Abstract
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital condition. It responds well to early diagnosis and treatment, but otherwise the prognosis is poor. We present our case series of 12 patients (mean age, 2 ± 2.58 yr; age range, 2 mo–8 yr), emphasizing the diagnostic process and discussing our surgical results. The diagnosis of ALCAPA should be suspected in infants who have dilated cardiomyopathy with electrocardiographic changes that suggest ischemia, and in older children who have isolated mitral regurgitation. When clinical suspicion is high, the results of 2-dimensional echocardiography combined with color-flow Doppler studies in expert hands can establish the diagnosis, thus avoiding angiography in critically ill infants. The treatment of choice in our patients was transfer and reimplantation of the left coronary artery onto the ascending aorta. There were 2 deaths: both were infants in extremis who underwent emergency surgery. An older child with severe ventricular dysfunction was given mechanical ventricular assistance and then heart transplantation. As of this report, all 10 survivors remained well and asymptomatic.
Keywords: Abnormalities, multiple/diagnosis; cardiac surgical procedures/methods; cardiomyopathy, dilated/etiology/physiopathology; collateral circulation/physiology; coronary vessel anomalies/diagnosis/pathology/radiography/surgery; echocardiography, Doppler, color/methods; heart arrest/etiology; pulmonary artery/abnormalities/pathology; time factors; treatment outcome
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA), a rare malformation, is the most prevalent coronary artery anomaly described in children. It occurs in approximately 1 in 300,000 live births (0.25%–0.5% of all congenital cardiac anomalies). It is usually an isolated cardiac malformation.1–4
The 2 clinical variants of ALCAPA, found in infants and older individuals, respectively, are distinguished by the presence or absence of adequate intercoronary collateral circulation that supplies blood from the right coronary artery (RCA) to the left side of the heart.5 The 80% to 85% of patients without an adequate collateral supply typically present with congestive heart failure (CHF) secondary to ischemia within the first few months of life; their mortality rate is 90% without early surgical correction.5 Patients with an adequate collateral supply can remain asymptomatic until adolescence or adulthood and then present with a murmur (usually continuous) on the left parasternal border. Alternatively, they can present with angina on exertion. They are at risk of sudden death.6–11
We present our experience with 12 pediatric patients who had ALCAPA, and we discuss the diagnostic process and our surgical results.
Patients and Methods
We retrospectively studied the cases of 12 patients (4 boys, 8 girls) who underwent surgical correction of ALCAPA from 1980 through 2009. Eleven were operated on at our hospital; one patient with particularly poor ventricular function was transferred to a hospital where heart transplantation was available. The patients' mean age at presentation was 2 ± 2.58 years (range, 2 mo–8 yr). Eight were infants (age, <2 yr); of these, 5 were 2 months old. The 12 patients' mean weight upon diagnosis was 10 ± 7.76 kg (range, 3–26.5 kg); 8 patients weighed less than 10 kg.
All patients were evaluated by means of chest radiography, electrocardiography (ECG), echocardiography, and cardiac catheterization. The mean duration of study was 46.6 ± 59.7 months (range, 2 d–14 yr). The echocardiographic method was exclusively 2-dimensional before 1988; color-flow Doppler evaluation was added thereafter (ATL® Ultramark®, 11XATP, and iE33 xMatrix successively; Koninklijke Philips N.V.; Best, The Netherlands). One patient took an exercise ECG test.
Postoperatively in our institution, upon a patient's arrival at the intensive care unit, ECG and echocardiography are performed. If the results are normal, if the patient has an uneventful postoperative course and progresses well, and if monitoring reveals no worrisome ECG developments, further ECG might not be performed until the patient's discharge from intensive care. Otherwise, ECG is performed at least daily.
In these patients, the ECG results were evaluated for signs of ischemia (alterations in the ST segments and T waves, and the presence or absence of Q waves). The echocardiographic studies involved left ventricular (LV) function, mitral insufficiency, increased echogenicity of the papillary muscles, morphology and diameter of the coronary arteries and the site and direction of flow within, and evidence of collateral coronary flow within the septum and free ventricular walls.
After the diagnosis of ALCAPA was made, the patients underwent surgical correction. The technique of choice was reimplantation of the left coronary artery (LCA) onto the ascending aorta, with use of extracorporeal circulation.
Results
Table I shows the findings in our 12 patients.
TABLE I.
Clinical, Electrocardiographic, Echocardiographic, and Outcome Data on the 12 Patients a
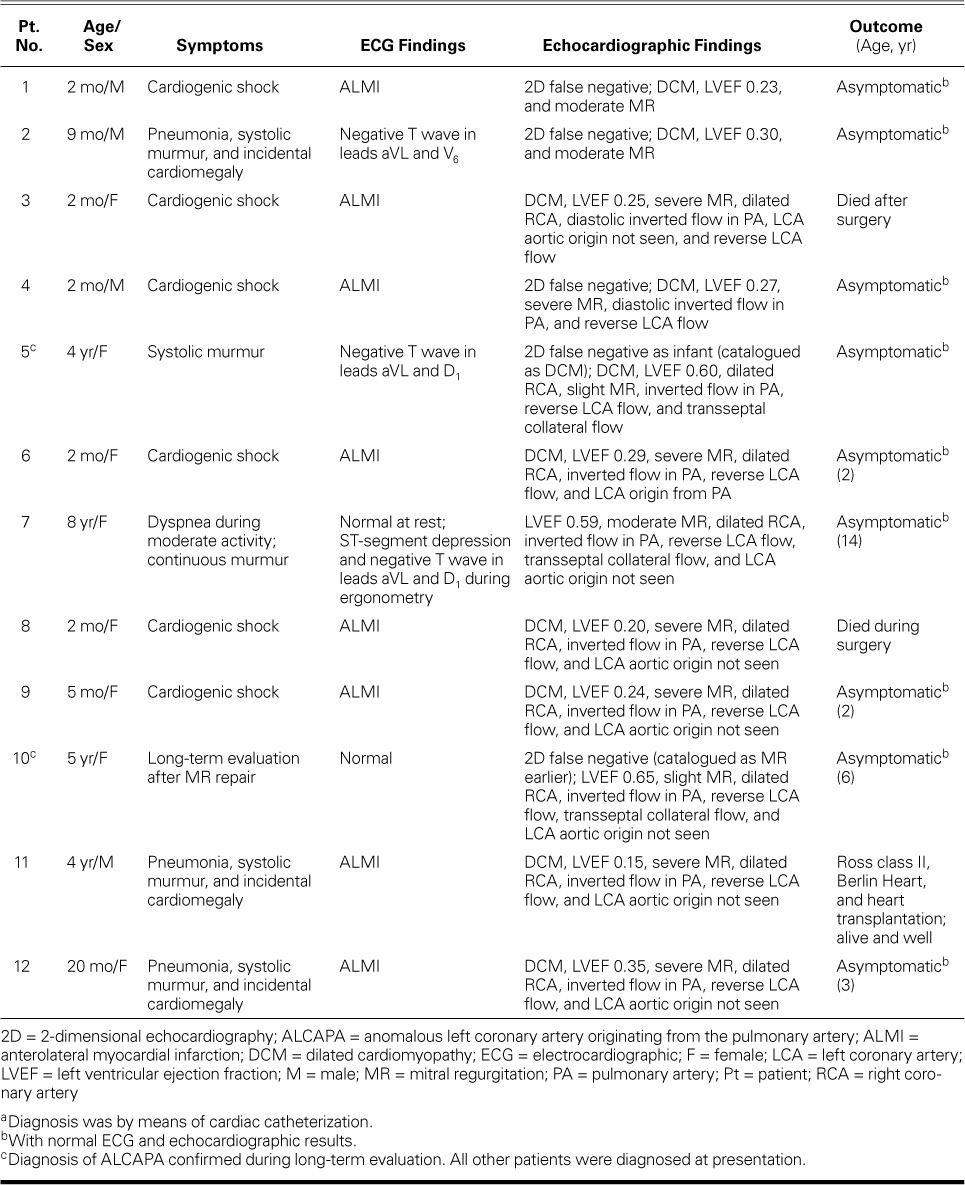
Clinical Findings. Six patients who had dilated cardiomyopathy (DCM) and severe CHF at presentation were younger than 1 year old, and 5 were 2 months old. Two older patients underwent long-term evaluation after original diagnoses of DCM and rheumatic mitral regurgitation; this last patient had undergone surgical mitral valve repair. One asymptomatic older child presented with a continuous murmur and dyspnea. Three patients (2 younger than 1 year of age) presented with incidental cardiomegaly and systolic murmurs in the context of pneumonia.
Electrocardiographic Findings. Ten patients had ECG evidence of ischemia; in 8 (7 infants), it signified acute myocardial infarction (MI) (Fig. 1). All the infants with CHF caused by DCM had ECG signs of acute MI. Of the 4 older patients, 2 had normal ECG results and 2 had T-wave inversions. The patient who took the exercise ECG test had ST-segment depression in the left anterior chest leads.
Fig. 1.
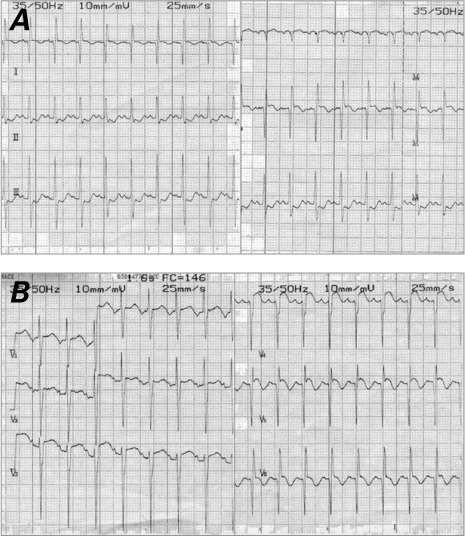
Electrocardiograms show signs of acute anterolateral myocardial infarction: deep Q waves, marked ST-segment elevation, and T-wave inversion in leads A) I and aVL (Patient 6) and B) V4 through V6 (Patient 8).
Echocardiographic Findings. The results of 2-dimensional echocardiography and color-flow Doppler study raised our suspicion of ALCAPA in 7 patients. The echocardiograms in 5 patients erroneously indicated that the LCA emerged from the aortic root (Figs. 2A and B), leading to false-negative diagnoses in those patients. The diagnosis of ALCAPA was firmly established when the origin of the LCA was actually seen (Fig. 2C). The most recent 7 patients, in all of whom color-flow Doppler mode was used, were diagnosed correctly from the outset.
Fig. 2.
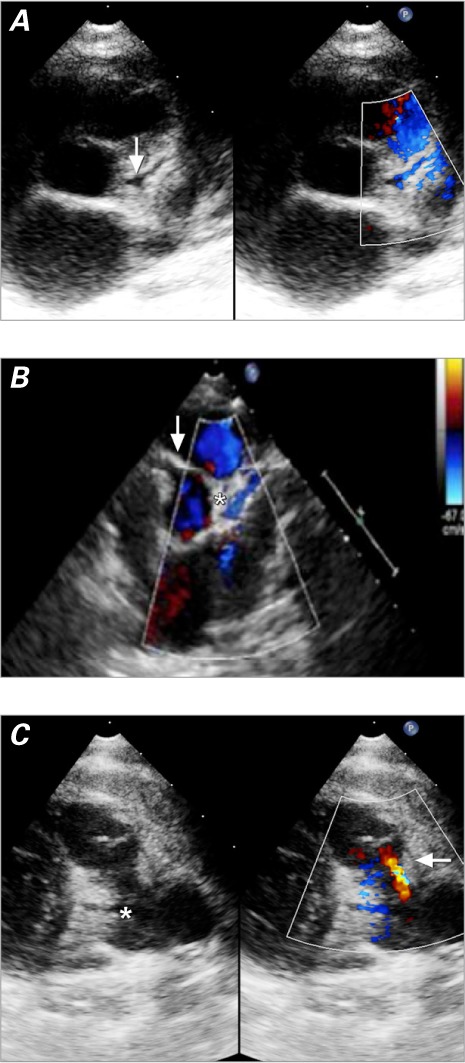
Two-dimensional echocardiograms. A) Patient 6. The parasternal short-axis view gives the erroneous impression that the left coronary artery emerges from the aortic root (arrow). In color-flow Doppler mode, diastolic flow from right to left (in blue) is seen inside the left coronary artery from the pulmonary artery. B) Patient 10. The parasternal short-axis view shows that the left coronary artery has no connection to the aorta despite their proximity and has retrograde diastolic flow (in blue) from right to left (asterisk). The dilated right coronary artery emerges from the aortic root (arrow). C) Patient 4. The parasternal short-axis view shows retrograde diastolic flow in the main pulmonary artery from the left coronary artery (in red) from left to right (arrow). The origin of the left coronary artery from the main pulmonary artery (asterisk) should not be mistaken for a persistent ductus arteriosus.
We identified 2 distinct ALCAPA groups, infant and older. In the infant group (characterized by CHF and ischemic ECG changes), we found severe LV dilation and dysfunction, moderate-to-severe mitral insufficiency, hyperechogenicity of the papillary muscles, retrograde diastolic flow in the LCA and main pulmonary artery (PA), a slightly dilated RCA, and little intraseptal collateral circulation. The 2nd group comprised older and asymptomatic children who had dilation but preserved function of the LV, mild-to-moderate mitral insufficiency, a severely dilated RCA (Fig. 2B), considerable transseptal blood flow from collateral vessels (Fig. 3), and retrograde flow in the LCA and main PA.
Fig. 3.
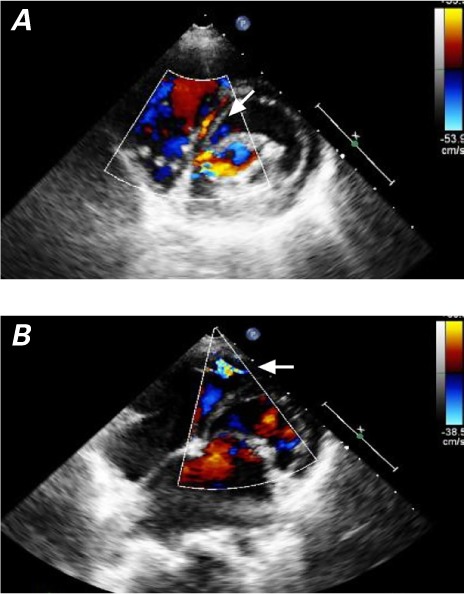
Patient 7. Two-dimensional echocardiograms (short-axis views in color-flow Doppler mode, with posterior angle to the apex) in A) mid septal and B) apical locations show diastolic or continuous flow (arrows) within the ventricular septum. These signs of intercoronary collateral vessels should not be mistaken for multiple ventricular septal defects.
Features common to both groups, although with different severity, were RCA dilation and retrograde flow in the LCA and main PA.
Catheterization and Angiography. Catheter and angiographic studies confirmed the diagnosis of ALCAPA in all our patients. In the older, asymptomatic children, a well-developed network of intercoronary collateral vessels was seen (Fig. 4A). In the infants with DCM, poor collateral circulation necessitated pulmonary angiography to identify the origin of the LCA from the PA (Fig. 4B).
Fig. 4.
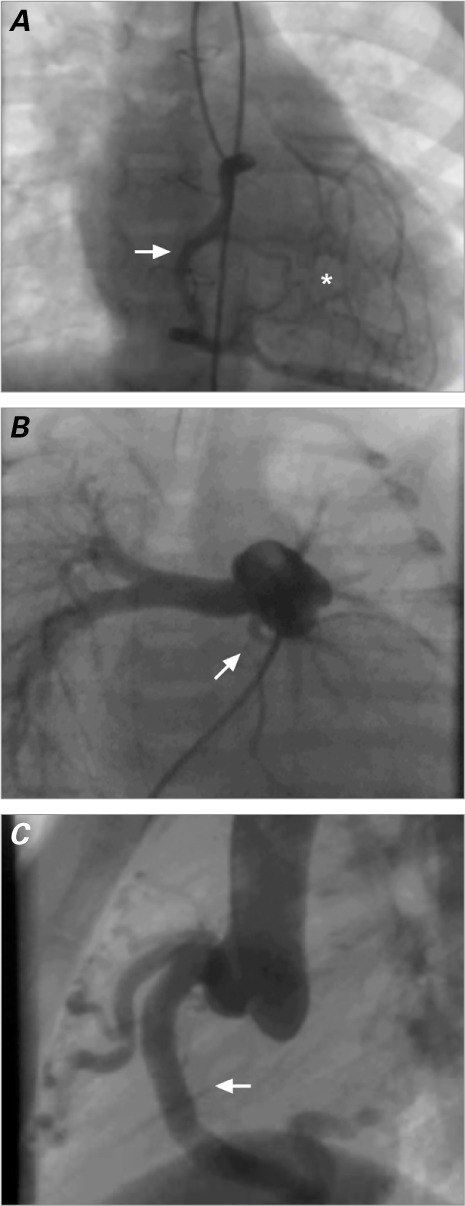
A) Patient 10. Right-sided coronary angiogram shows a well-developed network of intercoronary collateral vessels (asterisk) that enable flow from the right coronary artery to the left coronary artery (arrow). B) Patient 12. Pulmonary arteriogram (posteroanterior projection) shows the left coronary artery and its division into the left circumflex and left anterior descending coronary arteries (arrow). C) Patient 11. Aortogram shows a dilated right coronary artery (arrow).
Surgical Treatment. In the 11 surgical repairs in our hospital, the LCA ostium was removed from the PA and anastomosed to the ascending aorta. The anomalous LCA arose from the posterior aspect of the PA in all instances.
Two infants who presented with severe hemodynamic instability and ECG evidence of MI were diagnosed with ALCAPA too late for anything but emergency surgery as a desperate measure, and both died. One hemodynamically stable older patient who had particularly poor ventricular function was transferred to a hospital where ventricular assistance devices, extracorporeal membrane oxygenation (ECMO), and heart transplantation were available. The patient indeed needed mechanical assistance and subsequent transplantation.
Follow-Up Evaluation. Follow-up periods varied between 2 days and 14 years (mean, 46.6 ± 59.7 mo). There were no late sequelae in the 10 survivors, contrary to reported follow-up results of other surgical techniques. Therefore, the overall survival rate was 83%; in the older, asymptomatic children, it was 100%. The survivors progressed favorably, remained asymptomatic, and had normal ECG and echocardiographic values within a few months. However, RCA dilation persisted in the older children (Fig. 4C). As stated, the patient who was transferred underwent eventual heart transplantation.
Discussion
Unlike other cardiomyopathies in pediatric patients, the severe ischemic cardiomyopathy in ALCAPA can respond to surgical treatment, as evidenced by the 83% survival rate in our series.2–4 Early diagnosis of ALCAPA is crucial because of its grim progression of irreversible ischemic damage.
This report of our 12 ALCAPA patients emphasizes the diagnostic aspect. We saw both forms of presentation, as described earlier; 75% were of the infant type, consistent with the medical literature.1–11 Our suspicion of ALCAPA on the basis of the clinical and ECG findings proved to be crucial, because it prompted angiographic studies after nondiagnostic echocardiograms. In turn, the angiographic findings led to prompt surgical treatment.
In the infants, the chief symptom was irritability elicited by only slight effort, such as feeding, with signs of poor peripheral perfusion. These features started within 2 months of birth, coinciding with substantial reduction in pulmonary vascular resistance that resulted in coronary steal from the anterolateral aspect of the LV. The ECG signs of ischemia accompanied the deteriorating ventricular function. This progression created strong suspicion of ALCAPA.12
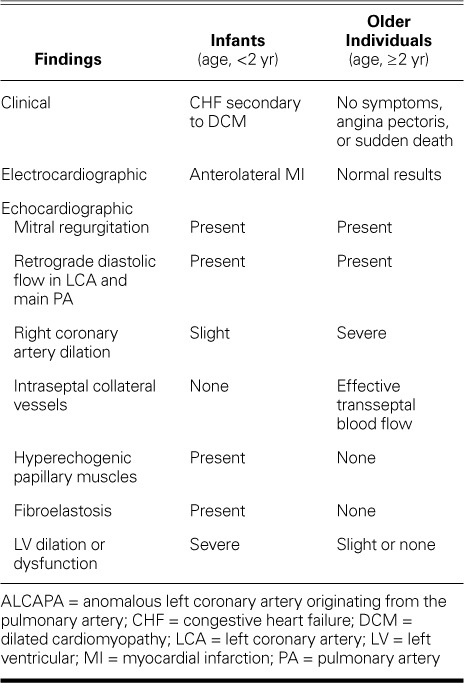
The older children were asymptomatic and might have remained so until adolescence or young adulthood, presumably because of effective collateral blood supply to the LV from the RCA. Such patients can have angina upon exertion and are at risk of sudden death. Alternatively, they can present with signs of CHF caused by decompensation of already-borderline cardiac function, secondary to acute infectious illness. Their baseline ECG usually shows ischemic changes only in cases of decompensation or during a stress test.6–11 Table II shows typical clinical, ECG, and echocardiographic findings in infants and older individuals who have ALCAPA.
TABLE II.
Typical Clinical, Electrocardiographic, and Echocardiographic Findings in ALCAPA
Color-flow Doppler echocardiography is currently the diagnostic method of choice. In experienced hands and with suspicion of ALCAPA, information of such high quality is obtained that some authors advocate not performing angiography, especially in severely ill infants.13 Before the use of the color-flow Doppler mode, the false-negative diagnostic rate was 50%,14 comparable with our series.
The chief diagnostic mistake occurs in the short-axis echocardiographic view, when the apparently vascular structure originating from the aortic root can be misinterpreted as bifurcation. This structure can be misidentified as the LCA.13 However, color-flow Doppler imaging clarifies that this structure and the aorta have no connection, despite their proximity, and that the structure has either retrograde flow or none. The retrograde flow corresponds to blood received by the anomalous LCA from the collateral arteries. This finding, together with the identification of a dilated RCA and diastolic or continuous retrograde flow in the PA near the site where the LCA would be, strongly points to ALCAPA—and should not be misidentified as persistent ductus arteriosus. Obviously, the diagnosis can be firmly established when the origin of the LCA is actually seen.15,16
Another contributory finding is diastolic or continuous flow within the ventricular septum, which suggests intercoronary collateral circulation. This phenomenon should not be confused with multiple ventricular septal defects.17
Mitral regurgitation caused by ischemia of the papillary muscles is so characteristic that its absence, even in the presence of DCM, rules out ALCAPA.18–20 Of note, one of our patients had been diagnosed elsewhere to have mitral regurgitation of rheumatic origin, and mitral valve repair had been performed. The diagnosis of ALCAPA was established during follow-up echocardiographic studies.
When collateral circulation is less developed, the echocardiographic findings are less visible—which means that diagnosing ALCAPA in severely ill infants can be difficult. Nevertheless, severe DCM with mitral regurgitation and ECG signs of ischemia suggests ALCAPA.20 Because of feeble collateral circulation, aortograms or right-sided angiograms might fail to reveal the LCA; nonetheless, the existence of only one coronary artery should raise suspicion.21 Pulmonary arteriography then becomes necessary and will establish the diagnosis of ALCAPA.
Cardiac catheterization and angiography, the classical gold standard for confirming the diagnosis of ALCAPA,21 achieved that end in all our patients. Performing angiography in severely ill infants is being questioned,20 although these infants are precisely those in whom diagnosis by other means might be difficult. In this context, Chang and Allada20 have devised a scoring system, with use of ECG and echocardiography but not angiography, for the diagnosis of ALCAPA and its differentiation from DCM. This system has a sensitivity of 100% and a specificity of 91%. In addition, advances in cardiac magnetic resonance and computed tomography might make these methods an alternative to catheterization when the diagnosis is in doubt.22–24
Progress in diagnosis, surgical techniques, and postoperative care has yielded dramatic prognostic improvements in ALCAPA patients: mortality rates have declined from 90% to ≤20%.25–29
We think that the best repair technique for ALCAPA is reimplantation of the LCA in the aortic root, and indeed this has produced results superior to those of other techniques.30–32 Our hospital's surgeons have extensive experience with the arterial switch operation and greatly prefer the more anatomic coronary-transfer technique to the Takeuchi procedure. Tension or kinking is possible when a coronary vessel is transferred; however, our surgeons have had no such technical problems in correcting ALCAPA, and the end results in the operating room have appeared to be morphologically satisfactory. Indeed, the mobilization of the LCA has been no more extensive than that in the arterial switch, so we do not think that this factor would explain the poor results in 3 of our ALCAPA patients. The 2 deaths and the eventual transplantation occurred because of late diagnoses and the grave prognostic consequences thereof. Our hospital had no ventricular assist devices or ECMO available during those clinical presentations. Of note, when ALCAPA presents with a patient in extremis, emergency surgery is appropriate. Placing such patients on ECMO might worsen the cardiac ischemia by decompressing the PA and increasing steal from the right coronary system, thus compromising the chances of recovery.
Conclusion
The early diagnosis and treatment of ALCAPA can overcome its otherwise poor prognosis. The diagnosis should be suspected in infants who have CHF that suggests DCM and who have ECG changes that suggest anterolateral myocardial ischemia, and in older children who have isolated mitral regurgitation. Two-dimensional echocardiograms combined with color-flow Doppler studies might establish the diagnosis without angiography in critically ill infants. Reimplantation of the anomalous LCA in the aortic root is our treatment of choice. We think that severely ill patients should undergo repair in centers that have mechanical ventricular assist devices, ECMO, and heart transplantation programs.
Footnotes
From: Department of Pediatric Cardiology, Virgen del Rocio Children's Hospital, 41013 Seville, Spain
References
- 1.Zemanek D, Veselka J. Coronary artery anomalies - a short review. Centr Eur J Med. 2007;2(2):140–53. [Google Scholar]
- 2.Cowles RA, Berdon WE. Bland-White-Garland syndrome of anomalous left coronary artery arising from the pulmonary artery (ALCAPA): a historical review. Pediatr Radiol. 2007;37(9):890–5. doi: 10.1007/s00247-007-0544-8. [DOI] [PubMed] [Google Scholar]
- 3.He XH, Li Y, Huang MR, Gao W, Li F, Yu ZQ et al. Anomalous origin of the left coronary artery from the pulmonary artery: report on 10 cases [in Chinese] Zhongguo Dang Dai Er Ke Za Zhi. 2007;9(1):25–7. [PubMed] [Google Scholar]
- 4.Walsh MA, Duff D, Oslizlok P, Redmond M, Walsh KP, Wood AE, Coleman DM. A review of 15-year experience with anomalous origin of the left coronary artery. Ir J Med Sci. 2008;177(2):127–30. doi: 10.1007/s11845-008-0146-y. [DOI] [PubMed] [Google Scholar]
- 5.Tan X, Sun K, Li F, Zhang YQ, Wu LP. Clinical diagnosis of anomalous origin of the left coronary artery from the pulmonary artery in 22 cases [in Chinese] Zhonghua Er Ke Za Zhi. 2008;46(12):881–4. [PubMed] [Google Scholar]
- 6.Aslanger E, Altun I, Umman B. Sudden cardiac arrest in a patient with an anomalous left main coronary artery originating from the pulmonary artery. Acta Cardiol. 2009;64(6):835–7. doi: 10.2143/AC.64.6.2044755. [DOI] [PubMed] [Google Scholar]
- 7.Irving C, Wren C. Asymptomatic anomalous origin of the left coronary artery from the pulmonary artery. Pediatr Cardiol. 2009;30(3):385–6. doi: 10.1007/s00246-008-9356-3. [DOI] [PubMed] [Google Scholar]
- 8.Ramana RK, Varga P, Leya F. Late presentation of an anomalous origin of the left coronary artery from the pulmonary artery: case report and review. J Invasive Cardiol. 2008;20(10):564–6. [PubMed] [Google Scholar]
- 9.Browne LP, Kearney D, Taylor MD, Chung T, Slesnick TC, Nutting AC, Krishnamurthy R. ALCAPA: the role of myocardial viability studies in determining prognosis. Pediatr Radiol. 2010;40(2):163–7. doi: 10.1007/s00247-009-1412-5. [DOI] [PubMed] [Google Scholar]
- 10.Allen DR, Shiecken RM, Donofrio MT. Hoarseness as the initial clinical presentation of anomalous left coronary artery from the pulmonary artery. Pediatr Cardiol. 2005;26(5):668–71. doi: 10.1007/s00246-004-0846-7. [DOI] [PubMed] [Google Scholar]
- 11.Grosse-Wortmann L, Wenzl T, Hoevels-Guerich HH. Anomalous origin of the left coronary artery from the pulmonary artery in a premature infant with preserved left ventricular function. Pediatr Cardiol. 2006;27(2):269–71. doi: 10.1007/s00246-004-0878-z. [DOI] [PubMed] [Google Scholar]
- 12.Kurup RP, Daniel R, Kumar RK. Anomalous origin of the left coronary artery from the pulmonary artery in infancy with preserved left ventricular function: potential pitfalls and clues to diagnosis. Ann Pediatr Cardiol. 2008;1(1):65–7. doi: 10.4103/0974-2069.41061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen MS, Herlong RJ, Silverman NH. Echocardiographic imaging of anomalous origin of the coronary arteries. Cardiol Young. 2010;20(Suppl 3):26–34. doi: 10.1017/S104795111000106X. [DOI] [PubMed] [Google Scholar]
- 14.Kudo Y, Suda K, Koteda Y. Pitfalls of echocardiographic evaluation of anomalous origin of the left coronary artery from the pulmonary trunk. Cardiol Young. 2008;18(5):537–8. doi: 10.1017/S1047951108002564. [DOI] [PubMed] [Google Scholar]
- 15.Alva C, Gomez FD, Jimenez-Arteaga S, Martinez-Sanchez A, Ortegon-Cardena J, Yanez L, Riera-Kinkel C. Anomalous origin of the left coronary artery from the pulmonary artery. Echocardiographic diagnosis. Arch Cardiol Mex. 2008;79(4):274–8. [PubMed] [Google Scholar]
- 16.Yang YL, Nanda NC, Wang XF, Xie MX, Lu Q, He L, Lu XF. Echocardiographic diagnosis of anomalous origin of the left coronary artery from the pulmonary artery. Echocardiography. 2007;24(4):405–11. doi: 10.1111/j.1540-8175.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 17.Hildreth B, Junkel P, Allada V, Sintec C, Sapin S. An uncommon echocardiographic marker for anomalous origin of the left coronary artery from the pulmonary artery: visualization of intercoronary collaterals within the ventricular septum. Pediatr Cardiol. 2001;22(5):406–8. doi: 10.1007/s002460010263. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad Z, Haw M, Vettukattil JJ. Congenital mitral regurgitation due to papillary muscle infarction: a case report and approach to evaluation. Eur J Pediatr. 2010;169(5):621–3. doi: 10.1007/s00431-009-1072-3. [DOI] [PubMed] [Google Scholar]
- 19.Dahle G, Flane AE, Lindberg HL. ALCAPA, a possible reason for mitral insufficiency and heart failure in young patients. Scand Cardiovasc J. 2007;41(1):51–8. doi: 10.1080/14017430601050348. [DOI] [PubMed] [Google Scholar]
- 20.Chang RR, Allada V. Electrocardiographic and echocardiographic features that distinguish anomalous origin of the left coronary artery from pulmonary artery from idiopathic dilated cardiomyopathy. Pediatr Cardiol. 2001;22(1):3–10. doi: 10.1007/s002460010142. [DOI] [PubMed] [Google Scholar]
- 21.Friedman AH, Fogel MA, Stephens P, Jr, Hellinger JC, Nykanen DG, Tweddell J et al. Identification, imaging, functional assessment and management of congenital coronary arterial abnormalities in children. Cardiol Young. 2007;17(Suppl 2):56–67. doi: 10.1017/S1047951107001163. [DOI] [PubMed] [Google Scholar]
- 22.Goo HW, Yang DH. Coronary artery visibility in free-breathing young children with congenital heart disease on cardiac 64-slice CT: dual-source ECG-triggered sequential scan vs. single-source non-ECG-synchronized spiral scan. Pediatr Radiol. 2010;40(10):1670–80. doi: 10.1007/s00247-010-1693-8. [DOI] [PubMed] [Google Scholar]
- 23.Jebelli M, Kernstine K, Mandegar MH, Sarzaeem MR, Rayatzadeh H. The 64-multislice computed tomogram averts misdiagnosis of an anomalous origin of the left main coronary artery. Pediatr Cardiol. 2009;30(8):1184–5. doi: 10.1007/s00246-009-9508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khatri S, Varma SK, Khatri P, Kumar RS. 64-slice multidetector-row computed tomographic angiography for evaluating congenital heart disease. Pediatr Cardiol. 2008;29(4):755–62. doi: 10.1007/s00246-008-9196-1. [DOI] [PubMed] [Google Scholar]
- 25.Ben Ali W, Metton O, Roubertie F, Pouard P, Sidi D, Raisky O, Vouhe PR. Anomalous origin of the left coronary artery from the pulmonary artery: late results with special attention to the mitral valve. Eur J Cardiothorac Surg. 2009;36(2):244–9. doi: 10.1016/j.ejcts.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Ojala T, Salminen J, Happonen JM, Pihkala J, Jokinen E, Sairanen H. Excellent functional result in children after correction of anomalous origin of left coronary artery from the pulmonary artery--a population-based complete follow-up study. Interact Cardiovasc Thorac Surg. 2010;10(1):70–5. doi: 10.1510/icvts.2009.209627. [DOI] [PubMed] [Google Scholar]
- 27.Kuroczynski W, Kampmann C, Kayhan N, Heinemann M, Pruefer D, Vahl CF. Anomalous origin of the left coronary artery from the pulmonary artery: mid-term results after surgical correction. Clin Res Cardiol. 2008;97(4):266–71. doi: 10.1007/s00392-007-0621-x. [DOI] [PubMed] [Google Scholar]
- 28.Chiu HH, Wang JK, Chen CA, Chiu SN, Lin MT, Lue HC et al. Resolution of pathologic Q wave, left ventricular dysfunction and mitral regurgitation after dual coronary repair of the anomalous origin of the left coronary artery from the pulmonary artery. Eur J Pediatr. 2008;167(11):1277–82. doi: 10.1007/s00431-008-0667-4. [DOI] [PubMed] [Google Scholar]
- 29.Lange R, Vogt M, Horer J, Cleuziou J, Menzel A, Holper K et al. Long-term results of repair of anomalous origin of the left coronary artery from the pulmonary artery. Ann Thorac Surg. 2007;83(4):1463–71. doi: 10.1016/j.athoracsur.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Muneer Amanullah M, Rostron AJ, Leslie Hamilton JR, Chaudhari MP, Hasan A. Towards an anatomically correct repair for anomalous left coronary artery arising from the pulmonary trunk. Cardiol Young. 2008;18(4):372–8. doi: 10.1017/S1047951108002369. [DOI] [PubMed] [Google Scholar]
- 31.Ono M, Goerler H, Boethig D, Breymann T. Surgical repair of anomalous origin of the left coronary artery arising from the left pulmonary artery. Ann Thorac Surg. 2009;88(1):275–6. doi: 10.1016/j.athoracsur.2008.11.069. [DOI] [PubMed] [Google Scholar]
- 32.Wu QY, Xu ZH. Surgical treatment of anomalous origin of coronary artery from the pulmonary artery. Chin Med J (Engl) 2008;121(8):721–4. [PubMed] [Google Scholar]


