Abstract
Background. Influenza disproportionately impacts older adults while current vaccines have reduced effectiveness in the older population.
Methods. We conducted a comprehensive evaluation of cellular and humoral immune responses of adults aged 50 years and older to the 2008–2009 seasonal trivalent inactivated influenza vaccine and assessed factors influencing vaccine response.
Results. Vaccination increased hemagglutination inhibition and neutralizing antibody; however, 66.3% of subjects did not reach hemagglutination inhibition titers ≥ 40 for H1N1, compared with 22.5% for H3N2. Increasing age had a minor negative impact on antibody responses, whereas prevaccination titers were the best predictors of postvaccination antibody levels. Preexisting memory B cells declined with age, especially for H3N2. However, older adults still demonstrated a significant increase in antigen-specific IgG+ and IgA+ memory B cells postvaccination. Despite reduced frequency of preexisting memory B cells associated with advanced age, fold-rise in memory B cell frequency in subjects 60+ was comparable to subjects age 50–59.
Conclusions. Older adults mounted statistically significant humoral and cell-mediated immune responses, but many failed to reach hemagglutination inhibition titers ≥40, especially for H1N1. Although age had a modest negative effect on vaccine responses, prevaccination titers were the best predictor of postvaccination antibody levels, irrespective of age.
Keywords: aging, immune response, influenza, older adults, vaccine
Seasonal influenza is responsible for an estimated 23 000 deaths (range, 3349–48 614) annually in the United States [1, 2]. The majority of these deaths occur among adults aged 65 years and older [1]. Adults age 65+ have 10 to 30 times more hospitalizations annually due to influenza-associated illness than younger adults and longer hospital stays [2–6]; these effects are even more pronounced with more advanced age [2].
Although vaccination remains the most cost-effective method to reduce influenza-associated morbidity and mortality, immunosenescence renders vaccines less effective in older adults [7, 8]. Contributing factors include impaired T and B cell functionality and narrowing of the T and B cell repertoire [9–15]. Although it is recognized that more effective vaccines are needed for older adults, the capacity of their immune systems to respond and the components of the immune system that should be targeted for improved efficacy are not well understood. The hemagglutination inhibition (HI) assay is currently used to evaluate vaccine immunogenicity, in part because an HI antibody titer ≥32 or 40 is generally considered a laboratory correlate of protection, having been associated with protection against influenza infection in 50% of the population in a number of studies in younger adults [16–19]. Findings from recent studies also suggest that cell-mediated immune responses may correlate better with protection in older individuals [20, 21]. However, alternative correlates to HI have not yet been established. Although multiple studies have compared immune responses of older adults to young healthy adults, relatively fewer have examined the progression in later life. This study examines factors influencing immune responses to seasonal trivalent inactivated influenza vaccine (TIV) progressing from middle-age (age 50–59 years) through advanced age (age 80+) using a comprehensive set of assessments for antibody and cell-mediated immune responses.
METHODS
Methods are presented in brief and described in detail in Supplementary Figure 1.
Study Design
Approximately 590 subjects were enrolled during September through October 2008 at Vanderbilt University Medical Center (Nashville, TN) and Marshfield Clinic Research Foundation (Marshfield, WI) [22]. Subjects ≥50 years old were eligible to participate at Vanderbilt, and subjects ≥65 years old were eligible to participate at Marshfield. Subjects were grouped by age; 50–59 (middle-aged), 60–69, 70–79, and 80+ years. Informed consent was obtained from all participants. Human experimentation guidelines of the Department of Health and Human Services were followed in the conduct of this trial. Procedures, informed consent documents, and data collection forms were reviewed and approved by Institutional Review Boards at each site. Participant characteristics are summarized in Table 1.
Table 1.
Participant Characteristics
| Age Group | Overall | 50–59 | 60–69 | 70–79 | 80+ |
|---|---|---|---|---|---|
| Subject no. | 90 | 26 | 23 | 27 | 14 |
| Sex (%) | |||||
| Male | 44.4 | 23.1 | 52.2 | 55.6 | 50.0 |
| Female | 55.6 | 76.9 | 47.8 | 44.4 | 50.0 |
| Race (%) | |||||
| White | 94.4 | 84.6 | 95.7 | 100.0 | 100.0 |
| Black | 4.4 | 11.5 | 4.3 | 0.0 | 0.0 |
| Other | 1.1 | 3.8 | 0.0 | 0.0 | 0.0 |
| Comorbiditya (%) | |||||
| Heart | 1.1 | 3.8 | 17.4 | 7.4 | 0.0 |
| Lung | 7.8 | 19.2 | 4.3 | 7.4 | 7.1 |
| Immune | 10.0 | 0.0 | 0.0 | 7.4 | 7.1 |
| HI ≥40 (%) | |||||
| H1N1 | |||||
| Day 0 | 10.2 | 8.0 | 13.0 | 7.7 | 14.3 |
| Day 28 | 33.7 | 40.7 | 39.1 | 28.0 | 21.4 |
| H3N2 | |||||
| Day 0 | 29.5 | 32.0 | 30.4 | 26.9 | 28.6 |
| Day 28 | 77.5 | 74.1 | 82.6 | 92.0 | 50.0 |
| ≥4-fold rise (%) | |||||
| H1N1 | 22.7 | 20.0 | 17.4 | 26.9 | 28.6 |
| H3N2 | 64.8 | 72.0 | 47.8 | 76.9 | 57.1 |
Abbreviations: HI, hemagglutination inhibition; HIV, human immunodeficiency virus.
a Comorbid conditions include heart and lung disease, and immunosuppression (Immune). Immunosuppression includes immune dysfunction (including HIV), transplant, chemotherapy, steroid, or other immune-modulating medications.
Participants received TIV from their usual caregiver, at vaccine clinics, or at the study site by study staff. The 2008–2009 TIV was composed of A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2), and B/Florida/4/2006 strains. Although HI titers were determined for all subjects, for this study, a subset of 130 randomly selected subjects underwent a more comprehensive evaluation which included HI and microneutralization (MN) assays, antibody binding by biolayer interferometry, and T and B cell analysis. Due to limited sample volumes and the number of assessments performed, only the influenza A strains were examined in this study. Evaluations and analysis were done by staff blinded to participant age. Serum samples were collected from all subjects and peripheral blood mononuclear cells (PBMCs) from a subset of subjects prior to vaccination and 21–28 days postvaccination.
Serological Assays
Microneutralization and HI assays were performed as previously described [23]. Hemagglutination inhibition testing was performed by Focus Diagnostics, Inc. (Cypress, CA). For both assays, serial 2-fold dilutions were tested in duplicate and expressed as the reciprocal of the highest dilutions giving 50% neutralization or complete inhibition of agglutination. Antibody binding to hemagglutinin (HA) was analyzed by biolayer interferometry on an Octet Red instrument (Fortebio, Inc., Menlo Park, CA). H3N2 and H1N1 recombinant HA was synthesized and purified as described previously [24].
Cell-Mediated Responses
Analysis of T-cell responses was performed on a subset of 60 subjects, randomly selected from each age group. Cells were stimulated overnight with live H3N2 or H1N1 virus. Brefeldin A (Golgi Plug; BD Biosciences, San Diego, CA) was added to cultures for the last 6 hours to block Golgi transport. Cells were stained with monoclonal antibodies recognizing CD4, CD8, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ (BD Biosciences) and analyzed using an LSRII Flow Cytometer (BD Biosciences).
For assessment of antigen-specific memory B cells, PBMCs were cultured with poke weed mitogen (Sigma-Aldrich, St. Louis, MO), Type B CpG oligonucleotide (Invivogen, San Diego, CA), and purified protein A (Sigma-Aldrich) for 5–6 days to induce polyclonal activation. A standard memory B cell enzyme-linked immunospot (ELISPOT) was performed [25] with minor modifications (Supplementary Figure 1). Spot-forming units were counted using ImmunoSpot (Cellular Technology Ltd., Cleveland, OH) and expressed as percentage antigen-specific IgG or IgA B cells of total IgG or IgA-secreting cells. Monovalent-inactivated vaccines were provided by Sanofi Pasteur to assess the frequency of influenza-specific antibody-secreting cells (ASCs).
Statistical Analysis
Log2-transformed HI and MN titers were used as dependent variables and summarized as geometric mean titers (GMT) after back-transformation. Means and differences in means were estimated using repeated measures linear mixed models [26, 27]. Back-transforming model-estimated means yielded GMTs and back-transforming differences between day 28 and day 0 means yielded GMT ratios (day 0 to day 28 fold-rise). Models contained indicator (1,0) variables representing serum draw day 28 vs day 0, indicator variables representing age groups, and product terms representing interaction between serum draw and age group. Interaction terms allowed us to (1) estimate fold-rises having separate slopes by age group and (2) test for differences in fold-rises between groups.
Linear regression models used continuous year of age as an explanatory variable. Dependent variables were log2 titers and log2 fold-rises for individual subjects. This allowed visualization of trends in the strength of the fold-rise across age. Postvaccination log2 titers were regressed against prevaccination titers. Pre- and postvaccination serum responses were also regressed against T-cell responses.
Antibody binding and T- and B-cell responses used models similar to serology data; however, because these data are expressed as percentages, we used log10 transformation similar to He et al [28]. Back-transforming model-estimated means yielded geometric mean percentages (GMPs), and back-transforming differences between day 28 and day 0 means yielded GMP ratios. Analyses were performed using SAS software, version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Serological Responses
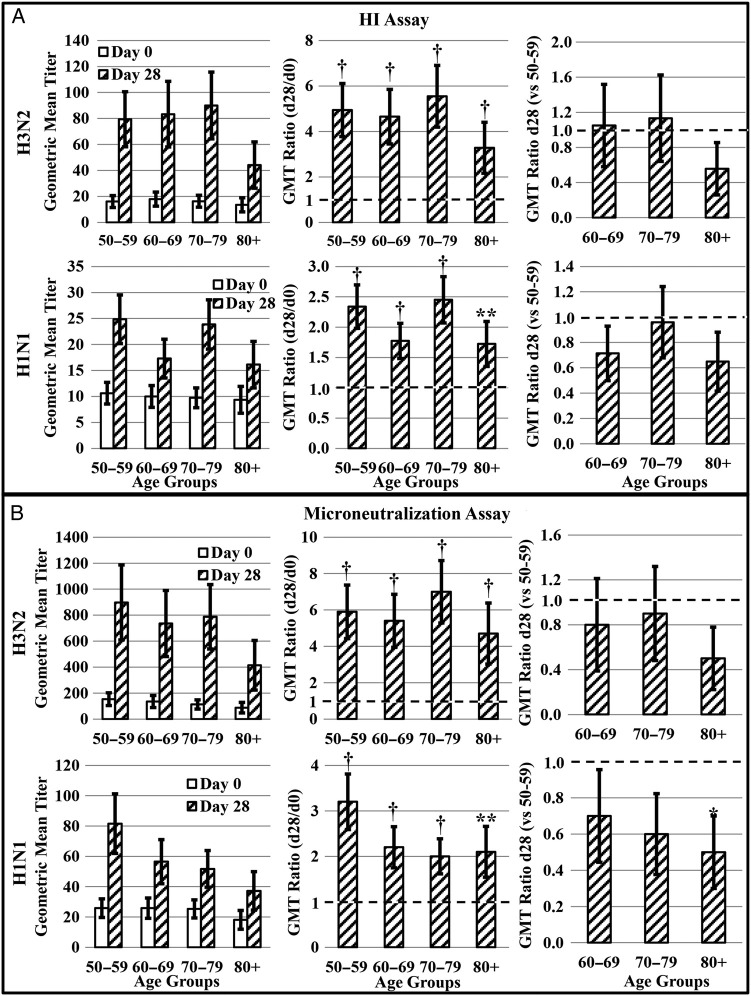
Hemagglutination inhibition responses for the entire cohort were previously reported [22]. Within the subset of 130 subjects that underwent comprehensive evaluation, 10.2% had HI titers ≥40 to H1N1 prior to vaccination with individual age groups ranging from 8.0% to 14.3% (Table 1). Subjects in this study mounted only modest humoral responses to H1N1. Although postvaccination HI and MN titers were marginally higher in each age group (ie, GMT ratio > 1; Figure 1A and B), the majority of subjects (66.3%; Table 1) did not reach HI titers ≥40 for H1N1. On average, subjects increased their HI titers 2.8-fold with individual age groups ranging from 2.3- to 3.4-fold. Hemagglutination inhibition titers of older adults to H1N1 were not significantly different than titers from middle-aged adults (Figure 1A). However, subjects age 80+ had lower MN titers (GMT ratio < 1) than middle-aged subjects postvaccination (Figure 1B).
Figure 1.
Assessment of antibody responses to 2008–2009 trivalent inactivated influenza vaccine. A, Serum hemagglutination inhibition (HI) antibody (n = 88) and B, microneutralization titers (n = 90) to H3N2 and H1N1 were assessed on day 0 prior to vaccination and day 28 after vaccination. The geometric mean titer (GMT) ratio (day 28 GMT/day 0 GMT) was calculated to determine the vaccine-associated change in antibody within each age group. A GMT ratio of 1 (dotted line) is indicative of no change. Day 28 GMT ratios were used to compare responses of older adult groups to middle-aged controls (day 28 GMT of older adult group/day 28 GMT of 50–59 age group). A GMT ratio less than 1 (dotted line) is indicative of a lower postvaccination response. Error bars represent 1 standard error. Significance is indicated by *P ≤ .05, **P ≤ .01, †P ≤ .001.
In contrast to humoral responses to H1N1, subjects mounted more robust responses to H3N2. More subjects (29.5% vs 10.2%) had preexisting HI titers ≥40 to H3N2 than H1N1 (Table 1), and H3N2 HI and MN titers increased in all age groups postvaccination (Figure 1A and B). A total of 77.5% of subjects achieved HI titers ≥40 to H3N2 postvaccination with individual age groups ranging from 50.0% to 92.0% (Table 1). The percentage of subjects demonstrating a 4-fold or greater rise in HI titer was within a similar range (Table 1). All older age groups showed postvaccination titers comparable to middle-aged counterparts (Figure 1A and B).
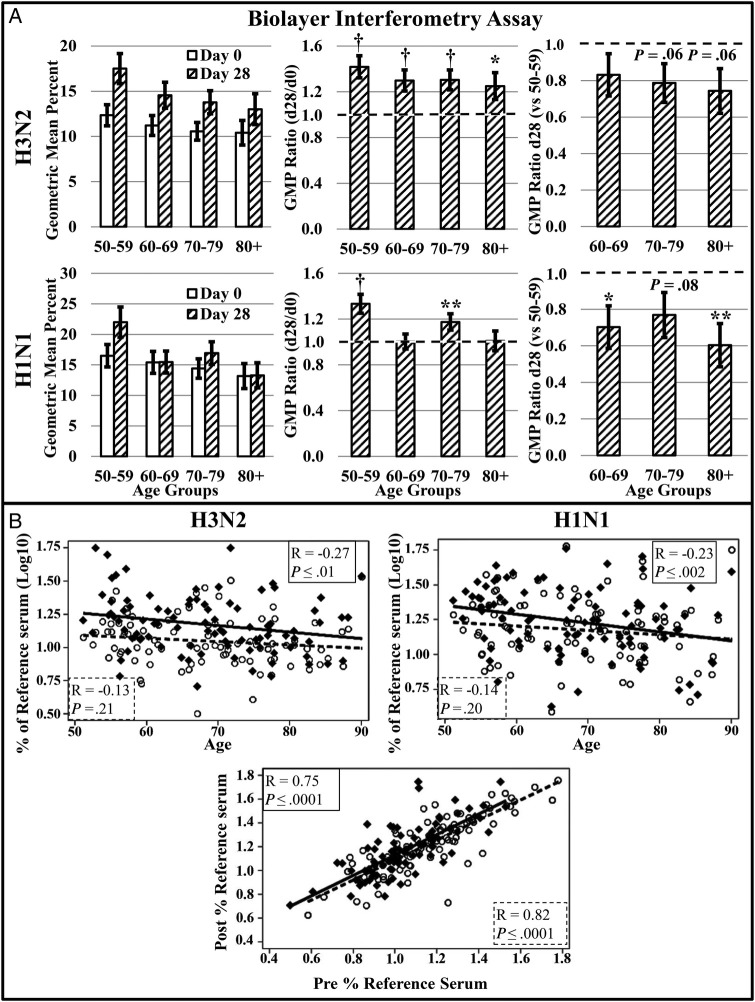
Serum antibody binding was assessed by biolayer interferometry. Antibody binding generally followed a similar trend to HI and MN titers. Changes in antibody binding induced by H1N1 were modest and variable by age group, with older subjects exhibiting lower antibody binding capacity than middle-aged subjects (Figure 2A). In contrast, subjects in all age groups increased antibody binding capacity to H3N2 postvaccination (Figure 2A). Adults in older age groups showed postvaccination binding comparable to middle-aged counterparts, although subjects in the 2 oldest age groups showed slightly lower binding, which approached significance (P = .06; Figure 2A).
Figure 2.
Assessment of antibody binding capacity by biolayer interferometry. A, Antibody binding capacity (n = 90) to H3N2 and H1N1 was assessed on day 0 prior to vaccination and day 28 after vaccination. The geometric mean percent (GMP) ratio (day 28 GMP/day 0 GMP) was calculated to determine the vaccine-associated change in antibody binding within each age group. A GMP ratio of 1 (dotted line) is indicative of no change. Day 28 GMP ratios were used to compare responses of older adult groups to middle-aged controls (day 28 GMP of older adult group/day 28 GMP of 50–59 age group). A GMT ratio less than 1 (dotted line) is indicative of lower postvaccination antibody binding. B, Antibody binding capacity was plotted by age and correlations calculated to determine the effect of age on preexisting ( ) and postvaccination antibody binding (
) and postvaccination antibody binding ( ). The correlation coefficient and P value are presented for preexisting (
). The correlation coefficient and P value are presented for preexisting ( ) and postvaccination (
) and postvaccination ( ) correlations with age. The influence of preexisting, cross-reactive antibody on the day 28 postvaccination response to H1N1 (
) correlations with age. The influence of preexisting, cross-reactive antibody on the day 28 postvaccination response to H1N1 ( ) and H3N2 (
) and H3N2 ( ) was also determined. The correlation of pre- and postvaccination antibody responses to H1N1 (
) was also determined. The correlation of pre- and postvaccination antibody responses to H1N1 ( ) and H3N2 (
) and H3N2 ( ) are depicted. The correlation coefficient and P value are presented for prevaccination H1N1 (
) are depicted. The correlation coefficient and P value are presented for prevaccination H1N1 ( ) and H3N2 (
) and H3N2 ( ) correlations with the postvaccination antibody binding capacity. Error bars represent 1 standard error. Significance is indicated by *P ≤ .05, **P ≤ .01, †P ≤ .001.
) correlations with the postvaccination antibody binding capacity. Error bars represent 1 standard error. Significance is indicated by *P ≤ .05, **P ≤ .01, †P ≤ .001.
Effect of Age on Antibody Titers
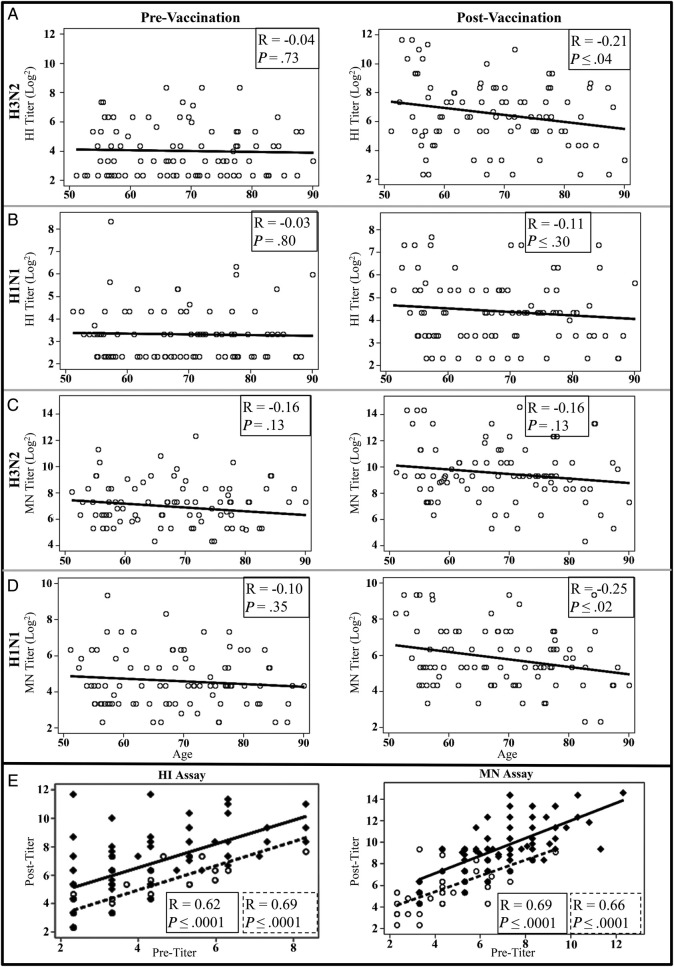
We found a modest and gradual decline in immune responses from middle-age through age 80+ years. The effect of age on humoral responses to TIV was not readily apparent with all assays and exhibited differences between strains. When correlations with age did achieve statistical significance, the correlations were relatively low with correlation coefficients ranging between −0.11 and −0.27 (Figures 2B and 3A–D). Postvaccination HI responses to H3N2 demonstrated a modest reduction in HI titers to H3N2 with increasing age (R = −0.21; Figure 3A), although the reduction demonstrated by MN assay did not reach significance (Figure 3C). Despite the reduced responses exhibited by all subjects to H1N1, MN titers to H1N1 were decreased modestly with increasing age (R = −0.25; Figure 3D). Postvaccination antibody binding capacity for both H1N1 and H3N2 also were negatively correlated with increasing age (Figure 2B). Furthermore, although postvaccination titers correlated with age, no effect of age was observed on prevaccination HI and MN titer or antibody binding (Figures 2B and 3A–D).
Figure 3.
Correlations with vaccine antibody response. Antibody to A and C, H3N2 and B and D, H1N1 were assessed from serum samples taken at prevaccination and 28 days postvaccination by A and B, HI (n = 88) and C and D, microneutralization ([MN] n = 90). Antibody levels were plotted by age, and correlations were calculated to determine the effect of age on preexisting and postvaccination antibody titers. The correlation coefficient and P value are presented for correlations with age. E, The influence of preexisting, cross-reactive antibody to H3N2 ( ) and H1N1 (
) and H1N1 ( ) on the postvaccination, day 28 response was also determined. The correlation of pre- and postvaccination antibody responses to H1N1 (
) on the postvaccination, day 28 response was also determined. The correlation of pre- and postvaccination antibody responses to H1N1 ( ) and H3N2 (
) and H3N2 ( ) are depicted. The correlation coefficient and P value are presented for prevaccination H1N1 (
) are depicted. The correlation coefficient and P value are presented for prevaccination H1N1 ( ) and H3N2 (
) and H3N2 ( ) correlations with the postvaccination titers. HI, hemagglutination inhibition.
) correlations with the postvaccination titers. HI, hemagglutination inhibition.
The best predictor of postvaccination response was prevaccination titers. All age groups significantly increased antibody titers in response to TIV (Figure 1A and B), but subjects with higher cross-reactive prevaccination HI and MN titers demonstrated higher postvaccination titers to H1N1 and H3N2 (Figure 3E) with correlation coefficients ranging from 0.62 to 0.69. Higher prevaccination binding capacity also correlated with higher postvaccination binding (Figure 2B) with correlation coefficients of 0.75 and 0.82 for H3N2 and H1N1, respectively.
Vaccine-Specific Memory B Cells
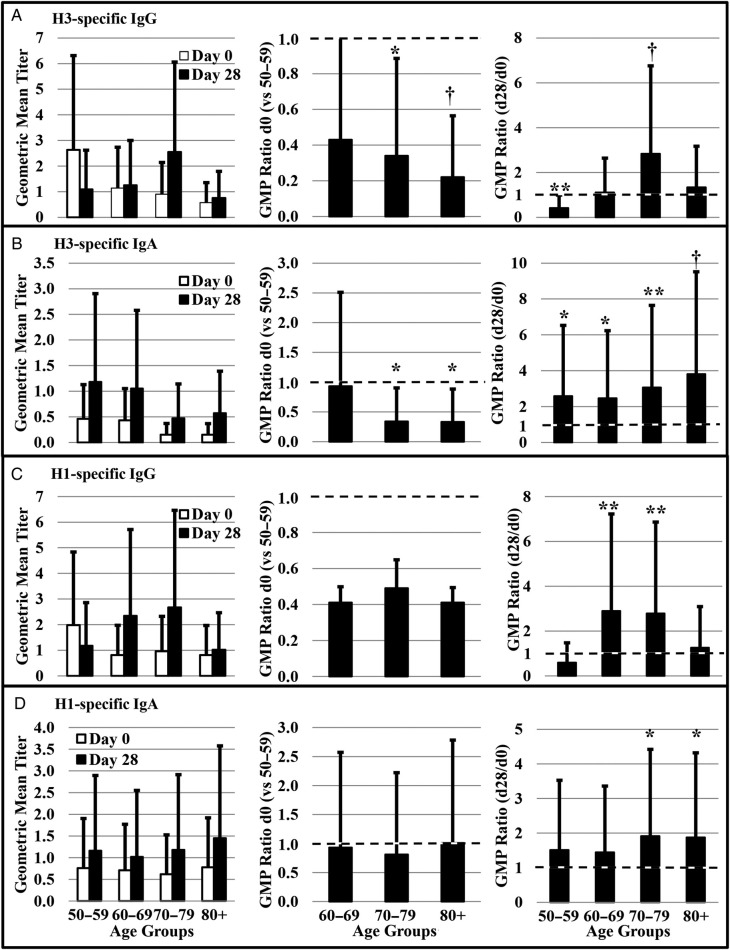
Frequencies of vaccine-specific memory B cells were evaluated by ELISPOT for 10 randomly selected individuals from each age group. Memory B cells were stimulated to selectively proliferate and differentiate into ASCs (Supplementary Figure 1), and isotype-specific ASCs were measured (Figure 4A–D, left panels). Subjects in the older age groups generally showed the lowest levels of preexisting memory B cells, but they also tended to demonstrate the largest expansion of memory B-cell populations in response to TIV vaccination (Figure 4). The frequencies of preexisting (d0) memory B cells in subjects aged 70+ years were lower than in middle-aged subjects (GMP ratio < 1) for H3-specific IgG and IgA memory B cells (Figure 4A and B). Although preexisting (d0) H1-specific IgG memory B cells tended to be lower in subjects over age 60, there were no significant differences over time in any of the age groups (Figure 4C). Postvaccination, H3- and H1-specific IgG memory B cells increased in the 70–79 age group as well as the 60–69 age group in the case of H1 (Figure 4A and C). It is interesting to note that middle-aged subjects had decreased numbers of H3-specific IgG memory B cells postvaccination (Figure 4A). All age groups exhibited increased numbers of H3-specific IgA memory B cells in response to vaccination. However, H1-specific IgA memory B cells increased only among subjects in the 2 oldest age groups (Figure 4B and D).
Figure 4.
Age-associated changes in preexisting memory B cells. The number of H3N2 (H3)-specific A, immunoglobulin (Ig)G+ and B, IgA+ as well as H1N1 (H1)-specific C, IgG+ and D, IgA+ memory B cells were assessed by B cell enzyme-linked immunospot at day 0 before vaccination and on day 28 postvaccination. The day 0 geometric mean percent (GMP) of memory B cells of older adults were compared with middle-aged controls by generating day 0 GMP ratios (day 0 GMP of older adult group/day 0 GMP of 50–59 age group). A d0 GMP ratio less than 1 (dotted line) is indicative of fewer antigen-specific, preexisting memory B cells. The GMP ratio (day 28/day 0% antigen-specific cells) was calculated to determine changes in the number of memory B cells within each age group associated with vaccination. A GMP ratio of 1 (dotted line) is indicative of no change. Error bars represent 1 standard error. Significance is indicated by *P ≤ .05, **P ≤ .01, †P ≤ .001; n = 42.
T-Cell Responses
Peripheral blood mononuclear cells were stimulated with live virus, and the proportions of IFN-γ and TNF-α-producing T cells were assessed. Although sampling closer to the time of vaccination would have been more beneficial for assessment of T-cell responses, it would not have been optimal for detection of changes in antibody titers. T-cell responses were highly variable, and the small number of subjects meant detection of potentially meaningful differences in T-cell responses was difficult (Supplementary Figure 2A–H). Similar to what was observed for memory B cells, subjects in the 50–59 year old age category generally had higher preexisting H1N1- and H3N2-specific CD4+ T-cell responses (Supplementary Figure 2I–L). However, after vaccination, CD4+ T-cell responses among older subjects appeared to be either comparable or lower but not statistically different from their middle aged counterparts (Supplementary Figure 2M–P). No significant CD8+ T-cell responses were found (data not shown); however, TIV is not known to effectively induce CD8+ T-cell responses.
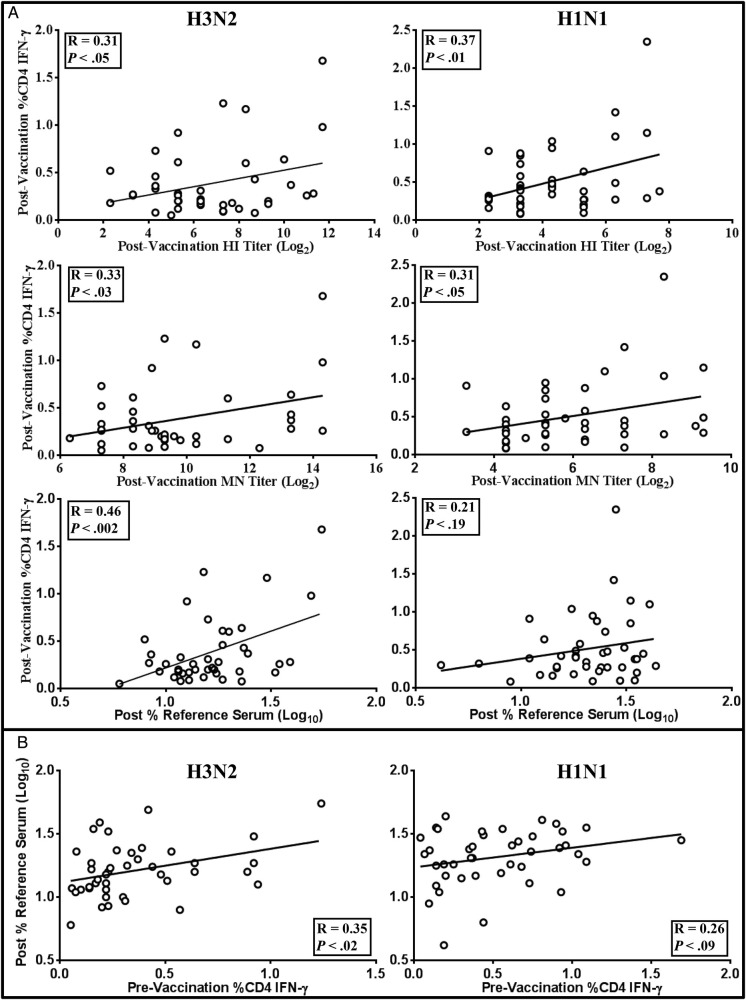
Postvaccination HI, MN, and antibody binding capacity in this study all correlated with IFN-γ-producing CD4+ T cells with correlation coefficients ranging from 0.31 to 0.46 (Figure 5A). Only antibody binding capacity for H1N1, which had demonstrated only low and variable changes to H1N1 (Figure 2A), did not correlate with IFN-γ-producing CD4+ T cells (Figure 5A). Tumor necrosis factor-α producing CD4+ T cells did not correlate with any of the serological responses, nor did CD8+ T cells demonstrate any correlation (data not shown). It is interesting to note that preexisting IFN-γ responses to H3N2 by CD4+ T cells correlated with postvaccination antibody binding (R = 0.35; Figure 5B), but not with HI or MN titers (data not shown). As with the postvaccination response, preexisting CD4+ T cell responses to H1N1 showed no correlation with antibody binding.
Figure 5.
Correlations with T-cell responses. A, Postvaccination interferon (IFN)-γ production by H1N1- or H3N2-stimulated CD4+ T cells correlated with postvaccination hemagglutination inhibition (HI), microneutralization (MN), and antibody binding capacity. B, correlations were also calculated between prevaccination IFN-γ+ CD4+ T cells and postvaccination antibody binding capacity to determine the influence of preexisting IFN-γ+ CD4+ T cells on antibody response to vaccination. The correlation coefficient and P values are presented for each comparison.
DISCUSSION
Immunosenescence is considered an impediment to protection of older adults from infectious diseases and a contributing factor to the observed reduced effectiveness of seasonal influenza vaccines in persons 65 years and older. Decreased T and B cell functionality and narrowing of the T and B cell repertoire results in reduced antibody titers, lower antibody affinity, and narrowing of the antibody repertoire [29–32].
All 2008–2009 TIV strains changed from the previous season's vaccine, resulting in the majority of the population studied here having a relatively low level of preexisting cross-reactive, neutralizing antibodies to the influenza A virus vaccine components. In our study, only 10.2% and 29.5% of subjects had preexisting HI titers ≥40 to H1N1 and H3N2, respectively (Table 1). During the 8 influenza seasons preceding the 2008–2009 season, there was only 1 change in the predominant H1N1 strain; the vaccine antigen changed from A/New Caledonia/20/1999 to A/Solomon Islands/3/2006 for the 2007–2008 season. In contrast, 4 different H3N2 strains predominated in the same period. In addition, the previous season was dominated by H3N2, with 60% of H3N2 samples tested being more antigenically similar to A/Brisbane/10 than to A/Wisconsin/67, that season's vaccine strain [33]. Thus, vaccination and/or natural infection with more antigenically diverse H3N2 strains in the recent past may have led to more cross-protective prevaccination titers for H3N2. Indeed, our memory B cell ELISPOT indicated that subjects had a larger number of preexisting H3N2-specific, IgG+ memory B cells than those against H1N1 (Figure 4A and C).
Three assays were used to comprehensively assess the effect of age on antibody responses. The HI assay has historically been used to assess protection from influenza and is the best understood correlate of protection against influenza infection in younger adults. However, HI is a surrogate assay and does not directly measure virus neutralization. The MN assay measures functional neutralization and has been suggested to be more sensitive than HI [34, 35], but a threshold MN titer associated with protection has not been described. Biolayer interferometry detects all antibody capable of binding to HA, not just those that neutralize virus infectivity, presumably including antibody with broader functionality such as opsonization, initiation of the complement cascade, and/or antibody-mediated cellular cytotoxicity. Thus, biolayer interferometry may give an indication of broader protection beyond simple virus neutralization, providing some indication of the quality of the antibody response.
Subjects in all age groups increased HI and MN titers postvaccination (Figure 1A and B); however, many did not exhibit HI titers ≥40. This was especially true for H1N1; only 33.7% of subjects reached titers ≥40, compared with 77.5% for H3N2 (Table 1). Subjects in all age groups demonstrated increased binding capacity to H3N2; however, only subjects age 50–59 and 70–79 increased H1N1 binding (Figure 2A). It should be noted, however, that H3N2 virus activity has been found consistently to be associated with more severe morbidity and mortality among older adults than H1N1 [2, 5]. Furthermore, as previously stated, cell-mediated immune responses may correlate better with protection in older adults than HI titers [20, 21]. Protection from influenza in older adults may be more complex than increased HI titers.
In general, we confirmed that antibody responses decreased with increasing age, although results varied by assay type and virus strain. Antibody binding capacity was negatively correlated with age for both strains, consistent with a potential impact on affinity maturation and possible narrowing of the antibody repertoire (Figure 2B). However, this effect was relatively modest; correlation coefficients ranged from −0.11 to −0.27. Prevaccination titers were the best predictors of postvaccination HI, MN, and antibody binding, with correlation coefficients between 0.62 and 0.82 (Figures 2B and 3E). Although postvaccination titers were impacted by age, prevaccination titers, the best predictors of postvaccination response, showed no correlation with age (Figures 2B and 3A–D). A meta-analysis by Voordouw et al [36] analyzing influenza clinical trial data from the European Union showed similar influences of age and prevaccination titers on postvaccination responses.
For a typical influenza season, some proportion of the population has at least partial immunity to circulating strains through prior vaccination or natural infection. Preexisting, antigen-specific or cross-reactive memory B cells are frequently the main source for protective antibody. Replacement of all 3 vaccine strains for the 2008–2009 influenza season provided a unique opportunity to assess changes in antigen-specific memory B cell frequency in association with age. Indeed, 2008–2009 TIV boosted the frequencies of class-switched memory B cells (antigen-specific IgG and IgA) in almost all age groups (Figure 4A–D). Subjects in all age groups significantly expanded H3-specific IgA memory B cells (Figure 4B), and despite the overall lower antibody titers to H1, there were significant expansions of H1-specific memory B cells in most age groups (Figure 4C and D). Although it is normally thought that HI activity and virus neutralization are primarily mediated by serum IgG, it has been speculated that IgA memory B cells may home to mucosal surfaces upon infection, providing protection of mucosal surface through local production of IgA [37]. Assessment of both IgA and IgG memory B cells may provide a more comprehensive picture of protection upon infection.
It is noteworthy that despite a reduced frequency of preexisting memory B cells associated with increasing age, fold-rises in memory B cell frequency among subjects aged 60+ was either comparable to or better than that found among subjects in the middle-age group (Figure 4A–D). These findings suggest that the gross function of B cells pertaining to memory B-cell generation was not drastically compromised. More importantly, however, binding capacity of post-vaccine serum antibody declined for both strains with increasing age (Figure 2B), indicating that the capacity of somatic hypermutation in antibody variable regions is affected by advancing age. Our data suggest that immunosenescence in advanced age is manifested more by qualitative changes than quantitative changes. This result is seemingly contradictory to recent studies by Sasaki et al [38] that showed frequencies of vaccine-specific plasmablasts and concentration of plasmablast-derived polyclonal antibody were lower in older adults than young adults, whereas yields of secreted IgG per plasmablast and overall vaccine-specific avidity or affinity of polyclonal antibodies were not different between age groups. In their study, subjects age 70–100 were compared with those aged 18–30, whereas our subjects were all 50 years and older. Although the quantity of the antibody response decreases in advanced age compared with young adults, this change could be gradual and subtle during transition from middle-age to older adults (60–79) to elderly adults (80+). Instead, qualitative changes (eg, affinity maturation, class switching, etc.) may start to compromise antibody responses. Consistent with this idea, the age-associated decline in HI titers was significant only for H3N2 (but not H1N1) and MN titer only for H1N1 (but not H3N2), yet antibody binding capacity for both antigens declined with age (Figure 2B).
Serological responses in this older adult population correlated with IFN-γ-producing CD4+ T cells, with subjects exhibiting higher numbers of IFN-γ+ CD4+ T cells also exhibiting higher HI, MN, and antibody binding capacity (Figure 5A). The role of IFN-γ in protection of all age groups against viral infection has long been recognized. McElhaney et al [20] previously demonstrated that older adults with higher IFN-γ/interleukin-4 ratios were less likely to exhibit influenza-like illness than those with lower ratios. Furthermore, preexisting IFN-γ+ CD4+ T cells correlated with postvaccination antibody binding capacity, whereas HI and MN titers did not (Figure 5B), suggesting that preexisting or cross-reactive IFN-γ+ CD4+ T cells may play a role in enhancement of antibody quality.
Preexisting immunity in this study had a significant effect on the ensuing immune response to vaccination. The constant evolution of the influenza virus makes yearly vaccine strains difficult to predict. Yet, despite the change in all 3 influenza vaccine strains for the 2008–2009 season, we found cross-reactivity to these strains in our study population. Although T cell epitopes are recognized as being much more highly conserved, we were also able to demonstrate low levels of preexisting or cross-reactive antibody. During the 2009 H1N1 pandemic, older adults were believed to be relatively more protected to the virus due to exposure to related viruses earlier in life [23]. This exemplifies the impact that prior immunity may have in providing protection from influenza and the potential longitude of this impact. The history of a person's influenza vaccination/infection likely influences their current responses, and the immune system of older adults may have an extensive lifetime of exposure from which to draw.
This study was limited by several factors. First, for several assays, it was feasible to test only a small number of samples, thus limiting the number of statistical comparisons that could be made and reducing the power to detect differences by age groups. Second, populations at the 2 sites were different. Participants at Vanderbilt were typically younger and more likely to be employed, whereas those at Marshfield were older and had previously received influenza vaccination. Differences observed between groups may not have been solely due to age but instead affected by differences in methods of recruitment or other site-specific differences. Finally, chronic preexisting medical conditions, sex, and race are all known to affect immune function, but variables representing these factors were not included in the analyses performed because of limited sample sizes.
CONCLUSIONS
Our findings underscore the importance of understanding not only immunosenescence as an obstacle for influenza vaccination of older adults but also other factors influencing vaccine responses. Older adults in this study were able to mount significant humoral and cell-mediated immune responses to TIV. Although age negatively affected these postvaccination responses, age was not the biggest influence. Prevaccination responses had a much larger impact, irrespective of age at vaccination. A better understanding of factors influencing preexisting, cross-reactive immunity would provide better insight into mechanisms for achieving better protection of older adults from yearly influenza epidemics.
Supplementary Material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank Dr. Michael Decker of Sanofi Pasteur (Swiftwater, PA) for providing TIV and monovalent influenza vaccines to assess cell-mediated immune responses.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry.
Financial support. This work was supported by the Centers for Disease Control and Prevention (CDC 1 U18 IP000184-01 and CDC 5 U18 IP000183-02) and in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and Centers for Disease Control and Prevention.
Potential conflicts of interest. H. K. T. has received research funding from Sanofi Pasteur and MedImmune. M. R. G. and M. S. have received research funding from MedImmune. J. M. K. has received research funding from GlaxoSmithKline and Juvaris, Inc. (now Colby Pharmaceuticals).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Thompson MG, Shay DK, Zhou H et al. . Estimates of deaths associated with seasonal influenza---United States, 1976--2007. MMWR 2010; 59:1057–62. [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E et al. . Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–86. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). Fact sheet N°211. April 2009. Available at: http://www.who.int/mediacentre/factsheets/fs211/en/index.html Accessed 3 March 2014.
- 4.Falsey AR, Hennessey PA, Formica MA et al. . Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 5.Thompson WW, Shay DK, Weintraub E et al. . Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–40. [DOI] [PubMed] [Google Scholar]
- 6.Mullooly JP, Bridges CB, Thompson WW et al. . Influenza- and RSV-associated hospitalizations among adults. Vaccine 2007; 25:846–55. [DOI] [PubMed] [Google Scholar]
- 7.Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine 2007; 25:3066–9. [DOI] [PubMed] [Google Scholar]
- 8.Aspinall R, Del Giudice G, Effros RB et al. . Challenges for vaccination in the elderly. Immun Ageing 2007; 4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whisler RL, Grants IS. Age-related alterations in the activation and expression of phosphotyrosine kinases and protein kinase C (PKC) among human B cells. Mech Ageing Dev 1993; 71:31–46. [DOI] [PubMed] [Google Scholar]
- 10.Weksler ME. Changes in the B-cell repertoire with age. Vaccine 2000; 18:1624–8. [DOI] [PubMed] [Google Scholar]
- 11.Naylor K, Li G, Vallejo AN et al. . The influence of age on T cell generation and TCR diversity. J Immunol 2005; 174:7446–52. [DOI] [PubMed] [Google Scholar]
- 12.Fulop T, Larbi A, Wikby A et al. . Dysregulation of T-cell function in the elderly: scientific basis and clinical implications. Drugs Aging 2005; 22:589–603. [DOI] [PubMed] [Google Scholar]
- 13.Gibson KL, Wu YC, Barnett Y et al. . B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell 2009; 8:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 2009; 9:185–94. [DOI] [PubMed] [Google Scholar]
- 15.Caraux A, Klein B, Paiva B et al. . Circulating human B and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal CD138− and CD138+ plasma cells. Haematologica 2010; 95:1016–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972; 70:767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull 1979; 35:69–75. [DOI] [PubMed] [Google Scholar]
- 18.Beare AS, Hobson D, Reed SE, Tyrrell DA. A comparison of live and killed influenza-virus vaccines. Report to the Medical Research Council's Committee on Influenza and other Respiratory Virus Vaccines. Lancet 1968; 2:418–22. [DOI] [PubMed] [Google Scholar]
- 19.Beare AS, Tyrrell DA, Hobson D et al. . Live influenza B vaccine in volunteers. A report to the Medical Research Council by their Committee on Influenza and Other Respiratory Virus Vaccines. J Hyg (Lond) 1969; 67:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McElhaney JE, Xie D, Hager WD et al. . T cell responses are better correlates of vaccine protection in the elderly. J Immunol 2006; 176:6333–9. [DOI] [PubMed] [Google Scholar]
- 21.McElhaney JE, Ewen C, Zhou X et al. . Granzyme B: correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine 2009; 27:2418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talbot HK, Coleman LA, Crimin K et al. . Association between obesity and vulnerability and serologic response to influenza vaccination in older adults. Vaccine 2012; 30:3937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancock K, Veguilla V, Lu X et al. . Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361:1945–52. [DOI] [PubMed] [Google Scholar]
- 24.Carney PJ, Lipatov AS, Monto AS et al. . Flexible label-free quantitative assay for antibodies to influenza virus hemagglutinins. Clin Vaccine Immunol 2010; 17:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods 2004; 286:111–22. [DOI] [PubMed] [Google Scholar]
- 26.Brown H, Prescott R. Applied Mixed Models in Medicine. 2nd ed Chichester, UK: J. Wiley & Sons; 2006. [Google Scholar]
- 27.Littell RC, Milliken GA, Stroup WW et al. . SAS for Mixed Models. 2nd ed Cary, NC: SAS Institute, Inc.; 2006. [Google Scholar]
- 28.He XS, Holmes TH, Zhang C et al. . Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol 2006; 80:11756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kogut I, Scholz JL, Cancro MP, Cambier JC. B cell maintenance and function in aging. Semin Immunol 2012; 24:342–9. [DOI] [PubMed] [Google Scholar]
- 30.Lefebvre JS, Haynes L. Aging of the CD4 T cell compartment. Open Longevity Science 2012; 6:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006; 24:1159–69. [DOI] [PubMed] [Google Scholar]
- 32.Allman D, Miller JP. B cell development and receptor diversity during aging. Curr Opin Immunol 2005; 17:463–7. [DOI] [PubMed] [Google Scholar]
- 33.Epperson S, Blanton L, Dhara R et al. . Influenza activity---United States and Worldwide, 2007–2008 season. MMWR 2008; 57:692–7. [PubMed] [Google Scholar]
- 34.Gitelman AK, Kaverin NV, Kharitonenkov IG et al. . Dissociation of the haemagglutination inhibition and the infectivity neutralization in the reactions of influenza A/USSR/90/77 (H1N1) virus variants with monoclonal antibodies. J Gen Virol 1986; 67(Pt 10):2247–51. [DOI] [PubMed] [Google Scholar]
- 35.Rowe T, Abernathy RA, Hu-Primmer J et al. . Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999; 37:937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voordouw AC, Beyer WE, Smith DJ et al. . Evaluation of serological trials submitted for annual re-licensure of influenza vaccines to regulatory authorities between 1992 and 2002. Vaccine 2009; 28:392–7. [DOI] [PubMed] [Google Scholar]
- 37.Czerkinsky C, Prince SJ, Michalek SM et al. . IgA antibody-producing cells in peripheral blood after antigen ingestion: Evidence for a common mucosal immune system in humans. Proc Natl Acad Sci U S A 1987; 84:2449–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki S, Sullivan M, Narvaez CF et al. . Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest 2011; 121:3109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.