51; 0.82) | <.001 |
| Maternal age squared (years) | 1.01 (1.00; 1.01) | .004 |
| Inflammatory process | 5.41 (3.90; 7.70) | <.001 |
| High socioeconomic status | 0.82 (0.69; 0.96) | .02 |
| Rain (mm) | 0.99 (0.96; 1.03) | .75 |
| Kato-Katz test positivity | 0.98 (0.56; 1.70) | .93 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; IPTp, intermittent preventive treatment; MQ, mefloquine; SP, sulphadoxine-pyrimethamine.
a Random intercept at the individual level and random slope for gestational age. Analysis on 2227 blood smears from 826 women.
Table 4.
Multilevel Model on Factors Associated With Plasmodium falciparum Parasitemia (in Logarithm) During Pregnancy: Iron Levels Analysisa
| Factor | Coefficient (95% CI) | P Value |
|---|---|---|
| Ferritin corrected on inflammation (logarithm of µg/L) | 0.22 (0.18; 0.27) | <.001 |
| Folate (logarithm of ng/mL) | −0.23 (−0.37; −0.08) | .002 |
| IPTp with MQ (SP = reference) | −0.01 (−0.09; 0.07) | .81 |
| Gestational age (weeks) | −0.01 (−0.01; −0.002) | .01 |
| Maternal age (years) | −0.15 (−0.21; −0.09) | <.001 |
| Maternal age squared (years) | 0.002 (0.001; 0.003) | <.001 |
| Inflammatory process | 0.62 (0.53; 0.71) | <.001 |
| High socioeconomic index | −0.05 (−0.09; −0.01) | .01 |
| Rain (mm) | −0.00 (−0.01; 0.01) | .98 |
| Kato-Katz test positivity | −0.01 (−0.15; 0.13) | .90 |
Abbreviations: CI, confidence interval; IPTp, intermittent preventive treatment; MQ, mefloquine; SP, sulphadoxine-pyrimethamine.
aRandom intercept at the individual level and random slope for gestational age. Analysis on 2227 blood smears of 826 women.
High iron levels were also significantly associated with PM and LBW. More precisely, high levels of ferritin corrected on inflammation at delivery was strongly associated with placental malaria (aOR = 2.02; 95% CI, 1.43–2.86; P < .01) (Table 5, placental malaria). Similarly, corrected high ferritin at the ANV2 and at delivery were significantly correlated with increased odds of LBW (aOR = 1.59; 95% CI, 1.12–2.26 and aOR = 1.69; 95% CI, 1.28–2.22, respectively) (Table 5, low birth weight at delivery [birth weight <2500 g]).
Table 5B.
Logistic Regression on the Possibility of Having Low Birth Weight at Delivery (Birth Weight <2500 g).a
| Factor | AOR (95% CI) | P Value |
|---|---|---|
| Socioeconomic index | 0.91 (0.72; 1.19) | .55 |
| Maternal BMI before pregnancy | 0.92 (0.84; 1.00) | .06 |
| Gestational age at the first ANV (and IPTp dose) | 0.90 (0.85; 0.96) | <.001 |
| Ferritin corrected on inflammation at ANV2 (logarithm) | 1.59 (1.12; 2.26) | .01 |
| Ferritin corrected on inflammation at delivery (logarithm) | 1.69 (1.28; 2.22) | <.001 |
| Positive blood smear at ANV2 | 2.88 (1.15; 7.22) | .02 |
Abbreviations: ANV, antenatal visit; AOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; IPTp, intermittent preventive treatment.
a Analysis on the birth weight of 763 infants. Pseudo R2 = 0.11
Table 5A.
Logistic Regression on the Possibility of Having Placental Malariaa
| Factor | AOR (95% CI) | P Value |
|---|---|---|
| Socioeconomic index | 1.26 (0.88; 1.79) | .20 |
| Maternal age | 0.94 (0.87; 1.00) | .06 |
| Ferritin corrected on inflammation at delivery (logarithm) | 2.02 (1.43; 2.86) | <.001 |
| Inflammatory process at delivery | 4.65 (2.32; 9.3) | <.001 |
| Folate (logarithm) at ANV2 | 0.16 (0.03; 0.86) | .03 |
| Number of maternal positive blood smears during pregnancy | 2.51 (2.00; 3.15) | <.001 |
Abbreviations: ANV, antenatal visit; AOR, adjusted odds ratio; CI, confidence interval.
aAnalysis on 689 placentas by blood smear. Pseudo R2 = 0.43
We investigated further the differences in malarial risk factors stratifying between anemic- and nonanemic, and iron-deficient and noniron-deficient women (Table 6). In this analysis, we included women with ID defined by serum ferritin <15 µg/L at the moment of the malaria measure. Multilevel models showed corrected ferritin and CRP were equally significant for parasite density among anemic and non anemic women. However, folate was not correlated to parasite density in anemic women. In addition, iron levels were no longer associated with P falciparum parasite density among iron-deficient women.
Table 6.
Multilevel Model on Factors Associated With Having Positive Blood Smears During Pregnancy Among Iron-Deficient Womena
| Factor | AOR (95% CI) | P Value |
|---|---|---|
| Ferritin corrected on inflammation (logarithm of µg/L) | 0.96 (0.63; 1.47) | .86 |
| Folate (logarithm of ng/mL) | 0.69 (0.28; 1.73) | .43 |
| Gestational age (weeks) | 0.96 (0.90; 1.03) | .27 |
| Maternal age (years) | 0.70 (0.51; 0.97) | .03 |
| Maternal age squared (years) | 1.01 (0.99; 1.01) | .06 |
| Inflammatory process | 5.86 (3.54; 10.00) | <.001 |
| Socioeconomic index | 0.85 (0.67; 1.07) | .16 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; IPTp, intermittent preventive treatment; MQ, mefloquine; SP, sulphadoxine-pyrimethamine.
a Random intercept at the individual level and random slope for gestational age. Analysis on 1605 blood smears from 747 women.
DISCUSSION
Benefits of iron supplementation during pregnancy for reducing iron related-diseases are undeniable. A Cochrane review showed supplementation was associated to a 70% decreased risk of anemia and to a 57% reduced risk of ID at delivery compared with controls [16]. However, epidemiological studies have questioned the benefits of iron supplementation in the context of malaria-endemic countries [24]. In a recent meta-analysis of the association between malaria and iron status or supplementation, data were reported to be insufficient for assessing the potential for an increased risk of P falciparum [25] infection. In addition, ID was associated with a decreased malarial risk in pregnancy when measured by ferritin, which is a robust indicator for iron levels [26, 27]. Indeed, the lack of complete follow-up of women through pregnancy is an important obstacle for the assessment of the influence of iron levels on P falciparum malaria. In the majority of the studies included in the meta-analysis, iron was only determined either at enrollment, at delivery, or both. In the only prospective cohort [28], malaria was analyzed solely with regard to the first episode of the pregnancy.
In our study, we have assessed for the first time the influence of iron levels on malarial risk in a prospective longitudinal cohort through pregnancy, considering the possibility of having a positive blood smear and P falciparum parasite density. Indeed, iron levels, measured by ferritin corrected for inflammation, were significantly associated with malarial episodes and P falciparum density through the pregnancy period in the context of IPTp and ITN use. Furthermore, this association is strongly significant even after adjustment on inflammatory status. Moreover, iron levels are significantly associated with placental malaria even after adjustment on maternal infection. Literature shows PM is associated with increased infant's susceptibility to the infection, translating into an increased number of clinical episodes [29–31]. Consequently, the association of high iron with placental malaria might contribute to enhance its consequences throughout the perinatal period. Finally, the association of maternal iron levels with LBW, possibly due to their relationship with PAM, suggests a broader impact of iron on infant health. Further details on the evolution of iron levels and anemia during pregnancy in this cohort are presented by Ouédraogo et al [19, 20, 32], but ID conferred protection against malaria through the entire follow-up. However, iron levels were no longer associated with P falciparum parasite density among iron-deficient women, which suggests the possible existence of a threshold level above which iron levels become deleterious. Indeed, there was significant increased malarial risk above 30 days of supplementation in the stratified analysis of 2 African surveys with high antimalarial preventive measures (relative risk = 1.42; 95% CI, 1.09–1.84) [25].
Our results are consistent with those in other studies. Although iron supplementation trials do not show augmented malaria morbidity associated with iron supplements, ID is correlated with lower odds of malarial episodes [25]. Iron deficiency was statistically linked to reduced risk of placental malaria in Tanzania [17]. Ferritin was also higher among placenta-infected mothers in Gabon [33] and zinc protoporphyrin in Malawi [34], but these differences were not statistically significant. Similar results were found in clinical trials in The Gambia [35] or Kenya [36]. The recent meta-analysis on malarial risk and iron status suggested a possible but not significant difference in placental malaria associated with iron supplementation depending on sickle cell genotype [25]. However, as stated previously, these studies report iron levels only at enrollment, at delivery, or both, and the limited sample might be insufficient to show a statistically significant effect.
Possible explanations for the increased malarial risk associated with iron levels found in our study are related to malaria pathophysiology in both the host and the parasite. At the host level, Plasmodium interferes with the physiological iron distribution and use through hemolysis, release of heme, dyserythropoiesis, anemia, deposition of iron in macrophages, and inhibition of dietary iron absorption [37]. Furthermore, the changes in iron metabolism during a malaria infection may modulate susceptibility to coinfections [37]. In addition, iron inhibits the synthesis of nitric oxide by inhibiting the expression of inducible nitric oxide synthase and thereby interferes with macrophage-mediated cytotoxicity against Plasmodium [38]. Moreover, nontransferrin-bound iron is involved in the severity of malaria [39–41]. Indeed, Plasmodium has the capacity of acquiring iron in a transferrin-independent pathway [42]. With regard to placenta, Penha-Gonçalves et al [43] described in their preliminary results that iron overload in trophoblasts of Plasmodium berghei-infected placenta is associated with fetal death.
Accurate assessment of iron levels is challenging and no gold standard exists at present. We used serum ferritin to measure iron levels because it is a robust iron indicator and its frequent use in clinical studies facilitates the comparison of our results with other cohorts. To attenuate the interference of inflammation on ferritin values (ferritin is an acute phase protein), we corrected ferritin upon inflammation (with correction factors according to CRP). Then, we included systematically inflammation in the statistical models to capture its independent association with malarial risk.
Another important finding of our study is the association between folate levels and PAM outcomes. High folate was correlated with reduced risk of malarial episodes, parasite density, and PM. Folate is an important cofactor used by (1) P falciparum in DNA synthesis and methylation and (2) mRNA translation. Therefore, antifolates have been extensively used against malaria for nearly 70 years [44]. Hence, it is expected that folate levels are inversely correlated with malarial outcomes.
CONCLUSIONS
The interaction between iron and PAM is daunting because of the iron requirements during pregnancy and the fact that iron contributes to P falciparum growth. In turn, this interaction is modified by malaria control interventions. Intermittent preventive treatment in pregnancy clears Plasmodium parasites and has a prophylactic effect on malarial episodes. Intermittent preventive treatment in pregnancy and iron and folate supplements are given only at precise moments of pregnancy, whereas the impact of malaria on pregnancy outcomes are different according to gestational age. For these reasons, it is important to show that iron and folate levels are associated with increased malarial risk in a prospective longitudinal cohort in the context of both supplements and IPTp.
We show for the first time that high ferritin and low folate levels are associated with increased malarial risk during pregnancy period with regard to malarial episodes and P falciparum parasite density in the context of IPTp and ITN use, even if positive smears diminish effectively after IPTp implementation. In addition, iron levels also have a significant association with important perinatal outcomes such as PM malaria and LBW. Our data also suggest there might be a threshold level above which iron has a deleterious impact on malarial risk. These results warrant additional epidemiological studies to evaluate the effect of different doses of iron and folate supplementation on maternal and infant health outcomes in malaria-endemic regions.
Acknowledgments
We thank Elizabeth Lim for reading and editing the manuscript and for making valuable linguistic corrections. We also thank the MiPPAD executive committee and Malaria in Pregnancy Consortium reviewers for valuable input in this work. Furthermore, we thank the women who participated in the study. Finally, we thank the midwives of the district of Allada and their assistants for help in conducting this study.
Financial support. This work was supported by the European and Developing Countries Clinical Trials Partnership (grant EDCTP-IP.07.31080.002; MiPPAD study “Malaria in Pregnancy Preventive Alternative Drugs,” http://clinicaltrials.gov/ct2/show/NCT00811421), and the Malaria in Pregnancy Consortium, which is funded through a grant from the Bill and Melinda Gates Foundation to the Liverpool School of Tropical Medicine). V. M.-A. was funded by the Réseau doctoral de l'Ecole des Hautes Etudes en Santé Publique and the Direction Générale de l'Armement grant.
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. World Malaria Report 2013. Available at: http://www.who.int/malaria/publications/world_malaria_report_2013/wmr2013_no_profiles.pdf?ua=1. Accessed 4 March 2015.
- 2.Maitra N, Joshi M, Hazra M. Maternal manifestations of malaria in pregnancy: a review. Indian J Matern Child Heal 1993; 4:98–101. [PubMed] [Google Scholar]
- 3.Desai M, Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007; 7:93–104. [DOI] [PubMed] [Google Scholar]
- 4.White NJ. Intermittent presumptive treatment for malaria. PLoS Med 2005; 2:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng G, Simpson JA, Chaluluka E, et al. Decreasing burden of malaria in pregnancy in Malawian women and its relationship to use of intermittent preventive therapy or bed nets. PLoS One 2010; 5:e12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisele TP, Larsen DA, Anglewicz PA, et al. Malaria prevention in pregnancy, birthweight, and neonatal mortality: a meta-analysis of 32 national cross-sectional datasets in Africa. Lancet Infect Dis 2012; 12:942–9. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Malaria Policy Advisory Committee to the WHO: conclusions and recommendations of September 2013 meeting. Malar J 2013; 12:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington WE, Mutabingwa TK, Kabyemela E, et al. Intermittent treatment to prevent pregnancy malaria does not confer benefit in an area of widespread drug resistance. Clin Infect Dis 2011; 53:224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maestre A, Carmona-fonseca J. Effect of submicroscopic or polyclonal Plasmodium falciparum infection on mother and gestation product: systematic review. Brazilian J Epidemiol 2010; 13:2008–9. [DOI] [PubMed] [Google Scholar]
- 10.Mohammed AH, Salih MM, Elhassan EM, et al. Submicroscopic Plasmodium falciparum malaria and low birth weight in an area of unstable malaria transmission in Central Sudan. Malar J 2013; 12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nosten F, ter Kuile F, Maelankirri L, et al. Malaria during pregnancy in an area of unstable endemicity. Trans R Soc Trop Med Hyg 1991; 85:424–9. [DOI] [PubMed] [Google Scholar]
- 12.Leenstra T, Phillips-Howard PA, Kariuki SK, et al. Permethrin-treated bed nets in the prevention of malaria and anemia in adolescent schoolgirls in western Kenya. Am J Trop Med Hyg 2003; 68:86–93. [PubMed] [Google Scholar]
- 13.Rogerson SJ, van den Broek NR, Chaluluka E, et al. Malaria and anemia in antenatal women in Blantyre, Malawi: a twelve-month survey. Am J Trop Med Hyg 2000; 62:335–40. [DOI] [PubMed] [Google Scholar]
- 14.Saute F, Menendez C, Mayor A, et al. Malaria in pregnancy in rural Mozambique: the role of parity, submicroscopic and multiple Plasmodium falciparum infections. Trop Med Int Health 2002; 7:19–28. [DOI] [PubMed] [Google Scholar]
- 15.Huynh BT, Fievet N, Gbaguidi G, et al. Influence of the timing of malaria infection during pregnancy on birth weight and on maternal anemia in Benin. Am J Trop Med Hyg 2011; 85:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peña-rosas JP, De-regil LM, Dowswell T, et al. Daily oral iron supplementation during pregnancy. Cochrane Collab 2012; 12:12–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabyemela ER, Fried M, Kurtis JD, et al. Decreased susceptibility to Plasmodium falciparuminfection in pregnant women with iron deficiency. J Infect Dis 2008; 198:163–6. [DOI] [PubMed] [Google Scholar]
- 18.González R, Mombo-Ngoma G, Ouédraogo S, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV-negative women: a multicentre randomized controlled trial. PLoS Med 2014; 11:e1001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouédraogo S, Koura GK, Accrombessi MM, et al. Maternal anemia at first antenatal visit: prevalence and risk factors in a malaria-endemic area in Benin. Am J Trop Med Hyg 2012; 87:418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouédraogo S, Koura GK, Bodeau-Livinec F, et al. Maternal anemia in pregnancy: assessing the effect of routine preventive measures in a malaria-endemic area. Am J Trop Med Hyg 2013; 88:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Planche T, Krishna S, Kombila M, et al. Comparison of methods for the rapid laboratory assessment of children with malaria. Am J Trop Med Hyg 2001; 65:599–602. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Iron Deficiency Anaemia: Assesment, Prevention, and Control. 2001. Available at: http://www.apps.who.int/iris/bitstream/10665/66914/1/WHO_NHD_01.3.pdf?ua=1. Accessed 4 March 2015. [Google Scholar]
- 23.Thurnham DI, Mccabe LD, Haldar S, et al. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr 2010; 92:546–55. [DOI] [PubMed] [Google Scholar]
- 24.Clark MA, Goheen MM, Cerami C. Influence of host iron status on Plasmodium falciparum infection. Front Pharmacol 2014; 5:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sangaré L, van Eijk AM, Ter Kuile FO, et al. The association between malaria and iron status or supplementation in pregnancy: a systematic review and meta-analysis. PLoS One 2014; 9:e87743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joint World Health Organization/Centers for Disease Control and Technical Consultation on the Assessment of Iron Status at the Population Level. Assessing the iron status of populations Geneva: 2004. Available at: http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9789241596107.pdf. Accessed 4 March 2015. [Google Scholar]
- 27.Burté F, Brown BJ, Orimadegun AE, et al. Circulatory hepcidin is associated with the anti-inflammatory response but not with iron or anemic status in childhood malaria. Blood 2013; 121:3016–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nacher M, McGready R, Stepniewska K, et al. Haematinic treatment of anaemia increases the risk of Plasmodium vivax malaria in pregnancy. Trans R Soc Trop Med Hyg 2003; 97:273–6. [DOI] [PubMed] [Google Scholar]
- 29.Le Hesran JY, Cot M, Personne P, et al. Maternal placental infection with Plasmodium falciparum and malaria morbidity during the first 2 years of life. Am J Trop Med Hyg 1997; 146:826–31. [DOI] [PubMed] [Google Scholar]
- 30.Schwarz NG, Adegnika AA, Breitling LP, et al. Placental malaria increases malaria risk in the first 30 months of life. Clin Infect Dis 2008; 47:1017–25. [DOI] [PubMed] [Google Scholar]
- 31.Tonga C, Kimbi HK, Anchang-Kimbi JK, et al. Malaria risk factors in women on intermittent preventive treatment at delivery and their effects on pregnancy outcome in Sanaga-Maritime, Cameroon. PLoS One 2013; 8:e65876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouédraogo S, Bodeau-Livinec F, Briand V, et al. Malaria and gravidity interact to modify maternal haemoglobin concentrations during pregnancy. Malar J 2012; 11:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Santen S, de Mast Q, Luty AJF, et al. Iron homeostasis in mother and child during placental malaria infection. Am J Trop Med Hyg 2011; 84:148–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senga EL, Koshy G, Brabin BJ. Zinc erythrocyte protoporphyrin as marker of malaria risk in pregnancy - a retrospective cross-sectional and longitudinal study. Malar J 2012; 11:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menendez C, Todd J, Alonso PL, et al. The response haemoglobin to iron supplementation of pregnant women with the genotype AA or AS. Trans R Soc Trop Med Hyg 1995; 89:289–92. [DOI] [PubMed] [Google Scholar]
- 36.Van Eijk AM, Ayisi JG, Slutsker L, et al. Effect of haematinic supplementation and malaria prevention on maternal anaemia and malaria in western Kenya. Trop Med Int Health 2007; 12:342–52. [DOI] [PubMed] [Google Scholar]
- 37.Spottiswoode N, Duffy PE, Drakesmith H. Iron, anemia and hepcidin in malaria. Front Pharmacol 2014; 5:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss G, Werner-Felmayer G, Werner ER, et al. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J Exp Med 1994; 180:969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner GD, Ly VC, Nguyen TH, et al. Systemic endothelial activation occurs in both mild and severe malaria. Correlating dermal microvascular endothelial cell phenotype and soluble cell adhesion molecules with disease severity. Am J Pathol 1998; 152:1477–87. [PMC free article] [PubMed] [Google Scholar]
- 40.Kartikasari AE, Georgiou NA, Visseren FL, et al. Endothelial activation and induction of monocyte adhesion by nontransferrin-bound iron present in human sera. FASEB J 2006; 20:353–5. [DOI] [PubMed] [Google Scholar]
- 41.Hurrell R. Iron and malaria: absorption, efficacy and safety. Int J Vitam Nutr Res 2010; 80:279–92. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Lopez R, Haldar K. A transferrin-independent iron uptake activity in Plasmodium falciparum-infected and uninfected erythrocytes. Mol Biochem Parasitol 1992; 55:9–20. [DOI] [PubMed] [Google Scholar]
- 43.Penha-Gonçalves C, Gozzelino R, de Moraes LV. Iron overload in Plasmodium berghei-infected placenta as a pathogenesis mechanism of fetal death. Front Pharmacol 2014; 5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salcedo-Sora JE, Ward SA. The folate metabolic network of Falciparum malaria. Mol Biochem Parasitol 2013; 188:51–62. [DOI] [PubMed] [Google Scholar]