Abstract
Background. Histoplasmosis-associated hemophagocytic lymphohistiocytosis (HLH) is a relatively rare disorder for which data are limited regarding optimal treatment and clinical outcomes in adults. We describe the clinical features, treatment, and outcomes of patients with histoplasmosis-associated HLH at our institution.
Methods. We performed a retrospective chart review of all inpatients at Parkland Hospital diagnosed with HLH associated with Histoplasma capsulatum from 2003 to 2013.
Results. Eleven cases of histoplasmosis-associated HLH over this time period were identified. Nine of eleven cases were males (82%). Nine of these patients had human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS), 1 was a renal transplant patient on immunosuppressants, and the other had no documented immunocompromise. The most common HLH criteria were splenomegaly (n = 10), fever (n = 10), and ferritin >500 ng/dL (n = 9). Urine Histoplasma antigen was positive in every patient tested (n = 9 of 9), and most antibodies for Histoplasma were positive if checked (n = 4 of 5). A majority of patients received liposomal amphotericin B (n = 9) with an average treatment duration of 11 days, and 5 patients also received prednisone, intravenous immunoglobulin (IVIG), or both. Overall, 5 patients died within 30 days (45.5%), and 7 patients died within 90 days (63.6%). Of the 5 patients that received immunosuppression, 4 died (80%), whereas in the group not given additional immunosuppression (n = 5), 2 died (40%).
Conclusions. Histoplasmosis-associated HLH among adults is a lethal disease of highly immunocompromised patients, especially patients with HIV/AIDS. Clinical features such as splenomegaly, elevated ferritin, and cytopenias should prompt evaluation for HLH in this population. Further data are needed to define the role of immunosuppression, IVIG, and highly active antiretroviral therapy in treating this condition.
Keywords: disseminated histoplasmosis, hemophagocytic syndrome, HIV
Hemophagocytic syndrome, also called hemophagocytic lymphohistiocytosis (HLH), is a rare syndrome characterized by a hyperstimulated but ineffective immune response with characteristic signs of fever, hepatosplenomegaly, and cytopenias. A majority of cases occur in children and are triggered by primary genetic disorders (“primary HLH”) that cause defects in cytotoxic functioning of natural killer (NK) and T lymphocytes. In adults, “secondary HLH” can be triggered by hematologic malignancies and autoimmune diseases, but more often it occurs with various bacterial infections, fungi, and viruses [1, 2]. Although aggressive chemotherapy treatment protocols and allogeneic stem cell transplant have been shown to decrease mortality in children with HLH due to a genetic predisposition, optimal treatment of infection-related HLH in adults is not clear [3, 4]. Some physicians treat only the underlying infection, whereas others use immune suppression in addition to antimicrobials. The literature presents scant evidence regarding the efficacy of chemotherapy in infection-related HLH.
Rather than a disease on its own, HLH can be viewed as an immune derangement and hypercytokinemia caused by various triggers. Under normal conditions, intracellular infections are controlled by a complex interaction of cell types, to which NK cells are central. In HLH, defects in NK cell cytotoxicity result in a positive feedback loop of uncontrolled intracellular infection, ongoing immune activation, and a lack of immune down-regulation. Without NK cell removal of cytotoxic T lymphocytes (CTLs), a sustained increase in activated lymphocytes generates a cytokine storm and unopposed macrophage activation that damage end organs of the host [5, 6].
Clinical and laboratory findings in HLH include markers of immune activation as well as end organ damage. High levels of tumor necrosis factor (TNF)-α production by macrophages stimulates fever and inhibits the ability of lipoprotein lipase to remove triglycerides from the serum. Activated macrophages phagocytose erythrocytes and scavenge heme, but they also secrete ferritin and contribute to hyperferritinemia. Cytopenias are not just a result of hemophagocytosis, but they also related to suppression of normal hematopoiesis by cytokines. Macrophages may also secrete plasminogen activators that accelerate the conversion of plasminogen to plasmin, causing hyperfibrinolysis and leading to characteristically low fibrinogen levels. Soluble interleukin (IL)-2 receptor, which is secreted by activated macrophages and lymphocytes, is extremely high in HLH as well [5].
In cases of secondary HLH, the pathophysiology is thought to be from temporary acquired immunodeficiency states that result in NK cell defects. In HLH, the perforin-mediated killing function is blocked leading to an accumulation of activated T lymphocytes and activated histiocytes with increasingly high levels of cytokines. Human immunodeficiency virus (HIV), for instance, is known to cause NK cell defects, which may explain the higher incidence of HLH in HIV patients [7–11].
With the understanding that HLH is a result of defective NK cell function, it becomes clear that intracellular organisms are uniquely positioned to trigger HLH, because NK cells are essential for clearing infected somatic cells and activated CTL. All of the pathogens currently described as triggers of HLH are either intracellular or facultatively intracellular, including viruses (Epstein-Barr virus [EBV], cytomegalovirus, HIV), parasites (malaria, leishmania), mycobacteria, fungi, and bacteria (Babesia, Listeria, Coxiella). Without functioning NK cells, a host suffers a double defect of an exuberant but ineffective immune response that damages the host without clearing the infection.
The existing treatment and outcomes data for histoplasmosis-associated HLH in adults consist of 27 reported cases (summarized in Table 1). Seventeen of the cases occurred in HIV patients, a majority from before the era of highly active antiretroviral therapy (HAART). The mortality rate for all reported cases was 10 of 26 (38%). Treatment is not always detailed, but half of cases report using antifungal therapy alone. Five of 26 cases report using antifungal therapy in addition to immune suppression. The remainder did not diagnose the condition pre-mortem or did not detail a treatment regimen. The paucity of data highlights the need for reporting of more cases in the current era to examine whether any treatments can aid in survival of these patients.
Table 1.
Previous Cases of Histoplasma-Associated HLH Reported in the Literaturea
| Author | Year | Underlying Disease | CD4 | Treatment | Outcome |
|---|---|---|---|---|---|
| Majluf-Cruz [12] | 1993 | HIV | NR | Fluconazole | Survived |
| HIV | NR | Amphotericin B | Survived | ||
| HIV | NR | none | Died | ||
| Keller [13] | 1994 | CMC | N/A | Amphotericin B | Survived |
| Koduri [14] | 1995 | None | N/A | Amphotericin B/solumedrol | Died |
| Koduri [15] | 1995 | HIV | 36 | ART/Amphotericin B/ IVIG × 2d | Died |
| HIV | 4 | ART/Amphotericin B/ IVIG × 2d | Died | ||
| HIV | 6 | ART/Amphotericin B/ IVIG × 2d | Died | ||
| HIV | 22 | ART/Amphotericin B/ IVIG × 2d | Survived | ||
| HIV | 32 | ART/Amphotericin B | Survived | ||
| HIV | 44 | ART/Amphotericin B | Survived | ||
| Chemlal [16] | 1997 | HIV | 34 | NR | NR |
| Kumar [17] | 2000 | None | N/A | None | Died |
| HIV | NR | None | Died | ||
| Rao [18] | 2002 | CLL | N/A | Amphotericin B | Survived |
| Masri [19] | 2003 | Heart transplant | N/A | Amphotericin B | Survived |
| Gil-Brusola [20] | 2007 | HIV | 39 | None | Died |
| Guiot [21] | 2007 | HIV | 66 | Abelcet × 36d --> itraconazole | Survived |
| Sanchez [22] | 2007 | HIV | NR | Amphotericin B × 6 wks | Survived |
| Wang [23] | 2007 | CKD/ fungal endocarditis | N/A | None | Died |
| Phillips [24] | 2008 | Sarcoidosis on chronic steroids | N/A | NR | Survived |
| De Lavaissiere [25] | 2009 | HIV | NR | ART/IVIG × 2 g/Amphotericin B × 4 wks --> itraconazole | Survived |
| Lo [26] | 2010 | Renal transplant | N/A | Ambisome × 2 wks --> itraconazole; reduced immunosuppression (IS) | Survived |
| Renal transplant | N/A | Amphotericin B × 1 wk --> itraconazole; reduced IS | Survived | ||
| Van Koeveringe [27] | 2010 | CLL | N/A | Amphotericin B | Survived |
| Vaid [28] | 2011 | HIV | 153 | Antifungal and ART | Died |
| Chandra [29] | 2012 | HIV | NR | Ketoconazole | Survived |
Abbreviations: ART, antiretroviral therapy; CKD, chronic kidney disease; CLL, chronic lymphocytic leukemia; CMC, chronic mucocutaneous candidiasis; HIV, human immunodeficiency virus; HLH, hemophagocytic lymphohistiocytosis; IVIG, intravenous immunoglobulin; N/A, not applicable; NR, not reported.
a Underlying disease, treatment, and outcomes.
In this study, we sought: (1) to identify cases of histoplasmosis-associated HLH at our large urban safety net hospital, (2) to describe the clinical characteristics of patients with histoplasmosis-induced HLH including diagnostic criteria, and (3) to describe the treatment regimens and clinical outcomes of this study population.
METHODS
We performed a retrospective chart review of cases of histoplasma-associated HLH at Parkland Hospital in Dallas, Texas, a large urban hospital that is the sole public safety net provider in Dallas County. Records were reviewed from December 2003 to February 2013. Cases were identified searching the results of bone marrow biopsy specimens from 2003 to 2013 for the key term “hemophagocytosis.” This was the time period during which the pathology department electronically coded cases in a searchable database. The reports of bone marrow specimens with mention of hemophagocytosis were reviewed for evidence of histoplasmosis infection and absence of malignancy. The last case that did not have a bone marrow specimen was included because the patient was seen by the authors as an inpatient consultation during the time of the review, and the patient was found to have evidence of HLH by laboratory criteria. This study was approved by the UT Southwestern Medical Center Institutional Review Board.
Patients were excluded from review if they were <18 years of age, and if no clinical records were available. Record review was via paper charts, which often contained limited data, and the electronic medical record for patients after 2006. Patients were considered to have possible (4 of 8 criteria) or confirmed (5 of 8 criteria) HLH according to the HLH-2004 criteria: (1) Fever; (2) Splenomegaly; (3) Cytopenias affecting 2/3 cell lines in the peripheral blood; (4) Hypertriglyceridemia and/or hypofibrinogenemia; (5) Hemophagocytosis in the spleen, bone marrow, or lymph nodes, with no evidence of malignancy; (6) Low or absent NK cell activity (not available at our facility); (7) Ferritin >500 ng/dL; (8) Soluble IL-2 receptor >2400 U/mL (not available at our facility) [3]. Patients were considered to have disseminated Histoplasma capsulatum if any of the following were true as per Infectious Diseases Society of America guidelines: (1) cultures from sputum or a sterile body site (bone marrow, lymph node, biopsy specimen) grew H capsulatum; (2) biopsy specimens contained granulomas with yeast morphologically consistent with H capsulatum; or (3) urine or serum Histoplasma antigen was positive [30].
For all patients meeting inclusion criteria, data were collected on patient demographics, comorbidities, presence of fever, presence of organomegaly, laboratory values (complete blood counts, ferritin, lactate dehydrogenase [LDH], triglycerides, fibrinogen), microbiologic data, diagnostic procedures (bone marrow biopsy, bronchoscopy), treatment course (antifungal medication, chemotherapy), and outcomes (survival to hospital discharge). Immunosuppression included corticosteroids, TNF inhibitors, calcineurin inhibitors, cytotoxic chemotherapy, intravenous immunoglobulin (IVIG), and methotrexate.
RESULTS
Eleven cases of histoplasmosis-associated HLH were identified: 10 patients with hemophagocytosis on bone marrow examination, and 1 patient reported by the Infectious Disease consult service at the time of our search who did not undergo a bone marrow biopsy but met other laboratory criteria for HLH. Cases occurred between December 2003 and February 2013. The demographics and clinical characteristics of these patients are presented in Table 2. A majority of the patients had HIV (9 of 11). One was a renal transplant recipient, and the other had no known immunosuppression. A majority were male (9 of 11), with a mean age of 43.9 years. The majority of HIV patients were not on HAART at diagnosis (6 of 9), and the mean CD4 count was very low at 14.3. The average time between admission and bone marrow biopsy was 9 days (range, 3–15). Antifungal start dates were not routinely available.
Table 2.
Characteristics of Patients With Histoplasmosis-Induced HLH, 2003–2013
| Case Number | Age | Gender | Ethnicity | Country of Origin | HIV | Immunosuppressive Medications at Diagnosis | CD4 Count | HIV VL | ART at Time of dx |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 31 | Female | Hispanic | Mexico | Yes | No | 1 | None | Yes |
| 2 | 53 | Male | Non-Hispanic/White | USA | Yes | No | 6 | 205 000 | Yes |
| 3 | 33 | Female | Non-Hispanic/Black | USA | Yes | No | 1 | 750 000 | No |
| 4 | 47 | Male | Hispanic | Mexico | No | Yes | N/A | N/A | N/A |
| 5 | 28 | Male | Non-Hispanic/Black | USA | Yes | No | Unknown | Unknown | Yes |
| 6 | 60 | Male | Hispanic | Unknown | No | Yes | N/A | N/A | N/A |
| 7 | 44 | Male | Hispanic | Unknown | Yes | No | 2 | 190 000 | No |
| 8 | 52 | Male | Non-Hispanic/White | USA | Yes | No | 16 | 6 440 000 | No |
| 9 | 52 | Male | Non-Hispanic/White | USA | Yes | No | 16 | 6 440 000 | No |
| 10 | 32 | Male | Hispanic | El Salvador | Yes | No | 50 | >10 000 000 | Yes |
| 11 | 51 | Male | Non-Hispanic/White | USA | Yes | No | 9 | 6036 | No |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; HLH, hemophagocytic lymphohistiocytosis; N/A, not applicable; VL, viral load.
Results of the microbiology and radiologic testing done during inpatient evaluation are shown in Table 3. The most consistent diagnostic finding was a positive urine Histoplasma antigen, which was positive in 100% of the specimens that were sent (9 of 9). Eight patients had H capsulatum visualized on bone marrow biopsy (Figure 1), 7 had positive blood cultures for H capsulatum, and 4 had intracellular yeast seen on peripheral smear (Figure 2). A bronchoalveolar lavage specimen from one of the patients is shown in Figure 3. On chest radiography, the most common finding was bilateral infiltrates (6 of 11), but other findings included pleural effusions (n = 5), pulmonary nodules (n = 3) (Figure 4), lymphadenopathy (n = 4), pneumothorax (n = 1), cardiomegaly (n = 3), and pulmonary edema (n = 1).
Table 3.
Microbiologic and Radiologic Findings in Patients With Histoplasma-Associated HLH
| Case Number | Bone Marrow With Yeast | Histoplasma on Peripheral Smear | Bone Marrow With Hemophagocytosis | Histoplasma Antibodya (<1:8) | Urine Histoplasma Ag (<2.0 EIA) | Sites Growing Histoplasma | CXR Findings |
|---|---|---|---|---|---|---|---|
| 1 | Yes | Yes | Yes | N/A | N/A | Blood, bone marrow, | BI, B effusions, CM |
| 2 | Yes | Yes | Yes | Positive | Positive | None | BI, LAD |
| 3 | No | No | Yes | N/A | >13 | Blood | Hemothorax, right pneumothorax |
| 4 | No | No | Yes | Positive | Positive | None | BI, L effusion |
| 5 | Yes | Yes | Yes | N/A | >13 | Blood, bone marrow | Clear |
| 6 | Yes | No | Yes | N/A | >13 | Bone marrow, skin | CM |
| 7 | Yes | No | Yes | N/A | 11 | Sputum, blood | Edema, BI, effusions |
| 8 | Yes | No | Yes | Positive | >19 | Bone marrow | Miliary nodules, effusions, LAD |
| 9 | Yes | No | Yes | N/A | >19 | Blood, bone marrow | Miliary nodules |
| 10 | Yes | No | Yes | Positive | N/A | Respiratory blood, bone marrow | CM, BI, nodules, L effusion |
| 11 | No | Yes | Not done | Negative | 4.9 | Respiratory, blood, gastric tissue | BI, nodules |
Abbreviations: Ag, antigen; B, bilateral; BI, bilateral infiltrates; CM, cardiomegaly; EIA, enzyme immunoassay; HLH, hemophagocytic lymphohistiocytosis; L, Left; LAD, lymphadenopathy; N/A, not applicable (not reported).
a Complement fixation titer, ARUP laboratories.
Figure 1.
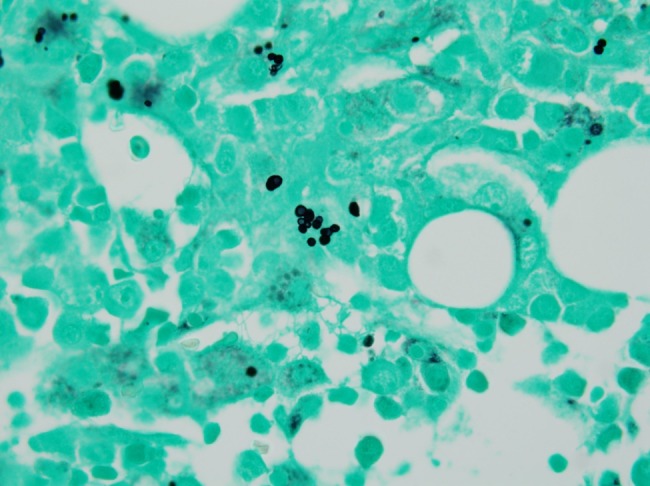
Gomori methenamine silver stain of bone marrow, 40×. Intracellular yeast consistent with Histoplasma capsulatum.
Figure 2.
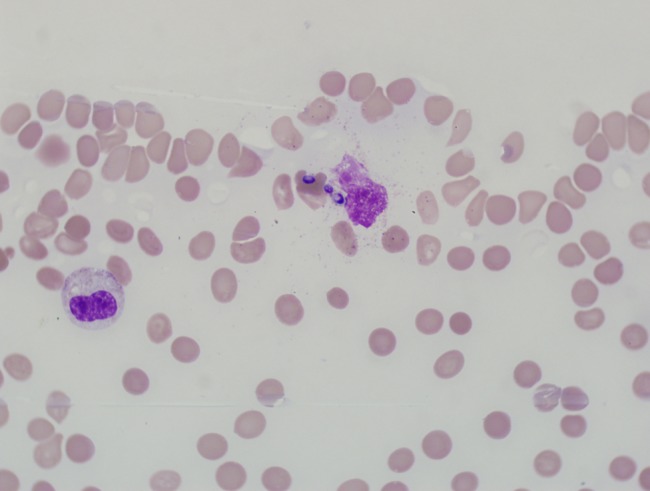
Peripheral blood smear showing intracellular yeast. 100× oil. Modified Wright-Giemsa stain.
Figure 3.
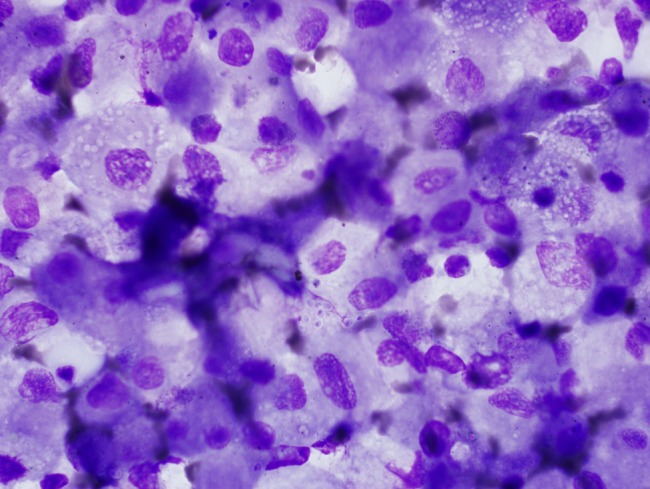
Bronchoalveolar lavage specimen showing intracellular yeast. Wright stain, 1000×.
Figure 4.
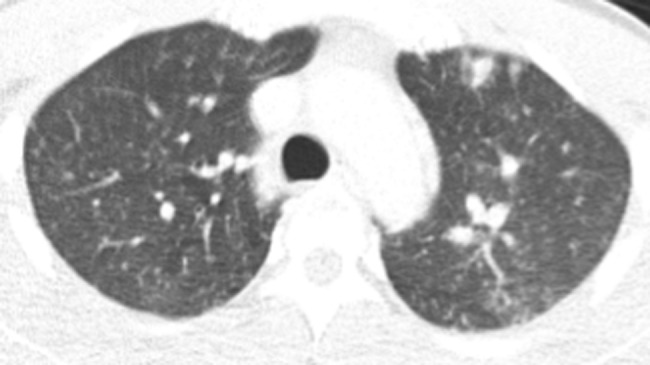
Chest computed tomography with mixed pattern of large and small nodules.
Specific laboratory values included in the HLH-2004 criteria are reported in Table 4. Soluble IL-2 receptor levels and NK cell activity were not available. All patients had fever documented except for 2 patients, who had sparse medical records available. Cytopenias were universal and severe, with most patients having platelet counts <50 × 103/µL. The mean ferritin level was extremely high at 30 722 ng/mL (range, 1713–100 000+). All but one patient had documented splenomegaly.
Table 4.
Clinical Features and Diagnostic Criteria for HLH-2004
| Case Number | Tmax During Admission | Rash | Splenomegaly | Hemoglobin Nadir (12.4–17.3 g/dL) | Platelet Nadir (150–450 ×103/µL) | Peak Ferritin (30.0–400.0 ng/mL) | LDH (135–225 U/L) | HLH Criteria |
|---|---|---|---|---|---|---|---|---|
| 1 | 39.8 | No | Yes | 6 | 12 | Unknown | 1336 | 5 of 8 |
| 2 | 38.6 | No | Yes | 7.1 | 9 | >16 500 | 971 | 5 of 8 |
| 3 | 37 | No | Yes | 6 | 48 | 1713 | 227 | 4 of 8 |
| 4 | 39.2 | No | Yes | 5.9 | 16 | >16 500 | 1815 | 6 of 8 |
| 5 | 37.3 | No | Yes | 7.3 | 70 | >16 500 | 1277 | 4 of 8 |
| 6 | 38.5 | Yes | No | 8.8 | 45 | Unknown | 971 | 5 of 8 |
| 7 | 39.9 | No | Yes | 6.3 | <5 | >16 500 | 1402 | 5 of 8 |
| 8 | 38.7 | No | Yes | 7 | 4 | 4392 | 402 | 6 of 8 |
| 9 | 39.4 | No | Yes | 7 | 4 | 4400 | 402 | 5 of 8 |
| 10 | 101.9 | No | Yes | 5.7 | 7 | >100 000 | 2517 | 5 of 8 |
| 11 | 39.5 | No | Yes | 5.7 | 10 | >100 000 | 8004 | 5 of 8 |
Abbreviations: HLH, hemophagocytic lymphohistiocytosis; LDH, lactate dehydrogenase; T, temperature.
From the available treatment data, the most commonly reported treatment regimen was liposomal amphotericin B (n = 9) for a mean of 11 days (range, 3–21), followed by oral antifungals (most commonly itraconazole) for 3–12 months (n = 5) (see Table 5). Three patients died during the initial amphotericin course.
Table 5.
Treatment and outcomes of Histoplasma-associated hemophagocytic lymphohistiocytosis
| Case Number | Antifungal drug used | Liposomal amphotericin B duration (days) | Oral antifungal duration (days) | Immuno suppressive treatment | HAART at time of diagnosis | Immuno suppressive duration | HAART started during admission | Outcome at 30 days | Survival from admission (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Liposomal amphotericin B | Unknown | 0 days | None | Yes | None | No | Died | 16 |
| 2 | Liposomal amphotericin B; itraconazole | 14 | Unknown | None | Yes | None | Yes | Alive | 2168 |
| 3 | Liposomal amphotericin B; fluconazole | 21 | >1 year | Prednisone 20 mg PO daily | No | Unknown | No | Died | 221 |
| 4 | Itraconazole | 0 | Unknown | None | N/A | None | N/A | Alive | 44 |
| 5 | Unknown | Unknown | Unknown | Unknown | Yes | Unknown | Unknown | Alive | 3560 |
| 6 | Liposomal amphotericin B; voriconazole | Unknown | Unknown | Prednisolone 10 mg po BID, Tacrolimus 1.5 mg PO daily | N/A | 5 months | N/A | Alive | 2849 |
| 7 | Liposomal amphotericin B; itraconazole | 16 | 12 weeks | None | No | None | No | Alive | 86 |
| 8 | Liposomal amphotericin B; itraconazole | 5 | 0 days | IVIG × 1; prednisone 40 BID | No | 9 days | No | Died | 9 |
| 9 | Liposomal amphotericin B; itraconazole | 3 | Unknown | IVIG 1gm × 1 | No | 1 day | No | Died | 9 |
| 10 | Liposomal amphotericin B; itraconazole | 18 | >1 year | None | Yes | None | No | Alive | 408 |
| 11 | Liposomal amphotericin B | 11 | 0 days | Prednisone 40 mg PO BID; solumedrol 60 mg IV q6hrs | No | 11 days | Yes | Died | 13 |
Abbreviations: HAART, Highly active antiretroviral therapy; IVIG, intravenous immunoglobulin.
In addition, 2 HIV patients received steroids, 1 received IVIG alone, and 1 received steroids in addition to IVIG. One renal transplant patient continued on his home regimen of prednisolone and tacrolimus, whereas the other renal transplant patient had no documented immunosuppressive treatment. One patient was started on HAART during the admission, whereas 4 others were continued on their outpatient HAART regimens. One patient presented with HLH within 3 weeks of starting HAART, which is consistent with an immune reconstitution inflammatory syndrome (IRIS).
Of the 5 patients that received immunosuppression during admission (including prednisone, IVIG, or both), 4 died (80%), whereas in the group not given additional immunosuppression (n = 5), 2 died (40%). The remaining patient survived but his treatment course was not available for review. Overall 30-day mortality was high (5 of 11 patients, 45.5%). Four patients died during the admission (within 16 days), while one patient died 7 months later. There was not a significant difference in mortality between patients who did or did not receive immunosuppression (P = .24, 2 tailed Fisher's exact test) although the comparison was underpowered.
DISCUSSION
We report eleven cases of Histoplasma-associated HLH, the largest case series of this clinical phenomenon that has been reported to date. The relatively large number of Histoplasma-associated HLH cases at our hospital, compared with the number of cases reported at other institutions in the literature, may be due to the geographic overlap of histoplasmosis and HIV as well as the under-recognition of this condition in other settings [31, 32]. We hypothesize that this condition may be even more prevalent than we found in our series, given our somewhat limited case finding strategy. In addition, while many clinicians recognize that disseminated histoplasmosis causes high fevers, an elevated LDH, and a high ferritin, they may not consider HLH in this clinical scenario. In our series, the mean time from presentation to bone marrow was >1 week, which may have led to treatment delays. It remains to be seen if early diagnosis could lead to improved outcomes with earlier institution of antimicrobial and possibly immunsuppressive therapy.
In our series, uncontrolled HIV was the dominant risk factor for this condition, while renal transplant patients are also at risk [33]. HIV patients may be uniquely vulnerable to HLH given the depletion of NK cells in HIV, which has been connected to multiple manifestations of immune dysregulation [20, 34, 35]. The predominance of males is difficult to explain, as no gender predilection has been reported for HLH or histoplasmosis, but may be related to a higher prevalence of HIV in males in Dallas. Guidelines do not recommend routinely screening for disseminated histoplasmosis in HIV patients before starting HAART [36], nor is this done in renal transplant patients. However, it may be reasonable to screen patients with severe anemia or thrombocytopenia with a urine histoplasma antigen, especially those planned for intense immunosuppression, so that their infection might be recognized and treated prior to immunosuppression.
One interesting clinical finding in our series that has not been described previously is the occurrence of a cardiopulmonary syndrome in patients with HLH. Several patients had either cardiomegaly, pleural effusions, or pulmonary edema on their chest radiographs. A high output state related to anemia in these patients may be contributing, possibly in addition to the cytokine storm causing leaky vasculature.
Treatment for the triggering condition is recommended as first line therapy for patients with HLH, although indications for initiating chemotherapy directed at HLH is less clear in the adult population with the sporadic (rather than inherited form) of the disease. Although all patients in our series received antifungal treatment with amphotericin products and/or azole treatment, there was wide variability on whether or not immunomodulation was used. None of the patients in this series were treated with the chemotherapy protocols such as those recommended for HLH in children (etoposide, dexamethasone with or without intrathecal therapy),although half of patients in our series received steroids, IVIG, or both. Given the small sample size, no benefit or harm could be attributed to IVIG or steroids. Since a majority of tissue damage is caused by cytotoxic lymphocytes, therapies such as corticosteroids and cytotoxic chemotherapies such as those used in children make sense as strategies to control the inflammation, but have not been established in adults. More targeted immunosuppression in macrophage activation syndrome (a similar pathologic process) is being evaluated in studies underway using cytokine antagonists and IL-1 receptor antagonists [37].
The role of HAART for treatment of this condition is not clear. Although HAART may improve outcomes in patients not receiving HIV treatment at the time of developing HLH, it also may precipitate HLH. In our series, one patient presented with HLH within 3 weeks of starting HAART, which is consistent with an immune reconstitution inflammatory syndrome (IRIS). Prior publications have reported HLH as a manifestation of IRIS in HIV-positive patients, 2 with HIV alone, 1 associated with leishmania, 1 with EBV, and 1 with lymphoma [25, 38–43]. As with other chronic fungal and mycobacterial diseases that have a strong potential to cause IRIS, it may be reasonable to wait for the acute phase of HLH to resolve before instituting HIV treatment.
There remains considerable controversy surrounding treatment of adult patients with HLH related to infectious triggers. Our comparison between patients who did or did not receive additional immunosuppression detected more survivors in the non-immunosuppression group, although the analysis is underpowered and may reflect treatment bias of individuals who were more ill. Additional data, even if retrospective, must be compiled to help guide decisions in this difficult-to-treat group.
This study has several limitations. First, our search strategy focused on bone marrow biopsy results, which may have missed patients with histoplasmosis-associated HLH who did not undergo bone marrow biopsy. Nonetheless, we were able to identify 11 cases over an approximately 10-year period, which is the largest case series of histoplasmosis-associated HLH to date. Second, the medical records from patients occurring before implementation of the electronic medical record had less detailed information about treatment regimens. Finally, because this is a retrospective review with a small sample size, no conclusions can be reached on the impact of different treatment regimens on clinical outcomes. Prospective treatment studies would be ideal, but they are unlikely given the rarity of this disease.
CONCLUSIONS
Histoplasma-associated HLH was relatively common at our large urban safety net hospital in Dallas, Texas, and uncontrolled HIV patients make up the majority of patients, whereas solid organ transplant patients also seem to be at risk. Key features that should prompt evaluation for this condition are splenomegaly, an extremely elevated ferritin, and cytopenias in an immunocompromised patient. Fungal blood cultures and urine Histoplasma antigen are high-yield tests in this setting for diagnosing H capsulatum as the underlying trigger for HLH. The mortality rate is high despite the use of antifungal agents and immune suppression. More data on treatment for this condition are needed in adults. The role and timing of HAART initiation in treatment of this condition remains unclear.
Acknowledgments
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (award Number KL2TR001103; to A. N.).
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rouphael NG, Talati NJ, Vaughan C et al. . Infections associated with haemophagocytic syndrome. Lancet Infect Dis 2007; 7:814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filipovich AH. The expanding spectrum of hemophagocytic lymphohistiocytosis. Curr Opin Allergy Clin Immunol 2011; 11:512–6. [DOI] [PubMed] [Google Scholar]
- 3.Henter JI, Horne A, Arico M et al. . HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007; 48:124–31. [DOI] [PubMed] [Google Scholar]
- 4.Tseng YT, Sheng WH, Lin BH et al. . Causes, clinical symptoms, and outcomes of infectious diseases associated with hemophagocytic lymphohistiocytosis in Taiwanese adults. J Microbiol Immunol Infect 2011; 44:191–7. [DOI] [PubMed] [Google Scholar]
- 5.Chandrakasan S, Filipovich AH. Hemophagocytic lymphohistiocytosis: advances in pathophysiology, diagnosis, and treatment. J Pediatr 2013; 163:1253–9. [DOI] [PubMed] [Google Scholar]
- 6.Meeths M, Chiang SC, Lofstedt A et al. . Pathophysiology and spectrum of diseases caused by defects in lymphocyte cytotoxicity. Exp Cell Res 2014; 325:10–7. [DOI] [PubMed] [Google Scholar]
- 7.Tincati C, Basilissi M, Sinigaglia E et al. . Invariant natural killer T (iNKT) cells in HAART-treated, HIV-positive patients with bone and cardiovascular impairment. PloS One 2014; 9:e110287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parasa VR, Selvaraj A, Sikhamani R, Raja A. Interleukins 15 and 12 in combination expand the selective loss of natural killer T cells in HIV infection in vitro. Clin Exp Med 2014; 15:205–13. [DOI] [PubMed] [Google Scholar]
- 9.Naluyima P, Eller MA, Laeyendecker O et al. . Impaired natural killer cell responses are associated with loss of the highly activated NKG2A(+)CD57(+)CD56(dim) subset in HIV-1 subtype D infection in Uganda. AIDS 2014; 28:1273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merino AM, Dugast AS, Wilson CM et al. . KIR2DS4 promotes HIV-1 pathogenesis: new evidence from analyses of immunogenetic data and natural killer cell function. PloS One 2014; 9:e99353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillon SM, Lee EJ, Bramante JM et al. . The natural killer cell interferon-gamma response to bacteria is diminished in untreated HIV-1 infection and defects persist despite viral suppression. J Acquir Immune Defic Syndr 2014; 65:259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majluf-Cruz AS, Hurtado Monroy R, Souto-Meirino C et al. . [Hemophagocytic syndrome associated with histoplasmosis in the acquired immunodeficiency syndrome: description of 3 cases and review of the literature]. Sangre (Barc) 1993; 38:51–5. [PubMed] [Google Scholar]
- 13.Keller FG, Kurtzberg J. Disseminated histoplasmosis: a cause of infection-associated hemophagocytic syndrome. Am J Pediatr Hematol Oncol 1994; 16:368–71. [PubMed] [Google Scholar]
- 14.Koduri PR, Carandang G, DeMarais P, Patel AR. Hyperferritinemia in reactive hemophagocytic syndrome report of four adult cases. Am J Hematol 1995; 49:247–9. [DOI] [PubMed] [Google Scholar]
- 15.Koduri PR, Chundi V, DeMarais P et al. . Reactive hemophagocytic syndrome: a new presentation of disseminated histoplasmosis in patients with AIDS. Clin Infect Dis 1995; 21:1463–5. [DOI] [PubMed] [Google Scholar]
- 16.Chemlal K, Andrieu-Bautru V, Couvelard A. Hemophagocytic syndrome during Histoplasma capsulatum infection. Haematologica 1997; 82:726. [PubMed] [Google Scholar]
- 17.Kumar N, Jain S, Singh ZN. Disseminated histoplasmosis with reactive hemophagocytosis: aspiration cytology findings in two cases. Diagn Cytopathol 2000; 23:422–4. [DOI] [PubMed] [Google Scholar]
- 18.Rao RD, Morice WG, Phyliky RL. Hemophagocytosis in a patient with chronic lymphocytic leukemia and histoplasmosis. Mayo Clin Proc 2002; 77:287–90. [DOI] [PubMed] [Google Scholar]
- 19.Masri K, Mahon N, Rosario A et al. . Reactive hemophagocytic syndrome associated with disseminated histoplasmosis in a heart transplant recipient. J Heart Lung Transplant 2003; 22:487–91. [DOI] [PubMed] [Google Scholar]
- 20.Gil-Brusola A, Peman J, Santos M et al. . Disseminated histoplasmosis with hemophagocytic syndrome in a patient with AIDS: description of one case and review of the Spanish literature. Rev Iberoam Micol 2007; 24:312–6. [DOI] [PubMed] [Google Scholar]
- 21.Guiot HM, Bertran-Pasarell J, Tormos LM et al. . Ileal perforation and reactive hemophagocytic syndrome in a patient with disseminated histoplasmosis: the role of the real-time polymerase chain reaction in the diagnosis and successful treatment with amphotericin B lipid complex. Diagn Microbiol Infect Dis 2007; 57:429–33. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez A, Celaya AK, Victorio A. Histoplasmosis-associated hemophagocytic syndrome: a case report. AIDS Read 2007; 17:496–9. [PubMed] [Google Scholar]
- 23.Wang Z, Duarte AG, Schnadig VJ. Fatal reactive hemophagocytosis related to disseminated histoplasmosis with endocarditis: an unusual case diagnosed at autopsy. South Med J 2007; 100:208–11. [DOI] [PubMed] [Google Scholar]
- 24.Phillips J, Staszewski H, Garrison M. Successful treatment of secondary hemophagocytic lymphohistiocytosis in a patient with disseminated histoplasmosis. Hematology 2008; 13:282–5. [DOI] [PubMed] [Google Scholar]
- 25.De Lavaissiere M, Manceron V, Bouree P et al. . Reconstitution inflammatory syndrome related to histoplasmosis, with a hemophagocytic syndrome in HIV infection. J Infect 2009; 58:245–7. [DOI] [PubMed] [Google Scholar]
- 26.Lo MM, Mo JQ, Dixon BP, Czech KA. Disseminated histoplasmosis associated with hemophagocytic lymphohistiocytosis in kidney transplant recipients. Am J Transplant 2010; 10:687–91. [DOI] [PubMed] [Google Scholar]
- 27.van Koeveringe MP, Brouwer RE. Histoplasma capsulatum reactivation with haemophagocytic syndrome in a patient with chronic lymphocytic leukaemia. Neth J Med 2010; 68:418–21. [PubMed] [Google Scholar]
- 28.Vaid N, Patel P. A case of haemophagocytic syndrome in HIV-associated disseminated histoplasmosis. Acute Med 2011; 10:142–4. [PubMed] [Google Scholar]
- 29.Chandra H, Chandra S, Sharma A. Histoplasmosis on bone marrow aspirate cytological examination associated with hemophagocytosis and pancytopenia in an AIDS patient. Korean J Hematol 2012; 47:77–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wheat LJ, Freifeld AG, Kleiman MB et al. . Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–25. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Texas - 2013 State Health Profile. State Health Profiles 2013; P. 1-2. Available at: http://www.cdc.gov/nchhstp/stateprofiles/pdf/Texas_profile.pdf. Accessed 30 October, 2014. [Google Scholar]
- 32.Prevention CfDCa. Sources of Histoplasmosis: Where does Histoplasma live? 2014. Available at: http://www.cdc.gov/fungal/diseases/histoplasmosis/causes.html. Accessed 30 October, 2014. [Google Scholar]
- 33.Nieto-Rios JF, Aristizabal-Alzate A, Ocampo C et al. . Disseminated histoplasmosis and haemophagocytic syndrome in two kidney transplant patients. Nefrologia 2012; 32:683–4. [DOI] [PubMed] [Google Scholar]
- 34.Naranbhai V, Altfeld M, Abdool Karim Q et al. . Natural killer cell function in women at high risk for HIV acquisition: insights from a microbicide trial. AIDS 2012; 26:1745–53. [DOI] [PubMed] [Google Scholar]
- 35.Adachi E, Koibuchi T, Imai K et al. . Hemophagocytic syndrome in an acute human immunodeficiency virus infection. Intern Med 2013; 52:629–32. [DOI] [PubMed] [Google Scholar]
- 36.National Institutes of Health. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. AIDSInfo 2015. Available at: http://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-oi-prevention-and-treatment-guidelines/0. Accessed 10 October, 2014. [Google Scholar]
- 37.Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annu Rev Med 2015; 66:145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo X, Chen N, Wang TY et al. . [Visceral leishmaniasis associated hemophagocytic lymphohistiocytosis: report of four childhood cases]. Zhonghua Er Ke Za Zhi 2011; 49:550–3. [PubMed] [Google Scholar]
- 39.Thoden J, Rieg S, Venhoff N et al. . Fatal hemophagocytic syndrome in a patient with a previously well-controlled asymptomatic HIV infection after EBV reactivation. J Infect 2012; 64:110–2. [DOI] [PubMed] [Google Scholar]
- 40.Safdar SM, Rehman JU, Samman EM, Bahabri NM. Fatal hemophagocytic syndrome as a manifestation of immune reconstitution syndrome in a patient with acquired immunodeficiency syndrome. Saudi Med J 2013; 34:861–4. [PubMed] [Google Scholar]
- 41.Patel KK, Patel AK, Sarda P et al. . Immune reconstitution visceral leishmaniasis presented as hemophagocytic syndrome in a patient with AIDS from a nonendemic area: a case report. J Int Assoc Physicians AIDS Care 2009; 8:217–20. [DOI] [PubMed] [Google Scholar]
- 42.Cuttelod M, Pascual A, Baur Chaubert AS et al. . Hemophagocytic syndrome after highly active antiretroviral therapy initiation: a life-threatening event related to immune restoration inflammatory syndrome? AIDS 2008; 22:549–51. [DOI] [PubMed] [Google Scholar]
- 43.Kanitez M, Kapmaz M, Alpay N et al. . Hemophagocytic syndrome associated with immune reconstitution inflammatory syndrome in a patient with AIDS related Burkitt's leukemia/lymphoma. Case Report Med 2014; 2014:308081. [DOI] [PMC free article] [PubMed] [Google Scholar]


