Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) blood stream infection (BSI) is a major healthcare burden in some but not all healthcare settings, and it is associated with 10%–20% mortality. The introduction of mandatory reporting in England of MRSA BSI in 2001 was followed in 2004 by the setting of target reductions for all National Health Service hospitals. The original national target of a 50% reduction in MRSA BSI was considered by many experts to be unattainable, and yet this goal has been far exceeded (∼80% reduction with rates still declining). The transformation from endemic to sporadic MRSA BSI involved the implementation of serial national infection prevention directives, and the deployment of expert improvement teams in organizations failed to meet their improvement trajectory targets. We describe and appraise the components of the major public health infection prevention campaign that yielded major reductions in MRSA infection. There are important lessons and opportunities for other healthcare systems where MRSA infection remains endemic.
Keywords: healthcare-associated infections
Why Did Methicillin-Resistant Staphylococcus aureus Become Such a Problem?
Staphylococcus aureus has been recognized for over a century as a major cause of infection, including healthcare-associated infections (HCAIs). In the early 1940s, almost all S aureus strains were susceptible to penicillin, but by 1960, 95% of hospital strains were resistant due to selection of penicillinase (β-lactamase)-producing strains. Meticillin (formerly methicillin), and later oxacillin, cloxacillin, and flucloxacillin, were developed to resist staphylococcal β-lactamase and restore treatment options. However, within 1 year of its introduction in 1960, the first methicillin-resistant S aureus (MRSA) strain was described [1, 2].
Methicillin-resistant S aureus infections were rare in the 1960s, but within a decade they were causing clinical concern, particularly in Australia, where multiple antibiotic-resistant strains were noted, particularly associated with infections in neonates [3, 4]. In the 1980s, some hospitals in England had major outbreaks due to strains that were designated “epidemic MRSA” (EMRSA), and they required a “search and destroy” approach for their control; ie, isolate and treat the patient, screen patient and staff contacts for colonization, and give decolonization treatment to anyone found to be positive [5, 6]. Two particular strains with enhanced epidemic potential (EMRSA-15, EMRSA-16) subsequently emerged in the United Kingdom (UK) in the early 1990s, causing excess morbidity and mortality [7–10]. The extent of the challenge posed by these 2 epidemic strains threatened to overwhelm the system so the search-and-destroy approach was abandoned in many UK institutions.
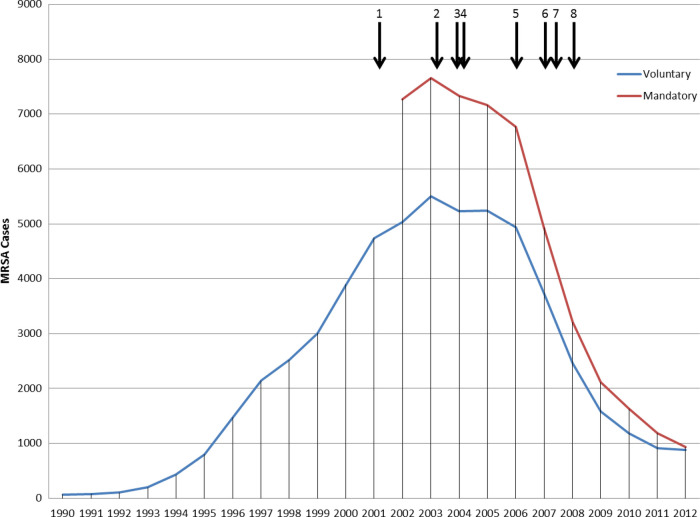
The MRSA epidemic was monitored by surveillance of S aureus blood culture-positive cases [11]. Because these represent the most severe infections and are straightforward to define, blood should be sterile, and so a positive blood culture likely indicates blood stream infection (BSI). The surveillance, which involved the reporting of cases by microbiology laboratories to a national database, was undertaken initially by the Public Health Laboratory Service and then, from 2003 to 2013, by its successor, the Health Protection Agency (HPA). Until 2001, this surveillance was voluntary, but, aware of rising numbers and public concern, the Government introduced mandatory reporting of MRSA BSIs for all National Health Service (NHS) hospitals in England in 2001 [12]. The total numbers of reported MRSA BSIs (Figure 1) peaked at 7700 in 2003–2004, and the numbers declined markedly from 2006 [13, 14]. Methicillin-resistant S aureus infection had also become the subject of considerable public and media attention: headlines referred to “MRSA massacres”, “plagues”, “superbugs”, and “squalid hospitals”. Thus, control of MRSA became a political priority.
Figure 1.
Numbers of methicillin-resistant Staphylococcus aureus (MRSA) blood stream infections (BSIs) reported in England, 1990–2012. Vertical arrows indicate notable interventions aimed at reducing MRSA BSIs: 1, introduction of mandatory reporting of MRSA BSIs; 2, launch of national report targeting 7 key areas for improvement (active surveillance and investigation, infection risks associated with medical devices, reservoirs of infection, standards of hygiene in clinical practice, prudent use of antimicrobials, management and organization, and research and development; 3, launch of the cleanyourhands campaign to improve the standard of hand hygiene, which required alcohol hand gel to be available, as a minimum, at all points of patient contact; 4, launch of MRSA/Cleaner Hospitals Improvement Programme and, in November 2004, announcement of mandatory target to halve the number of MRSA BSIs in hospitals in England by 2008; 5, legislation was introduced in 2006, which implemented a statutory Code of Practice on healthcare-associated infection (HCAI) that applied to all National Health Service (NHS) healthcare providers; 6, in late 2007 a series of additional measures was added, including a requirement for quarterly reporting on HCAIs to hospital Boards, an extension of the cleanyourhands campaign, and a legal requirement for hospital Chief Executives to report MRSA BSIs (and Clostridium difficile infections) centrally more frequently (within 2 weeks of each following month); 7, start of significant reductions in cephalosporin and fluoroquinolone prescribing in hospitals; 8, MRSA screening implementation guidance was issued during 2008, stating that screening of elective and emergency admissions should be occurring by March 2009 and December 2010, respectively.
Why did this happen? As MRSA BSIs increased in the 1990s, many assumed that these were replacing infections caused by methicillin-susceptible S aureus (MSSA) strains; in fact, they were additional to MSSA BSIs (which were also increasing) [15]. During the last quarter of the 20th century, infections (including HCAIs) were not regarded as priority areas. Antibiotics and vaccines were thought to provide the answer to infection, and clinical hygiene, aseptic practice, and other prevention and control measures received scant attention during clinical or management training. Crucially, there was a failure to appreciate that although medical progress was increasing life expectancy, many medical interventions (eg, surgery, catheter use, immunomodulation) increased the risk of nosocomial infection. Notably, there was a lack of robust data on the proportion of preventable HCAIs.
How Has Methicillin-Resistant Staphylococcus aureus Infection Been Controlled in England?
There has been no single “silver bullet” to address the challenge of MRSA infection. Instead, multiple infection prevention and control (IPC) initiatives, targeting different aspects and levels of healthcare, took place (Table 1) [16–57]. Senior managers and clinicians were required to ensure that interventions were a major priority across organizations, with a zero tolerance approach to MRSA and other HCAIs. Thus, a culture change was needed to place IPC at the heart of healthcare practice, including a shift from reactive to proactive surveillance and practice audit. However, a zero tolerance approach does not mean that there will be no infections. Instead, the aim was to minimize infection risks, with implementation of improved clinical practice protocols, particularly for hand hygiene (hand washing and use of alcohol hand rubs) [31], environmental cleaning, and disinfection [27, 32], including a series of high-impact interventions (HIIs) in the form of care bundles, particularly for invasive procedures with a high risk of infection [36, 39].
Table 1.
Chronology of Key Events in MRSA BSI Control Program in England
| Date | Initiative | Comment |
|---|---|---|
| March 1998 | House of Lords Select Committee on Science and Technology report “Resistance to antibiotics and other antimicrobial agents” [16]. | Recommended that the NHS should set itself targets for controlling MRSA in hospitals and publish its achievements; also that infection control and hygiene should be at the heart of good hospital management and practice, and that resources should be redirected accordingly. |
| February 2000 | National Audit Office (NAO) 1st report: The management and control of HAI in acute NHS Trusts in England [17]. | Surveyed infection control teams (ICTs) believed that 15% reduction in HAI was achievable. NAO observed that there “may be a growing mismatch between what is expected of ICTs in controlling infection and the staffing and other resources allocated to them”. |
| June 2000 | UK antimicrobial resistance strategy and action plan [18]. | Areas for action included surveillance, prudent antimicrobial use, and infection control. It was stated that the Department of Health would lead the development of performance standards and targets for HAI (including MRSA) for England and Wales. |
| November 2000 | Committee of Public Accounts 42nd report of session 1999–2000. The management and control of hospital acquired infection in acute NHS Trusts in England [19]. | Two main conclusions: (1) the NHS did not have a grip on the extant of HAI; and (2) a root and branch shift towards prevention was needed at all levels and that a philosophy that prevention is everybody's business not just the specialists. |
| January 2001 | National Evidence-Based Guidelines for Preventing Healthcare-Associated Infections in NHS Hospitals in England (EPIC 1) published [20]. | First systematic review of the evidence to support interventions to reduce HCAI. |
| April 2001 | National Standards of Cleanliness – NHS Estates [21]. | Outcome-based standards to improve cleanliness. It was recognized that cleaning staff had an important role in quality improvement. |
| April 2001 | Introduction of mandatory surveillance for MRSA BSIs. Department of Health. Surveillance of healthcare associated infections, CMO's Update 30 [22]. | Minimum dataset to be collected by acute hospitals in England: (1) the total number of blood culture sets taken; (2) the total number of positive blood cultures; (3) the total number of blood cultures positive for Staphylococcus aureus; (4) MRSA-positive blood cultures as a proportion of all S aureus-positive blood cultures. Each data set to be expressed as a proportion of all hospital activity. Data submitted on a quarterly basis to be published from April 2002. |
| January 2002 | Getting ahead of the curve: a strategy for infectious diseases [23]. | Recommended the establishment of the Health Protection Agency (HPA). Infectious disease control needed to be transformed from a “Cinderella service” by bringing into the mainstream of service development. Used the term “HCAI” instead of “HAI”. |
| June 2003 | Surveillance of healthcare associated infections [24]. | From July 2003, mandatory MRSA bacteremia data to be the basis of a performance management indicator. Data from the 2 most recent years were to provide each Trust with an “improvement score” as part of a balanced scorecard, which contributed to star ratings of each acute hospital Trust. |
| June 2003 | Hospital Pharmacy initiative for promoting prudent use of antibiotics in hospitals [25]. | ∼£12 million was allocated for 3 years from 2003 to 2004 to pump prime clinical pharmacy initiatives around antimicrobial prescribing in acute NHS hospital trust. Funding often used to employ “antimicrobial pharmacists”. |
| December 2003 | Winning Ways: working together to reduce healthcare associated infection in England [26]. | Set a clear direction for the NHS on actions to reduce the relatively high levels of particular HCAIs. Trusts were required to designate a Director for Infection Prevention and Control. Seven key action areas: active surveillance and investigation; reducing the infection risks associated with medical devices; reducing reservoirs of infection; high standards of hygiene in clinical practice; prudent use of antimicrobials; management and organization; research and development. |
| July 2004 | Towards cleaner hospitals and lower rates of infection [27]. | Hospital cleanliness remained a major patient concern, and MRSA was a growing problem. The 2 issues were considered to be linked (from a public perspective). |
| August 2004 | Rapid Review Panel established. Recommendation from Winning Ways [28]. | The panel provided a prompt assessment of new and novel equipment, materials, products, or protocols that may be of value to the NHS in improving hospital infection control and reducing HCAI. |
| July 2004 | National Standards Local Action - Health and Social Care Standards and Planning Framework 2005/2006–2007/2008 [29]. | Healthcare organizations were charged to keep patients, staff, and visitors safe by having systems to ensure that the risk of HCAI is reduced, with particular emphasis on high standards of hygiene and cleanliness, and achieving year-on-year reductions in MRSA (a target for MRSA reduction to be set for each NHS hospital Trust). |
| July 2004 | NAO 2nd report: Improving patient care by reducing the risk of HAI [30]. | Noted that: the number of MRSA bacteremias had increased since mandatory surveillance had been introduced, but the data did not enable clinicians to identify and reduce risks within their specialty; welcomed the publication of Winning Ways; 80% of Trust Chief Executives reported that they had made changes to their infection control arrangements since 2000, one key driver being controls assurance. |
| September 2004 | NPSA patient safety alert about hand hygiene [31]. | Instructed NHS trusts to install alcohol-based hand rub at the point of care. Signaled the piloting of the cleanyourhands campaign. |
| October 2004 | A Matron's Charter: an action plan for cleaner hospitals [32]. | Emphasized the role of hospital cleanliness as an integral part of infection control. All staff working in healthcare to receive education in infection control. |
| November 2004 | Introduction of mandatory target to reduce MRSA bloodstream infections in hospitals [33]. | The Secretary of State for Health announces a target to halve the number of MRSA BSIs in hospitals by March 2008, using 2003–2004 as baseline. (Actually, this was a 60% reduction target for most trusts [20% per year for 3 consecutive years] because those with few or none could contribute little to national [50% reduction] target.) |
| January 2005 | MRSA/HCAI/Cleanliness Improvement Programme [34]. | Originally focused on the 20 most challenged Trusts with respect to MRSA BSI rates. Became the HCAI and Cleanliness Improvement Programme, aiming to (1) support hospital trusts and NHS organizations to reduce MRSA BSIs and (2) improve public and patient confidence in the NHS. |
| April 2005 | Office for National Statistics (ONS) Report: Deaths involving MRSA: England and Wales, 1999–2003 [35]. | The first ONS report on deaths relating to MRSA. MRSA accounted for 66% of death certificates that mentioned S aureus in 2002 compared with only 12% in 1993. |
| June 2005 | Saving Lives – a delivering program to reduce healthcare associated infection, including MRSA [36]. | Focused on practice measures to reduce MRSA BSIs: high-impact interventions were produced to improve the reliability of clinical practice in a number of areas, including antimicrobial prescribing and the insertion and maintenance of invasive devices, to minimize the risk of HCAI. |
| June 2005 | Introduction of monthly MRSA BSI reporting [37]. | Reporting required by 15th of each following month. Publication of data summaries increased in frequency from annual to twice a year. |
| October 2005 | Introduction of Mandatory MRSA bacteremia enhanced surveillance scheme [38]. | MRSA surveillance scheme enhanced to capture comprehensive data via a web-enabled data capture system on individual cases of MRSA bacteremia, including: patient demographics; date of admission; date of bacteremia; location at time of blood culture; consultant specialty; type of clinical care at the time the blood sample was taken. |
| May 2006 | Going further faster: implementing the Saving Lives delivery program [39]. | Guidance to support the delivery of the MRSA target and the Saving Lives program. The focus was on actions that could impact on MRSA, but these would also support system-wide improvement in HCAI. |
| 1 June 2006 | Introduction of root cause analysis toolkit (NPSA) [40]. | An action tool for clinicians and risk managers to use when a patient had a life-threatening infection, such as MRSA bacteremia. This helped clinical teams to identify (1) what factors or events led to the infection and (2) how to reduce the risks of it happening again. |
| October 2006 | The Health Act 2006: Code of Practice for the prevention and control of healthcare associated infections [41]. | Legal requirement for managers of NHS organizations to deliver low HCAI risk for patients. Failure to observe the Code could result in either an Improvement Notice issued by the Healthcare Commission or the organization being reported for significant failings and placed on special measures. |
| November 2006 | Summary of best practice for MRSA screening [42]. | Trusts recommended to consider models for screening and decolonization of high-risk patients (A&E and ICU admissions, and some groups preoperative surgical patients, eg, elective orthopedic, cardiothoracic, neurosurgical). |
| February 2007 | EPIC 2 published [43]. | Updated evidence-based guidance on standard precautions and the insertion and optimal management of CVCs and UCs. |
| June 2007 | Clean, safe care: reducing infections and saving lives [44]. | A progress report on what had been achieved and what still needed to be done. The NHS was on track to achieve the MRSA target. The Healthcare Commission begins its program of unannounced inspections of NHS trusts against the Code of Practice. |
| July 2007 | £50 million additional funding for reducing healthcare associated infections [45]. | Additional one-off funding to be spent promptly to achieve rapid results on reducing HCAIs. Front-line staff were expected to be involved in the decision making and be kept informed of where the additional money was been spent. Each Strategic Health Authority received £5 m to allocate to NHS organizations. |
| September 2007 | Uniforms and work wear: An evidence base for developing local policy [46]. | Widely known as the “bare below the elbows” guidance, this was widely adopted throughout the NHS. It supported the requirements of the Health and Social Care Act 2008 Code of Practice relating to the need for uniform and workwear policies to support effective hand hygiene. |
| November 2007 | Deep Clean campaign announced [47]. | The government announced £57.5 m for deep cleaning throughout Trusts between December 2007 and March 2008. Later NAO report estimated that £62.6 m was spent on Deep Clean campaign. Trust Directors of Nursing were asked to agree jointly with Directors of Estates and Facilities what was needed and how it would be evaluated. |
| December 2007 | Operating framework for NHS, 2008/2009 [48]. | Recognized that meeting the HCAI challenge required additional actions across the NHS for 2008/2009, including: introducing MRSA screening for all elective admissions from 2008/2009, and for all emergency admissions as soon as practicable within the next 3 years; and implementing the forthcoming HCAI and Cleanliness Strategy. The tariff uplift for 2008/2009 recognizes the importance of tackling HCAI and improving cleanliness, and the NHS contract sets out sanctions for failing to achieve agreed improvements. |
| July 2008 | MRSA screening operational guidance [49]. | Supported NHS trusts in introducing MRSA screening for all elective patients by March 2009. |
| September 2008 | Clean Hands Save Lives. NPSA updated and reissued their Patient Safety Alert [50]. | The alert recognized the reduction in MRSA bacteremias; however, it stated to maintain this and other improvements, it was vital that hand hygiene remains high on the patient safety agenda. |
| December 2008 | Operating framework for NHS, 2009/2010 [51]. | Noted that the NHS had achieved the ambitious 50% reduction of MRSA nationally, although not in every organization: this objective should remain their immediate goal. From April 2009, all elective admissions had to be screened for MRSA, and this was to be extended to emergency admissions as soon as possible and no later than 2011. |
| January 2009 | NHS Constitution published [52]. | Recognized that the NHS had met the national target to halve the number of MRSA bloodstream infections by 2008/2009 and that it was expected to continue to reduce HCAIs. Setting a national minimum standard for these infections would be considered. |
| December 2009 | Operating framework for NHS, 2010/2011 [53]. | After a recommendation from the National Quality Board, from April 2010, NHS organizations were to set an objective for reducing MRSA infections, relative to the median, with the best performers setting their objectives locally. The objective reflected a zero-tolerance approach to preventable infections and aimed to reduce variation in performance on MRSA bloodstream infections. The MRSA objectives for 2010–2011 were calculated against yearly baseline data from October 2008– September 2009. |
| March 2010 | MRSA screening operational guidance 3 [54]. | Supported NHS organizations in introducing MRSA screening for all relevant emergency admissions by end of 2010. |
| December 2010 | Operating framework for NHS, 2011/2012 [55] | MRSA objectives for 2011–2012 were set using the same methodology as the previous year. Clostridium difficile objectives for 2011–2012 also introduced (again used a methodology to be used for 3 years). |
| 2011 | National One Week Audit of MRSA screening in NHS acute trusts [56]. | Found that screening all admissions according to current guidance is not clinically or cost effective; recommended that all patients admitted to high-risk specialties and all critical care units, whether elective or emergency admissions, should be screened for MRSA. Patients identified as carrying MRSA should be isolated and given decolonization/suppressive therapy. |
| November 2011 | Start Smart Then Focus launched [57]. | Guidance to NHS acute Trusts on antimicrobial stewardship. |
Abbreviations: A&E, accident and emergency; BSI, blood stream infections; CMO, Chief Medical Officer; CVC, central venous catheter; HAI, hospital-acquired infection; HCAI, healthcare-associated infection; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; NHS, National Health Service; NPSA, National Patient Safety Agency; UC, urinary catheter; UK, United Kingdom.
The response to the public health threat posed by MRSA infection was underpinned by an improvement program and a target to halve MRSA BSIs by 2008 (baseline 2003–2004) [33, 34]. Legislation was introduced in 2006, which implemented a statutory Code of Practice on HCAI that applied to all NHS and independent (2008) healthcare providers [41, 58].
The Early Years (2000–2005): The New Millennium Challenge
The (voluntary) Nosocomial Infection National Surveillance Scheme (NINSS) for England and Wales, established in 1996, covered several infections including BSIs and postoperative infections; NINSS extended a long-standing system for reporting all clinically significant bacteremias [11, 59]. In April 2001, mandatory surveillance of MRSA BSIs was introduced in England [12, 22]. All MRSA-positive blood cultures had to be reported, excepting duplicates from an individual within 2 weeks. Methicillin-resistant S aureus BSIs were apportioned to community or hospital acquisition according to whether the blood culture was collected within the first 48 hours of admission or later, respectively. Methicillin-resistant S aureus BSIs occurring in each hospital were initially reported annually, but no reduction target was set.
A report by the Chief Medical Officer (CMO) for England led to the creation of the HPA in 2003, and it identified priority areas in infectious diseases, including HCAIs and antimicrobial resistance [23]. The HPA had responsibility for surveillance, including the mandatory surveillance of MRSA BSIs in England. A second CMO report, specifically on HCAI [26], established the need for improved surveillance and more stringent IPC measures, with an emphasis on hand hygiene using alcohol hand gel and aseptic practice. National Health Service hospitals were each required to appoint a Director of IPC: that is, a senior clinician (medical or nursing) who reports directly to the Chief Executive and Board. In 2004, the National Patient Safety Agency (NPSA) launched the “cleanyourhands” campaign to improve the poor standard of hand hygiene that had been identified in NHS hospitals [31]. This campaign was linked to the international World Health Organization hand hygiene program [60]. The NPSA program included publicity and educational materials for staff, patients and relatives, and crucially required alcohol hand gel to be available, as a minimum, at all points of patient contact.
The Department of Health's Active Program and a Government Target
A National Audit Office (NAO) report in 2004 was very critical of progress (since 2000) in reducing HCAIs, particularly MRSA BSIs [19, 30]. There was increasing public and media concern about MRSA, heightened by increasing numbers of associated deaths; MRSA accounted for 12% and 66% of all death certificates that mentioned S aureus in 1993 and 2002, respectively [35]. In 2004, an action plan focused on both hospital cleanliness and infection control [27], and it led to a MRSA/Cleaner Hospitals Improvement Program (CHIP) [34]. Notably, in November 2004, the Secretary of State for Health announced a target to halve the number of MRSA BSIs in hospitals in England by March 2008 [33]. This target placed MRSA infection reduction on the same basis as other NHS targets, including those for waiting times in Accident or Emergency departments and for receiving elective hospital care. This was the first time that this approach had been applied to a clinical rather than financial or organizational outcome. Each hospital was given an expected trajectory to reduce MRSA BSIs, which mandated a 20% reduction in each of 3 consecutive 12-month periods (2005–2008).
The MRSA/CHIP had 2 main arms. First, “improvement teams” (ITs), comprising experienced nurses, doctors, and managers with a good track record in IPC, visited NHS hospitals to review practices and advise on improvements and implementation. Improvement teams did not have enforcement powers, but they were highly influential, particularly because they initially focused on hospitals (n = 20 of 170) that did not achieve their MRSA BSI reduction trajectories. Second, a series of guidance documents and clinical HII care bundles were produced to guide clinical practice in IPC (particularly for MRSA) and to support education and training of clinical staff [36]. The HII care bundles clearly established the 5–6 essential elements to minimize infection risks. All elements were to be followed consistently, and the bundles incorporated simple audit tools. For MRSA infection, the bundles focused on central and peripheral venous catheters, renal dialysis catheters, surgical sites (wounds), ventilated patients, and urinary catheters [44]. Additional bundles were added for Clostridium difficile infection (CDI), optimal blood culture sampling, MRSA screening, and prudent antimicrobial prescribing [44].
In late 2007, a series of additional measures was added, including a requirement for quarterly reporting on HCAIs by matrons and clinical directors to hospital Boards, an extension of the cleanyourhands campaign, and a legal requirement on hospital Chief Executives to report MRSA BSIs (and CDIs) to the HPA more frequently (within 2 weeks of each following month) [47]. The Care Quality Commission was given new powers, including annual IPC inspections of all hospitals. Compliance by NHS staff with HCAI measures was given a new focus by the introduction of a dress code [48]. Commonly known as “bare below the elbows”, clinical staff were required to wear short sleeves and not to wear wrist watches, bracelets, or rings (other than plain wedding rings); doctors were not to wear ties that could come into contact with patients and were to dispense with the “white coat” uniform. The dress code aimed to facilitate good hand hygiene practice, and staff were encouraged to challenge noncompliant colleagues.
Screening for Methicillin-Resistant Staphylococcus aureus Carriage and Colonization
Screening of patients for MRSA had been routine practice in some hospitals in England (in high-risk surgical specialties) for many years, but it had not been applied to all admissions. In November 2006, MRSA screening guidance was issued [42], linked to specialist society recommendations [61], and advised wider screening of patients admitted to high-risk specialties. However, implementation of this risk-based approach was patchy. One year later, a wider approach to screening was announced, effectively meaning that most NHS admissions should be screened; implementation guidance was issued during 2008, stating that screening of elective and emergency admissions should be occurring by March 2009 and December 2010, respectively [46]. However, as discussed below, implementation of MRSA screening was slow to be implemented and inconsistently applied.
Where Are We Now?
During the first half of the 3-year MRSA target program in England (2005–2008), numbers of MRSA BSIs began to decrease, albeit slowly and not on trajectory to meet the 50% reduction target [14]. However, as the focus on IPC measures increased, the rate of reduction increased markedly. Thus, by 2008–2009 there had been a 62% decrease in MRSA BSIs from 7700 in 2003–2004 (baseline) to 2932 in 2008–2009. The reduction continued to 1898 in 2009–2010 (75% reduction), 1481 in 2010–2011 (81% reduction), and 1114 (86% reduction) in 2011–2012 [14]. The MRSA BSI rates (per 100 000 population have declined from 8.7 to 5.7 to 3.6 to 2.8 to 2.1 and to 1.7 in 2007–2008, 2008–2009, 2009–2010, 2010–2011, 2011–2012, and 2012–2013, respectively. There has also been a change in pattern of the underlying causes of MRSA BSIs, and a shift in their distribution [12, 14]. Since 2010, more cases were associated with nonhospital care than in-patient care. There has been an emphasis on preventing cases associated with intravascular lines and other implanted devices. These accounted for more than half of hospital-associated cases, with another 20% related to skin and soft tissue infections (SSTIs). In the wider community, <30% are associated with implanted or indwelling devices, with a similar proportion associated with SSTIs. Notably, the reduction in BSIs has been accompanied by a decrease in deaths in England in which MRSA appeared on the death certificate from 1556 (480 as underlying cause) in 2006 to 485 (82) in 2010 [62].
DISCUSSION
There is an approximate north-south gradation in Europe of increasing MRSA infection rates [63, 64]. In 2011, MRSA prevalence (as a proportion of all invasive S aureus infections, which is a proxy indicator of the incidence of MRSA infection) was <25%, 25%–50%, or >50% in 16, 6, and 2 countries, respectively [63]. Between 2008 and 2011, MRSA prevalence decreased significantly in 6 countries (UK, Denmark, Belgium, France, Spain, and Ireland), and it increased significantly in Luxembourg, Hungary, Poland, and Romania [63]. In the remaining 17 European countries, no significant changes in MRSA prevalence were recorded [63]. There are few systematically collected surveillance data on MRSA infection across the United States; a recent estimate found no statistically significant increase in the hospitalization rate due to MRSA between 2005 and 2009, although the total number of infections increased [65]. In Asia, MRSA has remained endemic in many hospitals over the past decade with the majority of S aureus infections caused by MRSA [66]. Notably, there has been a large, significant reduction in incidence of hospital-onset MRSA and MSSA BSIs in Australia since 2002, which coincided with the introduction of a range of IPC activities [67].
The contrasting epidemiology of MRSA infection across countries likely reflects differences in the dynamics of epidemic strains [68–71], qualitative and quantitative variation in antimicrobial prescribing [72–74], and the reach of healthcare-improvement projects [65, 66, 75]. The prevalence of MRSA clones may follow a wave trajectory (initial expansion, relative stasis, then decline) [71]. If such clones dominate in a particular area or country, then, depending on their natural cycle of expansion or contraction, the incidence of MRSA infection may alter accordingly. Longer observations of consistent changes in MRSA incidence make such a phenomenon less likely to be the sole explanation for marked changes in infection rates. A notable example of the extent of differences in MRSA BSI incidence is seen in Northern Germany versus the Netherlands, which have similar sized populations and share a national border [76]. There was a 32-fold higher incidence of MRSA bacteremia in the German Northern Rhine-Westphalia region, possibly due to differences in healthcare structures and MRSA control strategies. Dutch MRSA control strategy has historically been interventionist and based on rigorous search-and-destroy methods [75].
In the first decade of the new millennium, there was public health requirement to address MRSA infection that became both a public and political imperative in England. The control of MRSA BSI in England has no identifiable key intervention, such as the claimed role of the Broad Street pump handle over 150 years ago [77]. However, historians now argue that controlling the cholera outbreak was more complicated than the simple disablement of a water pump [77]. Controlling and indeed preventing infection is a multimodal process. After a 57% reduction in MRSA BSIs in England had been achieved, the NAO noted that there had been “a cultural change in the way that organisations tackle IPC and the priority that it is afforded” [78]. Such statements are consistent with a very marked decrease in incidence of CDI in England that occurred over a similar timescale. Healthcare-associated infection control became a remit for whole organizations rather than the prerogative of IPC teams alone. This shift saw an increased focus on “old-fashioned” practices of hand hygiene, asepsis and cleanliness, and practice changed from being reactive to proactive; hence, from infection control to IPC. Use of root cause analysis became commonplace in the NHS, and this enhanced not only a sense of shared ownership of HCAI, but it facilitated learning and a culture where suboptimal practice would be challenged [79]. Even if a root cause of a MRSA BSI could not always be identified, the process encouraged team ownership of IPC, and it placed an emphasis on optimizing patient journeys through the healthcare system.
There was some skepticism whether national targets were either desirable or achievable [80]. Nevertheless, infection targets and reduction trajectories for all NHS hospital were instrumental in changing organizational approaches to HCAI control [33]. The use of clinical endpoints in organizational performance management was new to the NHS. The MRSA/CHIP identified that the first and major priorities were to address clinical protocols, hand hygiene, and aseptic practice aspects of MRSA IPC.
Increased purchasing of alcohol hand products was independently associated with reduced MRSA BSIs in England and Wales, but this was established only in 2007–2008 (as opposed to the previous 36 months) of the cleanyourhands campaign [81]. This lag is in line with increased staff compliance with bundles of interventions to reduce HCAI risk. A recent review confirmed the association between increased consumption of alcoholic hand rub and reduced MRSA rates [82]. Notably, the introduction of legislation in 2006 was strongly associated with reduced MRSA BSIs (and CDI) [41, 81]. Review visits by ITs were also significantly associated with reduced MRSA BSIs (and CDI) for at least 6 months after each visit [81].
These examples illustrate how it is possible to draw some conclusions about which interventions were likely to have been more important in the control of MRSA. However, given the (1) multimodal and often bundled interventions that were used and (2) limited data to quantify the extent of their implementation, there are clear limitations to drawing definitive conclusions regarding their effectiveness. Nevertheless, it should be noted that there is no evidence that the reduction in recorded MRSA BSIs was due to fewer blood cultures being taken. Indeed, in 2009, a report concluded that significant reductions in MRSA BSIs (and CDIs) had occurred, but the rates of other healthcare-associated BSIs remained unaltered (Supplementary Table).
The role of screening and decolonization in the control of MRSA infection, and BSI specifically, is less clear [83]. Colonization of the skin, nose, or throat with MRSA (and other S aureus strains) often precedes invasive infection, and a colonized patient can be at risk of developing an endogenous infection or of being a source for transmission [84]. Therefore, screening patients on admission, coupled with decolonization (or suppression) treatment of MRSA carriers, may have a part to play in preventing MRSA infections. As of November 2008, only 18% and 34% of hospitals were screening all admissions and elective patients, respectively [78]. Furthermore, a point prevalence study in 2011 (encompassing 86% of hospitals) found that only 61% of acute admissions were being screened, despite this being national policy; and yet these were more than twice as likely as elective admissions (2.1% vs 0.9%) to be MRSA positive [56]. Thus, MRSA screening was unlikely to have been a primary cause of the major reductions in MRSA BSIs in England seen between 2006 and 2009.
There is evidence that exposure to antibiotics such as cephalosporins or fluoroquinolones can increase the risk of MRSA infection [72–74]. Progress in improving information and tracking of hospital antibiotic prescribing has been limited in England, largely because of delays in developing electronic prescribing. Nevertheless, marked shifts in antibiotic prescribing did occur post-2007, probably largely in response to guidance to reduce the risk of CDI [85, 86]. There were significant successive decreases between 2007 and 2010 in the proportions of patients with CDI in England who had received either cephalosporins or fluoroquinolones [85]. Hence, at a time of increased compliance with IPC measures to reduce MRSA transmission, the selective pressure for MRSA due to antimicrobial prescribing was also changing. As mentioned earlier, the multimodal nature of the interventions precludes us drawing conclusions about the relative importance of individual components. In reality, this limitation is shared by most infection prevention campaigns [67].
CONCLUSIONS
In conclusion, we emphasize that multiple major changes in practice occurred in hospitals in England during the first decade of the present millennium, in response to an extensive IPC program. It is now commonplace for hospitals to diagnose no MRSA BSIs for months at a time. However, the success story of the control of MRSA BSI (and CDI) is tempered by emergent HCAI threats, notably caused by Gram-negative bacilli, including multiple antibiotic-resistant strains. For example, numbers of Escherichia coli BSIs in England are increasing [87], and they are now approximately 4 times more common than was MRSA BSI at its peak incidence [14]. Thus, there remain considerable challenges in the prevention of infection that will require targeted measures and continued comprehensive surveillance.
Supplementary Material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
Author contributions. All the authors contributed equally to the writing of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Phillips I. MRSA: a historical perspective. In: Gould I, ed. MRSA in Practice. London: Royal Society of Medicine Press Ltd, 2007: 1–12. [Google Scholar]
- 2.Jevons MP. Celbelinin-resistant staphylococci. BMJ 1961; i:564–7. [Google Scholar]
- 3.Pavillard R, Harvey K, Douglas D, et al. Epidemic of hospitalised infection due to methicillin-resistant Staphylococcus aureus in major Victorian hospitals. Med J Aust 1982; I:451–4. [PubMed] [Google Scholar]
- 4.Gilbert GL, Asche V, Hewstone AS, et al. Methicillin-resistant Staphylococcus aureus in neonatal nurseries. Med J Aust 1982; I:455–9. [PubMed] [Google Scholar]
- 5.Spicer WJ. Three strategies in the control of staphylococci including methicillin-resistant Staphylococcus aureus. J Hosp Infect 1984; 5(Suppl A):45–9. [DOI] [PubMed] [Google Scholar]
- 6.Kerr S, Kerr GE, Mackintosh CA, et al. A survey of methicillin-resistant Staphylococcus aureus affecting patients in England and Wales. J Hosp Infect 1990; 16:35–48. [DOI] [PubMed] [Google Scholar]
- 7.Richardson JF, Reith S. Characterization of a strain of methicillin-resistant Staphylococcus aureus (EMRSA-15) by conventional and molecular methods. J Hosp Infect 1993; 25:45–52. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. EMRSA-16: a new epidemic strain of Staphylococcus aureus. Commun Dis Rep CDR Wkly 1993; 3:25. [PubMed] [Google Scholar]
- 9.Cox RA, Conquest C, Mallaghan C, et al. A major outbreak of methicillin-resistant Staphylococcus aureus caused by a new phage-type (EMRSA-16). J Hosp Infect 1995; 29:87–106. [DOI] [PubMed] [Google Scholar]
- 10.Johnson AP, Aucken HM, Cavendish S, et al. Dominance of EMRSA-15 and -16 among MRSA causing nosocomial bacteraemia in the UK: analysis of isolates from the European Antimicrobial Resistance Surveillance System (EARSS). J Antimicrob Chemother 2001; 48:143–4. [DOI] [PubMed] [Google Scholar]
- 11.Reacher MH, Shah A, Livermore DM, et al. Bacteraemia and antibiotic resistance of its pathogens reported in England and Wales between 1990 and 1998: trend analysis. BMJ 2000; 320:213–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson AP, Davies J, Guy R, et al. Mandatory surveillance of methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia in England: the first 10 years. J Antimicrob Chemother 2012; 67:802–9. [DOI] [PubMed] [Google Scholar]
- 13.Health Protection Agency. Results of the first three and a half years of the Department of Health's mandatory methicillin resistant Staphylococcus aureus (MRSA) surveillance system in acute trusts in England. Available at: http://www.hpa.org.uk/topics/infectiousdiseases/infectionsaz/staphylococcusaureus/epidemiologicaldata/mandatorysurveillance/staphresultsofthefirstthreeandahalfyearsofthedept/. Accessed February 6, 2015.
- 14.Health Protection Agency. Mandatory surveillance of Staphylococcus aureus bacteraemia. Available at: http://www.hpa.org.uk/web/hpaweb&page&hpawebautolistname/page/1191942169773. Accessed February 6, 2015.
- 15.Health Protection Agency. Staphylococcus aureus bacteraemia past publications (voluntary surveillance). Available at: http://www.hpa.org.uk/topics/infectiousdiseases/referencelibrary/staphylococcusaureusreferences/staph_15_pastpubsvoluntarysurveillance/. Accessed February 6, 2015.
- 16.House of Lords Select Committee on Science and Technology. Resistance to antibiotics and other antimicrobial agents (HL Paper 81-1, 7th Report Session 1997–98). London: The Stationary Office, 1998. Available at: http://www.parliament.the-stationery-office.co.uk/pa/ld199798/ldselect/ldsctech/081vii/st0702.htm. Accessed February 6, 2015. [Google Scholar]
- 17.Report by the Comptroller and Auditor General – HC 230 session 1999–2000. The management and control of hospital acquired infection in acute NHS trusts in England. London: The Stationary Office, 2000. Available at: http://www.nao.org.uk/report/the-management-and-control-of-hospital-acquired-infection-in-acute-nhs-trusts-in-england/. Accessed February 6, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Department of Health. UK antimicrobial resistance strategy and action plan. London: DH, 2000. Available at: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4078448.pdf. Accessed February 6, 2015. [Google Scholar]
- 19.Committee of Public Accounts 42nd Report of Session 1999–2000. The management and control of hospital acquired infection in acute NHS Trusts in England 2000. London: The Stationary Office, 2000. Available at: http://www.publications.parliament.uk/pa/cm199900/cmselect/cmpubacc/306/30602.htm. Accessed February 6, 2015. [Google Scholar]
- 20.Pratt RJ, Pellowe C, Loveday HP, et al. The epic project: developing national evidence-based guidelines for preventing healthcare-associated Infections. J Hosp Infect 2001; 47(Supplement): S3–82. [DOI] [PubMed] [Google Scholar]
- 21.NHS Estates. National Standards of Cleanliness. London: The Stationary Office, 2001. [Google Scholar]
- 22.Chief Medical Officer's Update 30. Surveillance of healthcare associated infections. London: DH, 2001. Available at: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4013652.pdf. Accessed February 6, 2015. [Google Scholar]
- 23.Department of Health. Getting ahead of the curve: a strategy for combating infectious diseases (including other aspects of health protection). A report from the Chief Medical Officer. London: Department of Health, 2002. Available at: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4060875.pdf. Accessed February 6, 2015. [Google Scholar]
- 24.Department of Health. Chief Medical Officer letter - PL CMO (2003)4: surveillance of healthcare associated infections. London: Department of Health, 2003. Available at: http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/publicationsandstatistics/lettersandcirculars/professionalletters/chiefmedicalofficerletters/dh_4003782. Accessed February 6, 2015. [Google Scholar]
- 25.Department of Health. Chief Medical Officer letter - PL CMO (2003)3: Hospital pharmacy initiative for promoting prudent use of antibiotics in hospitals. London: Department of Health, 2003. Available at: http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Publicationsandstatistics/lettersandcirculars/professionalletters/chiefmedicalofficerletters/dh_4004614. Accessed February 6, 2015. [Google Scholar]
- 26.Department of Health. Winning ways: working together to reduce healthcare associated infection in England. London: Department of Health, 2003. Available at: http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_4064682. Accessed February 6, 2015. [Google Scholar]
- 27.Department of Health. Towards cleaner hospitals and lower rates of infection: a summary of action. London: Department of Health, 2004. Available at: http://webarchive.nationalarchives.gov.uk/+/dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_4085649. Accessed February 6, 2015. [Google Scholar]
- 28.Gush C, Borriello P. An overview of the UK Department of Health's Rapid Review Panel. J Hosp Infect 2007; 65(S2):27–9. [DOI] [PubMed] [Google Scholar]
- 29.Department of Health. National Standards Local Action - Health and Social Care Standards and Planning Framework 2005/06-2007/08. London: Department; of Health, 2004. Available at: http://webarchive.nationalarchives.gov.uk/+/dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_4086057. Accessed February 6, 2015. [Google Scholar]
- 30.Report by the Comptroller and Auditor General - HC 876 Session 2003–2004. Improving patient care by reducing the risk of hospital acquired infection: a progress report. London: The Stationary Office, 2004. Available at: http://www.nao.org.uk/wp-content/uploads/2004/07/0304876.pdf. Accessed February 6, 2015. [Google Scholar]
- 31.National Patient Safety Agency. Clean hands help to save lives. Patient safety alert 4. London: National Patient Safety Agency, 2004. [Google Scholar]
- 32.Department of Health. A matron's charter: an action plan for cleaner hospitals. London: Central Office of Communication, 2004. [Google Scholar]
- 33.Department of Health. Bloodborne MRSA infection rates to be halved by 2008 - Press notice. London: Department of Health, 2004. [Google Scholar]
- 34.MRSA/HCAI/Cleanliness Improvement Programme. 2005. Available at: http://webarchive.nationalarchives.gov.uk/20101125133833/http://clean-safe-care.nhs.uk/index.php?pid=1. Accessed February 6, 2015.
- 35.Office for National Statistics. Deaths involving MRSA: England and Wales, 1999–2003. Health Statistics Quarterly 25 London: The Stationary Office, 2005. Available at: http://cedadocs.badc.rl.ac.uk/291/1/health_stats.pdf. Accessed February 6, 2015. [Google Scholar]
- 36.Department of Health. Saving Lives – a delivering programme to reduce healthcare associated infection, including MRSA. London: Department of Health, 2005. Available at: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_4113888. Accessed February 6, 2015. [Google Scholar]
- 37.Department of Health. Mandatory surveillance of methicillin resistant Staphlylococcus aureus bacteraemias. Professional letter from the Chief Medical Officer (PL/CMO/2005/4). London: Department of Health, 2005. Available at: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4112590.pdf. Accessed February 6, 2015. [Google Scholar]
- 38.Department of Health. Introduction of the mandatory MRSA bacteraemia enhanced surveillance scheme. London: Department of Health, 2005. (CDR 6 October 2005) Available at: http://webarchive.nationalarchives.gov.uk/+/http://www.hpa.org.uk/cdr/archives/archive05/news/news4005.htm#mrsa. Accessed February 6, 2015. [Google Scholar]
- 39.Department of Health. Going further faster: implementing the Saving Lives delivery programme. Sustainable change for cleaner, safer care. London: Department of Health, 2006. Available at: http://webarchive.nationalarchives.gov.uk/20081105143757/http://dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_4134547?idcservice=get_file&did=8143&rendition=web. Accessed February 6, 2015. [Google Scholar]
- 40.National Patient Safety Agency. Learning through action to reduce infection. London: National Patient Safety Agency, 2006. Available at: http://www.nrls.npsa.nhs.uk/resources/?EntryId45=61832. Accessed February 6, 2015. [Google Scholar]
- 41.Department of Health. Code of practice for the prevention and control of healthcare associated infections (Health Act 2006). London: Department of Health, 2006. Available at: http://webarchive.nationalarchives.gov.uk/+/dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_4139336. Accessed February 6, 2015. [Google Scholar]
- 42.Department of Health. A summary of best practice for MRSA screening. Professional letter from the Chief Medical Officer (PL/CMO/2006/4). London: Department of Health, 2006. [Google Scholar]
- 43.Pratt RJ, Pellowe CM, Wilson JA, et al. epic2: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 2007; 65(Suppl 1):S1–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Department of Health. Clean, safe care: reducing infections and saving lives. London: Department of Health, 2007. Available at: http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_078134. Accessed February 6, 2015. [Google Scholar]
- 45.Department of Health. £50 million additional funding for reducing healthcare associated infections. Professional letter from the Chief Medical Officer (PL/CMO/2007/6) London: Department of Health, 2007. [Google Scholar]
- 46.Department of Health. Uniforms and workwear: an evidence base for developing local policy. London: Department of Health, 2007. Available at: http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_078433. Accessed February 6, 2015. [Google Scholar]
- 47.Department of Health. Improving infection control and cleanliness. Professional letter from the Chief Nursing Officer (PL/CNO/2007/6) London: Department of Health, 2007. Available at: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/en/publicationsandstatistics/lettersandcirculars/professionalletters/chiefnursingofficerletters/dh_080053?idcservice=get_file&did=153132&rendition=web. Accessed February 6, 2015. [Google Scholar]
- 48.Department of Health. The NHS in England: the operating framework for NHS, 2008/9. London: Department of Health, 2007. Available at: http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_081094. Accessed February 6, 2015. [Google Scholar]
- 49.Department of Health. MRSA screening operational guidance. London: Department of Health, 2008. Available at: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/en/publicationsandstatistics/lettersandcirculars/dearcolleagueletters/dh_086687?idcservice=get_file&did=170026&rendition=web. Accessed February 6, 2015. [Google Scholar]
- 50.National Patient Safety Agency. Clean hands save lives. Patient Safety Alert. Second Edition. London: National Patient Safety Agency, 2008:1–4. [Google Scholar]
- 51.Department of Health. Operating framework for 2009/10 for the NHS in England. London: Department of Health, 2008. Available at: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_091445. Accessed February 6, 2015. [Google Scholar]
- 52.Department of Health. The NHS Constitution. London: Department of Health, 2009. Available at: http://www.constitution.nhs.uk/downloads/nhs_constitution_document.pdf. Accessed February 6, 2015. [Google Scholar]
- 53.Department of Health. The NHS in England: Operating framework for NHS, 2010/11. London: Department of Health, 2009. Available at: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/en/publicationsandstatistics/publications/PublicationsPolicyAndGuidance/DH_110107. Accessed February 6, 2015. [Google Scholar]
- 54.Department of Health. MRSA screening - operational guidance 3. London: Department of Health, 2010. [Google Scholar]
- 55.Department of Health. The Operating Framework for the NHS in England 2011/12. London: Department of Health, 2010; Available at: https://www.gov.uk/government/publications/the-operating-framework-for-the-nhs-in-england-2011-12. Accessed February 6, 2015. [Google Scholar]
- 56.Fuller C, Robotham J, Savage J, et al. The National One Week Prevalence Audit of MRSA screening. Final report prepared for the Department of Health by the NOW study team. 2013. Available at: http://idrn.org/documents/resources/Final%20report.pdf. Accessed February 6, 2015.
- 57.Department of Health Advisory Committee on Antimicrobial Resistance and Healthcare Associated Infection. Antimicrobial stewardship ‘Start Smart Then Focus’. London: Department of Health, 2011. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/215308/dh_131181.pdf. Accessed February 6, 2015. [Google Scholar]
- 58.UK Parliament. Health and Social Care Act. 2008. http://services.parliament.uk/bills/2007-08/healthandsocialcare.html. Accessed February 6, 2015.
- 59.Cooke EM, Coello R, Sedgwick J, et al. A national surveillance scheme for hospital associated infections in England. Team of the Nosocomial Infection National Surveillance Scheme. J Hosp Infect 2000; 46:1–3. [DOI] [PubMed] [Google Scholar]
- 60.World Health Organization. Guidelines on hand hygiene in health care: a summary. First Global Patient Safety Challenge: Clean Care Is Safer Care, 2005. Available at: http://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=0CDUQFjAB&url=http%3A%2F%2Fwww.who.int%2Fentity%2Fgpsc%2F5may%2Ftools%2Fwho_guidelines-handhygiene_summary.pdf&ei=kf1TUqmLC46×XR8YCwDA&usg=AFQjCNEnOv-kBFzG12prtGTocqRErCjxIA&bvm=bv.53537100,d.d2k&cad=rja Accessed February 6, 2015. [PubMed] [Google Scholar]
- 61.Coia JE, Duckworth GJ, Edwards DI, et al. Guidelines for the control and prevention of meticillin-resistant Staphylococcus aureus (MRSA) in healthcare facilities. J Hosp Infect 2006; 63(Suppl 1):S1–44. [DOI] [PubMed] [Google Scholar]
- 62.Office for National Statistics. Deaths involving MRSA, 2006 to 2010. Available at: http://www.ons.gov.uk/ons/rel/subnational-health2/deaths-involving-mrsa/2006-to-2010/index.html. Accessed February 6, 2015.
- 63.European Centre for Disease Control. Antimicrobial resistance surveillance in Europe. 2011. Available at: http://www.ecdc.europa.eu/_layouts/CopyUtil.aspx?Use=id&Action=dispform&ItemId=719&ListId=4f55ad51-4aed-4d32-b960-af70113dbb90&WebId=270275b7-419a-4352-a8fb-f0c757d92e66&SiteId=ffe386b2-8461-4318-8856-32714ec41f3a. Accessed February 6, 2015.
- 64.de Kraker ME, Jarlier V, Monen JC, et al. The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin Microbiol Infect 2013; 19:860–8. [DOI] [PubMed] [Google Scholar]
- 65.Klein EY, Sun L, Smith DL, et al. The changing epidemiology of methicillin-resistant Staphylococcus aureus in the United States: a national observational study. Am J Epidemiol 2013; 177:666–74. [DOI] [PubMed] [Google Scholar]
- 66.Song JH, Hsueh PR, Chung DR, et al. Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J Antimicrob Chemother 2011; 66:1061–9. [DOI] [PubMed] [Google Scholar]
- 67.Mitchell BG, Collignon PJ, McCann R, et al. A major reduction in hospital-onset Staphylococcus aureus bacteremia in Australia-12 years of progress: an observational study. Clin Infect Dis 2014; 59:969–75. [DOI] [PubMed] [Google Scholar]
- 68.Grundmann H, Aanensen DM, van den Wijngaard CC, et al. Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular-epidemiological analysis. PLoS Med 2010; 7:e1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ellington MJ, Hope R, Livermore DM, et al. Decline of EMRSA-16 amongst methicillin resistant Staphylococcus aureus causing bacteraemias in the UK between 2001 and 2007. J Antimicrob Chemother 2010; 65:446–8. [DOI] [PubMed] [Google Scholar]
- 70.Wyllie DH, Walker AS, Miller R, et al. Decline of meticillin-resistant Staphylococcus aureus in Oxfordshire hospitals is strain-specific and preceded infection-control intensification. BMJ Open 2011; 1:e000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wyllie D, Paul J, Crook D. Waves of trouble: MRSA strain dynamics and assessment of the impact of infection control. J Antimicrob Chemother 2011; 66:2685–8. [DOI] [PubMed] [Google Scholar]
- 72.Wilcox MH. Recent initiatives to reduce the spread of meticillin-resistant Staphylococcus aureus. Br J Hosp Med (Lond) 2009; 70:399–401. [DOI] [PubMed] [Google Scholar]
- 73.Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2013; 4:CD003543 10.1002/14651858.CD003543.pub3. [DOI] [PubMed] [Google Scholar]
- 74.MacKenzie FM, Bruce J, Struelens MJ, et al. Antimicrobial drug use and infection control practices associated with the prevalence of methicillin-resistant Staphylococcus aureus in European hospitals. Clin Microbiol Infect 2007; 13:269–76. [DOI] [PubMed] [Google Scholar]
- 75.Wertheim HFL, Vos MC, Boelens HAM, et al. Low prevalence of methicillin-resistance Staphylococcus aureus (MRSA) at hospital admission in the Netherlands: the value of search-and-destroy and restrictive antibiotic use. J Hosp Infect 2004; 56:321–5. [DOI] [PubMed] [Google Scholar]
- 76.van Cleef BA, Kluytmans JA, van Benthem BH, et al. Cross border comparison of MRSA bacteraemia between The Netherlands and North Rhine-Westphalia (Germany): a cross-sectional study. PLoS One 2012; 7:e42787 10.1371/journal.pone.0042787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brody H, Rip MR, Vinten-Johansen P, et al. Map-making and myth-making in Broad Street: the London cholera epidemic, 1854. Lancet 2000; 356:64–8. [DOI] [PubMed] [Google Scholar]
- 78.Report by the Comptroller and Auditor General - HC 560 Session 2008–2009. Reducing healthcare associated infection in hospitals in England. London: The Stationary Office, 2009. Available at: http://www.nao.org.uk/wp-content/uploads/2009/06/0809560.pdf. Accessed February 6, 2015. [Google Scholar]
- 79.Department of Health. Root cause analysis. Available at: http://nhschoicestraining.spinningclock.com/index.php?pid=99. Accessed February 6, 2015.
- 80.Millar M. Are national targets the right way to improve infection control practice? J Hosp Infect 2009; 73:408–13. [DOI] [PubMed] [Google Scholar]
- 81.Stone SP, Fuller C, Savage J, et al. Evaluation of the national Cleanyourhands campaign to reduce Staphylococcus aureus bacteraemia and Clostridium difficile infection in hospitals in England and Wales by improved hand hygiene: four year, prospective, ecological, interrupted time series study. BMJ 2012; 344:e3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sroka S, Gastmeier P, Meyer E. Impact of alcohol hand-rub use on meticillin-resistant Staphylococcus aureus: an analysis of the literature. J Hosp Infect 2010; 74:204–11. [DOI] [PubMed] [Google Scholar]
- 83.Wenzel RP, Bearman G, Edmond MB. Screening for MRSA: a flawed hospital infection control intervention. Infect Control Hosp Epidemiol 2008; 29:1012–8. [DOI] [PubMed] [Google Scholar]
- 84.Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 2005; 5:751–62. [DOI] [PubMed] [Google Scholar]
- 85.Wilcox MH, Shetty N, Fawley WN, et al. Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis 2012; 55:1056–63. [DOI] [PubMed] [Google Scholar]
- 86.Ashiru-Oredope D, Sharland M, Charani E, et al. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart--Then Focus. J Antimicrob Chemother 2012; 67(Suppl 1):i51–63. [DOI] [PubMed] [Google Scholar]
- 87.Public Health England. Voluntary surveillance of Escherichia coli bacteraemia. Available at: http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/EscherichiaColi/VoluntarySurveillance/. Accessed February 6, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.