Abstract
Aims and objectives
The aim of the study was to investigate and compare the financial impact of Schmallenberg disease for different dairy production types in the United Kingdom and France.
Materials and methods
Integrated production and financial models for dairy cattle were developed and applied to Schmallenberg virus (SBV) disease in a British and French context. The five main production systems that prevail in these two countries were considered. Their respective gross margins measuring the holding's profitability were calculated based on public benchmarking, literature and expert opinion data. A partial budget analysis was performed within each production model to estimate the impact of SBV in the systems modelled. Two disease scenarios were simulated: low impact and high impact.
Results
The model gross margin obtained per cow space and year ranged from £1014 to £1484 for the UK and from £1037 to £1890 for France depending on the production system considered. In the UK, the net SBV disease costs in £/cow space/year for an average dairy farm with 100 milking spaces were estimated between £16.3 and £51.4 in the high-impact scenario and between £8.2 and £25.9 in the low-impact scenario. For France, the net SBV disease costs in £/cow space/year ranged from £19.6 to £48.6 in the high-impact scenario and £9.7 to £22.8 in the low-impact scenario, respectively.
Conclusion
The study illustrates how the combination of production and financial models allows assessing disease impact taking into account differing management and husbandry practices and associated price structures in the dairy sector. It supports decision-making of farmers and veterinarians who are considering disease control measures as it provides an approach to estimate baseline disease impact in common dairy production systems in the UK and France.
Keywords: Economics, Cattle, Infectious diseases, Schmallenberg virus, Production model
Introduction
Each year millions of pounds are spent globally on managing, preventing and researching animal diseases. The current allocation of money is largely based on biological processes of transmission and infection, and the application of biological knowledge to improve disease management. There is limited reference to animal disease impacts, which should be a fundamental aspect of prioritisation and efficient resource allocation, be it at the farm, national, international or global level. A principal reason for this is the lack of standardised, rigorous methods for the financial impact assessment of animal disease. At the farm level, estimates on the financial impact of disease provide important information for farm decision-making. However, disease impact studies at the farm level are highly variable due to the complex nature of the effects a disease exerts on production (Jericho 1974), augmented by numerous variables such as species, breed or management.
Several authors elaborated a theoretical methodological basis to estimate disease impact. In the conceptual framework of Mclnerney and others (1992), disease costs are split into output losses following disease occurrence and expenditures made to treat disease or prevent its occurrence. The economically optimal level of disease costs is obtained using a loss-expenditure frontier. This concept was applied (Yalcin and others 1999, Chi and others 2002, Alarcon and others 2013), at times criticised (e.g. Tisdell 1995) and further developed (e.g. Bennett and IJpelaar 2005, Howe and others 2013) in a range of studies. Commonly, disease impact models only look at one or very few specified production types (e.g. Alarcon and others 2013) and do not consider finer details such as differences in production type (e.g. dairy v beef), calving patterns (e.g. block calving v all-year-round calving), reproductive services (e.g. use of bull, synchronised insemination), feeding practices (e.g. intensive v extensive) or breed and associated price structures (e.g. different market prices due to product differentiations, distinct geographic locations or selling times). Because farm-specific factors influence disease impact and consequently the most appropriate decision on cost-effective disease management, disease cost estimates should reflect the farm situation as closely as possible. One way of providing tailored information for farmers is to establish a farm-level decision support system where the user can change herd input data (e.g. herd size, cull rate) and disease-specific assumptions (e.g. mortality rate due to disease). Such disease control calculators for selected diseases were developed and made available online by the University of Reading (www.fhpmodels.reading.ac.uk) and the Royal Veterinary College (http://www.bpex.org.uk/articles/303060, Alarcon and others 2013) in the UK.
Schmallenberg virus (SBV) affects ruminants and appears exclusively transmitted by insect vectors of the Culicoides species group and vertically in utero (Beer and others 2012, Garigliany and others 2012). Virus detection in blood lasts up to 15 days after natural infection (Claine and others 2013), and animals infected with SBV could develop immunoprotection, which may prevent repeated infection for at least two months (Wernike and others 2013). In ruminants, clinical signs are mainly associated with reproductive disorders. Depending on the time of infection, abortion, stillborn animals, premature deliveries and various intrauterine congenital malformations may occur (Conraths and others 2012, Steukers and others 2012, Veldhuis and others 2014). SBV has been detected in malformed fetuses, stillborn or newborn calves born at term but with signs of neurological disorders, such as blindness, deafness, recumbency, an inability to suck and convulsions (Lievaart-Peterson and others 2012, Steukers and others 2012). Dystocia in cattle may lead to negative impacts on animal welfare, culling and cow deaths (European Food Safety Authority 2012). In adult cows, the acute infection can result in transient and non-specific symptoms, namely diarrhoea, inappetence, fever and a reduction in milk yield, usually followed by a full recovery (Hoffmann and others 2012, Muskens and others 2012). While several studies were published on the biological effects of the disease, to date no scientific literature is available that estimates the financial impact of SBV at the farm level. Moreover, there is no information on how different production systems in the dairy sector are affected. Servicing or parturition in certain periods of the year may coincide with increased susceptibility of herds and/or other factors such as vector presence that may impact on exposure to pathogens and disease transmission (e.g. cows in autumn-calving herds are expected to be in middle to late gestation during the period of high-vector activity—a time when the fetus is more susceptible to malformations and abortions). The quantity of milk produced fluctuates during the year depending on the production system, that is, it is more or less constant for all-year-round-calving herds, whereas highest production occurs in autumn for autumn-calving herds and in spring/early summer for spring-calving herds. Thus, effects on milk yield due to clinical disease in adult animals during the period of high-vector activity would be more prominent in spring-calving herds. Costs and revenues are further influenced by seasonal changes in prices for milk and concentrates. These effects are schematically illustrated in online supplement 1. Finally, management practices impact on the flexibility of farmers’ decision-making. For example, a reproductive disease in closed herds may result in higher numbers of ‘empty’ breeding animals, thus the herd fails to achieve self-sustaining replacement rates, thereby causing suboptimal production. The same problem in an open herd can be balanced by buying in replacement stock.
To examine disease effects in different production systems and support decision-making on the farm, this study presents integrated production and financial models to estimate SBV disease costs in dairy cattle in the UK and France. The objectives were (1) to develop production models as a basis for financial analysis of SBV in dairy farms, (2) to apply the models to estimate the impact of SBV in the UK and France, and (3) to investigate potential differences in model variables and disease estimates between the two countries. This study provides a first step towards the development of a farm decision calculator for the control of SBV.
Methodology
Development of production models
Available benchmarking data and expert opinion was used to identify the most common and representative dairy cattle systems in the UK and France. In total, five dairy production systems each were identified for the UK and France (Table 1) based on relevant benchmarking literature for the UK (Scottish Agricultural College 2010, Agro Business Consultants 2012, Nix 2013) and France (Vignau-Loustau and Huyghe 2008). Literature data were complemented by the authors’ knowledge and experience of these systems and information shared by expert colleagues.
TABLE 1:
Description of the dairy production types in the UK and France considered in this study
| Country | Farm types | Description |
|---|---|---|
| The UK | Spring-calving closed farms (SC) | The majority of cows (80%) calve between February and May. Cows have a lower than national average milk yield per year and a 'hardier constituency' compared with very high-yielding herds. Feeding is predominantly grass with few concentrates. Most herds are closed, i.e. replacement is done using own calves. For this, all cows are mated with dairy bulls and most female heifers are used for replacement |
| Autumn-calving open farms (AOC) | The majority of cows (80%) calve between August and November. These farms require an essential housing infrastructure and an increased amount of concentrate. Cows have a higher than national average milk yield, and milk price during the period of highest production is at the annual peak. Replacement 50% from own heifers and 50% buying heifers from outside. The first two services a cow receives are done using dairy bull semen; subsequent services using beef bull semen (about 40% of calves born from beef bulls) | |
| Autumn-calving closed farms (ACC) | Similar to AOC, but with the difference that all replacements are bred and raised in the same farm. Therefore, all cows are inseminated by dairy bulls and nearly all heifers used for replacement. The 365-day calving rate in these farms is assumed to be higher than in open herds. | |
| All-year-round open farms (AYOC) | Cows calve anytime during the year. Fertility is poorer than in the autumn- and spring-calving systems and the replacement rate is consequently higher. Replacement through own heifers (50% of replacements) and by purchasing heifers from other farms. The first two services a cow receives are done using dairy bull semen; subsequent services using beef bull semen (about 40% of calves born are from beef bulls) | |
| All-year-round closed farms (AYCC) | Similar to AYOC, but with the difference that the replacement is done using only own heifers. All cows are mated with dairy bulls and almost all heifers are used for replacement. These farms are assumed to have better breeding performance and therefore a higher calving rate than open farms | |
| France | Lowland with corn silage (LC) | All-year-round calving; sometimes a calving peak may be seen in a proportion of the herd. High milk yield and high cost of production. Corn silage as main forage, normally all year long. Often zero-grazing system for cows in milk. A big part of concentrates needed is soy bean meal or derivates (bought). Poor fertility and high replacement rate. Replacement is mainly done using own heifers, but also purchasing of heifers from other farms. Only cows with reproduction issues are mated with beef artificial insemination (AI) (negligible) |
| Lowland with corn silage and grass (LCG) | Similar to LC, but grass is used in spring and fall, and sometimes all summer long (depending on area). It allows reducing the feed costs. Production remains high | |
| Upland with corn silage and grass (UCG) | Relies predominantly on grass, even if corn silage is available at least for winter (quantity available is limited). Production level is lower compared with LCG. Beef AI can be used to increase the calf selling prices. Variable price of milk compared with national level | |
| Upland with grass Massif Central (UGMC) | Located in mountains, centre of France. Mainly based on grass/hay. None or limited use of grass or corn silage. Both Holstein (with low production) and Montbéliarde breeds are used. Standard production (i.e. the same price applies for milk here and in the lowlands) and high-quality production (Protected Area Designation cheese, higher price of milk). Use of beef bull or bull AI is important (often up to 50% of cows) | |
| Upland with grass Franche-Comté (UGFC) | Located in mountains, east of France. Mainly based on grass/hay. Silage forbidden for cheese production. The production is highly specialised in high-quality products (Protected Area Designation cheese Comté) with Montbéliarde only and no use of beef bull crossing or AI. Higher price of milk compared with national level |
For the UK, only Holstein farm models were developed as these account for over 95 per cent of dairy production in the UK (Agro Business Consultants 2012). Autumn and all-year-round dairy systems were stratified into open and closed herds, while spring-calving herds were assumed to be mainly closed herds. In France, the different dairy production systems are described primarily by location and feeding type (Table 1). All French systems apart from the Upland with grass Franche-Comté system (UGFC) match the UK all-year-round open dairy system. The UGFC system matches the UK dairy closed system that uses all-year-round calving.
For the development of the production models, which simulated one production cycle of one year, benchmarking data from different independent sources based on farm surveys and actual expenditures made by farmers were used for both the UK (Scottish Agricultural College 2010, Agro Business Consultants 2012, EBLEX 2012, DairyCo 2013, Nix 2013) and France (Institut Elevage Bovin lait 2013, Institut Elevage Bovin viande 2013, Reuillon and others 2013, Institut Elevage Ovin lait 2012, Institut Elevage Ovin viande 2013). Some of the available benchmarking data needed to be complemented by other sources, such as the authors’ expertise and published statistics on market prices. For example, expenditures in France were available for the whole farm, but not disaggregated by the different classes of animals and thus needed to be broken down using the authors’ professional judgement.
Estimation of annual gross margins
The production models were used to estimate the annual gross margins for the different production systems (Eq. 1):
 |
1 |
A summary of calculations of revenues and costs is given in Table 2. All input parameters associated with each system are shown in online supplement 2. Detailed calculations of revenues and costs are given in online supplement 3.
TABLE 2:
Revenues and costs calculated in production models for dairy cattle in the UK and France (FR)
| Revenues and costs | Equations |
|---|---|
| Revenues | |
| UK-FR: milk sold | Total milk produced (l) x price of milk per litre (£) |
| UK-FR: dairy x dairy male calves (DDMCV) sold | NDDMCV sold x DDMCV value (£) |
| UK-FR: dairy x beef male calves (DBMCV) sold | NDBMCV sold x DBMCV value (£) |
| UK-FR: dairy x dairy female calves (DDFCV) sold | NDDFCV sold x DDFCV value (£) |
| UK-FR: dairy x beef female calves (DBFCV) sold | NDBFCV sold x DBFCV value (£) |
| UK: non-calving heifers sold | NH not calving and sold x cull value cow (£) |
| UK-FR: revenues from cows culled | NC culled x cull value cow (£) |
| Replacement costs | |
| FR: costs of purchasing heifer calves | NH bought x price of a replacement heifer calf (£) |
| UK: costs of purchasing in-calf heifers | NH bought x price of an in-calf replacement heifer (£) |
| UK-FR: costs of raising own heifers | NH that need to be raised to maintain the 100 milking spaces given the production parameters specified x costs of heifer rearing (£) |
| Feeding costs | |
| UK-FR: costs of feed concentrate in cows | Concentrates (kg) per litre of milk produced x price of dairy concentrate per tonne/1000 (£) x total milk produced (l) |
| UK: costs of forage in cows | NC x [Forage costs (£) per hectare/(NC per hectare of forage)] |
| UK: costs of bulk feed | NC x costs of bulk feed per cow (£) |
| FR: costs of forage in cows | NC x forage costs per cow (£) |
| UK-FR: costs of milk replacer in calves sold | NCV reared x price of milk per litre (£) x milk replacer (kg) per calf |
| Veterinary and disposal costs | |
| UK-FR: veterinary costs in cows | NC x total veterinary costs per cow (£) |
| UK: costs of disposing dead cows | NC that die x disposal costs dead calf, cow or heifer (£) |
| UK: costs of disposing dead heifers | NH that die x disposal costs dead calf, cow or heifer (£) |
| UK: costs of disposing dead calves | NCV that die x disposal costs dead calf, cow or heifer (£) |
| Other variable costs | |
| UK-FR: costs of artificial insemination | NC x costs of artificial insemination per cow (£) |
| UK-FR: costs of bedding | NC x costs of bedding per cow (£) |
| UK-FR: miscellaneous costs | NC x miscellaneous costs per cow (£) |
Input values are specific to each production system. Number (N) and quantities of animals/products indicated in the equations are obtained from the production models
C, cow; CV, calf; H, heifer
The revenues, that is, the amount of money a farm receives in exchange for its goods or outputs, included the return from selling milk and animals. Milk price took into account accuracy bonus, forecast bonus and penalties when production drops below a certain threshold. Animal outputs included calves for fattening, calves for breeding, culled cows and, for the UK, non-calving heifers. The latter represents those heifers that failed to conceive, and that therefore have to be sold (block-calving herds only). For France, non-calving heifers were not considered because of the absence of block-calving herds. Feed costs included the costs of concentrate, forage, bulk feed and milk replacer for calves sold. Forage costs were calculated differently for the UK and France (Table 2). For the UK, forage costs were calculated my multiplying the 'forage costs per hectare' by the number of cows per hectare. For France, estimations of forage costs for cows were based on the average daily dry matter intake depending on the lactation stage, the nature of the main forages used and the forage price. In France, disposing costs are paid through a tax when slaughtering animals and were therefore not included in the French models. Other variable costs included the costs of artificial insemination (AI), bedding, veterinary and miscellaneous costs, as reported in benchmarking data of both countries. Replacement costs accounted for the costs of purchasing new heifers-in calf (open farms) or raising own heifers (closed farms). For France, heifers purchased were young animals (not in calf) in accordance with the main practices observed. The residual value of cows was accounted for as 'revenues from cows culled'. The costs of raising own heifers were calculated by estimating the number of heifers to be raised in the herd (accounting for heifer mortality) and multiplying this by the variable costs of heifer rearing (VCHR) (Eq. 2):
 |
The heifer feeding costs were the average daily dry matter intake of concentrates and forage multiplied by the respective costs, for each rearing period from weaning to the first calving.
Assessment of disease impact using partial budget models
The scientific literature was screened to identify the biological effects of SBV in cattle, which included clinical manifestations (diarrhoea, milk drop and fever) and reproductive disorders (late abortion or malformations and related dystocia). Assumptions were made on general management practices and farmers’ reactions to these biological effects (Box 1). In terms of disease effects, it was assumed that milk drop would occur during the duration of the clinical episode and that milk production would return to normal after recovery. SBV reproduction problems were assumed to occur in the last trimester only (stillborn or malformed calves or abortion) when cows are already dry. Due to the lack of scientific evidence of early abortion or empty cows, these effects were not included.
BOX 1: Assumptions made on general management practices and reactions to Schmallenberg virus (SBV)-related disorders in the UK and France (FR) to estimate the financial impact of SBV.
General management practices (without SBV)
UK-FR: 100 milk cow spaces used to full capacity. Lactation period will be extended for some of the cows not conceiving to cover empty spaces.
UK: Farmers need to purchase extra in-calf heifers or use their own heifers to reach 80 calvings per 100 milk cow spaces because of low calving rates. Assumption: fertility higher in closed than open herds.
FR: The high replacement rate of dairy farms in France leads to consider 85 calvings per year for 100 inseminated cows. In open herds, a proportion of young heifers are purchased for replacement, whereas in closed herds replacement is done using own calves only.
UK-FR: All cows in closed herds inseminated with dairy bulls.
UK: 40 per cent of cows in open herds inseminated with beef bulls. Fifty per cent own stock replacement, 50 per cent purchase from outside.
FR: 0 per cent (LC, UCG), 20 per cent (lowland with corn silage and grass) and 40 per cent (upland with grass Massif Central) of cows in open herds inseminated with beef bulls. Low percentage: no replacement heifers are bought as not needed; high percentage: young heifers can be purchased if needed. No purchasing of in calf heifers.
Farmers’ reaction to clinical disease
UK-FR: A very small proportion of SBV affected cows will receive treatment (anti-inflammatory) to suppress fever.
Reproductive SBV disorders and related management practices
UK-FR: In case of abortion, the cow will be culled. Open farms will buy in-calf cows to replace those who have aborted. Closed farms will cull the cow and
replace it the following year (the UK, block calving) with the exception of all-year-round farms
replace it with older cows kept longer (FR and the UK, all-year-round calving)
UK-FR: When the number of heifers produced is not enough to achieve the required replacement rate,
in closed herds, farmers will keep older cows longer; the farmer has no longer the possibility of disposing cows with poor milk yield and therefore a reduction in milk production will occur;
in open herds, in-calf heifers are bought and replacement heifers not sold (the UK).
FR-UK: In case of late abortion, the veterinarian will be called out if the fetus presents signs of malformation. Antibiotic treatment will be applied to aborted cows.
FR-UK: When malformations lead to dystocia, the veterinarian will be called out. In few cases of dystocia, farmers will agree to conduct a caesarean. Milk loss due to dystocia was considered negligible and was not included.
UK-FR: When there is no dystocia, the veterinarian will not be called out and there will not be any medical treatment.
FR-UK: The costs of culling a malformed calf are negligible, but not the disposal costs (the UK).
FR-UK: A small proportion of aborted fetuses and calves stillborn and malformed will be submitted for SBV testing.
The production and gross margin models were run with and without SBV disease parameters (Table 3), and the differences obtained between a herd with SBV and a herd without SBV were used to estimate the net value using standard partial budget analysis (PBA), an economic method used to calculate the extra cost or benefit of a change (Eq. 3):
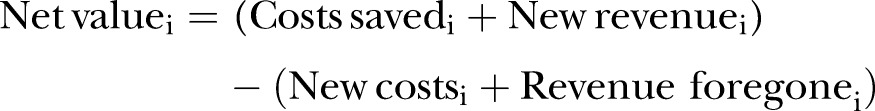 |
3 |
TABLE 3:
Parameters and values used for high-impact (HI) and low-impact (LI) Schmallenberg virus (SBV) disease scenarios
| Parameters | HI | LI | References | Reasoning |
|---|---|---|---|---|
| Number of calves stillborn, malformed or that need culling due to SBV out of 100 calves born | 1–10 ML=2 |
0–1 ML=1 |
Martinelle and others (2012) and expert opinion | Martinelle and others (2012): Median SBV morbidity rate in calves was 2%, which was taken as the most likely value. The minimum reported by Martinelle and others was taken as the lower range value and the median value plus 1 sd as the upper range value |
| Number of cows out of 100 cows that suffer from dystocia when they give birth to a stillborn or malformed calf due to SBV | 30 | 30 | Mee (2012), Meijering (1984) and expert opinion | Baseline dystocia rates reported for the UK were 6.9% in heifers and 2% in cows with abnormal presentations being the cause in 19.8% on average. With an increased proportion of malformations, dystocia rate was assumed to be considerably higher |
| Number of cows that need caesarean out of 100 cows with dystocia due to SBV | 5–7 ML=6 |
5–7 ML=6 |
Day (2007), Haskell (2008) and expert opinion | The proportion of caesareans conducted in the case of dystocia was reported to be between 5% and 7% |
| Number of cows with clinical episodes due to SBV out of 100 cows in a herd | 3–31 ML=7.5 |
0 | Martinelle and others (2012) and expert opinion | Martinelle and others (2012): Median SBV morbidity rate in cattle was 7.5%, which was taken as the most likely value. The minimum reported by Martinelle and others was taken as the lower range value and the median value plus 1 sd as the upper range value |
| Number of cows that require treatment out of 100 cows with clinical episodes due to SBV | 10–20 ML=15 |
10–20 ML=15 |
Expert opinion | This figure reflects the regular need for treatment of dairy cattle in the UK presented with unspecific diarrhoea, fever, general depression and/or inappetence |
| Number of cows with SBV abortions out of 100 cows in a herd | 0–2 ML=2 |
0–1 ML=1 |
Expert opinion | The proportion of abortions due to SBV is uncertain. There is lack of studies on this and experts agreed on these approximated figures based on abortion rates seen in other diseases |
| Number of aborted fetuses presenting signs of malformation out of 100 fetuses aborted | 60 | 60 | Expert opinion | This estimate was based on unpublished UK data communicated in the expert group |
| Probability of aborted fetuses, stillborn, malformed and calves culled to be tested for SBV | 0.05 | 0.05 | Expert opinion | The Animal Health and Veterinary Laboratories Agency recommends investigation of abortions when the incidence rises above 3% in a herd per year or if several abortions occur in quick succession (http://www.defra.gov.uk/ahvla-en/files/pub-cattle-abortion.pdf). Due to the absence of 'abortion storms' owing to SBV and farmers suspecting the disease, it was assumed that only a small proportion submit aborted fetuses, stillborn or malformed calves to be tested for SBV |
| Duration of clinical episode in a cow (days) | 14–21 ML=14 |
14–21 ML=14 |
Martinelle and others (2012) and expert opinion | Martinelle and others (2012): Duration of clinical signs was recorded in seven cattle herds and lasted on average 12 days (from 10 to 15). Experts reported that they had observed longer durations of clinical episodes and increased the range to 2–3 weeks |
| Number of cows culled out of 100 after an abortion | 100 | 100 | Expert opinion | Experts concluded that cows with a late abortion are highly likely to be culled and replaced because the milk yield is seriously reduced after an abortion (in the following lactation) |
| Number of cows culled out of 100 after giving birth to a malformed calf | 0 | 0 | Expert opinion | Because cows giving malformation can still produce the normal milk yield, they are highly likely to be retained in the flock for the next insemination. This reflects common management practices in the UK |
ML, most likely.
Net value (or net SBV disease costs) represents the financial impact of SBV for a year cycle and a defined disease scenario i. The individual partial budget items can be found in the results tables and the associated calculations in online supplement 3. For the PBAs, disease parameters were introduced in the production models and the differences between gross margin parameters of disease and no disease situations were obtained. For example, the number of ‘dairy x dairy male calves sold’ in a herd without SBV was higher than in an SBV-affected herd as SBV infection can cause reproductive problems. Consequently, the difference between the two was recorded as 'dairy x dairy male calves not sold' under revenues foregone in the PBA.
Data on the within-herd SBV incidence, the incidence of various disease effects (e.g. rate of diarrhoea, drop in milk yield, fever) and the magnitude of those effects (e.g. proportion of milk loss) are sparse. Consequently, only two disease scenarios were considered:
Scenario 1: A high impact in a herd that is highly susceptible to disease, which may, for example, be a management system where the susceptible gestation period falls into a season of high-vector activity.
Scenario 2: A low impact in a herd that is less susceptible to disease, which may, for example, be a management system in an area with low-vector density.
To complement the values derived from the scientific literature, the input values for the model were discussed and agreed on in the workshop described in the section ‘Software, input values, sensitivity analysis and validation’. For the most variable and uncertain parameters, minimum, most likely and maximum values were agreed upon (Table 3).
Software, input values, sensitivity analysis and validation
All models were built in Microsoft Excel. Apart from the parameter values derived from published literature, a workshop with 10 experts representing members of the SBV surveillance team at the Animal Health and Veterinary Laboratories Agency, industry representatives, veterinary clinicians and academic researchers was held to present and discuss the structure of the production models, input variables and assumptions. Before the meeting, experts were requested to complete a table with their opinion on the values of specific disease parameters (Table 3), and their ranges, for high- and low-impact scenarios. The different expert estimates obtained and their averages were presented to the experts during the workshop for discussion. For the parameters with major differences and uncertainties, all workshop participants were encouraged to explain why they disagreed and a discussion was stimulated to get to an agreement on the most appropriate values. Further, the structure of the production models, gross margin and partial budget analyses was presented and discussed until an agreement was reached. The second workshop was held at the end of the study, where the models developed and their results were presented. Experts were asked for their opinion on the validity of the results obtained. Similarly, animal health professionals in France looked at the inputs and the assumptions made and provided recommendations for improvement if deemed necessary. Gross margin results were compared with literature estimates for validation purposes.
Sensitivity analysis
The sensitivity analyses were done by varying simultaneously the two variables' proportion of SBV abortions (varied between 0 and 3.5 per cent in steps of 0.5 per cent) and proportion of stillborn and malformed calves (varied at values from 0 to 5 per cent in steps of 1 per cent). These variables were selected taking into account the uncertainty attached to them and their hierarchical position in the model. Uncertainty was determined considering the range of estimates collated from the literature and experts, and the input from discussions during the first expert workshop. In addition, the models were run with all lowest and all highest values as defined in Table 3 to estimate the range of disease impact.
Results
Production models and gross margin analyses
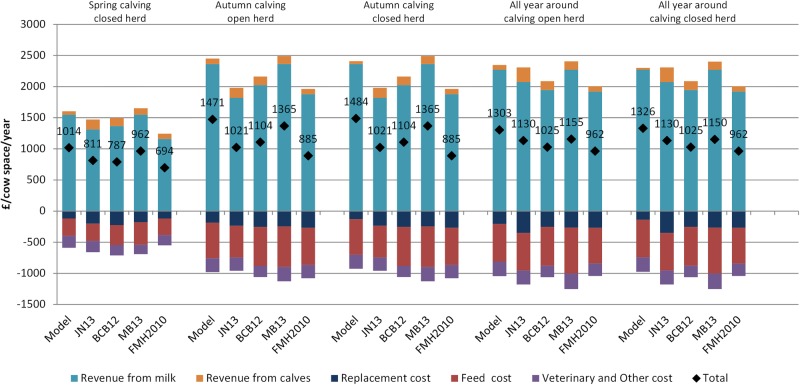
Summarised results of the gross margin analyses are shown in Figs 1 and 2, while the detailed structure and results of the production models and gross margin analyses of non-SBV-infected farms are shown in online supplements 2 and 3. The model gross margins obtained for the UK's spring-calving closed farms (SC), autumn-calving open farms (AOC), autumn-calving closed farms (ACC), all-year-round open farms (AYOC) and all-year-round closed farms (AYCC) were £1014, £1471, £1484, £1303 and £1326 per cow space per year, respectively (Fig 1). Main differences observed between the model gross margin and the industry gross margin were largely caused by differences in the estimation of replacement and feed costs (Fig 1). Overall model estimates were higher than the estimates reported in the literature.
FIG 1:
Dairy cattle production systems in the UK: break-down of revenues and variable costs in the models and gross margin analyses in the literature. BCB12, Budgeting and Costing Book 2012; FMH2010, Farm Management Handbook 2010; JN13, John Nix 2013; MB13, Milkbench Report 2013
FIG 2:
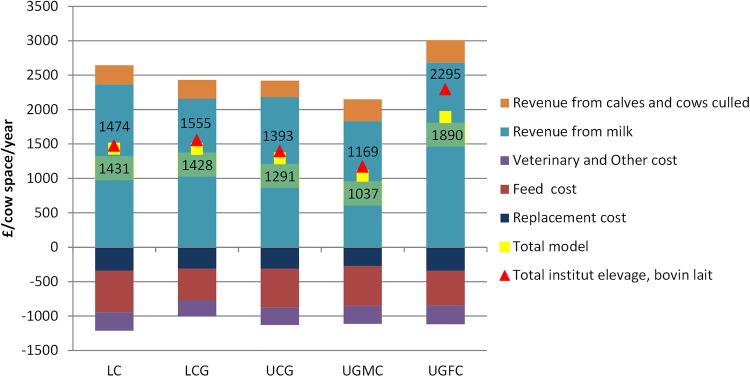
Dairy cattle production systems in France: break-down of revenues and variable costs in the models and gross margin analyses in the literature. LC, lowland with corn silage; LCG, lowland with corn silage and grass; UCG, upland with corn silage and grass; UGFC, upland with grass Franche-Comté; UGMC, upland with grass Massif Central
The model gross margins obtained for the French lowland with corn silage (LC), lowland with corn silage and grass (LCG), upland with corn silage and grass (UCG), upland with grass Massif Central (UGMC) and UGFC systems were £1431, £1428, £1291, £1037 and £1890 per cow space and year, respectively. The model gross margin estimations for the LC, LCG and UCG systems showed differences of 3 per cent and 8 per cent, respectively, to published gross margins (Fig 2). The differences for the UGMC and UGFC systems were 13 per cent and 21 per cent, respectively, due to lower operational costs in the present work compared with references for these two upland systems (Fig 2).
Financial impact of SBV
Results of the SBV disease costs for dairy farms are shown in Tables 4 and 5.
TABLE 4:
Schmallenberg virus (SBV) disease costs (£) for five dairy production types in the UK, high-impact (HI) and low-impact (LI) scenarios
| Spring-calving closed farms |
Autumn-calving open farms | Autumn-calving closed farms | All-year-round open farms |
All-year-round closed farms |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HI | LI | HI | LI | HI | LI | HI | LI | HI | LI | ||
| Additional expenditures | Veterinary assistance on cows that have dystocia due to SBV | 44 | 22 | 44 | 22 | 44 | 22 | 44 | 22 | 44 | 22 |
| Treatment of cows that need caesarean due to SBV dystocia | 5 | 3 | 5 | 3 | 5 | 3 | 5 | 3 | 5 | 3 | |
| Treatment of cows that have clinical SBV episodes | 12 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | |
| Treatment of cows that have aborted due to SBV | 99 | 50 | 99 | 50 | 99 | 50 | 99 | 50 | 99 | 50 | |
| SBV testing of aborted fetuses, stillborn or malformed calves | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | |
| Costs of purchasing and raising heifers for replacement | 1392 | 703 | 2195 | 1109 | 1392 | 703 | 2195 | 1109 | 1392 | 703 | |
| Disposal costs of dead calves and fetuses due to SBV | 135 | 68 | 135 | 68 | 135 | 68 | 135 | 68 | 135 | 68 | |
| Revenues forgone | Dairy x dairy male calves not sold | 129 | 65 | 78 | 39 | 129 | 65 | 46 | 23 | 76 | 38 |
| Dairy x beef male calves not sold | 0 | 0 | 137 | 69 | 0 | 0 | 122 | 61 | 0 | 0 | |
| Dairy x dairy heifer calves not sold | 639 | 322 | 182 | 92 | 639 | 322 | 182 | 92 | 639 | 322 | |
| Dairy x beef heifer calves not sold | 0 | 0 | 116 | 58 | 0 | 0 | 103 | 52 | 0 | 0 | |
| Milk not sold from cows with clinical episode | 3 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | |
| Milk not sold from cows with abortion | 2429 | 1227 | 0 | 0 | 3701 | 1870 | 0 | 0 | 0 | 0 | |
| Milk not sold due to less efficiency of old cows | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Milk not produced due to less efficiency of heifers | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Revenues foregone on cows culled | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Sum of costs | 4890 | 2461 | 3008 | 1510 | 6163 | 3104 | 2948 | 1481 | 2408 | 1208 | |
| Expenditures saved | Concentrate feed saved in cows with clinical episodes | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| Concentrate feed saved on cows that aborted | 233 | 118 | 0 | 0 | 243 | 123 | 0 | 0 | 0 | 0 | |
| Concentrate saved on old cows used as replacement | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Concentrate feed saved in heifers | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Feed saved on calves dead due to SBV | 23 | 12 | 15 | 7 | 23 | 12 | 15 | 7 | 23 | 11 | |
| Extra revenues | Extra revenues from cows culled due to SBV abortion | 627 | 317 | 627 | 317 | 627 | 317 | 627 | 317 | 627 | 317 |
| Extra revenues from culling heifers that did not conceive | 125 | 63 | 0 | 0 | 125 | 63 | 0 | 0 | 125 | 63 | |
| Sum of benefits | 1008 | 509 | 643 | 324 | 1019 | 515 | 642 | 324 | 776 | 392 | |
| Net SBV disease costs (£)/farm (n cow spaces=100) | 3882 | 1952 | 2365 | 1186 | 5143 | 2589 | 2306 | 1156 | 1632 | 816 | |
| Net SBV disease costs (£)/cow space/year | 38.8 | 19.5 | 23.7 | 11.9 | 51.4 | 25.9 | 23.1 | 11.6 | 16.3 | 8.2 | |
TABLE 5:
Schmallenberg virus (SBV) disease costs (£) for five dairy production types in France, high-impact (HI) and low-impact (LI) scenarios
| Open LC |
Open LCG |
Open UCG |
Open UGMC |
Closed UGFC |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HI | LI | HI | LI | HI | LI | HI | LI | HI | LI | ||
| Additional expenditures | Veterinary assistance on cows that have dystocia due to SBV | 41 | 20 | 41 | 20 | 57 | 29 | 57 | 29 | 57 | 29 |
| Treatment of cows that need caesarean due to SBV dystocia | 5 | 3 | 5 | 3 | 5 | 3 | 5 | 3 | 5 | 3 | |
| Treatment of cows that have clinical SBV episodes | 33 | 0 | 33 | 0 | 33 | 0 | 33 | 0 | 33 | 0 | |
| Treatment of cows that have aborted due to SBV | 141 | 106 | 141 | 106 | 141 | 106 | 141 | 106 | 141 | 106 | |
| SBV testing of aborted fetuses, stillborn or malformed calves | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Costs of purchasing and raising heifers for replacement | 2881 | 1441 | 2997 | 1498 | 2881 | 1441 | 2990 | 1495 | 1669 | 784 | |
| Disposal costs of dead calves and fetuses due to SBV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Revenues forgone | Dairy x dairy male calves not sold | 69 | 34 | 55 | 27 | 69 | 34 | 78 | 39 | 242 | 121 |
| Dairy x beef male calves not sold | 0 | 0 | 27 | 14 | 0 | 0 | 101 | 51 | 0 | 0 | |
| Dairy x dairy heifer calves not sold | 144 | 72 | 0 | 0 | 144 | 72 | 0 | 0 | 69 | 69 | |
| Dairy x beef heifer calves not sold | 0 | 0 | 18 | 9 | 0 | 0 | 75 | 37 | 0 | 0 | |
| Milk not sold from cows with clinical episode | 81 | 0 | 75 | 0 | 75 | 0 | 64 | 0 | 92 | 0 | |
| Milk not sold from cows with abortion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Milk not sold due to less efficiency of old cows | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 542 | 220 | |
| Milk not produced due to less efficiency of heifers | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 936 | 341 | |
| Revenues foregone due to penalty in milk | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Revenues foregone on cows culled | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1369 | 684 | |
| Sum of costs | 3396 | 1676 | 3392 | 1677 | 3406 | 1684 | 3543 | 1758 | 5156 | 2408 | |
| Expenditures saved | Concentrate feed saved in cows with clinical episodes | 11 | 0 | 8 | 0 | 13 | 0 | 15 | 0 | 13 | 0 |
| Concentrate feed saved on cows that aborted | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Concentrate saved on old cows used as replacement | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 73 | 30 | |
| Concentrate feed saved in heifers | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 126 | 46 | |
| Feed saved on calves dead due to SBV | 79 | 39 | 47 | 24 | 79 | 39 | 55 | 27 | 88 | 52 | |
| Extra revenues | Extra revenues from cows culled due to SBV abortion | 1325 | 663 | 1383 | 692 | 1225 | 613 | 1369 | 685 | 0 | 0 |
| Sum of benefits | 1415 | 702 | 1438 | 715 | 1316 | 652 | 1438 | 712 | 300 | 127 | |
| Net SBV disease costs (£)/farm (n cow spaces=100) | 1988 | 974 | 1953 | 963 | 2085 | 1033 | 2104 | 1047 | 4856 | 2281 | |
| Net SBV disease costs (£)/cow space/year | 19.9 | 9.7 | 19.5 | 9.6 | 20.9 | 10.3 | 21.0 | 10.5 | 48.6 | 22.8 | |
LC, lowland with corn silage; LCG, lowland with corn silage and grass; UCG, upland with corn silage and grass; UGFC, upland with grass Franche-Comté; UGMC, upland with grass Massif Central
In the high-impact scenario in the UK, the net SBV disease costs in £/cow space/year for an average dairy cattle farm were estimated at 38.8 in SC, 23.7 in AOC, 51.4 in ACC, 23.1 in AYOC and 16.3 in AYCC systems. In the low-impact scenario in the UK, the net SBV disease costs in £/cow space/year for an average dairy cattle farm were estimated at 19.5 in SC, 11.9 in AOC, 25.9 in ACC, 11.6 in AYOC and 8.2 in AYCC systems (Table 4).
In the high-impact scenario in France, the net SBV disease costs in £/cow space/year were estimated at 19.9 in LC, 19.6 in LCG, 20.9 in UCG, 21.0 in UGMC and 48.6 in UGFC systems. In the low-impact scenario in France, the net SBV disease costs in £/cow space/year were estimated at 9.7 in LC, 9.6 in LCG, 10.3 in UCG, 10.5 in UGMC and 22.8 in UGFC systems (Table 5). If LC, LCG, UCG and UGMC were considered as closed, the net SBV disease costs in £/cow space/year were estimated at 42.5, 50.5, 39.9 and 55.4 for the high-impact scenario and 20.7, 25.6, 19.7 and 28.0 for the low-impact scenario, respectively. If UGFC was considered as open, the net total costs £/cow space/year were estimated at 20.8 and 10.2 for the high- and low-impact scenarios, respectively.
For all open systems (the UK and France), the main costs of disease were associated with purchasing or raising extra heifers for replacement (74–89 per cent of the sum of costs). For closed farms in the UK, milk loss due to culling cows with abortion represented the major costs of disease (50–61 per cent of the sum of costs). For the UGFC closed system in France, the replacement costs and the revenues foregone from cows not culled highly contributed to the sum of costs (32 and 27 per cent, respectively).
Sensitivity analyses performed for two of the most sensitive and uncertain disease parameters expressed as net SBV disease costs in £/cow space/year showed that an increase in abortion rate influenced disease costs more than a rise in number of calves stillborn or malformed (see online supplement 4). For the UK, the net SBV disease costs per cow space and year ranged from £0.1 to £69.9 for SC, £0.1 to £44.1 for AOC, £0.1 to £91.6 for ACC, £0.1 to £42.8 for AYOC and £0.1 to £41.0 for AYCC farms. The ranges from the best case (using the minimum values for all disease inputs as defined in Table 3) to the worst case (using the maximum values for all disease inputs as defined in Table 3) for the high-impact scenario were £2.2 to £56 for SC, £2.4 to £42.5 for AOC, £2.2 to £68.6 for ACC, £2.2 to £40.6 for AYOC and £2 to £41.30 for AYCC farms. For the low-impact scenario, the net costs per cow and year ranged from £0 to £19.5 for SC, £0 to £11.9 for AOC, £0 to £25.9 for ACC, £0 to £11.6 for AYOC and £0 to £8.2 for AYCC farms.
For France, the net SBV disease costs per cow space and year ranged from £0 to £35.2 for LC, £0 to £34.5 for LCG, £0 to £36.8 for UCG, £0 to £38.4 for UGMC and £0 to £86.6 for UGFC farms. The net costs ranges from the best case to the worst case for the high-impact scenario per cow space and year were between £1.9 and £54.2 for LC, £2.1 and £53.2 for LCG, £2.0 and £55.9 for UCG, £2.9 and £75.8 for UGMC, and £2.6 and £86.6 for UGFC systems. For the low-impact scenario, the net costs per cow and year ranged from £0.7 to £9.7 for LC, £0.7 to £9.7 for LCG, £0.7 to £10.3 for UCG, £0.7 to £10.5 for UGMC and £0.7 to £22.8 for UGFC systems.
The impact of SBV on the farm profitability was assessed by comparing the gross margins without SBV and low- and high-impact SBV infection, respectively. In the UK, the reduction in gross margin per cow space and year was between 1.6 per cent (AYCC system) and 4.3 per cent (SC system) for the high-impact scenario, and between 0.9 per cent (AOC system) and 1.3 per cent (SC and AYOC systems) for the low-impact scenario. In France, the reduction in gross margin per cow space and year was between 1.4 per cent (open LC and UCG) and 2.6 per cent (closed UGFC) for the high-impact scenario and between 0.7 per cent (open LC and LCG) and 1.2 per cent (closed UGFC) for the low-impact scenario. Detailed results are given in online supplement 5.
Discussion
In the present study, farm-level models were developed that combine herd dynamics and gross margin analysis of different production systems as a basis for PBA to estimate SBV disease costs in the UK and France. The production models developed allow to account for financial consequences that are related to specific management practices that can be difficult to capture otherwise. The results clearly illustrate that the disease impact differs substantially depending on the underlying production system, based on closed or open farms, block calving and all-year-round calving, heifer replacement management and replacement rate, and various combinations thereof. The production models proved particularly useful in estimating the effects of disease parameters that have a cascade effect. For example, an increase in abortions means that fewer calves are born, which does not only accrue a loss in the form of less calves sold, but also reflects a cost due to more adult animals dying or being culled because of the abortion. In the case of a closed farm, this meant that either more calves needed to be kept for replacement in the model or, if the calving rate was not high enough, adult cattle earmarked for removal from the herd due to production reasons were kept longer. Consequently, block-calving closed herds were the systems with higher disease impact due to limited flexibility in maintaining the same level of milk production and the need to retain dairy heifer calves for the extra replacement needed due to SBV (revenue foregone from sales lost). On the other hand, major costs associated with open farms accrued from purchasing or raising heifers for replacement, which was almost double for open farms compared with closed farms due to the high market prices of replacement stock. Disease impact was found to be generally higher for autumn-calving herds due to higher replacement rates and milk price.
For France, the disease impact was also considerably higher when closed herds were considered. This was mainly due to the high replacement rate of French herds (between 28 and 35 per cent compared with the 20 and 25 per cent replacement rate of UK herds). In disease situations that affect the number of calves being raised, French closed farms cannot produce enough heifers to achieve target replacement rates. Consequently, they have to keep old cows that leads to inefficient production and a higher disease impact in such herds. While closed management does exist, it is likely that such farms will switch to open management to be able to use the existing milking spaces when facing replacement difficulties. Certain situations, particularly the scarcity of affordable replacement heifer or cows, mimic the challenges closed herds face. This could, for example, happen when demand for replacement animals is high because of high milk prices and/or there is limited supply of specific breeds (e.g. only few farmers sell Montbéliarde animals). The LCG and UGMC systems in France are open systems that are heavily reliant on purchasing young heifers because they commonly use beef bull or beef AI in their herds. The LC and UCG systems may be open or closed, depending on farmers’ behaviour.
Building on the gross margin models, costs saved, new revenues, new costs and revenues foregone could be calculated by comparing the values between farms modelled to be healthy and those modelled to be diseased. Importantly, the net value obtained in the PBA is equivalent to the difference of the gross margins with and without disease. The partial budget approach helps to understand what the magnitude of the additional costs and benefit items are due to disease incursion and which items most influence the impact and therefore should be the focus of potential intervention measures. The impact of SBV on the gross margin, on the other hand, shows how profitability changes. This information is also important for farmers as it may indicate sustainability issues, reduction in capacity for investment and determine the importance farmers will attribute to disease. Consequently, both the PBA and gross margin analysis provide critical information in relation to SBV (or disease in general).
The partial budget could have been calculated without the underlying production model by, for example, using equations that take into account animal numbers, prices and the cascade effects of disease parameters. However, validation of such calculations would be difficult in the absence of empirical data. In this study, the creation of the production models allowed straightforward validation of the costs, revenues and gross margins by comparing them to published values on gross margins. For some production systems, particularly in the UK models, some model costs (e.g. replacement costs) were found to be higher than those calculated by the industry, and therefore the overall gross margins were higher. One main reason for this was that milk prices used were taken from the DairyCo benchmarking data, which were considerably higher than those used by other benchmarking reports. The DairyCo milk prices were used because they reflected the actual prices, whereas other references used projected estimates. The second main reason was that the calculations of replacement costs were similar to the latter references, but dissimilar to DairyCo data. Consequently, the UK models showed higher revenues for milk than most references and lower replacement costs than the DairyCo benchmarking report, which may have led to a slight overestimation of SBV disease impact. Nonetheless, the method and comparative approach used represent a useful way to understand the possible model deviations and their repercussion on the results.
The present models could also include situations that were not accounted for in the example used. For example, revenues foregone due to milk penalties associated with low milk production did not apply because milk drop and percentage of cows affected were too low for that. However, such effects could be substantial in the case of diseases that have a strong negative effect on milk yield.
The SBV disease costs were presented for a low-impact scenario and a high-impact scenario without providing any information about the likelihood of a farm being in the high-impact or low-impact category. The likelihood of being in the SBV high- or low-impact category may be different for the different production systems. For example, all-year-round-calving farms are expected to be less at risk of suffering from high impact due to their flexibility on the time of calving. The French systems, which are based on all-year-round calving, have therefore greater scope to influence the risk of exposure. However, only observational studies and/or epidemiological transmission models would allow estimating the probability of disease on a farm with some level of precision; this was not within the scope of this study and remains open to further research.
One of the main limitations of this study was the lack of data available in the literature on SBV disease effects, which may be partly due to a lack of reporting and the absence of incentives for reporting. Most of the published scientific literature described the situation on Schmallenberg-affected farms, but only in some exceptional cases compared them with non-affected farms or previous years before SBV emergence. In consequence, attribution of disease estimates was not possible from those studies. Experimental studies or epidemiological studies comparing affected and non-affected farms are needed in order to obtain more accurate disease estimates. The disease estimates used in this study were derived from scientific publications when possible and complemented by expert opinion consultation. Sensitivity analyses on disease estimates were used to account for this uncertainty and demonstrate the influence of the most uncertain input values used.
Because of the biological and economic consequences of SBV, there is a demand for effective animal health interventions (Trickett 2013). Given the epidemiology of the virus and infection in wildlife, elimination of the virus from populations may prove challenging and the focus therefore lies on interventions to avoid the negative impact of disease. Vaccines for SBV in ruminants have been developed in Europe and licences have been approved in some EU countries for marketing of the vaccine (Anonymous 2013a, b). The purpose of SBV vaccines is to induce an immune response that prevents the virus from reaching the fetus. Farmers will need to make a judgement whether the additional investment needed to protect their herds or flocks is justified by the resulting loss avoidance. Apart from vaccination, there are limited options to control the disease effectively. Some authors suggested measures to reduce exposure to the vector through disruption of vector breeding sites, pesticide use, housing and protection of ruminants by repellents (Baylis and others 2010, British Cattle Veterinary Association 2012). Additional measures could be a breeding system that manages the timing of service or insemination of animals depending on the season and concentration of midges and thereby reduces exposure to the virus in the critical period. However, such a strategy may prove difficult where production and management systems are targeted towards the seasonality of grass growth and market demand.
The integrated production and partial budget models could be used to assess the economic efficiency of potential interventions at the farm level. There is scope to convert them into a farm-level decision tool where veterinarians or farmers could enter farm-level-specific data and thereby estimate disease impact and the net value of potential interventions for their farms.
Acknowledgments
The authors thank the experts who made time to attend the expert workshop to discuss the models developed and the inputs used and various colleagues in the UK and France for their willingness to provide information on production systems, management and husbandry practices, and potential disease impact.
Footnotes
Funding: This project has been funded by Merial France. BH acknowledges funding from the Leverhulme Centre for Integrative Research on Agriculture and Health.
Contributors: The authors fulfil the authorship requirements and any contribution made by other people are listed in the ‘Funding’ section.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The authors are working on a web version of the models used to make them accessible to the public. In the meantime, the Excel models are available from the authors upon request.
References
- Agro Business Consultants (2012) The Agricultural Budgeting and Costing Book. 74th edn ABC books, Melton Mowbray [Google Scholar]
- Alarcon P., Rushton J., Nathues H., Wieland B. (2013) Economic efficiency analysis of different strategies to control post-weaning multi-systemic wasting syndrome and porcine circovirus type 2 subclinical infection in 3-weekly batch system farms. Preventive Veterinary Medicine 110, 103–118 doi:10.1016/j.prevetmed.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous (2013a) VMD authorises SBV vaccine for use in the UK. The Veterinary Record 172, 54323709393 [Google Scholar]
- Anonymous (2013b) Merial Receives Approval For New Vaccine To Prevent Schmallenberg Disease In Livestock. www.merial.com/EN/PressRoom/PressRelease/Pages/MerialApprovalSchmallenbergVaccine.aspx. Accessed August 2014
- Baylis M., Parkin H., Kreppel K., Carpenter S., Mellor P. S., McIntyre K. M. (2010) Evaluation of housing as a means to protect cattle from Culicoides biting midges, the vectors of bluetongue virus. Medical and Veterinary Entomology 24, 38–45 doi:10.1111/j.1365-2915.2009.00842.x [DOI] [PubMed] [Google Scholar]
- Beer M., Conraths F. J., van der Poel W. H. M. (2012) Schmallenberg virus - a novel orthobunyavirus emerging in Europe. Epidemiology and Infection 141, 1–8 doi:10.1017/S0950268812002245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R., IJpelaar J. (2005) Updated estimates of the costs associated with thirty four endemic livestock diseases in great Britain: a note. Journal of Agricultural Economics. 56, 135–144 doi:10.1111/j.1477-9552.2005.tb00126.x [Google Scholar]
- British Cattle Veterinary Association (2012) Possible actions to reduce the impact of Schmallenberg virus in cattle herds. www.bcva.eu/bcva/cattle-health-welfare/schmallenberg-virus-sbv. Accessed 5.10.13
- Chi J., VanLeeuwen J. A., Weersink A., Keefe G. P. (2002) Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum. Preventive Veterinary Medicine 55, 137–153 doi:10.1016/S0167-5877(02)00094-6 [DOI] [PubMed] [Google Scholar]
- Claine F., Coupeau D., Wiggers L., Muylkens B., Kirschvink N. (2013) Schmallenberg Virus among female lambs, Belgium, 2012. Emerging Infectious Diseases 19, 2012–2014 doi:10.3201/eid1907.121768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conraths F. J., Peters M., Beer M. (2013) Schmallenberg virus, a novel orthobunyavirus infection in ruminants in Europe: Potential global impact and preventive measures. New Zealand Veterinary Journal 61, 63–67 [DOI] [PubMed] [Google Scholar]
- DairyCo (2013) Milkbench report 2013. www.dairyco.org.uk/resources-library/market-information/milkbenchplus/milkbench-report-2012/. Accessed 3.5.13
- Day C. (2007) Birth difficulties (dystocia) in cattle, clinical trial. www.alternativevet.org/Clinical Trial - Birth Cattle WS006-07.pdf. Accessed March 2014
- EBLEX (2012) EBLEX business pointer. www.eblex.org.uk/returns/business-pointers-2012/. Accessed 3.5.13
- European Food Safety Authority (2012) “Schmallenberg” virus: analysis of the epidemiological data and assessment of impact. EFSA Journal 10, 1–89 [Google Scholar]
- Garigliany M.-M., Bayrou C., Kleijnen D., Cassart D., Jolly S., Linden A., Desmecht D. (2012) Schmallenberg virus: a new Shamonda/Sathuperi-like virus on the rise in Europe. Antiviral Research 95, 82–87 doi:10.1016/j.antiviral.2012.05.014 [DOI] [PubMed] [Google Scholar]
- Haskell S. R. (2008) Blackwell's Five-Minute Veterinary Consult: Ruminant. Iowa, USA: Wiley-Blackwell [Google Scholar]
- Hoffmann B., Scheuch M., Höper D., Jungblut R., Holsteg M., Schirrmeier H., Eschbaumer M., Goller K. V., Wernike K., Fischer M., Breithaupt A., Mettenleiter T. C., Beer M. (2012) Novel orthobunyavirus in cattle, Europe, 2011. Emerging Infectious Diseases 18, 469–472 doi:10.3201/eid1803.111905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K. S., Häsler B., Stärk K. D. C. (2013) Economic principles for resource allocation decisions at national level to mitigate the effects of disease in farm animal populations. Epidemiology and Infection 141, 91–101 doi:10.1017/S095026881200060X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institut Elevage Bovin lait (2013) [Résultats 2011 et estimations 2012 pour les exploitations bovins lait. Résultats nationaux. Collection résultats annuels. Réseaux d’élevage pour le conseil et la prospective]. June, pp 1–52, ISBN: 978-2-36343-401-2
- Institut Elevage Bovin viande (2013) [Résultats 2011 des exploitations bovins viande. Résultats nationaux. Collection résultats annuels. Réseaux d’élevage pour le conseil et la prospective]. June, pp 1–40, ISBN: 978-2-36343-409-8
- Institut Elevage Ovin lait (2012) [Résultats 2010 des exploitations ovins lait. Résultats nationaux. Collection résultats annuels. Réseaux d’élevage pour le conseil et la prospective]. September, pp 1–24, ISBN: 978-2-36343-313-8
- Institut Elevage Ovin viande (2013) [Résultats 2011 et estimations 2012 pour les exploitations ovins viande. Résultats nationaux. Collection résultats annuels. Réseaux d’élevage pour le conseil et la prospective]. April, pp 1–23, ISBN: 978-2-36343-245-2
- Jericho K. W. (1974) Summary of past assessments of the effects of disease on farm economics. The Canadian Veterinary Journal 18, 214–215 [PMC free article] [PubMed] [Google Scholar]
- Lievaart-Peterson K., Luttikholt S. J. M., Van den Brom R., Vellema P. (2012) Schmallenberg virus infection in small ruminants – First review of the situation and prospects in Northern Europe. Small Ruminant Research. 106, 71–76 doi:10.1016/j.smallrumres.2012.03.006 [Google Scholar]
- Meijering A. (1984). Dystocia and stillbirth in cattle — A review of causes, relations and implications. Livestock Production Science 11 (2), 143–177. [Google Scholar]
- Martinelle L., Dal Pozzo F., Gauthier B., Kirschvink N., Saegerman C. (2012) Field veterinary survey on clinical and economic impact of Schmallenberg Virus in Belgium. Transboundary and Emerging Diseases 61, 285–288 doi:10.1111/tbed.12030 [DOI] [PubMed] [Google Scholar]
- Mclnerney J. P., Howe K. S., Schepers J. A. (1992) A framework for the economic analysis of disease in farm livestock. Preventive Veterinary Medicine 13, 137–154 [Google Scholar]
- Mee J. (2012) Prevalence and risk factors for dystocia in dairy cattle – with emphasis on confinement systems. WCDS Advances in Dairy Technology 24, 113–125 [Google Scholar]
- Muskens J., Smolenaars A. J. G., van der Poel W. H. M., Mars M. H., van Wuijckhuise L., Holzhauer M., van Weering H., Kock P. (2012) [Diarrhea and loss of production on Dutch dairy farms caused by the Schmallenberg virus]. Tijdschrift voor Diergeneeskunde 137, 112–115 [PubMed] [Google Scholar]
- Nix J. (2013) The John Nix farm management pocketbook 2013. 43rd edn Agro Business Consultants Ltd, Melton Mowbray [Google Scholar]
- Reuillon J., Fagon J., Charroin T., Laurent M. (2013). Cout de production en élevage bovin lait. idele.fr/recherche/publication/idelesolr/recommends/cout-de-production-en-elevage-bovins-lait.html. Accessed August 2014
- Scottish Agricultural College (2010) Farm Management Book 2009/2010. 30th edn, SAC. SAC consulting [Google Scholar]
- Steukers L., Bertels G., Cay A. B., Nauwynck H. J. (2012) Schmallenberg virus:emergence of an Orthobunyavirus among ruminants in Western Europe. Vlaams Diergeneeskundig Tijdschrift 81, 119–127 [Google Scholar]
- Tisdell C. (1995) Assessing the approach to cost-benefit analysis of controlling livestock diseases of McInerney and others (No. 3), Research papers and reports in animal health economics. Brisbane
- Trickett S. (2013) “Low impact” Schmallenberg may be final straw for some producers. www.fwi.co.uk/articles/11/01/2013/137119/schmallenberg-virus-39costing-farms-thousands39.htm. Accessed 3.5.13
- Veldhuis A. M. B., Santman-Berends I. M. G. A., Gethmann J. M., Mars M. H., van Wuyckhuise L., Vellema P., Holsteg M., Höreth-Böntgen D., Conraths F. J., van Schaik G. (2014) Schmallenberg virus epidemic: Impact on milk production, reproductive performance and mortality in dairy cattle in the Netherlands and Kleve district, Germany. Preventive Veterinary Medicine 116, 412–422 doi:10.1016/j.prevetmed.2014.04.015 [DOI] [PubMed] [Google Scholar]
- Vignau-Loustau L., Huyghe C. (2008) Chapter 1: the forage system (Le système fourrager). In L Vignau-Loustau, C Huyghe, eds.Forage Strategies (Stratégies Fourragères). France Agricole: Paris (France),pp. 50–65 [Google Scholar]
- Wernike K., Eschbaumer M., Schirrmeier H., Blohm U., Breithaupt A., Hoffmann B., Beer M. (2013) Oral exposure, reinfection and cellular immunity to Schmallenberg virus in cattle. Veterinary Microbiology 165, 155–159 doi:10.1016/j.vetmic.2013.01.040 [DOI] [PubMed] [Google Scholar]
- Yalcin C., Stott A. W., Logue D. N., Gunn J. (1999) The economic impact of mastitis-control procedures used in Scottish dairy herds with high bulk-tank somatic-cell counts. Preventive Veterinary Medicine 41, 135–149 doi:10.1016/S0167-5877(99)00052-5 [DOI] [PubMed] [Google Scholar]