Abstract
Background. Few data exist on the incidence and risk factors of Staphylococcus aureus colonization and skin and soft tissue infections (SSTIs) among patients infected with human immunodeficiency virus (HIV).
Methods. Over a 2-year period, we prospectively evaluated adults infected with HIV for incident S aureus colonization at 5 body sites and SSTIs. Cox proportional hazard models using time-updated covariates were performed.
Results. Three hundred twenty-two participants had a median age of 42 years (interquartile range, 32–49), an HIV duration of 9.4 years (2.7–17.4), and 58% were on highly active antiretroviral therapy (HAART). Overall, 102 patients (32%) became colonized with S aureus with an incidence rate of 20.6 (95% confidence interval [CI], 16.8–25.0) per 100 person-years [PYs]. Predictors of colonization in the final multivariable model included illicit drug use (hazard ratios [HR], 4.26; 95% CI, 1.33–13.69) and public gym use (HR 1.66, 95% CI, 1.04–2.66), whereas antibacterial soap use was protective (HR, 0.50; 95% CI, 0.32–0.78). In a separate model, perigenital colonization was associated with recent syphilis infection (HR, 4.63; 95% CI, 1.01–21.42). Fifteen percent of participants developed an SSTI (incidence rate of 9.4 cases [95% CI, 6.8–12.7] per 100 PYs). Risk factors for an SSTI included incident S aureus colonization (HR 2.52; 95% CI, 1.35–4.69), public shower use (HR, 2.59; 95% CI, 1.48–4.56), and hospitalization (HR 3.54; 95% CI, 1.67–7.53). The perigenital location for S aureus colonization was predictive of SSTIs. Human immunodeficiency virus-related factors (CD4 count, HIV RNA level, and HAART) were not associated with colonization or SSTIs.
Conclusions. Specific behaviors, but not HIV-related factors, are predictors of colonization and SSTIs. Behavioral modifications may be the most important strategies in preventing S aureus colonization and SSTIs among persons infected with HIV.
Keywords: behaviors, colonization, HIV, human immunodeficiency virus, MRSA, risk factors, skin and soft tissue infections, Staphylococcus aureus
Staphylococcus aureus is the leading cause of skin and soft tissue infections (SSTIs) [1]. Persons infected with the human immunodeficiency virus (HIV) are at increased risk for several conditions including both S aureus colonization and infections [2–7]. Studies have shown that the incidence of S aureus infections is 6- to 18-fold higher among HIV-infected compared with HIV-uninfected persons [6, 8].
Although the precise reason for this increased risk is unclear, proposed reasons have included immune suppression, comorbid conditions, and certain lifestyle behaviors such as illicit drug use and high-risk sexual behaviors [9]. Studies before the advent of highly active antiretroviral therapy (HAART) largely attributed the increased risk to immunosuppression [3, 10, 11]; however, studies during the HAART era have shown that antiretroviral medication use and/or CD4 cell counts are often not associated with S aureus infections [9]. Furthermore, despite the availability of HAART in the developed world, persons infected with HIV continue to have elevated incidence rates of S aureus infections [6, 8], suggesting that factors beyond immunosuppression, such as specific behaviors (eg, drug use), may be the most important risk factors.
Additional data are needed to define the incidence rates and contemporary risk factors for staphylococcal colonization and SSTIs among persons infected with HIV. Because many prior studies have focused on cross-sectional or retrospective data [5, 12–18], intravenous drug-using populations [14], or specifically on methicillin-resistant S aureus (MRSA) [7, 19–22], a prospective study of staphylococcal (both methicillin-susceptible S aureus [MSSA] and MRSA) colonization among persons infected with HIV is needed. Furthermore, because S aureus colonization significantly increases the risk for subsequent infections [2, 3, 10, 23–25], data evaluating colonization at multiple body sites beyond the nares are warranted because such carriage may be important in the development of SSTIs [19]. Therefore, we used a large, prospective study of adults infected with HIV to determine the incidence of and risk factors for staphylococcal colonization at 5 body sites as well as the subsequent development of SSTIs.
METHODS
Study Population
A prospective study was designed to evaluate staphylococcal colonization among HIV-infected adults during a 2-year period. The primary outcome was efficacy of a decolonization strategy in reducing carriage among those who screened positive for MRSA carriage as previously reported [26]. The study also aimed to determine the incidence of and risk factors for staphylococcal (MRSA and MSSA) colonization and SSTIs over time among those who initially screened negative at baseline.
We evaluated 516 adults infected with HIV for incident S aureus colonization at 5 body sites (ie, bilateral nares, pharynx, bilateral axilla, bilateral groin areas, and perirectal area). After excluding those with S aureus colonization at the baseline visit (n = 161) or those lost to follow-up (n = 33), 322 participants were prospectively observed over a 2-year period for incident colonization. We also evaluated participants with no prior history of SSTIs (n = 293) for the outcome of incident SSTIs. Participants were enrolled between May 2007 and May 2010 with study completion in 2012. Clinical sites were located in geographically diverse locations across the United States and included the Naval Medical Center San Diego (San Diego, CA), Walter Reed Army Medical Center (Washington DC), and Naval Medical Center Portsmouth (Portsmouth, VA).
Study Procedures
After the baseline visit to assess for prevalent S aureus colonization, participants who screened negative for S aureus at all body sites were observed every 6 months over a 2-year period. During each study visit, participants underwent screening for S aureus carriage at 5 body sites. Because prior studies demonstrated the importance of extranasal colonization [27–30], perirectal and throat cultures were collected along with the 3 standard sites (nares, axilla, and groin). Swabs (n = 5; BBL CultureSwab Plus; Becton Dickinson and Company, Sparks Glencoe, MD) were collected by a physician, and the presence of S aureus was determined by College of American Pathologists-accredited laboratories at each clinical site using standard microbiological methods [31]. We included both MSSA and MRSA colonization in our study outcome, and we did not specifically evaluate MRSA colonization given its low incidence rate. Skin and soft tissue infections were recorded at each study visit using detailed questions by research study coordinators who inquired about the occurrence of cellulitis, abscess, carbuncles, furuncles, pustules, or folliculitis during the past 6 months. Patients underwent a physical examination at each study visit including a skin examination. Any active SSTI with a drainable collection was cultured. Participants were also educated regarding SSTIs and instructed to present to clinic for evaluation with any signs of an SSTI, including during times between scheduled study visits.
During each study visit, participants completed a questionnaire assessing several factors of interest including demographics and specific behaviors over the past 6 months. Behaviors assessed included use of antimicrobial soap, drug use (illicit drugs, alcohol, and tobacco), use of public gyms and showers, contact sports, sharing personal items (eg, towels, razors), shaving practices, receipt of a tattoo, and sexual activity including condom use and number of partners. We also assessed number of household contacts and pet ownership during each 6-month period.
Research coordinators collected data from the medical records during every 6-month visit regarding HIV-specific information including HIV duration (time from the first HIV-positive test until study enrollment), diagnosis of acquired immune deficiency syndrome (AIDS), CD4 cell count, HIV RNA level, and use of HAART (defined as the use of 3 or more full-dose antiretroviral medications). In addition, medical conditions (eg, cancer, diabetes, liver disease, kidney disease, skin conditions, and recent sexually transmitted infections), medication use (including trimethoprim-sulfamethoxazole and therapies requiring injections), and healthcare encounters (emergency department visits and hospitalizations) were abstracted from the medical records and entered on standard case report forms.
All participants provided voluntary written informed consent. The study was approved by the governing military institutional review boards at each clinical site and conducted in accordance with the principles of the Declaration of Helsinki and standards of Good Clinical Practice (as defined by the International Conference on Harmonization).
Statistical Methods
Descriptive statistics were performed evaluating the baseline characteristics of the study population and presented as numbers (percentages) and medians (interquartile ranges [IQRs]) for categorical and continuous variables, respectively. Participants were observed until the first occurrence of S aureus colonization or censor; those without colonization were censored at last study visit. Incidence rates were calculated as the number of events divided by person-years (PYs) of follow-up.
At each 6-month visit, colonization status and exposures of interest were assessed and used in the models as time-updated variables. Univariable and multivariable Cox proportional hazard models with time-updated covariates assessed the predictors of S aureus colonization. The final multivariable model was created using a stepwise approach and included only variables that were statistically significant. All statistical tests were 2 sided, and P value < .05 was considered statistically significant. Hazard ratios (HR) are reported with 95% confidence intervals (CIs). Likewise, a separate model was created for the outcome of incident SSTIs using the same methodology. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Study Population
Three hundred twenty-two participants were studied with a median age of 42 years (IQR, 32–49) and 93% were male. The median CD4 count was 525 cells/mm3 (IQR, 404–716), 57% had an undetectable HIV RNA level (<50 copies/mL), and 58% were receiving HAART. Other characteristics of the study population are shown in Table 1.
Table 1.
Baseline Descriptive Characteristics Among HIV-Infected Adults by Incident Staphylococcus aureus Colonization
| Variablea | Total N (%) | MRSA/MSSA Colonization N (%) | No MRSA/MSSA Colonization N (%) |
|---|---|---|---|
| Total | 322 | 102 | 220 |
| Demographics | |||
| Age, yearsb | 42 (32–49) | 42 (35–49) | 42 (31–50) |
| Race | |||
| Caucasian | 175 (54.3) | 52 (51.0) | 123 (55.9) |
| African-American | 126 (39.1) | 41 (40.2) | 85 (38.6) |
| Other | 21 (6.5) | 9 (8.8) | 12 (5.5) |
| Gender, male | 298 (92.5) | 95 (93.1) | 203 (92.3) |
| Behaviors | |||
| Antibacterial soap use | 150 (46.6) | 37 (36.3) | 113 (51.4) |
| Illicit drug use | 10 (3.1) | 2 (2.0) | 8 (3.6) |
| Alcohol use | 229 (71.1) | 76 (74.5) | 153 (69.5) |
| Tobacco use | 96 (29.8) | 34 (33.3) | 62 (28.2) |
| Public gym use | 188 (58.4) | 64 (62.7) | 124 (56.4) |
| Public shower use | 141 (43.8) | 48 (47.1) | 93 (42.3) |
| Plays football | 19 (5.9) | 6 (5.9) | 13 (5.9) |
| Plays soccer | 15 (4.7) | 4 (3.9) | 11 (5.0) |
| Shares personal items | 11 (3.4) | 2 (2.0) | 9 (4.1) |
| Body shaving | 151 (46.9) | 47 (46.1) | 104 (47.3) |
| Tattoo | 60 (18.6) | 22 (21.6) | 38 (17.3) |
| Sexually active, yes | 180 (55.9) | 58 (56.9) | 122 (55.5) |
| Number of sexual partners | |||
| 0 | 117 (36.3) | 36 (35.3) | 81 (36.8) |
| 1 | 98 (30.4) | 31 (30.4) | 67 (30.5) |
| ≥2 | 65 (20.2) | 17 (16.7) | 48 (21.8) |
| Lives alone | 119 (37.0) | 30 (29.4) | 89 (40.5) |
| Owns a pet | 146 (45.3) | 51 (50.0) | 95 (43.2) |
| HIV-related factors | |||
| Duration of HIV, yearsb | 9.4 (2.7–17.4) | 10.3 (2.4–18.2) | 8.6 (3.0–17.1) |
| AIDS diagnosis | 72 (22.4) | 25 (24.5) | 47 (21.4) |
| CD4 countb, cells/mm3 | 525 (404–716) | 521 (373–711) | 528 (414–719) |
| CD4 count, cells/mm3 | |||
| <350 | 58 (18.0) | 22 (21.6) | 36 (16.4) |
| ≥350 | 264 (82.0) | 80 (78.4) | 184 (83.6) |
| Viral load, undetectable <50 copies/mL | 183 (56.8) | 56 (54.9) | 127 (57.7) |
| Current HAART use | 185 (57.5) | 56 (54.9) | 129 (58.6) |
| Medical Conditions | |||
| Cancer | 13 (4.0) | 0 (0.0) | 13 (5.9) |
| Diabetes | 17 (5.3) | 3 (2.9) | 14 (6.4) |
| Kidney disease | 8 (2.5) | 1 (1.0) | 7 (3.2) |
| Liver disease | 13 (4.0) | 3 (2.9) | 10 (4.5) |
| Skin disease | 34 (10.6) | 14 (13.7) | 20 (9.1) |
| Chlamydia | 35 (10.9) | 13 (12.7) | 22 (10.0) |
| Gonorrhea | 87 (27.0) | 28 (27.5) | 59 (26.8) |
| Syphilis | 74 (23.0) | 19 (18.6) | 55 (25.0) |
| Medication Use | |||
| TMP-SMX | 25 (7.8) | 9 (8.8) | 16 (7.3) |
| Injectable medication | 13 (4.0) | 4 (3.9) | 9 (4.1) |
| Healthcare Encounters | |||
| ED visit | 96 (29.8) | 24 (23.5) | 72 (32.7) |
| Hospitalized | 49 (15.2) | 12 (11.8) | 37 (16.8) |
Abbreviations: ED, emergency department; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; MRSA, methicillin-resistant S aureus; MSSA, methicillin-susceptible S aureus; TMP-SMX, trimethoprim-sulfamethoxazole.
a Factors were ascertained within the last 6 months before each study visit. All data represent n = 322, except there were missing data for the following variables: antibacterial soap use (n = 3), illicit drug use (3), alcohol use (3), tobacco (2), public gym (4), public shower (2), football (3), soccer (2), shared personal items (10), shaving (5), tattoo (1), sexually active (21), number of sexual partners (42), lives alone (2), owns a pet (3), HAART use (9), cancer (3), diabetes (4), kidney disease (5), liver disease (7), skin disease (3), chlamydia (3), gonorrhea (2), syphilis (4), TMP-SMX use (3), injection drug use (4), ED visit (7), and recent hospitalization (2).
b Median, interquartile range.
Incidence of and Risk Factors for Staphylococcus aureus Colonization
Overall, 102 (32%) participants became colonized with S aureus during the study period for an incidence rate of 20.6 (95% CI, 16.8–25.0) per 100 PYs. Among those colonized, the most common site of colonization was the nares in 72.5% (n = 74), followed by the throat (14.7%, n = 15), groin (13.7%, n = 14), perirectal area (13.7%, n = 14), and axilla (9.8%, n = 10). Some patients had multiple sites of incident colonization with 87 participants having a single site of colonization, whereas 9 had 2 sites, 2 had 3 sites, and 4 had 4 sites; no patient had colonization at all 5 body sites.
A nares-only culture survey would have missed 27.4% of S aureus colonization in the study. Overall, 23% of colonized participants had perigenital (groin and/or perirectal) carriage, and if perigenital cultures had not been performed, 13% of all colonization would have been missed. Exclusive throat colonization occurred in 10% of those colonized, whereas exclusive axilla positivity was found in only 3%. The overlap of colonization (nares, throat, and perigenital) is shown in Figure 1. Regarding the type of S aureus, colonization was MSSA in 89 cases, MRSA in 10 cases, and both MSSA and MRSA in 3 cases.
Figure 1.
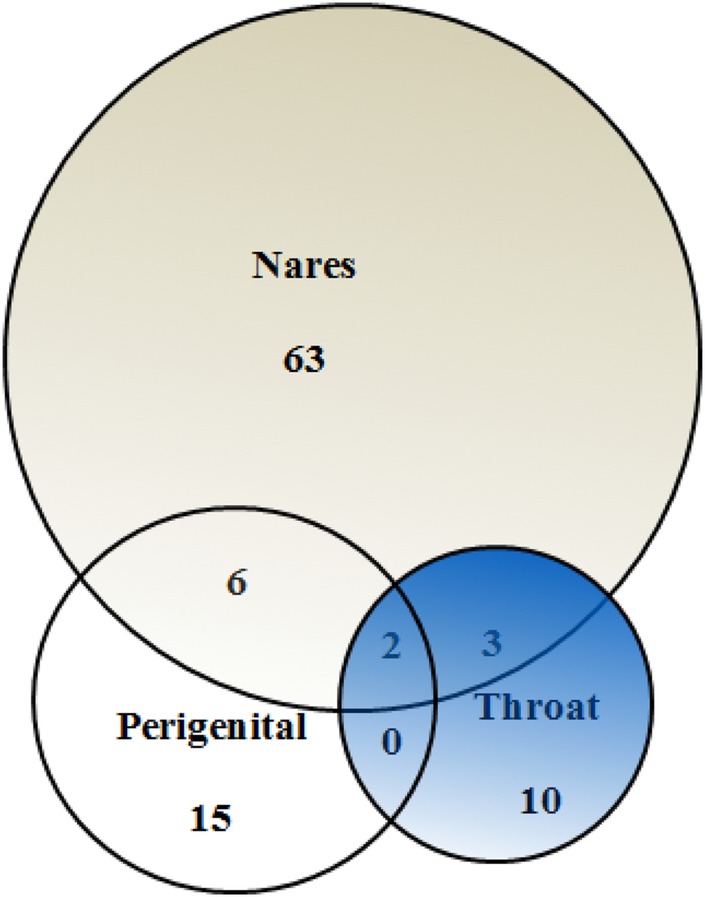
Venn diagram of the overlap of incident Staphylococcus aureus colonization at various body sites. Each circle size is proportional to the number colonized at each anatomic site. Perigenital is groin and/or perirectal colonization. The axilla site was omitted for simplicity.
In the univariable models, S aureus colonization was predicted by recent (in the last 6 months) illicit drug use, with borderline significant associations (P ≤ .10) with public gym use, having a skin condition, and hospitalization. Self-reported antibacterial soap use was associated with a reduced risk (Table 2). In the final multivariable model, predictors of S aureus colonization included illicit drug use (HR, 4.26; 95% CI, 1.33–13.69) and public gym use (HR, 1.66; 95% CI, 1.04–2.66). In addition, antibacterial soap use was found to be protective of colonization (HR, 0.50; 95% CI, 0.32–0.78) (data not shown).
Table 2.
Univariable Risk Factors for Staphylococcus aureus Colonization
| Variablea | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Demographics | ||
| Age, per 1 yr | 1.00 (0.98, 1.02) | .81 |
| Race | ||
| Caucasian | 1.00 | .84 |
| African-American | 1.04 (0.69, 1.57) | .20 |
| Other | 1.60 (0.79, 3.25) | |
| Gender, male | 1.07 (0.50, 2.31) | .86 |
| HIV-related factors | ||
| Duration of HIV, per 1 yr | 1.00 (0.98, 1.03) | .99 |
| AIDS diagnosis | 1.10 (0.70, 1.74) | .67 |
| CD4 count, cells/mm3 | ||
| <350 | 1.00 | .86 |
| ≥350 | 1.05 (0.62, 1.76) | |
| Viral load, undetectable <50 copies/mL | 1.07 (0.72, 1.61) | .73 |
| Current HAART use | 1.03 (0.63, 1.66) | .91 |
| Behaviors | ||
| Illicit drug use | 3.75 (1.37, 10.26) | .01* |
| Alcohol use | 0.98 (0.64, 1.51) | .94 |
| Tobacco use | 1.08 (0.69, 1.69) | .73 |
| Lives alone | 1.00 (0.65, 1.52) | .98 |
| Owns a pet | 1.14 (0.76, 1.72) | .54 |
| Public gym use | 1.50 (0.98, 2.29) | .06** |
| Public shower use | 1.07 (0.71, 1.61) | .76 |
| Plays football | 0.74 (0.27, 2.02) | .56 |
| Plays soccer | 1.13 (0.42, 3.09) | .81 |
| Sexually active, yes | 0.86 (0.57, 1.31) | .48 |
| Number of sexual partners | ||
| 0 | 1.00 | .99 |
| 1 | 1.00 (0.62, 1.61) | .95 |
| ≥2 | 1.02 (0.56, 1.85) | |
| Antibacterial soap use | 0.54 (0.34,0.83) | .01* |
| Shares towels | 1.49 (0.55, 4.08) | .43 |
| Body shaving | 1.21 (0.8, 1.82) | .37 |
| Tattoo | 1.67 (0.8, 3.5) | .17 |
| Medical Conditions | ||
| Cancer | 2.35 (0.74, 7.43) | .15 |
| Diabetes | 1.59 (0.50, 5.03) | .43 |
| Kidney disease | ||
| Liver disease | 1.20 (0.30, 4.89) | .80 |
| Skin disease | 2.00 (0.87, 4.58) | .10 |
| Sexually transmitted infections | ||
| Chlamydia | 1.53 (0.21, 10.98) | .68 |
| Gonorrhea | 1.41 (0.35, 5.73) | .64 |
| Syphilis | 1.04 (0.33, 3.28) | .95 |
| Medication Use | ||
| TMP-SMX | 0.67 (0.25, 1.84) | .44 |
| Injectable medication | 0.80 (0.29, 2.17) | .66 |
| Healthcare Encounters | ||
| ED visit | 1.11 (0.65, 1.91) | .70 |
| Hospitalized | 1.75 (0.91, 3.39) | .10 |
Abbreviations: AIDS, acquired immune deficiency syndrome; CI, confidence interval; ED, emergency department; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; TMP-SMX, trimethoprim-sulfamethoxazole.
a Factors were ascertained within the last 6 months before each study visit.
* P < .05.
** P < .10.
Because incident colonization at the perigenital location may have unique risk factors, we performed a separate model for colonization at this specific site. In the final multivariable model, perigenital S aureus colonization was associated with recent syphilis (HR, 4.63; 95% CI, 1.01–21.42), public shower use (HR, 4.33; 95% CI, 1.35–13.90), and hospitalization (HR, 5.11; 95% CI, 1.35–19.35). No relationships were noted between perigenital S aureus colonization and self-reported sexual activity or self-reported condom use (data not shown).
Incidence of and Risk Factors for Skin and Soft Tissue Infections
Among the study population with no history of SSTIs at baseline (n = 293), 15% developed an SSTI during the study period (incidence rate of 9.4 cases [95% CI, 6.8–12.7] per 100 PYs). Culture specimens were obtained from 15 of 43 (35%) of SSTI cases, yielding S aureus in 4 cases (27% of infections with a culture). Other organisms identified included beta-hemolytic streptococci (4) and Gram-negative bacilli (3), whereas the remainder showed normal skin flora (eg, coagulase-negative staphylococci). Skin and soft tissue infections included furuncles or pustules in 20 cases, abscesses in 5 cases, cellulitis in 5 cases, folliculitis in 3 cases, carbuncles in 2 cases, and other localized skin infections in 8 cases. The location of the infection was most commonly on the trunk (n = 14), followed by upper extremity (n = 11), perigenital area (n = 8), lower extremity (n = 8), and head/neck (n = 8); some infections involved multiple sites.
Risk factors for developing an SSTI in the time-updated univariable models are shown in Table 3. In the final multivariable model, incident S aureus colonization (HR, 2.52; 95% CI, 1.35–4.69), public shower use (HR, 2.59; 95% CI, 1.48–4.56), and hospitalization in the last 6 months (HR, 3.54; 95% CI, 1.67–7.53) were predictors for SSTIs. HIV-related factors (history of AIDS, CD4 cell count, HIV RNA level, and HAART use) were not predictive. In a separate multivariate model, we evaluated if incident S aureus colonization at the perigenital site was a predictor of subsequent development of an SSTI and found a significant relationship (HR, 3.95; 95% CI, 1.94–8.03).
Table 3.
Univariable Risk Factors for SSTIs
| Variablea | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Staphylococcus aureus colonization | 2.91 (1.59, 5.34) | .001* |
| Demographics | ||
| Age, per 1 yr | 0.97 (0.94, 0.99) | .02* |
| Race | ||
| Caucasian | 1.00 | .13 |
| African-American | 0.61 (0.32, 1.16) | .33 |
| Other | 0.37 (0.05, 2.75) | |
| Gender, male | 1.58 (0.38, 6.51) | .53 |
| HIV-related factors | ||
| Duration of HIV, per 1 yr | 0.92 (0.88, 0.97) | <.001* |
| AIDS diagnosis | 0.49 (0.19, 1.24) | .13 |
| CD4 count, cells/mm3 | ||
| <350 | 1.00 | .27 |
| ≥350 | 0.67 (0.33, 1.37) | |
| Viral load, undetectable <50 copies/mL | 1.61 (0.88, 2.92) | .12 |
| Current HAART use | 0.66 (0.33, 1.32) | .24 |
| Behaviors | ||
| Illicit drug use | 5.61 (1.71, 18.36) | .004* |
| Alcohol use | 0.93 (0.5, 1.74) | .81 |
| Tobacco use | 1.77 (0.96, 3.26) | .07** |
| Lives alone | 1.01 (0.54, 1.87) | .99 |
| Owns a pet | 1.68 (0.9, 3.11) | .10 |
| Public gym use | 2.12 (1.09, 4.19) | .03* |
| Public shower use | 2.72 (1.45, 5.10) | .002* |
| Plays football | 0.41 (0.06, 3.00) | .38 |
| Sexually active, yes | 1.59 (0.82, 3.10) | .17 |
| Number of sexual partners | ||
| 0 | 1.00 | |
| 1 | 0.50 (0.22, 1.12) | .09** |
| ≥2 | 0.59 (0.26, 1.34) | .21 |
| Antibacterial soap use | 0.72 (0.37, 1.39) | .33 |
| Shares towels | 0.72 (0.1, 5.24) | .75 |
| Body shaving | 1.41 (0.78, 2.57) | .26 |
| Tattoo | 3.07 (1.29, 7.32) | .01* |
| Medical Conditionsb | ||
| Kidney disease | 1.89 (0.26, 13.85) | .53 |
| Liver disease | 1.39 (0.19, 10.12) | .75 |
| Skin disease | 1.46 (0.35, 6.02) | .61 |
| Sexually transmitted infectionsb | ||
| Syphilis | 1.02 (0.14, 7.46) | .99 |
| Medication Use | ||
| TMP-SMX | 1.25 (0.38, 4.08) | .71 |
| Injectable medication | 4.14 (1.91, 8.95) | <.001* |
| Healthcare Encounters | ||
| ED visit | 2.28 (1.16, 4.47) | .02* |
| Hospitalized | 3.17 (1.40, 7.16) | .01* |
Abbreviations: AIDS, acquired immune deficiency syndrome; CI, confidence interval; ED, emergency department; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; SSTIs, skin and soft tissue infections; TMP-SMX, trimethoprim-sulfamethoxazole.
a Factors were ascertained within the last 6 months before each study visit.
b Unable to examine diabetes, cancer, gonorrhea, or chlamydia given no diagnosis among the SSTI cases.
* P < .05.
** P < .10.
DISCUSSION
Adults infected with HIV continue to have a high incidence of S aureus colonization and SSTIs during the HAART era. This study found that specific behaviors were significant predictors for S aureus colonization, and that incident colonization, particularly in the perigenital area, increased the risk for subsequent SSTIs. More importantly, HIV-related factors such as level of immunosuppression and HAART use were not significantly associated with either colonization or SSTIs. These data may be important for understanding the continued increased risk of S aureus colonization and SSTIs among adults infected with HIV and for development of preventive strategies.
The incidence rate of S aureus colonization of 20 per 100 PYs in our HIV cohort is higher than described among HIV-negative persons [32]. Before the HAART era, the increased rates of S aureus colonization, and subsequent infections, were mainly attributed to immunosuppression and frequent healthcare encounters [3, 10, 11]. However, despite the availability of effective HAART, rates of S aureus colonization have remained elevated.
Predictors for S aureus colonization in our study included specific behaviors. These data suggest that the increased colonization rates during the HAART era may no longer be largely attributed to immunosuppression [2, 19] but rather to specific behaviors among persons infected with HIV. In our cohort, illicit drug and public gym use were significantly associated with incident S aureus colonization; these specific behaviors may lead to exposure to S aureus via person-to-person contact or fomites such as shared drug paraphernalia or exercise equipment. We also found that patients with HIV who reported the use of antibacterial soap had a reduced risk for S aureus colonization. Whether this was directly related to the use of these products or indirectly related as a marker of improved hygiene is unknown; a previous study showed low rates of showering was associated with staphylococcal colonization and infection [33].
When examining the specific body site of S aureus colonization, we found that incident colonization in the perigenital area was associated with a recent diagnosis of syphilis. These data suggest the possibility of S aureus transmission during sexual activity. Prior cross-sectional studies have found associations between some sexual behaviors (eg, STIs, condom use) and MRSA carriage [16, 27]; our data confirms this relationship using prospective data. We did not find an association with self-reported sexual risk factors; however, this could be related to underreporting of these behaviors and/or missing data for these specific questions.
The current study highlights the value of 5-site testing for detection of incident S aureus colonization including the importance of throat and perigenital sites—adding these sites increased detection by 29%. Our study found low rates of incident MRSA colonization. Previous data suggest that MRSA may be declining in persons infected with HIV [34–36], similar to that seen in the general population [37]. Because both MSSA and MRSA carriage are associated with subsequent infections [10], these data are important in understanding S aureus in this vulnerable population.
Skin and soft tissue infections occurred in 15% of our HIV cohort during the 2-year study period with an incidence rate of 9.4 cases per 100 PYs, higher than estimates in the general population (3.2–4.9/100 PYs) [38, 39] but similar to a prior study among persons infected with HIV (11.7/100 PYs) [19]. Because some SSTIs in our study did not have purulent collections and hence lacked culture data, their precise etiology was unknown; however, it is likely that several SSTIs were due to S aureus since colonization with this organism in the prior 6 months was significantly associated with the development of SSTIs. This also concurs with prior publications showing a relationship between S aureus colonization and subsequent infections [2, 3, 10, 11, 19, 23–25, 40]. Our study is unique because we evaluated the specific location of S aureus colonization and subsequent development of SSTIs. We found that perigenital colonization was related to subsequent infection, a finding concurrent with a recent study among men who have sex with men that found that perianal MRSA colonization was a risk factor for SSTIs [19].
In addition to incident S aureus colonization, our study also found that specific behaviors including public shower use in the past 6 months was associated with SSTIs in multivariable models. Predictors of SSTIs also included factors related to breaches in skin integrity that may allow S aureus access to deeper tissues including recent hospitalization, perhaps due to concurrent placement of intravenous lines or surgical procedures compromising skin integrity.
The findings of this study may have clinical implications. For example, many HIV patients inquire about the pathogenesis of S aureus colonization or infection, and these data provide avenues for education and potential preventive guidance. Prospective studies on interventions to modify behavioral factors are needed to determine whether these specific strategies could limit future S aureus transmission and infection.
Our study had several limitations. We were unable to specifically examine incident MRSA colonization or SSTIs due to the low numbers of events; however, our data mirror studies showing a decline in MRSA among patients infected with HIV [34–36]. The objective of the current study was incident colonization and SSTIs; future studies may examine other outcomes such as colonization persistence and SSTI recurrence. Our study was performed among military members and veterans who were mostly men; hence, study findings may not be generalizable to women or other HIV populations. Furthermore, few participants had low CD4 counts (<200 cells/mm3) or were receiving trimethoprim-sulfamethoxazole prophylaxis [4, 11, 15, 20, 21]; however, in the HAART era these potential factors may be less common. Our study did not collect data on HIV acquisition risk factors due to the US military's policy, “don't ask, don't tell,” which was in effect at the time of study initiation; other studies have shown that most HIV infections in the US military are related to sexual activities (with at least 50% reporting homosexual risk factors), with very low rates due to intravenous drug use [41]. Molecular characterization of isolates was not available in our study. Finally, some data was based on self-report; however, when possible we used medical records to verify information including medical conditions and STIs.
Strengths include being one of the largest and longest prospective studies among HIV-infected persons providing contemporary data on S aureus colonization and SSTI incidence rates and risk factors. Furthermore, we assessed colonization using 5 different body locations. We evaluated the temporal associations between potential risk factors and subsequent incident colonization and SSTIs using time-updated 6-month intervals of data collection over a 2-year period. Finally, the study examined a large number of self-reported and objective factors.
CONCLUSIONS
In summary, persons infected with HIV remain at a higher risk for several health conditions during the HAART era, including incident S aureus colonization and SSTIs. Specific behaviors, rather than HIV-related factors, were risk factors for S aureus colonization and SSTIs. Risky sexual behavior as indicated by recent syphilis infection was associated with perigenital S aureus colonization, which in turn was associated with incident SSTIs. In addition, behaviors such as illicit drug use and public gym use were significant risk factors for incident S aureus colonization. These data suggest that behavioral modifications may be the most important strategies in preventing S aureus colonization and SSTIs among persons infected with HIV.
Acknowledgments
Disclaimer. The content and views expressed in this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the National Institutes of Health or the Department of Health and Human Services, or the Departments of the Army, Navy, Air Force, Department of Defense, nor the US Government. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government. The authors from the IDCRP have no financial interest in this work. All authors contributed to the content of the manuscript and concurred with the decision to submit it for publication.
Financial support. Support for this work (IDCRP-003) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed through the Uniformed Services University of the Health Sciences. This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Inter-Agency Agreement Y1-AI-5072.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 2006; 355:666–74. [DOI] [PubMed] [Google Scholar]
- 2.Shet A, Mathema B, Mediavilla JR, et al. Colonization and subsequent skin and soft tissue infection due to methicillin-resistant Staphylococcus aureus in a cohort of otherwise healthy adults infected with HIV type 1. J Infect Dis 2009; 200:88–93. [DOI] [PubMed] [Google Scholar]
- 3.Weinke T, Schiller R, Fehrenbach FJ, et al. Association between Staphylococcus aureus nasopharyngeal colonization and septicemia in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis 1992; 11:985–9. [DOI] [PubMed] [Google Scholar]
- 4.Hidron AI, Kourbatova EV, Halvosa JS, et al. Risk factors for colonization with methicillin-resistant Staphylococcus aureus (MRSA) in patients admitted to an urban hospital: emergence of community-associated MRSA nasal carriage. Clin Infect Dis 2005; 41:159–66. [DOI] [PubMed] [Google Scholar]
- 5.Seybold U, Supthut-Schroder B, Draenert R, et al. Prevalence and risk factors of nasal colonization with Staphylococcus aureus - association with HIV infection in older patients. Scand J Infect Dis 2009; 41:63–6. [DOI] [PubMed] [Google Scholar]
- 6.Popovich KJ, Weinstein RA, Aroutcheva A, et al. Community-associated methicillin-resistant Staphylococcus aureus and HIV: intersecting epidemics. Clin Infect Dis 2010; 50:979–87. [DOI] [PubMed] [Google Scholar]
- 7.Popovich KJ, Hota B, Aroutcheva A, et al. Community-associated methicillin-resistant Staphylococcus aureus colonization burden in HIV-infected patients. Clin Infect Dis 2013; 56:1067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crum-Cianflone NF, Burgi A, Hale BR. Increasing rates of community-acquired MRSA infections among HIV-infected persons. Int J STD AIDS 2007; 18:521–6. [DOI] [PubMed] [Google Scholar]
- 9.Shadyab AH, Crum-Cianflone NF. Methicillin-resistant Staphylococcus aureus (MRSA) infections among HIV-infected persons in the era of highly active antiretroviral therapy: a review of the literature. HIV Med 2012; 13:319–32. [DOI] [PubMed] [Google Scholar]
- 10.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 1997; 10:505–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen MH, Kauffman CA, Goodman RP, et al. Nasal carriage of and infection with Staphylococcus aureus in HIV-infected patients. Ann Intern Med 1999; 130:221–5. [DOI] [PubMed] [Google Scholar]
- 12.Onorato M, Borucki MJ, Baillargeon G, et al. Risk factors for colonization or infection due to methicillin-resistant Staphylococcus aureus in HIV-positive patients: a retrospective case-control study. Infect Control Hosp Epidemiol 1999; 20:26–30. [DOI] [PubMed] [Google Scholar]
- 13.Villacian JS, Barkham T, Earnest A, et al. Prevalence of and risk factors for nasal colonization with Staphylococcus aureus among human immunodeficiency virus-positive outpatients in Singapore. Infect Control Hosp Epidemiol 2004; 25:438–40. [DOI] [PubMed] [Google Scholar]
- 14.Holbrook KA, Klein RS, Hartel D, et al. Staphylococcus aureus nasal colonization in HIV-seropositive and HIV-seronegative drug users. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 16:301–6. [DOI] [PubMed] [Google Scholar]
- 15.McDonald LC, Lauderdale TL, Lo HJ, et al. Colonization of HIV-infected outpatients in Taiwan with methicillin-resistant and methicillin-sensitive Staphylococcus aureus. Int J STD AIDS 2003; 14:473–7. [DOI] [PubMed] [Google Scholar]
- 16.Crum-Cianflone NF, Shadyab AH, Weintrob A, et al. Association of methicillin-resistant Staphylococcus aureus (MRSA) colonization with high-risk sexual behaviors in persons infected with human immunodeficiency virus (HIV). Medicine (Baltimore) 2011; 90:379–89. [DOI] [PubMed] [Google Scholar]
- 17.Chacko J, Kuruvila M, Bhat GK. Factors affecting the nasal carriage of methicillin-resistant Staphylococcus aureus in human immunodeficiency virus-infected patients. Indian J Med Microbiol 2009; 27:146–8. [DOI] [PubMed] [Google Scholar]
- 18.Sissolak D, Geusau A, Heinze G, et al. Risk factors for nasal carriage of Staphylococcus aureus in infectious disease patients, including patients infected with HIV, and molecular typing of colonizing strains. Eur J Clin Microbiol Infect Dis 2002; 21:88–96. [DOI] [PubMed] [Google Scholar]
- 19.Szumowski JD, Wener KM, Gold HS, et al. Methicillin-resistant Staphylococcus aureus colonization, behavioral risk factors, and skin and soft-tissue infection at an ambulatory clinic serving a large population of HIV-infected men who have sex with men. Clin Infect Dis 2009; 49:118–21. [DOI] [PubMed] [Google Scholar]
- 20.Cenizal MJ, Hardy RD, Anderson M, et al. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus (MRSA) nasal colonization in HIV-infected ambulatory patients. J Acquir Immune Defic Syndr 2008; 48:567–71. [DOI] [PubMed] [Google Scholar]
- 21.Ramsetty SK, Stuart LL, Blake RT, et al. Risks for methicillin-resistant Staphylococcus aureus colonization or infection among patients with HIV infection. HIV Med 2010; 11:389–94. [DOI] [PubMed] [Google Scholar]
- 22.Zervou FN, Zacharioudakis IM, Ziakas PD, et al. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus colonization in HIV infection: a meta-analysis. Clin Infect Dis 2014; 59:1302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis MW, Hospenthal DR, Dooley DP, et al. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis 2004; 39:971–9. [DOI] [PubMed] [Google Scholar]
- 24.Peters PJ, Brooks JT, McAllister SK, et al. Methicillin-resistant Staphylococcus aureus colonization of the groin and risk for clinical infection among HIV-infected adults. Emerg Infect Dis 2013; 19:623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Eiff C, Becker K, Machka K, et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 2001; 344:11–6. [DOI] [PubMed] [Google Scholar]
- 26.Weintrob A, Bebu I, Johnson E, et al. Randomized, double-blinded study on decolonization procedures for methicillin-resistant Staphylococcus aureus (MRSA) among HIV-infected adults. In: Infectious Disease Society of America Meeting/IDWeek, San Francisco, CA: October 2–5, 2013. [Google Scholar]
- 27.Peters PJ, Brooks JT, Limbago B, et al. Methicillin-resistant Staphylococcus aureus colonization in HIV-infected outpatients is common and detection is enhanced by groin culture. Epidemiol Infect 2011; 139:998–1008. [DOI] [PubMed] [Google Scholar]
- 28.Yang ES, Tan J, Eells S, et al. Body site colonization in patients with community-associated methicillin-resistant Staphylococcus aureus and other types of S. aureus skin infections. Clin Microbiol Infect 2010; 16:425–31. [DOI] [PubMed] [Google Scholar]
- 29.Wertheim HF, Verveer J, Boelens HA, et al. Effect of mupirocin treatment on nasal, pharyngeal, and perineal carriage of Staphylococcus aureus in healthy adults. Antimicrob Agents Chemother 2005; 49:1465–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller LG, Eells SJ, Taylor AR, et al. Staphylococcus aureus colonization among household contacts of patients with skin infections: risk factors, strain discordance, and complex ecology. Clin Infect Dis 2012; 54:1523–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown DF, Edwards DI, Hawkey PM, et al. Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphylococcus aureus (MRSA). J Antimicrob Chemother 2005; 56:1000–18. [DOI] [PubMed] [Google Scholar]
- 32.Miller M, Cespedes C, Bhat M, et al. Incidence and persistence of Staphylococcus aureus nasal colonization in a community sample of HIV-infected and -uninfected drug users. Clin Infect Dis 2007; 45:343–6. [DOI] [PubMed] [Google Scholar]
- 33.Maree CL, Eells SJ, Tan J, et al. Risk factors for infection and colonization with community-associated methicillin-resistant Staphylococcus aureus in the Los Angeles County jail: a case-control study. Clin Infect Dis 2010; 51:1248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giuliani M, Longo B, Latini A, et al. No evidence of colonization with community-acquired methicillin-resistant Staphylococcus aureus in HIV-1-infected men who have sex with men. Epidemiol Infect 2010; 138:738–42. [DOI] [PubMed] [Google Scholar]
- 35.Hidron AI, Moanna A, Rimland D. The rise and fall of MRSA infections in HIV patients. AIDS 2011; 25:1001–3. [DOI] [PubMed] [Google Scholar]
- 36.Madariaga MG, Ullrich F, Swindells S. Low prevalence of community-acquired methicillin-resistant Staphylococcus aureus colonization and apparent lack of correlation with sexual behavior among HIV-infected patients in Nebraska. Clin Infect Dis 2009; 48:1485–7. [DOI] [PubMed] [Google Scholar]
- 37.Kallen AJ, Mu Y, Bulens S, et al. Health care-associated invasive MRSA infections, 2005–2008. JAMA 2010; 304:641–8. [DOI] [PubMed] [Google Scholar]
- 38.Ray GT, Suaya JA, Baxter R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a U.S. population: a retrospective population-based study. BMC Infect Dis 2013; 13:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hersh AL, Chambers HF, Maselli JH, et al. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008; 168:1585–91. [DOI] [PubMed] [Google Scholar]
- 40.Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 2005; 5:751–62. [DOI] [PubMed] [Google Scholar]
- 41.Brodine SK, Shaffer RA, Starkey MJ, et al. Drug resistance patterns, genetic subtypes, clinical features, and risk factors in military personnel with HIV-1 seroconversion. Ann Intern Med 1999; 131:502–6. [DOI] [PubMed] [Google Scholar]


