Abstract
We sought to validate the hypothesis that the development of atherosclerosis can be suppressed by the interleukin-1 receptor antagonist (IL-1Ra) in murine models of atherosclerosis in vivo, noninvasively seen by means of high-resolution ultrasound biomicroscopy, and we studied changes in inflammatory markers such as IL-1 and C-reactive protein (CRP) plasma levels in these models of atherosclerosis.
We divided IL-1Ra+/−/apolipoprotein-E (apoE)−/− and IL-1Ra+/+/apoE−/− mice into 2 age groups, used as atherosclerotic models. The control groups were age-matched IL-1Ra+/+/apoE+/+ mice. Plaque thickness was measured in the ascending aorta in short-axis images by means of ultrasound and histology. Plasma levels of IL-1 and CRP were quantified in the 3 murine groups.
At 16 weeks, plaque thickness in the ascending aortas of the IL-1Ra+/−/apoE−/− mice was significantly greater than that in the IL-1Ra+/+/apoE−/− mice, on ultrasound and histology (P <0.01). In contrast, at 32 weeks, the differences between these 2 genotypes were not statistically significant. Serum IL-1 levels were lower in the IL-1Ra+/−/apoE−/− mice than in the IL-1Ra+/+/apoE−/− mice at 16 and 32 weeks (P <0.05). At 16 weeks, serum CRP levels in the IL-1Ra+/−/apoE−/− mice were higher than in the IL-1Ra+/+/apoE−/− mice (P <0.01).
Our results suggest that ultrasound biomicroscopy enables evaluation of atherosclerotic lesions in vivo, noninvasively and in real-time, in apoE−/− mice. Partial IL-1Ra deficiencies might promote early plaque development in 16-week-old apoE−/− mice. The balance of IL-1 and IL-1Ra might influence atherosclerotic development. Finally, CRP might affect the initiation of atherosclerosis, rather than its progression.
Keywords: Apolipoproteins E/deficiency/genetics; atherosclerosis/diagnosis/genetics; disease models/animal; inflammation/physiopathology; interleukin-1/genetics/physiology; interleukin-1 receptor antagonist protein; mice, knockout; microscopy/methods; time factors
Atherosclerosis is the leading cause of cardiovascular morbidity and death in the world. Vascular inflammation is important in the development of atherosclerosis. The balance between pro- and anti-inflammatory cytokines is decisive in atherosclerotic progression.1 Interleukin (IL)-1, a prototypical inflammatory cytokine predominantly produced by macrophages and dendritic cells, plays a central role in inflammation.2,3 Interleukin-1 activity is counterbalanced by the IL-1 receptor antagonist (IL-1Ra), which is a secreted acute-phase reactant capable of binding competitively to IL-1R.4,5 Interleukin-1 and IL-1Ra have been suggested to be an important pathogenic pair in atherosclerotic development, and their balance has been reported to influence this process. Reduced IL-1Ra levels might also lead to the aggravation of atherosclerosis. In addition, coronary artery disease has a substantial inflammatory component, which is in part genetic.6 In human beings, IL-1Ra gene polymorphism has been significantly associated with coronary artery disease.7
To learn whether a deficiency of IL-1Ra promotes the development of atherosclerotic lesions, we obtained IL-1Ra+/− mice. Using apolipoprotein E−/− (apoE−/−) mice as an animal model of atherosclerosis, we established 3 murine genotypes (IL-1Ra+/+/apoE−/−, IL-1Ra+/−/apoE−/−, and IL-1Ra+/+/apoE+/+) by means of cross-breeding. In this study, we tested whether ultrasound biomicroscopy (UBM) enables the evaluation of murine atherosclerotic lesions in vivo, in a noninvasive, real-time fashion. Interleukin-1Ra deficiency had been found to promote early plaque development in apoE knockout mice with use of this novel imaging approach.8,9 In addition, we studied changes in inflammatory markers such as IL-1 and C-reactive protein (CRP) plasma levels in these models of atherosclerosis.
Materials and Methods
The apoE−/− and IL-1Ra+/− mice were obtained from the Jackson Laboratory (Bar Harbor, Maine). They were crossbred to produce IL-1Ra+/−/apoE+/− mice. Mice from this last group were crossbred in order to obtain IL-1Ra+/−/apoE−/−, IL-1Ra+/+/apoE−/−, and IL-1Ra+/+/apoE+/+ mice in the same mixed background.
We divided male IL-1Ra+/−/apoE−/− and IL-1Ra+/+/apoE−/− mice into 2 age groups of 16 and 32 weeks (n=11 in each group of IL-1Ra+/−/apoE−/− mice and n=8 in each group of IL-1Ra+/+/apoE−/− mice) for use as atherosclerotic models. Age-matched IL-1Ra+/+/apoE+/+ mice (n=8 each) were used as control groups. All the mice were fed chow without dietary manipulation and received care in accordance with national guidelines. All procedures conformed to our institutional animal-study guidelines.
Ultrasound Biomicroscopy
We performed UBM in all mice. Baseline ultrasonographic imaging values of the aortic root and ascending aorta were measured with use of the Vevo® 770 (VisualSonics Inc., a division of SonoSite Inc.; Toronto, Canada) at the beginning of the experiment. Imaging involved a 30-MHz scan head, a 12.7-mm focus, and a high resolution of 40 μm. Before examination, each mouse was given a 0.10–0.12-mL/10-g intraperitoneal injection of 0.5% pentobarbital sodium (45 mg/kg, in a volume of 0.2 mL of phosphate-buffered saline solution) as anesthesia, which yielded heart rates of 300 to 400 beats/min. The neck hair of the mice was carefully shaved, and warm ultrasound transmission gel was liberally applied to ensure optimal image quality. Electrocardiography with a lead II configuration was used for monitoring.
Right parasternal long-axis images captured the ascending aorta, aortic arch, and neck vessels in one plane. Use of the aortic valve ring and the brachiocephalic artery branch as anatomic landmarks enabled precise location of the measurement site of the ascending aorta during longitudinal studies. Thereafter, parasternal short-axis images yielded a cross-sectional view of the same arterial site immediately proximal to the branch of the brachiocephalic artery. Here, the intima-media complex thickness (IMT) or atherosclerotic lesions could be readily seen by adjusting the distance between the transducer and the target vascular site. An optimal freeze-frame image, which captured the largest cross-sectional vessel area, was selected and was taken manually to view and measure IMT or maximal plaque thickness.
A 10-s cine loop was stored digitally for offline examination on a VisualSonics image-analysis system. All measurements were performed 3 times at the same vascular site, and all images were analyzed by an operator who was unaware of the classifications of the mice. The IMT measurements were performed in accordance with validated protocols in human beings; IMT was defined as the distance from the leading edge of the lumen–intima interface to the leading edge of the media–adventitia interface of the far wall.10,11 It was measured in the lesser curvature of the ascending aorta wall; the vascular lumen–intimal interface was selected as the internal measurement site and the media–adventitial interface as the external limit.11,12 Atherosclerotic plaques in the ascending aorta were viewed by means of micro-ultrasound imaging. First, a long-axis view was used to see the aortic plaque. Thereafter, a short-axis view was taken in order to see the largest plaque site. The maximal plaque thickness was measured at the maximally thick point at the border with the vascular lumen and the adventitial layer. It was averaged from 3 lesion sites around the thickest part of a plaque, approximately 100 μm apart from each other.
Intra- and Interobserver Variability
Intra- and interobserver coefficients of variation for IMT or maximal plaque thickness measurements were analyzed, first by one operator on 2 different occasions for validation of intraobserver variability, and then by a different operator for evaluation of interobserver variability. Intra- and interobserver variability were calculated with use of coefficient of variation in accordance with the following formula: S.D. (x - y) / mean (x, y) × 100.
Histologic Measurements
After the UBM data were collected, the mice were killed. Each right atrium was cut open for exsanguination. The left ventricular apex was punctured with use of a butterfly needle, and the mouse was perfused with phosphate-buffered saline solution and was perfusion-fixed in 4% paraformaldehyde. The ascending aorta with neck vessels was removed and fixed in 4% paraformaldehyde overnight and was then embedded in paraffin for histologic study. Starting at the aortic root (at 100 μm above the aortic valve ring or, for morphologic reasons, at the site of maximal plaque thickness), 5-μm serial cross-sections were cut at 50-μm intervals along the ascending aorta, and the sections were stained with hematoxylin and eosin. Maximal plaque thickness in the ascending aorta and plaque area in the aortic sinus were measured with use of a Motic Med 6.0 automated image-analysis system (Motic China Group Co., Ltd.; Xiamen, PRC). Plaque area was delineated in the cross-sectional view by manually outlining each lesion as defined by the lumen boundary and the internal elastic lamina. Plaque thickness and plaque area values represent the mean values from 5 sections for each mouse.
Lipid, Lipoprotein, and Inflammatory Biomarker Analysis
The concentrations of total cholesterol (TC), triglycerides (TG), high-density-lipoprotein cholesterol (HDL-C), and low-density-lipoprotein cholesterol (LDL-C) were measured, and their levels in each group were recorded.
Levels of plasma IL-1 and CRP were quantified in each murine group. All measurements were performed independently by 2 researchers who used standard, commercially available enzyme-linked immunosorbent assay kits for mice. The plasma specimens were mixed thoroughly before assay.
Statistical Analysis
All analyses were performed with use of SPSS 13.0 software (IBM Corporation; Armonk, NY). Resulting values are expressed as mean ± SD. Normality tests were used to determine whether the data were well-modeled by means of normal distribution. If the data were normally distributed, unpaired one-way analysis of variance was used to test IMT and plaque thickness, levels of plasma lipids, and inflammatory biomarkers in the 3 murine groups. The Pearson test was used in correlating UBM and histologic values. A P value <0.05 was considered statistically significant.
Results
No significant differences in body weights were observed among the 3 murine groups at the same week of age.
Intima-Media and Maximal Plaque Thicknesses
We found various degrees of atherosclerosis in the IL-1Ra+/−/apoE−/− and IL-1Ra+/+/apoE−/− mice in the aortic sinus and ascending aorta, as seen by means of UBM and histology at 16 and 32 weeks. We found IMT thickening and the presence of atherosclerotic plaque in the arterial posterior wall. No atherosclerotic lesions were found in the control mice.
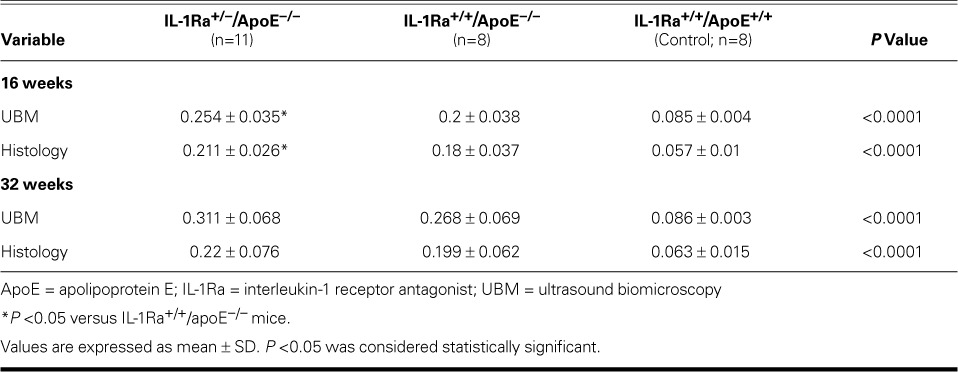
At 16 weeks, plaque thickness in the ascending aortas of the IL-1Ra+/−/apoE−/− mice was significantly greater than that in the IL-1Ra+/+/apoE−/− mice, as measured by UBM and histology (P <0.01). In contrast, at 32 weeks, the differences in plaque thickness and plaque area between the IL-1Ra+/−/apoE−/− mice and IL-1Ra+/+/apoE−/− mice were not statistically significant (Figs. 1 and 2; Table I).
Fig. 1.
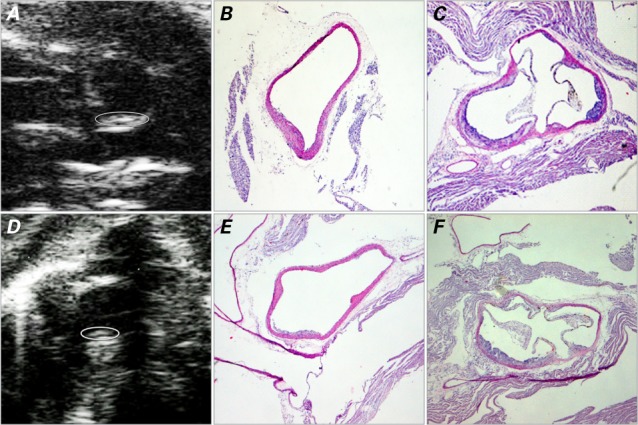
Images at 16 weeks show plaque development in the vascular lumen. In the IL-1Ra+/−/apoE−/− mice, the ascending aorta is shown on A) ultrasound biomicroscopy (UBM) (short-axis view) with B) the corresponding histologic image; C) histologic image of the aortic sinus (short-axis view). In the IL-1Ra+/+/apoE−/− mice, the ascending aorta is shown in D) UBM short-axis view with E) the corresponding histologic image; F) histologic image of the aortic sinus. The UBM images show the border of the plaque (circled) within the curvature of the ascending aorta. Histologic images are H & E stains, orig. ×4.
apoE = apolipoprotein E; IL-1Ra = interleukin-1 receptor antagonist
Fig. 2.
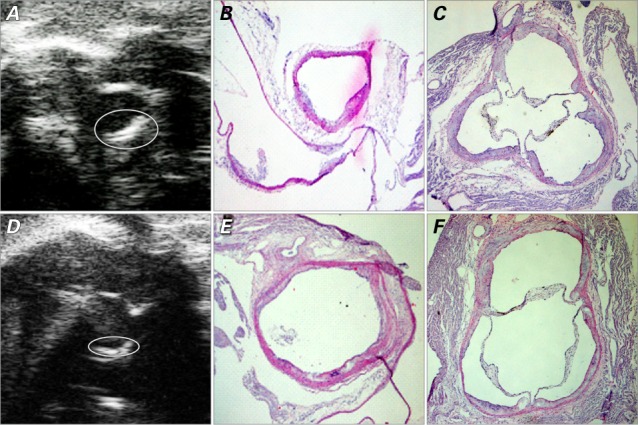
Images at 32 weeks show plaque in the vascular lumen. In the IL-1Ra+/−/apoE−/− mice, the ascending aorta is shown on A) ultrasound biomicroscopy (UBM) (short-axis view) with B) the corresponding histologic image; C) histologic image of the aortic sinus (short-axis view). In the IL-1Ra+/+/apoE−/− mice, the ascending aorta is shown in D) UBM short-axis view with E) the corresponding histologic image; F) histologic image of the aortic sinus. The UBM images show the border of the plaque (circled). Histologic images are H & E stains, orig. ×4.
apoE = apolipoprotein E; IL-1Ra = interleukin-1 receptor antagonist
TABLE I.
Intima–Media Thickness (mm) at the Ascending Aorta as Measured by Means of UBM and Histology in the 3 Murine Genotypes
At 16 weeks, plaque area in the aortic sinuses of the IL-1Ra+/−/apoE−/− mice as measured by histology was greater than that in the IL-1Ra+/+/apoE−/− mice (P <0.01). At 32 weeks, there was no significant difference between these 2 genotypes (Fig. 3).
Fig. 3.
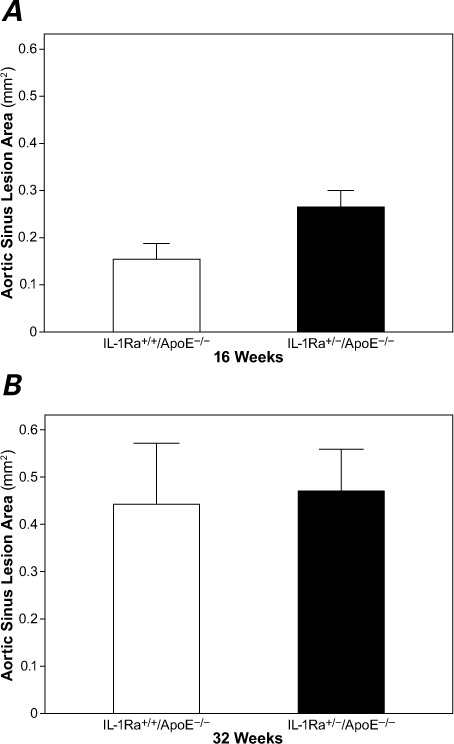
A) At 16 weeks, the aortic sinus plaque area in the IL-1Ra+/−/apoE−/− mice was significantly larger than in the IL-1Ra+/+/apoE−/− mice (P <0.01). B) There was no significant difference in plaque area between the groups at 32 weeks.
apoE = apolipoprotein E; IL-1Ra = interleukin-1 receptor antagonist
P <0.05 was considered statistically significant.
Plaque thickness in the ascending aorta as measured by UBM in the IL-1Ra+/−/apoE−/− mice and IL-1Ra+/+/apoE−/− mice was correlated with histologic measurements from the same vascular region (r =0.79 and r =0.75, respectively; both P <0.01) (Fig. 4).
Fig. 4.
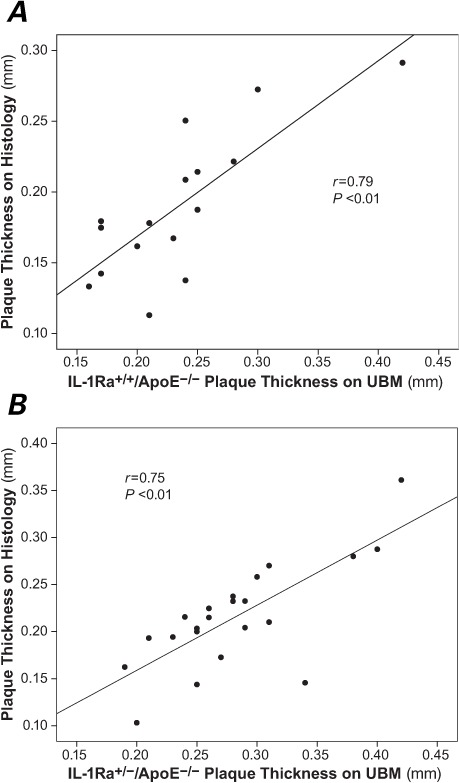
Plaque thickness measurements on ultrasound biomicroscopy (UBM) significantly correlated to those on histopathology in both A) the IL-1Ra+/+/apoE−/− mice and B) the IL-1Ra+/−/apoE−/− mice.
apoE = apolipoprotein E; IL-1Ra = interleukin-1 receptor antagonist
P <0.05 was considered statistically significant.
Intra- and Interobserver Variability
Intra- and interobserver variability was 5.2% and 7.1%, respectively, for UBM measurements of the ascending aortic IMT or plaque thickness.
Lipid, Lipoprotein, and Inflammatory Biomarker Measurements
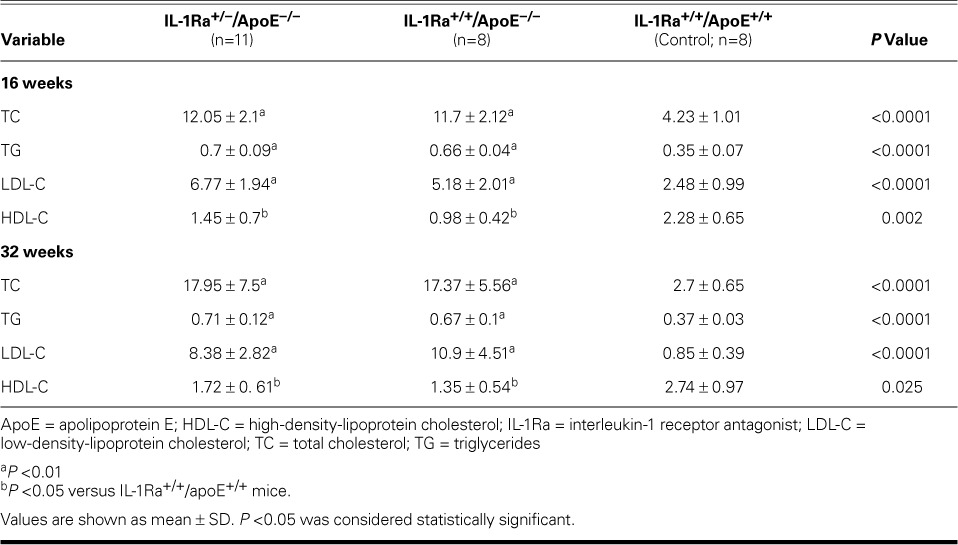
Serum TC, LDL-C, and TG levels were significantly higher in the IL-1Ra+/−/apoE−/− and IL-1Ra+/+/apoE−/− mice than in the control mice at the same weeks of age (P <0.01), and the HDL-C level was lower in the atherosclerotic mice than in the control mice at the same weeks of age (P <0.05) (Table II). However, no significant differences in plasma lipid levels were observed between the IL-1Ra+/−/apoE−/− and IL-1Ra+/+/apoE−/− mice at the same weeks of age.
TABLE II.
Comparison of Plasma Lipid Levels (mmol/L) among the 3 Study Groups
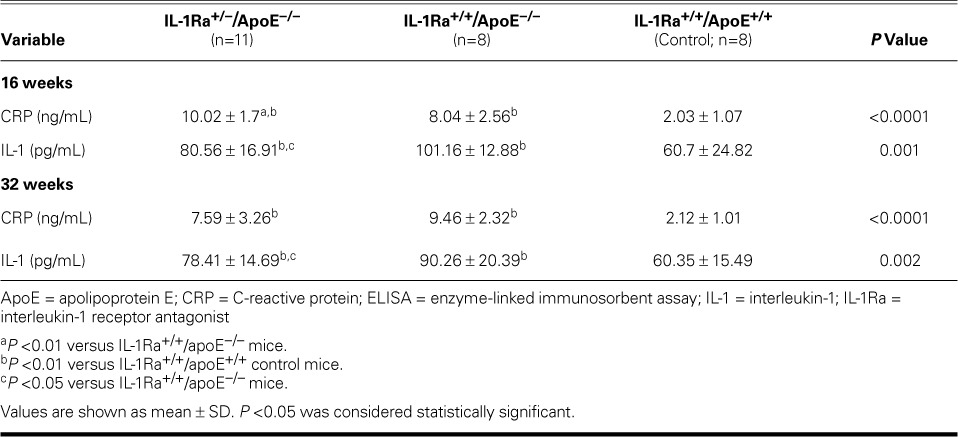
Serum IL-1 and CRP levels in the IL-1Ra+/−/apoE−/− and IL-1Ra+/+/apoE−/− mice were higher than in the control mice (P <0.01). The serum IL-1 level was lower in the IL-1Ra+/−/apoE−/− mice than in the IL-1Ra+/+/apoE−/− mice at 16 and 32 weeks (P <0.05). At 16 weeks, serum CRP levels in the IL-1Ra+/−/apoE−/− mice were higher than those in the IL-1Ra+/+/apoE−/− mice (P <0.01). However, CRP levels were not significantly different at 32 weeks between the IL-1Ra+/−/apoE−/− and IL-1Ra+/+/apoE−/− mice (Table III).
TABLE III.
Comparison of Plasma IL-1 and CRP Levels by Means of ELISA among the 3 Study Groups
Discussion
Our interest in the role of IL-1Ra in atherosclerosis arises from recent observations suggesting a role for IL-1 and IL-1Ra in early stages of the atherosclerotic process. However, the role of IL-1Ra in regard to established atherosclerotic lesions is unknown. By virtue of rapid imaging speed, reproducibility, and high resolution, UBM is an improved means of evaluating murine vascular atherosclerosis.13,14 In the current study, we think that we are the first to use UBM in combination with histopathology to evaluate the anti-atherosclerotic potential of IL-1Ra on the initiation and progression of atherosclerosis.
Atherogenesis is a complex process in which the activation of endothelial cells (ECs) and smooth-muscle cells appears to play a central role.15 Interleukin-1 is one of the most potent proinflammatory cytokines to act on both ECs and smooth-muscle cells.16–18 The activity of IL-1 is counterregulated by its endogenous inhibitor, IL-1Ra,19,20 and IL-1Ra is expressed in ECs and atherosclerotic lesions.21 Treatment with recombinant IL-1Ra has been an effective therapy for atherosclerosis in apoE−/− mice.22 To determine whether IL-1Ra deficiency promotes the development of atherosclerotic lesions, we compared the atherosclerotic lesions in IL-1Ra+/+apoE−/− and IL-1Ra+/−/apoE−/− mice.
Mice that lack IL-1Ra (IL-1Ra−/−) are highly susceptible to lipopolysaccharide-induced death.23 These mice develop various spontaneous inflammatory diseases in accordance with their genetic background.24 Double-knockout mice (IL-1Ra−/−/apoE−/−) have shown signs of severe aortic inflammation and a high mortality rate.25 Moreover, in our study, so few IL-1Ra−/−/apoE−/− mice were produced that we chose IL-1Ra+/−/apoE−/−/mice as the study group.
In our study, we observed no significant differences in body weights or plasma lipid levels between the IL-1Ra+/−/apoE−/− and IL-1Ra+/+/apoE−/− murine genotypes. This study therefore compared atherosclerotic lesions between them, to exclude differences in body weight or lipid levels as confounding factors. At 16 weeks, plaque thickness in the ascending aortas of the IL-1Ra+/−/apoE−/− mice as measured by UBM and histology was greater than that in the IL-1Ra+/+/apoE−/− mice. The plaque area measured by means of histology in the aortic sinus showed the same trend. Accordingly, partial IL-1Ra deficiency might be sufficient to promote early atherogenesis in the context of particular genetic disorders associated with hypercholesterolemia. In another study,26 IL-1β+/+/apoE−/− mice showed opposite results: the size of atherosclerotic lesions in the aortic sinus in IL-1β+/+/apoE−/− mice at 12 and 24 weeks of age showed a significant decrease of 30% when compared with the size in the IL-1 β+/+/apoE−/− mice. That report26 might support our results, and the investigators further suggested that the balance of inflammatory cytokine IL-1 and IL-1Ra influences the early development of atherosclerosis. Although lesion size in our IL-1Ra+/−/apoE−/− mice at 32 weeks tended to be larger than that in the IL-1Ra+/+/apoE−/− mice, the differences did not achieve statistical significance.
Inflammation is thought to play a major role in the initiation and progression of atherosclerosis and in the development of its sequelae.27 In our study, the apoE−/− mice had significantly higher plasma IL-1 levels than did the nonatherosclerotic control mice. This indicates a relationship between IL-1 and atherosclerosis in apoE−/− mice. Interleukin-1 activity is counterbalanced by IL-1Ra.4,5 Interleukin-1 and IL-1Ra have been suggested to be an important pathogenic pair in this process, and their balance has been reported to influence atherosclerotic development. Our study showed that serum IL-1 levels were lower in IL-1Ra+/−/apoE−/− mice than in IL-1Ra+/+/apoE−/− mice at 16 and 32 weeks. This might be related to the deficiency of IL-1Ra in the IL-1Ra+/−/apoE−/− mice, and the balance between IL-1 and IL-1Ra.
Plasma CRP level has been recommended as a risk marker of choice in predicting atherosclerotic development,28 and numerous results from in vitro experiments strongly point toward a proatherogenic role for CRP. However, until the current study, no evidence supported an active proatherogenic action of CRP in vivo. We found higher CRP concentrations in our atherosclerosis-model mice than in our nonatherosclerotic control mice. This suggests that higher CRP concentration is significantly correlated with atherosclerosis. However, plasma CRP levels did not increase significantly by age in our apoE-knockout mice. In addition, in comparison with IL-1Ra+/+/apoE−/− mice, our IL-1Ra+/−/apoE−/− mice had higher plasma CRP concentrations at 16 weeks, but not at 32 weeks, when there were no significant differences between the 2 groups. These results suggest that CRP might affect the initiation of atherosclerosis, rather than its progression.
The main limitation of this study is the lack of evidence of how a deficiency of IL-1Ra promotes the development of atherosclerotic lesions. After subsequent experimentation, we plan to provide mechanistic evidence of the anti-atherosclerotic effects of IL-1Ra on the initiation and progression of atherosclerosis.
In conclusion, our results suggest that UBM enables the evaluation of atherosclerotic lesions in vivo, non-invasively, and in real-time in apoE−/− mice. Partial IL-1Ra deficiency might promote early plaque development in apoE−/− mice at 16 weeks of age. The balance of IL-1 and IL-1Ra might influence atherosclerotic development. Finally, CRP might affect the initiation of atherosclerosis, rather than its progression.
Acknowledgments
We are indebted to the medical and technical staff of the ultrasound departments of Beijing Anzhen Hospital, Capital Medical University, and Beijing Institute of Heart and Lung Disease.
Footnotes
From: Department of Echocardiography, Beijing Anzhen Hospital, Capital Medical University, Beijing 100029, People's Republic of China
This work was supported by grants from the Natural Science Foundation (No. 81171351 and 81201110) and the Basic-Clinical Scientific Research Foundation Program of the Capital Medical University in China (No. 12JL55).
References
- 1.Ramos KS, Partridge CR, Teneng I. Genetic and molecular mechanisms of chemical atherogenesis. Mutat Res. 2007;621(1–2):18–30. doi: 10.1016/j.mrfmmm.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Bevilacqua MP, Pober JS, Majeau GR, Cotran RS, Gimbrone MA., Jr. Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984;160(2):618–23. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 4.Ohsuzu F. The roles of cytokines, inflammation and immunity in vascular diseases. J Atheroscler Thromb. 2004;11(6):313–21. doi: 10.5551/jat.11.313. [DOI] [PubMed] [Google Scholar]
- 5.Loppnow H, Werdan K, Reuter G, Flad HD. The interleukin-1 and interleukin-1 converting enzyme families in the cardiovascular system. Eur Cytokine Netw. 1998;9(4):675–80. [PubMed] [Google Scholar]
- 6.Dewberry RM, Crossman DC, Francis SE. Interleukin-1 receptor antagonist (IL-1RN) genotype modulates the replicative capacity of human endothelial cells. Circ Res. 2003;92(12):1285–7. doi: 10.1161/01.RES.0000078172.52740.9B. [DOI] [PubMed] [Google Scholar]
- 7.Andreotti F, Porto I, Crea F, Maseri A. Inflammatory gene polymorphisms and ischaemic heart disease: review of population association studies. Heart. 2002;87(2):107–12. doi: 10.1136/heart.87.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni M, Zhang M, Ding SF, Chen WQ, Zhang Y. Micro-ultrasound imaging assessment of carotid plaque characteristics in apolipoprotein-E knockout mice. Atherosclerosis. 2008;197(1):64–71. doi: 10.1016/j.atherosclerosis.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 9.Phoon CK, Turnbull DH. Ultrasound biomicroscopy-Doppler in mouse cardiovascular development. Physiol Genomics. 2003;14(1):3–15. doi: 10.1152/physiolgenomics.00008.2003. [DOI] [PubMed] [Google Scholar]
- 10.Foster FS, Pavlin CJ, Harasiewicz KA, Christopher DA, Turnbull DH. Advances in ultrasound biomicroscopy. Ultrasound Med Biol. 2000;26(1):1–27. doi: 10.1016/s0301-5629(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 11.Wendelhag I, Gustavsson T, Suurkula M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles and description of a computerized analysing system. Clin Physiol. 1991;11(6):565–77. doi: 10.1111/j.1475-097x.1991.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 12.Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74(6):1399–406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Yang Y, Xie J, Li Z, Zhang X, Li R. Noninvasive assessment of atherosclerosis in apolipoprotein-E knockout mice by ultrasound biomicroscopy. Ultrasound Med Biol. 2011;37(6):892–9. doi: 10.1016/j.ultrasmedbio.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Li RJ, Yang Y, Wang YH, Xie JJ, Song L, Wang Z et al. Micro-ultrasonographic imaging of atherosclerotic progression and correlation with inflammatory markers in apolipoprotein-E knockout mice. Tex Heart Inst J. 2011;38(4):364–70. [PMC free article] [PubMed] [Google Scholar]
- 15.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8(11):1249–56. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 16.Tamaru M, Tomura K, Sakamoto S, Tezuka K, Tamatani T, Narumi S. Interleukin-1beta induces tissue- and cell type-specific expression of adhesion molecules in vivo. Arterioscler Thromb Vasc Biol. 1998;18(8):1292–303. doi: 10.1161/01.atv.18.8.1292. [DOI] [PubMed] [Google Scholar]
- 17.Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA., Jr. Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am J Pathol. 1985;121(3):394–403. [PMC free article] [PubMed] [Google Scholar]
- 18.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87(6):2095–147. [PubMed] [Google Scholar]
- 19.Worrall BB, Azhar S, Nyquist PA, Ackerman RH, Hamm TL, DeGraba TJ. Interleukin-1 receptor antagonist gene polymorphisms in carotid atherosclerosis. Stroke. 2003;34(3):790–3. doi: 10.1161/01.STR.0000057815.79289.EC. [DOI] [PubMed] [Google Scholar]
- 20.Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77(8):1627–52. [PubMed] [Google Scholar]
- 21.Dewberry R, Holden H, Crossman D, Francis S. Interleukin-1 receptor antagonist expression in human endothelial cells and atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20(11):2394–400. doi: 10.1161/01.atv.20.11.2394. [DOI] [PubMed] [Google Scholar]
- 22.Elhage R, Maret A, Pieraggi MT, Thiers JC, Arnal JF, Bayard F. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation. 1998;97(3):242–4. doi: 10.1161/01.cir.97.3.242. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch E, Irikura VM, Paul SM, Hirsh D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc Natl Acad Sci U S A. 1996;93(20):11008–13. doi: 10.1073/pnas.93.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191(2):313–20. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merhi-Soussi F, Kwak BR, Magne D, Chadjichristos C, Berti M, Pelli G et al. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein E-knockout mice. Cardiovasc Res. 2005;66(3):583–93. doi: 10.1016/j.cardiores.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23(4):656–60. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 27.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 28.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]


