Abstract
Percutaneous coronary intervention is established as an effective treatment for patients with ischemic heart disease; in particular, drug-eluting stent implantation is known to suppress in-stent restenosis. Diabetes mellitus is an independent risk factor for restenosis, so reducing insulin resistance is being studied as a new treatment approach. In this prospective study, we sought to clarify the factors associated with in-stent restenosis after percutaneous coronary intervention, and we evaluated the homeostasis model assessment of insulin resistance (HOMA-IR) index as a predictor of restenosis.
We enrolled 136 consecutive patients who underwent elective percutaneous coronary intervention at our hospital from February 2010 through April 2013. All were implanted with a 2nd-generation drug-eluting stent. We distributed the patients in accordance with their HOMA-IR index values into insulin-resistant Group P (HOMA-IR, ≥2.5; n=77) and noninsulin-resistant Group N (HOMA-IR, <2.5; n=59). Before and immediately after stenting, we measured reference diameter, minimal lumen diameter, and percentage of stenosis, and after 8 months we measured the last 2 factors and late lumen loss, all by means of quantitative coronary angiography.
After 8 months, the mean minimal lumen diameter was smaller in Group P than that in Group N (1.85 ± 1.02 vs 2.37 ± 0.66 mm; P=0.037), and the mean late lumen loss was larger (0.4 ± 0.48 vs 0.16 ± 0.21 mm; P=0.025). These results suggest that insulin resistance affects neointimal tissue proliferation after 2nd-generation drug-eluting stent implantation.
Keywords: Blood glucose/analysis, cardiovascular diseases/complications, coronary angiography, coronary artery disease/physiopathology, coronary restenosis/prevention & control, diabetic angiopathies/prevention & control, insulin resistance/physiology, predictive value of tests, recurrence, stents
Percutaneous coronary intervention (PCI) has been established as an effective treatment approach in patients who have ischemic heart disease. The implantation of 2nd-generation drug-eluting stents (DES) has reduced the rates of in-stent restenosis; however, restenosis is still observed, especially in patients who have diabetes mellitus (DM).
In diabetic patients, it is necessary to prevent the occurrence of cardiovascular events caused by ischemic heart disease: approximately 75% of diabetic patients die of a cardiovascular event, and DM treatment and prevention pose substantial challenges.1 However, the prognosis in these patients is not improved solely by treating hyperglycemia2–4; insulin resistance should also be considered.5 (Insulin resistance refers to decreased tissue sensitivity to glucose metabolism in type 2 DM.) Diabetes mellitus is an independent factor of restenosis,6 and insulin resistance is reportedly a predictor of coronary artery restenosis in nondiabetic patients.7
In this study, we sought to clarify the factors associated with coronary restenosis after PCI, with use of the homeostasis model assessment of insulin resistance (HOMA-IR) index as a predictor of restenosis.
Patients and Methods
We enrolled 136 consecutive patients who underwent successful elective PCI at our hospital from February 2010 through April 2013. All gave their written informed consent to participate in this prospective study, which our institutional review board approved. Coronary angiography was performed with use of the Axiom™ Artis™ dBC (Siemens Healthcare GmbH; Erlangen, Germany) in all patients. The PCI was performed in all lesions that caused significant stenosis in accordance with the modified American College of Cardiology/American Heart Association (ACC/AHA) grading system: types A, B1, B2, and C.
All patients were taking aspirin (100 mg) and ticlopidine (200 mg) or clopidogrel (75 mg) daily. They were given a 5,000-IU heparin bolus intravenously before PCI, in the absence of contraindications. Medical therapy for risk factors such as hypertension, dyslipidemia, and DM was provided in accordance with each patient's condition. The study population excluded individuals who were taking a pioglitazone or insulin preparation, and those whose fasting glucose level exceeded 200 mg/dL.
The HOMA-IR index is determined on the basis of levels of fasting glucose and plasma insulin, and we used it as an index of insulin resistance in accordance with the following equation8,9:
HOMA-IR = [fasting glucose (mg/dL) × plasma insulin (μU/mL)] / 405.
In Japanese subjects, a HOMA-IR index value >2.5 has been defined as insulin resistance.10 Therefore, we distributed the 136 patients in accordance with their HOMA-IR index value into positive Group P (HOMA-IR, ≥2.5; n=77), and negative Group N (HOMA-IR, <2.5; n=59). There were no significant differences between the groups in demographic characteristics, risk factors, or medication for hypertension, dyslipidemia, or DM (Table I); nor were there significant differences in lesion-related variables, number of diseased vessels, ACC/AHA lesion grade, or DES characteristics (Table II).
TABLE I.
Baseline Characteristics of the Groups
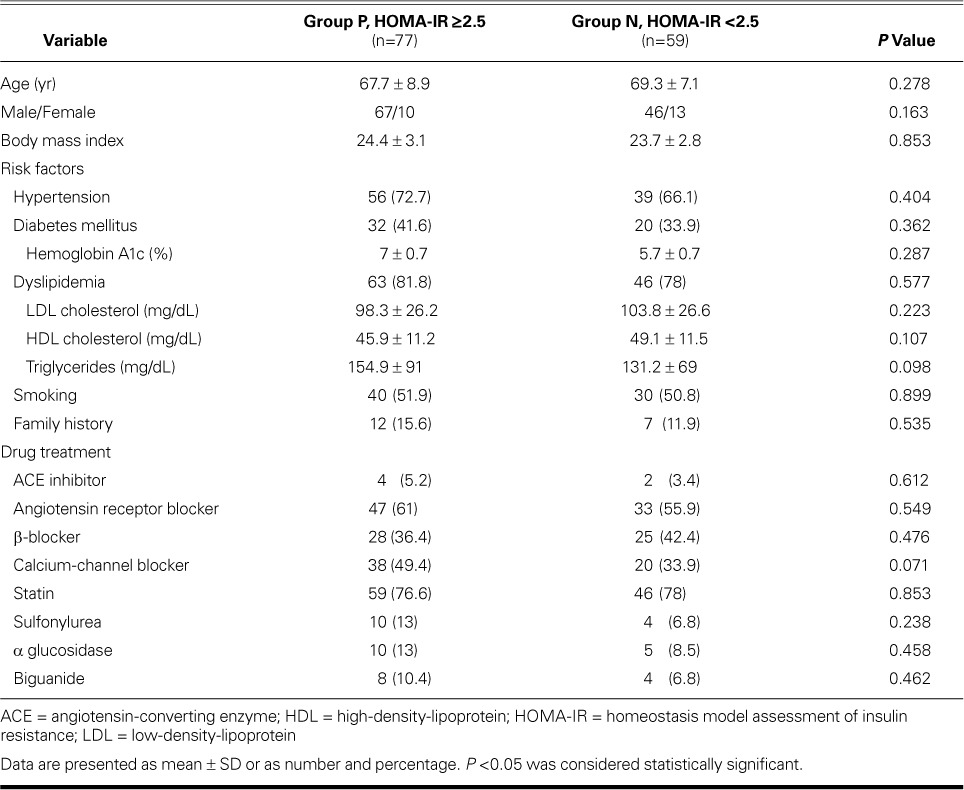
TABLE II.
Angiographic Characteristics of the Groups
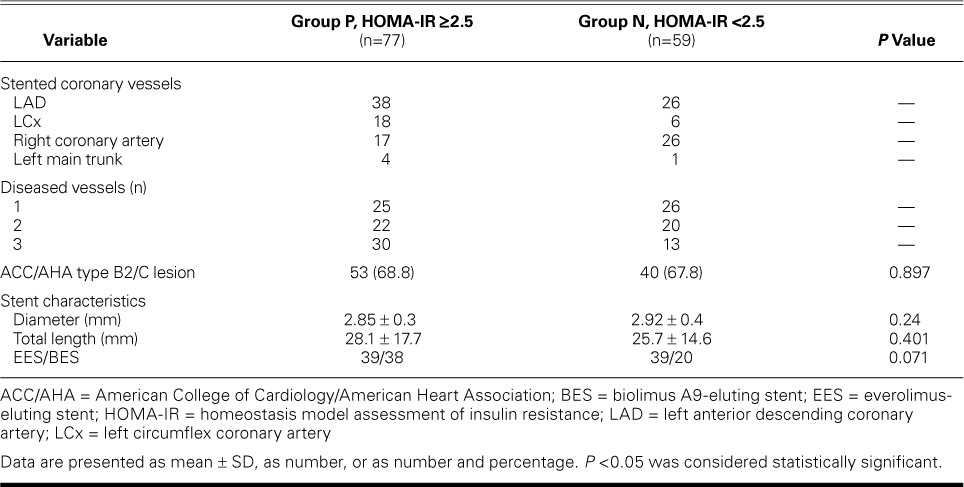
All patients were successfully implanted with a 2nd-generation DES—either everolimus-eluting, or biolimus A9™-eluting (Biosensors Japan, part of Biosensors International Group, Inc.; Tokyo, Japan). We measured luminal diameters of the coronary arteries and degrees of stenosis before PCI, immediately after PCI, and 8 months later. Quantitative coronary analysis (QCA) was then performed with use of QCA-CMS® version 6.0 (Medis Medical Imaging Systems B.V.; Leiden, The Netherlands), by several investigators. Restenosis was defined as stenosis of 50% or more of the luminal diameter.
Statistical Analysis
Data are expressed as mean ± SD. The groups were compared by means of the Mann-Whitney U test for continuous variables and the χ2 test for categorical variables. All analyses were performed with use of StatView 5.0 (SAS Institute Inc.; Cary, NC). A P value <0.05 was considered statistically significant.
Results
No patient died, had a major cardiac event (acute myocardial infarction, congestive heart failure coronary artery bypass grafting, severe arrhythmia, or stroke) or sustained a procedural sequela (stent thrombosis, fracture, or nondeployment).
At 8 months, the comparative restenosis rates of 7.8% in Group P (6 patients) and 3.4% in Group N (2 patients) were not statistically significant (Table III).
TABLE III.
Restenosis and Quantitative Coronary Angiographic Analysis
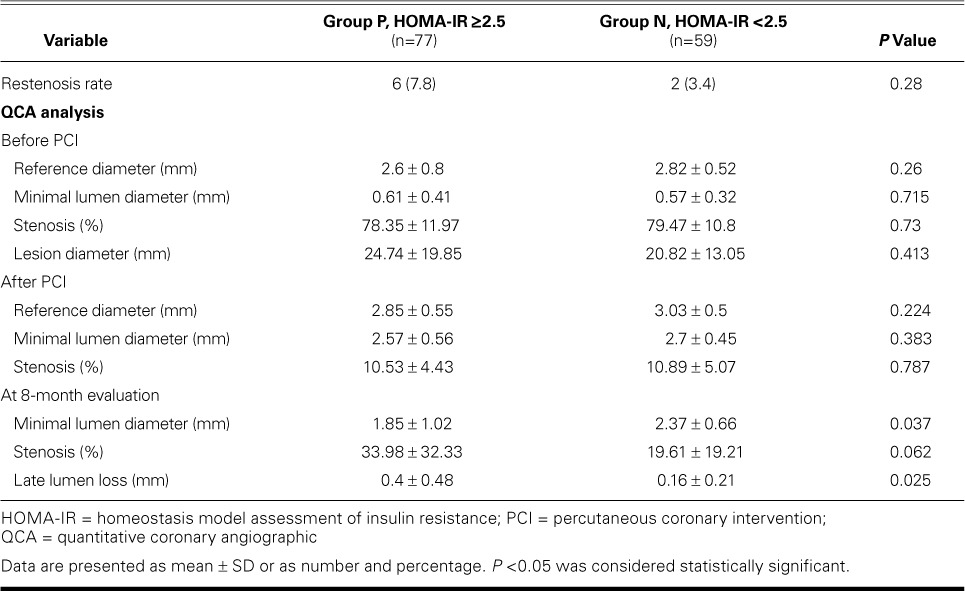
Before and immediately after PCI, the reference diameter, minimal lumen diameter (MLD), and percentage of stenosis were not significantly different between the groups. After 8 months, the mean MLD was significantly smaller in Group P than in Group N (1.85 ± 1.02 vs 2.37 ± 0.66 mm; P=0.037), and the mean late lumen loss was significantly larger (0.4 ± 0.48 vs 0.16 ± 0.21 mm; P=0.025) (Table III; Figs. 1 and 2).
Fig. 1.
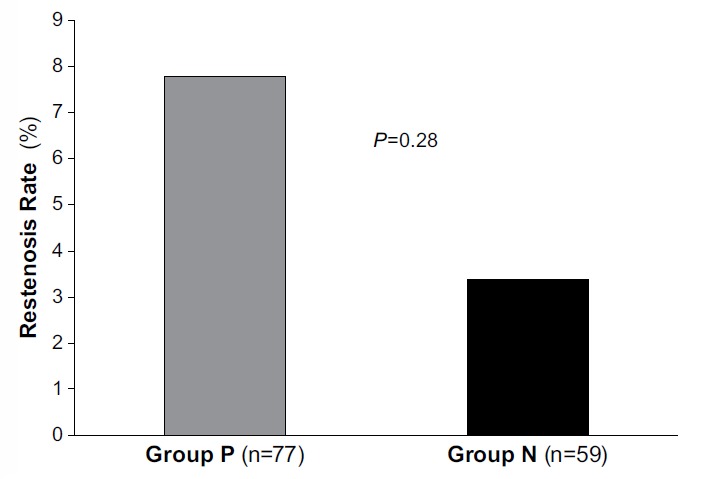
Graph shows restenosis rates of 7.8% in the insulin-resistant group P (6 patients) and 3.4% (2 patients) in the nonresistant group N. P <0.05 was considered statistically significant.
Fig. 2.
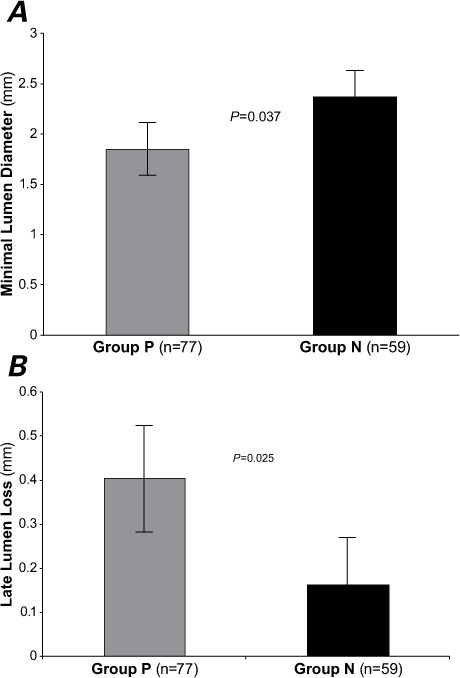
Graphs show the results of quantitative coronary angiographic analysis after 8 months in terms of A) minimal lumen diameter and B) late lumen loss. In insulin-resistant Group P, the minimal lumen diameter was less than that in nonresistant Group N (1.85 ± 1.02 vs 2.37 ± 0.66 mm; P=0.037), and late lumen loss was significantly larger (0.4 ± 0.48 vs 0.16 ± 0.21 mm; P=0.025). P <0.05 was considered statistically significant.
Discussion
We observed significant differences after 8 months between the groups in MLD, percentage of stenosis, and late lumen loss, but the restenosis rates were similar. These outcomes suggest that insulin resistance affects neointimal tissue proliferation after DES implantation.
Stent Restenosis and Diabetes Mellitus
The most important challenge after PCI is restenosis. The procedure causes laceration and dissection of the coronary vessel wall. The vessel wall recovers from this injury through rebuilding of the neointimal tissue, and restenosis is caused by the proliferation of smooth-muscle cells.11,12 This proliferative process is especially serious in diabetic patients, and DM has been determined to be a predictor of restenosis.
After DES were developed and used instead of bare-metal stents, overall restenosis rates after PCI declined. The DES have yielded excellent outcomes even in diabetic patients.13–15 Nevertheless, DES do not eliminate restenosis. As a remedy, we propose improving insulin resistance in addition to using DES.
A high-insulin state is considered to be one cause of neointimal proliferation. In the presence of a high-insulin state, mitogen-activated protein kinase is activated, which causes overproliferation.16 Smooth-muscle cells proliferate in the vessels because of the high-insulin state under insulin resistance.17 Therefore, it is necessary to treat DM by both lowering glycemic levels and improving insulin resistance.
Insulin resistance occurs in diabetic patients and in patients with hypertension, hypercholesterolemia, and metabolic syndrome. According to Hedblad and associates,5 insulin-resistant diabetic patients had higher mortality rates and more ischemic adverse coronary events than did patients without insulin resistance.5 However, the authors did not evaluate patients by means of coronary angiography and therefore could not examine restenosis. Sekiguchi and colleagues7 reported that insulin resistance was a predictor of restenosis in nondiabetic patients. In our study, MLD and late lumen loss showed significant changes upon QCA. Therefore, insulin resistance might hamper neointimal tissue proliferation after DES implantation. We evaluated insulin resistance by using the HOMA-IR index, which is easy to calculate and which serves to predict neointimal tissue proliferation after DES implantation.
Insulin has growth-promoting and protective vascular effects in vivo. Breen and colleagues18 showed that insulin increases neointimal growth after arterial injury. Hoffmann and associates19 found no significant differences in the occurrence of major adverse cardiac events from the use of bare-metal stents versus DES in patients with metabolic syndrome. Although metabolic syndrome is characterized by high insulin resistance, Hoffmann and associates did not evaluate that factor. Our Group P had a smaller MLD, a higher percentage of stenosis, and a larger late lumen loss. An imbalance of insulin effects would promote endothelial disadvantage in the human body.
Pioglitazone has been used in diabetic patients to improve insulin resistance and thereby prevent the occurrence of cardiovascular events.20 However, insulin resistance occurs not just in patients with DM. Therefore, we suggest that treatment for insulin resistance in the early phase will reduce the future rate of restenosis in a broad group of patients who are given a DES.
Limitations of the Study
This was a single-center, nonrandomized study. A core laboratory was not used for QCA. The relatively small number of patients in this study limits the power of estimation. Furthermore, the patients were evaluated only in the early postoperative period. We think that long-term follow-up in a larger number of patients is necessary to confirm our findings. In addition, this study evaluated only in-stent restenosis. In the future, we wish to evaluate coronary flow reserve.
Finally, the HOMA-IR index cannot be calculated in patients who are taking a pioglitazone or insulin preparation and who have a fasting glucose level exceeding >200 mg/dL, so another index of insulin resistance is necessary in those cases.
Conclusion
We evaluated the HOMA-IR index as a predictor of restenosis and found a similar restenosis rate in the groups with positive and negative HOMA-IR values. However, neointimal tissue proliferation was a severe problem in our positive-value group, as shown by means of QCA analysis. Accordingly, we suggest that insulin resistance affects neointimal tissue proliferation after 2nd-generation DES implantation.
Footnotes
From: Department of Cardiology, Dokkyo Medical University Koshigaya Hospital, Koshigaya City, 343-8555 Saitama, Japan
References
- 1.Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US adults. JAMA. 1999;281(14):1291–7. doi: 10.1001/jama.281.14.1291. [DOI] [PubMed] [Google Scholar]
- 2.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 3.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group [published erratum appears in Lancet 1999;354(9178):602] Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 5.Hedblad B, Nilsson P, Engstrom G, Berglund G, Janzon L. Insulin resistance in non-diabetic subjects is associated with increased incidence of myocardial infarction and death. Diabet Med. 2002;19(6):470–5. doi: 10.1046/j.1464-5491.2002.00719.x. [DOI] [PubMed] [Google Scholar]
- 6.Carrozza JP, Jr, Kuntz RE, Fishman RF, Baim DS. Restenosis after arterial injury caused by coronary stenting in patients with diabetes mellitus. Ann Intern Med. 1993;118(5):344–9. doi: 10.7326/0003-4819-118-5-199303010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Sekiguchi M, Kurabayashi M, Adachi H, Hoshizaki H, Oshima S, Taniguchi K. Usefulness of insulin resistance measured by homeostasis model assessment in predicting restenosis after coronary stent placement in nondiabetic patients. Am J Cardiol. 2004;93(7):920–2. doi: 10.1016/j.amjcard.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 8.Sinha DP, Ahmed S, Baneerjee AK, Das M, Hassan H. Significance of an index of insulin resistance in non-diabetic patients with impaired fasting glucose with acute myocardial infarction and its correlation to short term outcome. Indian Heart J. 2009;61(1):40–3. [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 10.Kanauchi M. A new index of insulin sensitivity obtained from the oral glucose tolerance test applicable to advanced type 2 diabetes. Diabetes Care. 2002;25(10):1891–2. doi: 10.2337/diacare.25.10.1891. [DOI] [PubMed] [Google Scholar]
- 11.Ueda M, Becker AE, Tsukada T, Numano F, Fujimoto T. Fibrocellular tissue response after percutaneous transluminal coronary angioplasty. An immunocytochemical analysis of the cellular composition. Circulation. 1991;83(4):1327–32. doi: 10.1161/01.cir.83.4.1327. [DOI] [PubMed] [Google Scholar]
- 12.Austin GE, Ratliff NB, Hollman J, Tabei S, Phillips DF. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985;6(2):369–75. doi: 10.1016/s0735-1097(85)80174-1. [DOI] [PubMed] [Google Scholar]
- 13.Holmes DR, Jr, Leon MB, Moses JW, Popma JJ, Cutlip D, Fitzgerald PJ et al. Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation. 2004;109(5):634–40. doi: 10.1161/01.CIR.0000112572.57794.22. [DOI] [PubMed] [Google Scholar]
- 14.Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation. 2004;109(16):1942–7. doi: 10.1161/01.CIR.0000127110.49192.72. [DOI] [PubMed] [Google Scholar]
- 15.Sabate M, Jimenez-Quevedo P, Angiolillo DJ, Gomez-Hospital JA, Alfonso F, Hernandez-Antolin R et al. Randomized comparison of sirolimus-eluting stent versus standard stent for percutaneous coronary revascularization in diabetic patients: the diabetes and sirolimus-eluting stent (DIABETES) trial. Circulation. 2005;112(14):2175–83. doi: 10.1161/CIRCULATIONAHA.105.562421. [DOI] [PubMed] [Google Scholar]
- 16.Low Wang CC, Goalstone ML, Draznin B. Molecular mechanisms of insulin resistance that impact cardiovascular biology. Diabetes. 2004;53(11):2735–40. doi: 10.2337/diabetes.53.11.2735. [DOI] [PubMed] [Google Scholar]
- 17.Bruemmer D. C-Peptide in insulin resistance and vascular complications: teaching an old dog new tricks. Circ Res. 2006;99(11):1149–51. doi: 10.1161/01.RES.0000251785.83860.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breen DM, Chan KK, Dhaliwall JK, Ward MR, Al Koudsi N, Lam L et al. Insulin increases reendothelialization and inhibits cell migration and neointimal growth after arterial injury. Arterioscler Thromb Vasc Biol. 2009;29(7):1060–6. doi: 10.1161/ATVBAHA.109.185447. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann R, Stellbrink E, Schroder J, Grawe A, Vogel G, Blindt R et al. Impact of the metabolic syndrome on angiographic and clinical events after coronary intervention using bare-metal or sirolimus-eluting stents. Am J Cardiol. 2007;100(9):1347–52. doi: 10.1016/j.amjcard.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298(10):1180–8. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]


