Abstract
Aims and objectives
To date assessment of changes in ocular temperature, as a hallmark of uveitis in horses has not been determined. Therefore the aim of the current study was to determine whether ocular temperature is increased in acute uveitic eyes compared with non-uveitic eyes, and to compare an affordable thermometry device with a thermography device.
Material and methods
Ocular temperatures of both eyes of five horses with acute unilateral uveitis and 10 normal horses were measured using thermometry and thermography. Presence and absence of acute uveitis were diagnosed through a complete ophthalmological examination. Ambient temperature and core body temperature were also recorded.
Results
The difference in temperatures between uveitic eyes and non-uveitic eyes was marked but not statistically significant (mean thermography temperature 34.0°C sd±1.6°C and 32.7°C sd±2.4°C, respectively v mean thermometry temperature 34.0°C sd±1.9°C and 31.6°C sd±3.1°C, respectively). No influence of core body temperature on either method was detected. Thermography was less influenced by ambient temperature than was thermometry.
Conclusion
In conclusion uveitic eyes are not significantly warmer than non-uveitic eyes. Despite the lack of significance, a tendency towards increased ocular temperature in uveitic eyes, compared with non-uveitic eyes was noted. Therefore more research on this topic is warranted.
Keywords: horse, uveitis, thermography
Introduction
The hallmarks of inflammation, for example, heat, redness, swelling, pain and loss of function, first described by Celsus more than 2000 years ago, apply to all tissues in the body. However the topic of ocular temperature has only been studied for 130 years (Purslow and Wolffsohn 2005). Since that time clinical applications of ocular thermometry have expanded to the diagnosis and research of ocular dry eye syndrome (Morgan and others 1993, Purslow and Wolffsohn 2005, Kamao and others 2011, Su and others 2011, Kawali 2013, Biondi and others 2015), and corneal ulcerations in human beings (Klamann and others 2013), as well as systemic diseases in large animals (Schaefer and others 2004, Johnson and others 2011). Limited reports of ocular thermography in cases of scleritis, keratitis and blepharitis are available in physician medicine (Kawali 2013). Furthermore ocular thermometry has been used as an accurate method to determine time of death in forensic medicine (Kaliszan 2012).
However research on ocular thermography to diagnose uveitis is limited to few case studies in physician medicine (Morgan and others 1993, Kawali 2013); to the authors’ knowledge there are no reports of increases in ocular temperature in the field of veterinary ophthalmology. If this tool proved to be an objective method in the diagnosis of ocular inflammation, it would greatly benefit non-specialist veterinary practitioners in early diagnosis of subtle cases of uveitis and facilitate faster therapeutic intervention. It was therefore the purpose of this study to determine ocular temperature in uveitic and non-uveitic eyes in horses by means of an established method of thermography as well as a commercially available thermometer.
Materials, animals and methods
Five client-owned horses with a history of acute onset uveitis and 10 normal horses owned by the Equine Clinic of the Veterinary University Vienna were included in the study. Verbal consent of the owners of the horses was acquired prior to image acquisition. The examination of the University owned horses was in agreement with the University Ethics committee. Information regarding breed, sex and age were collected. A complete history of the client-owned horses was taken, with special emphasis on the duration of ophthalmological symptoms, prior ocular disease, treatment by the referring vet and concurrent systemic disorders. Horses which had been pretreated by the referring vet, prior to presentation were excluded from the study. All normal horses included in the study were free of ophthalmological disorders based on the history since ownership of the Veterinary University Vienna, and a complete ophthalmological examination.
Measurement of ocular, rectal and ambient temperatures
Prior to the ophthalmological examination measurements of ocular temperatures of both eyes by means of thermography and thermometry were taken. All horses were subjected to an acclimatisation duration of approximately 30 minutes prior to temperature measurements. Thermographic images were taken using a portable infrared camera (VarioCam, Infratec GmbH, Germany), equipped with a 12.5-mm focal length lens, an uncooled microbolometer, and a focal plane array infrared detector with a spectral range between 7.5 µm and 14 µm. Emissivity was adjusted to 1.00 (unitless value). A bubble level was fixed on the camera to assure horizontal orientation. A total of five images of each eye were taken at 90° angle (from the horizontal axis of the eye) at 1 m distance (Fig 1). These settings were reported to achieve the highest reproducibility (Westermann and others 2013). Ocular thermometry measurements were taken using a commercially available thermometer (Voltcraft InfraRed Thermometer IR 260-8S, Germany). Accuracy of aim of the thermometer was achieved by activating the inbuilt laser pointer, which was directed towards the peripheral iris. Care was taken not to point the laser beam at the pupil, in order to avoid retinal damage (Kandari and others 2010). The method of temperature measurement and number of recorded measurement values was the same as described for ocular thermography. Ambient temperature in the examination room and rectal temperature of the horses were recorded prior to measurement of ocular temperature.
FIG 1:
The photograph illustrates the image acquisition distance (approximately 1 m) from the horse's head. The distance was maintained using medical tape of 1 m length. The images were taken approximately perpendicular to the horizontal axis of the equine orbit
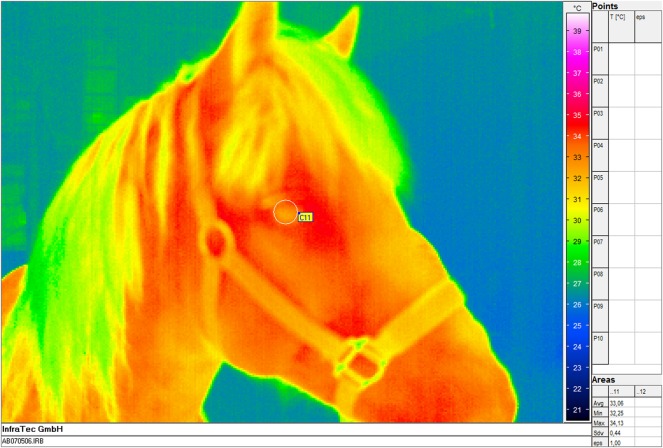
The images of ocular thermography were analysed with commercially available software (IRBIS, InfraTec GmbH, Germany). Maximum surface temperature was calculated from a region of interest which was calculated from a circle overlying the region of the eye (Fig 2).
FIG 2:
The image illustrates the evaluation of the thermography image using IRBIS. The minimum, maximum and mean temperatures are calculated from the circle overlying the equine eye in °C. The mean temperature of each image was noted and used for further evaluation
Ophthalmological examination
After acquisition of all temperature measurements, both eyes of all horses were examined by a single examiner (JOR) under supervision of an ECVO diplomate (BN) in a darkened room under standard conditions. The ocular adnexa, anterior and posterior segments of both eyes were examined using slit lamp biomicroscopy (Kowa portable slit-lamp SL-14, CR Medical, Austria) and direct and indirect ophthalmoscopy (Heine Omega 2C, Heine Optotechnik GmbH KG, Germany). Mydriasis was induced using one per cent tropicamide (Mydriatikum, Agepha, Austria) to examine the posterior ocular segments. Intraocular pressures were taken using rebound tonometry (TonoVet, TV 01, Dioptrix, France). Schirmer tear test and fluorescein staining were performed in all horses.
Statistical analysis
Image data were recorded and analysed with statistical software. The data were analysed using a commercial statistical software package (SPSS V.19, IBM SPSS Statistics, USA). Frequency distributions of sex and age were calculated. Mean temperatures of the right and left eyes of all horses were compared using a paired t test, since the data were normally distributed (Kolmogorov-Smirnov test). t Test for independent samples was performed to determine differences in mean ocular temperatures between groups. The influence of ambient temperature and rectal temperature on mean temperature of the eye was determined using paired t test. Pearson’s correlation was performed in order to correlate age with mean ocular temperatures. Values of P<0.05 were considered statistically significant.
Results
The normal group comprised of 10 horses, 8 mares and 2 geldings. Five horses fulfilled the inclusion criteria of the uveitic group, two mares, two geldings and one stallion. The median age in the normal group was 18.5 years (sd±5.0 years), and 4.0 years (sd±6.5 years) in the uveitic group. The body weight of all horses was not recorded. The breeds of the normal group included two Standardbreds, two Haflingers, three Warmbloods, one Arabian, one Trotter and one Iceland horse. The breeds of the uveitic group included two Iceland horses, one Standardbred, one Shetland pony and one Noriker. All horses of the normal group were housed in similar conditions (closed boxes at night and access to a large paddock throughout the day). Horses included in the uveitic group were kept in boxes and were turned out to a pasture or a paddock on a daily basis.
Ophthalmological findings
All horses in the normal group were free of ophthalmic diseases based on the complete ophthalmic examination as well as the history since ownership of the Veterinary University Vienna.
All horses in the uveitic group were diagnosed with acute onset uveitis, with no topical or systemic treatment prior to the ophthalmic examination at the Veterinary University Vienna. All horses had unilateral uveitis, three in the right eye (OD), two in the left eye (OS). Detailed findings of the ophthalmic examination are listed in Table 1.
TABLE 1:
Table listing gender, age, examination date, affected eye, duration of clinical symptoms, presence of aqueous flare, intraocular pressure and mean ocular temperatures using ocular thermography and thermometry in each eye
| Patient number | Sex | Age (years) | Examination date | Eye affected | Duration of symptoms (d) | Aqueous flare (0-+++) | IOP OS (mm Hg) | IOP OD (mm Hg) | Mean oc. temp. OS (TG) (°C) | Mean oc. temp. OD (TG) (°C) | Mean oc. temp. OS (TM) (°C) | Mean oc. temp. OD (TM) (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mare | 2 | 07.06.2012 | OS | 2 | ++ | 12 | 22 | 33.56 | 33.50 | 34.66 | 34.88 |
| 2 | Gelding | 14 | 07.30.2012 | OS | 3 | ++ | 14 | 22 | 34.82 | 33.60 | 37.32 | 35.72 |
| 3 | Stallion | 4 | 07.02.2013 | OD | 1 | ++ | 21 | 10 | 33.16 | 32.94 | 34.24 | 34.22 |
| 4 | Mare | 3 | 12.30.2012 | OD | 3 | +++ | 18 | 8 | 34.98 | 36.20 | 31.78 | 32.88 |
| 5 | Gelding | 18 | 04.10.2013 | OD | 2 | ++ | 25 | 20 | 33.56 | 33.90 | 32.90 | 32.56 |
IOP (intraocular pressure); OS (left eye); OD (right eye); oc. (ocular); TG (thermography); TM (thermometry).
Ophthalmic examinations of the normal group were performed on a single day in July. The uveitic group was examined throughout the year, three horses in July, one in December and one in April. Outside environmental temperature was not recorded, however the ambient temperature in the examination room was noted in all cases.
Agreement of ocular thermography and thermometry
Mean ocular temperature using ocular thermography in the normal group was 33.5°C (sd±0.4°C) and 33.3°C (sd±0.5°C) for OS and OD, respectively. Mean ocular temperature using thermometry in the normal group was 34.7°C (sd±0.5°C) for both eyes (OU). No correlation between the two measurement methods was evident (r=0.091) (Fig 3).
FIG 3:
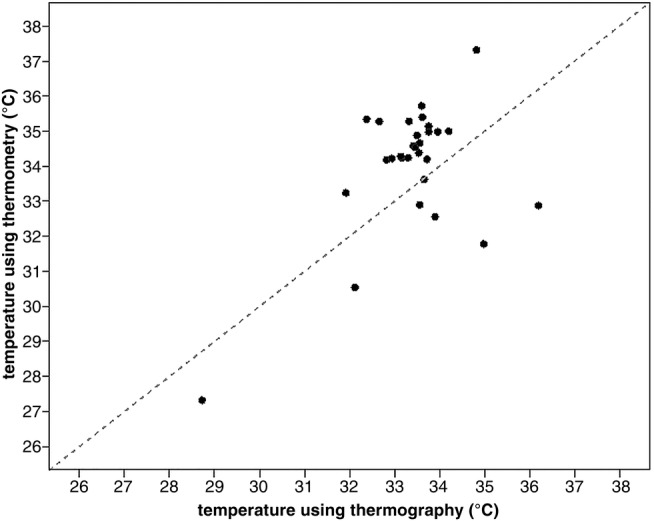
Graph illustrating the correlation between measurements from ocular thermography and thermometry
Mean ocular temperature of the affected eye versus the non-affected eye in the uveitic group was 34.0°C (sd±1.6°C) and 32.7°C (sd±2.4°C), respectively, using ocular thermography. Mean ocular temperatures of the affected eye versus the non-affected eye in the uveitic group was 34.0°C (sd±1.9°C) and 31.6°C (sd±3.1°C), respectively, using thermometry.
The difference between ocular temperatures OS and OD using thermography and thermometry was significant for the normal group (P<0.001). The difference in ocular temperatures between affected and non-affected eyes in the uveitic group was not significant using ocular thermography and thermometry (P=0.072 and P=0.071, respectively).
The overall coefficient of variation in the uveitic group was higher than in the normal group for both measurement methods.
Association of ophthalmic findings or age with ocular temperature
The temperature of eyes with acute onset uveitis was not significantly higher than the contralateral eye (free of disease) by means of ocular thermography and thermometry (P=0.299 and P=0.246, respectively). The difference between both non-affected eyes in the normal group however revealed a statistically significant difference in temperatures between OD and OS using ocular thermography (P<0.001). This difference was not significant using thermometry (P=0.36). The influence of age on ocular temperature was not significant for any group (P=0.199 and P=0.164).
Influence of ambient temperature and rectal temperature on ocular temperature
Mean ambient temperature at the time of examination of the normal group was 27.3°C (sd±0.6°C), and 20.1°C (sd±5.0°C) for the uveitic group. The difference between the groups was statistically significant (P<0.001). Mean rectal temperature of horses in the normal group was 37.3°C (sd±0.4°C), and 38.3°C (sd±0.4°C) in the uveitic group. The difference between the groups was not statistically significant (P=0.452). Using t test analysis no statistically significant influence of rectal temperature on ocular temperature was determined in either measurement method. However a statistically significant influence of ambient temperature on the results of ocular thermometry was determined (P<0.001) (Fig 4). A statistically non-significant influence of ambient temperature was noted in thermography (P=0.367).
FIG 4:
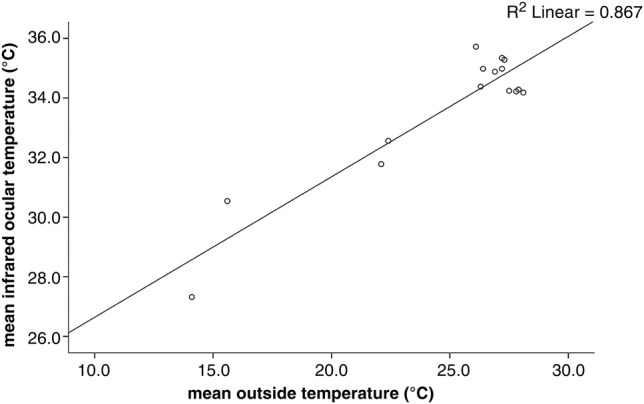
Graph illustrating the influence of ambient temperature on results of thermometry. A positive correlation of increased ocular temperature at higher ambient temperatures was noted
Discussion
The results of this pilot study, to introduce ocular thermography in the diagnosis of acute uveitis in horses and compare this method with a more practically feasible method of ocular thermometry, suggest that uveitic eyes are markedly warmer than non-uveitic eyes (>2°C), however the difference did not reach statistical significance.
There are many potential explanations for the achievement of these results, many of which have been discussed in the vast body of literature of influencing factors on ocular thermography. The influence of environmental temperature and air current on the results of ocular thermography has been found to significantly change the measurement results (Freeman and Fatt 1973). In the present study air current was not measured, however this factor was minimised by the fact that all horses were examined in the same closed stall with no draft or noticeable air current subjectively evident at the time of examination. However lacking objective data regarding this factor, no assumptions can be made towards the influence of subtle air currents. Furthermore all horses were given a 30-minute acclimatisation period in order to minimise changes in environmental temperature as a confounding factor. Ambient air temperature was noted in all cases and was deemed a significantly influencing factor of ocular thermometry. This observation was also made in a study on ocular thermometry in cattle (Church and others 2014). However a more dated study from human ophthalmology found that ocular thermometry was highly reproducible and variations were independent of environmental temperature (Koçak and others 1999). One explanation for these contradictory results of the respective studies may be the difference in equipment used for temperature measurement.
Further reports have determined age to be an influencing factor on the results of ocular temperature measurement, with mean absolute temperatures significantly decreasing with progressing age (Alió and Padron 1982). Based on the results of this study no significant influence of progressive age on ocular temperature could be determined.
The significant difference of ocular temperature between both eyes in the normal group was surprising and cannot be explained based on the body of literature on ocular temperature measurement in veterinary medicine. In human medicine the influence of local hemispherical activity, evidenced by ear and eye preference, on tympanic membrane temperature has been reported (Jackson 2011). However to the authors’ knowledge there is no evidence of the influence of hemispherical activity on tympanic membrane temperature, or ocular temperature in animals.
Studies investigating ocular temperature depending on the ocular lipid layer thickness have determined higher ocular surface temperatures in normal subjects with a thicker tear lipid layer (Giraldez and others 2009). This parameter was not measured in the current study, due to the lack of validated technical equipment to measure the tear film lipid layer.
Also the effect of interspecies differences in anterior chamber depth on the results of ocular thermography has been determined in a study conducted on human and rabbit eyes. In that study corneal temperature was measured in a cold chamber on rabbits and human beings using a thermography device. The more rapid cooling of the human cornea in comparison to the rabbit’s, was attributed to the shallower anterior chamber of human beings, with less aqueous humour available to compensate corneal outside cooling (Rysa and Sarvaranta 1974). This discussion point is in contrast to a study on anterior chamber depth between human races depending on their geographical location, with peoples living in colder areas, having shallower anterior chamber depths than peoples from southern regions. In that study heat transmission to the cornea, emitted by the iris is proposed (Forsius and others 1991). However extensive studies on interspecies and intraspecies differences of the effect of anterior chamber depth and globe size on the ocular temperature are still lacking. Furthermore reference values of ocular temperatures in different species are limited to ocular surface temperatures in human beings (Efron and others 1989).
Finally changes in eye temperature as a result of the sympathetic component of the autonomous nervous system was established in an experimental study in cattle, in which adrenaline infusions were administered to a group of cattle. The results of ocular thermography revealed a mean decrease in the ocular temperature of 1.4±0.05°C (Stewart and others 2010). The proposed explanation in that study was that the decrease in ocular temperature resulted from vasoconstriction of conjunctival blood vessels. However whether aqueous humour turnover was affected due to changes in blood flow of uveal blood vessels could not be concluded from that study. In the current study cortisol levels were not determined as part of the examination, but the fact that horses were held in stocks in an unfamiliar environment may have resulted in high enough stress levels to alter the results of ocular thermography to a degree where the difference between temperatures was no longer significant. Further studies on the influence of increased cortisol levels on ocular temperature in horses are therefore warranted.
The body of literature on ocular temperature measurement is somewhat confusing to the point that no algorithm has been established between measurements of ocular surface temperature and the temperature inside the eye. Thus no gold standard for non-invasive ocular temperature measurement has been established. It seems plausible that ocular surface temperature is influenced by anatomical and physiological traits such as ocular size, size of the palpebral fissure, presence of retrobulbar fat, anterior chamber depth, vitreal size, lens to globe ratio, corneal thickness and scleral thickness, uveal anatomy, tear film and aqueous humour turnover. It is empirically difficult to determine the exact influence of each of these factors on ocular temperature, even more so if ocular physiology is disturbed by pathological changes such as qualitative and quantitative tear film instabilities, corneal disease, uveitis, etc. Regarding the equipment used a variety of different thermography as well as thermometry devices have been employed by researchers (Mapstone 1970, Rysa and Sarvaranta 1974, Khvatova and others 1991, Koçak and others 1999, Johnson and others 2011, Su and others 2011, Kaliszan 2012, Klamann and others 2012, Biondi and others 2015). However no gold standard measurement technique has been established to date. Despite the lack of a validated technique each measurement method has advantages and disadvantages. The greatest disadvantage of most thermography devices is the high cost of a thermography camera. The camera used in this study had an initial purchase price in excess of €21 000 (approximately £16 797). Since it was the purpose of this study to find out whether measurement of ocular temperature is a feasible tool for practitioners, the authors decided to compare the measurement results of an easily available and low cost thermometer (approximate purchase price €31/£29) with the already established method of ocular temperature measurement, using ocular thermography. Due to the lack of information of the actual temperature within the eye, assertions cannot be made regarding the correlation between the actual intraocular temperature and the results of thermography readings.
There were also other potentially influencing factors to this study, not yet described in the literature. The measurement technique is extremely dependent on the equipment used. In this study the same device was used as in the study by Westermann and others (2013). The authors therefore used the same settings as in the before mentioned study to acquire the images, which were later evaluated using the designated software programme. Lacking recommendations from the literature of ocular thermometry measurement using the thermometry device, the authors employed the same settings as in ocular thermography for this study. The authors were therefore able to statistically compare both measurement techniques with no other confounding factors to take into account. A final note regarding technically influencing factors, the software programme, to evaluate the acquired images is also subject to human bias, since the areas of temperature measurement are manually programmed by the evaluator. The authors tried to limit this bias factor by having every image evaluated by a single researcher (JOR).
Other limitations of this study regardless of the technical equipment used include the small study group examined. However the inclusion criteria dictated that only horses, with no prior treatment were included in the trial. Since most cases of uveitis are pretreated by the referring veterinarians, the minimum population of five horses in the uveitic group was used. An additional factor, which may have had a severe influence on the results was the variety in stages of the disease. All horses were presented with acute onset uveitis, however in some cases it was not clear how long exactly the clinical signs were present. It would therefore be interesting to follow a case of experimentally induced uveitis over several days and record variations in ocular temperature, to determine at which stage of disease ocular temperature is highest.
Also, since no reference values of normal ocular temperature in the horse is available, there was no range of values to compare the results with. Finally since all normal horses were examined on a single day, whereas the study population was examined throughout various seasons of the year, the impact of ambient temperature on ocular temperature in the small study population was significant. However this confounding factor is hard to eliminate in a stable environment. Standard ambient temperatures would more easily be achieved in a consultation room, as used for small animals.
In conclusion, uveitic eyes are not significantly warmer than non-uveitic eyes upon ocular thermography, however a mild tendency towards increased ocular temperature in uveitic eyes was noted. The authors therefore aim to continue the research on this topic since the study of ocular temperature in eye diseases may increase their knowledge about the pathophysiology of ocular diseases, as well as ocular drug interactions of topical medications.
Acknowledgments
The authors thank Dr Simone Westermann for help and advice with the handling of the thermography equipment and data analysis.
Footnotes
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- Alió J., Padron M. (1982) Influence of age on the temperature of the anterior segment of the eye. Measurements by infrared thermometry. Ophthalmic Research 14, 153–159 doi:10.1159/000265187 [DOI] [PubMed] [Google Scholar]
- Biondi F., Dornbusch P. T., Sampaio M., Montiani-Ferreira F. (2015) Infrared ocular thermography in dogs with and without keratoconjunctivitis sicca. Veterinary Ophthalmology 18, 28–34 doi:10.1111/vop.12086 [DOI] [PubMed] [Google Scholar]
- Church J. S., Hegadoren P. R., Paetkau M. J., Miller C. C., Regev-Shoshani G., Schaefer A. L., Schwartzkopf-Genswein K. S. (2014) Influence of environmental factors on infrared eye temperature measurements in cattle. Research in Veterinary Science 96, 220–226 doi:10.1016/j.rvsc.2013.11.006 [DOI] [PubMed] [Google Scholar]
- Efron N., Young G., Brennan N. A. (1989) Ocular surface temperature. Current Eye Research 8, 901–906 [PubMed] [Google Scholar]
- Forsius H., Fellman J., Eriksson A. (1991) Anterior chamber depth in Arctic and tropical populations. Arctic Medical Research 1991(Suppl), 505–508 [PubMed] [Google Scholar]
- Freeman R. D., Fatt I. (1973) Enviromental influence on ocular temperature. Investigative Ophthalmology 12, 596–602 [PubMed] [Google Scholar]
- Giraldez M. J., Naroo S. A., Resua C. G. (2009) A preliminary investigation into the relationship between ocular surface temperature and lipid layer thickness. Contact Lens Anterior Eye 32, 177–180 doi:10.1016/j.clae.2009.06.004 [DOI] [PubMed] [Google Scholar]
- Jackson C. J. (2011) Evidence of a relationship between asymmetries in tympanic membrane temperature and lateralised sensory preferences. Laterality 16, 107–124 [DOI] [PubMed] [Google Scholar]
- Johnson S. R., Rao S., Hussey S. B., Morley P. S., Traub-Dargatz J. L. (2011) Thermographic eye temperature as an index to body temperature in ponies. Journal of Equine Veterinary Science 31, 63–66 doi:10.1016/j.jevs.2010.12.004 [Google Scholar]
- Kaliszan M. (2012) First practical applications of eye temperature measurements for estimation of the time of death in casework. Report of three cases. Forensic Science International 219, e13–e15 doi:10.1016/j.forsciint.2011.11.027 [DOI] [PubMed] [Google Scholar]
- Kamao T., Yamaguchi M., Kawasaki S., Mizoue S., Shiraishi A., Ohashi Y. (2011) Screening for dye eye with newly developed ocular surface thermographer. American Journal of Ophthalmology 151, 782–791 doi:10.1016/j.ajo.2010.10.033 [DOI] [PubMed] [Google Scholar]
- Kandari J. A., Raizada S., Razzak A. A. (2010) Accidental Laser Injury to the Eye. Ophthalmic Surgery, Lasers & Imaging 9, 1–5 [DOI] [PubMed] [Google Scholar]
- Kawali A. A. (2013) Thermography in ocular inflammation. Indian Journal of Radiologic Imaging 23, 281–283 [PMC free article] [PubMed] [Google Scholar]
- Khvatova A. V., Katargina L. A., Lokhmanov V. P., Zibarov L. N. (1991) Use of distant thermography in uveitis in children. Vestnik Oftalmologii 107, 46–49 [PubMed] [Google Scholar]
- Klamann M. K., Maier A. K., Gonnermann J., Klein J. P., Bertelmann E., Peyer U. (2013) Ocular surface temperature gradient is increased in eyes with bacterial corneal ulcers. Ophthalmic Research 49, 52–56 doi:10.1159/000343774 [DOI] [PubMed] [Google Scholar]
- Klamann M. K., Maier A. K., Gonnermann J., Klein J. P., Pleyer U. (2012) Measurement of dynamic ocular surface temperature in healthy subjects using a new thermography device. Current Eye Research 37, 678–683 doi:10.3109/02713683.2012.674610 [DOI] [PubMed] [Google Scholar]
- Koçak I., Orgül S., Flammer J. (1999) Variability in the measurement of corneal temperature using a noncontact infrared thermometer. Ophthalmologica 213, 345–349 doi:10.1159/000027452 [DOI] [PubMed] [Google Scholar]
- Mapstone R. (1970) Ocular thermography. British Journal of Ophthalmology 54, 751–754 doi:10.1136/bjo.54.11.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan P. B., Soh M. P., Efron N., Tullo A. B. (1993) Potential applications of ocular thermography. Optometry and Visual Science 70, 568–576 doi:10.1097/00006324-199307000-00008 [DOI] [PubMed] [Google Scholar]
- Purslow C., Wolffsohn J. S. (2005) Ocular surface temperature: a review. Eye Contact Lens 31, 117–123 doi:10.1097/01.ICL.0000141921.80061.17 [DOI] [PubMed] [Google Scholar]
- Rysa P., Sarvaranta J. (1974) Corneal temperature in man and rabbit. Observations made using an infrared camera and a cold chamber. Acta Ophthalmologica (Copenh) 52, 810–816 doi:10.1111/j.1755-3768.1974.tb01117.x [DOI] [PubMed] [Google Scholar]
- Schaefer A. L., Cook N., Tessaro S. V., Deregt D., Desroches G., Dubeski P. L., Tong A. K. W., Godson D. L. (2004) Early detection and prediction of infection using infrared thermography. Canadian Journal of Animal Science 84, 73–80 doi:10.4141/A02-104 [Google Scholar]
- Stewart M., Webster J. R., Stafford K. J., Schaefer A. L., Verkerk G. A. (2010) Technical note: effects of an epinephrine infusion on eye temperature and heart rate variability in bull calves. Journal of Dairy Science 93, 5252–5257 doi:10.3168/jds.2010-3448 [DOI] [PubMed] [Google Scholar]
- Su T. Y., Hwa C. K., Liu P. H., Wu M. H., Chang D. O., Su P. F., Chang S. W., Chiang H. K. (2011) Noncontact detection of dye eye using a custom designed infrared thermal image system. Journal of Biomedical Optics 16, 046009 doi:10.1117/1.3562964 [DOI] [PubMed] [Google Scholar]
- Westermann S., Buchner H. H. F., Schramel J. P., Tichy A., Stanek C. (2013) Effects of infrared camera angle and distance on measurement and reproducibility of thermographically determined temperatures of the distolateral aspects of the forelimbs in horses. Journal of the American Veterinary Medical Association 242, 388–39 doi:10.2460/javma.242.3.388 [DOI] [PubMed] [Google Scholar]