Abstract
Introduction
While clinical respiratory disease is considered a main cause of poor performance in horses, the role of subclinical respiratory virus infections is less clear and needs further investigation.
Aims and objectives
In this descriptive longitudinal study the relationship of markers of subclinical respiratory viral activity to occurrence of poor performance in racing Standardbred trotters was investigated.
Material and methods
66 elite Standardbred trotters were followed for 13 months by nasal swabs analysed with qPCR for equine influenza virus, equine arteritis virus, equine rhinitis B virus (ERBV), equine herpesvirus type 1(EHV-1) and equine herpesvirus type 4 (EHV-4) and serology to equine rhinitis A virus (ERAV), ERBV, EHV-1 and EHV-4, as well as the acute phase protein serum amyloid A (SAA). Findings on lab analyses were subsequently assessed for possible correlations to workload performance and trainer opinion measures of poor performance.
Results
Despite occurrence of poor performance and subclinical viral activity the authors were unable to detect association neither between subclinical viral activity and poor performance, nor between SAA elevations and either viral activity or poor performance.
Conclusions
Consistent with earlier study results, antibody titres to ERBV remained high for at least a year and few horses two years or older were seronegative to either ERAV or ERBV. In absence of clinical signs, serology to common respiratory viruses appears to have little diagnostic benefit in evaluation of poor performance in young athletic horses.
Keywords: Viruses, PCR, Serology
Introduction
Viral respiratory infections are among the most common equine health issues worldwide (Traub-Dargatz and others 1991) and cause impaired health and performance of the horses as well as financial losses for the owners and the equine industry. In the early1980s outbreaks of equine respiratory disease often remained undiagnosed (Mumford and Rossdale 1980). Despite several decades of research since then, the authors’ understanding of equine respiratory infections remains incomplete.
For the athletic horse, lack of participation in competitions is a clear indication of career impairment. However, when horses do compete, it is even more challenging to define true poor performance. Since there are no widely accepted standard definitions for poor performance with various studies having used different criteria, comparison of results between studies is seldom possible (Leleu and others 2005, Richard and others 2010). Compounding the difficulties in defining poor performance, the challenge remains in identifying the underlying cause of poor performance, since it is often multifactorial (Morris and Seeherman 1991). For Thoroughbred horses, locomotor and respiratory problems have been incriminated as the main causes for disruption of training and racing (Wilsher and others 2006). Specifically, viral infections may play a key role in the respiratory component of poor performance (Mumford and Rossdale 1980). While subclinical airway inflammation has been identified in Standardbred trotters with impaired performance (Richard and others 2010) the contribution by possible viral infections was not investigated. Unfortunately, since viral infections are largely refractory to diagnosis using stall side testing, there is a need for alternative biomarkers that help avert training or racing of a horse with underlying viral infections. Changes in the major acute phase protein serum amyloid A (SAA) appear to correlate to clinical equine influenza infection (Hulten and others 1999). However, it is unclear whether levels are altered in subclinical equine influenza or other respiratory viral infections, and hence its potential role as a diagnostic tool for viral associated equine poor performance remains unknown.
Subclinical infections as causes of poor performance in the equine athlete have been described earlier (Leleu and others 2005, Richard and others 2010, Fraipont and others 2011). However, while viral activity (Powell and others 1978, Carman and others 1997, Pusterla and others 2011) and antibodies to rhinitis viruses (Black and others 2007) in competition horses has been studied before there is a lack of studies investigating the relationship between viral infection status and athletic performance in horses. The aim of this longitudinal study was to investigate the relationship of subclinical respiratory viral activity on the athletic performance in Standardbred trotters. In addition, the authors also evaluated whether changes in SAA could be related to subclinical viral activity in the horse or to episodes of poor performance.
Materials and methods
Description of the cohort
A cohort of 66 trotters from four different training yards (TYs) with geographical proximity to the National Veterinary Institute (SVA) in Uppsala, Sweden was followed over 13 consecutive months between August 2010 and August 2011. Horses were selected based on following inclusions criteria: more than two years of age, in active racing and training, healthy on clinical examination and expected to remain with the same trainer for the duration of the study. The clinical examination included assessing rectal temperature, and presence of nasal discharge or cough, palpation of submandibular lymph nodes and a lameness examination. The numbers of horses included from each trainer was predetermined to reflect the distribution across the age groups at each trainer (i.e. proportional sample). The health status of all horses was monitored by the same veterinarian weekly throughout the study period. As long as horses remained with the same trainer, all horses trained and raced according to their normal schedule. However, unexpected injury could interrupt their schedule.
Sampling
Horses were sampled at monthly intervals. Each sampling occasion took place immediately following a standardised field exercise test (SFE, see below), with nasal secretions obtained using an Eswab (Eswab, Copan, Murrieta, USA) (Daley and others 2006) and serum samples collected by direct venepuncture into Vacutainer tubes (Becton, Dickinson and Company, Franklin Lakes, USA). The resting body temperature and clinical signs of respiratory disease such as fever, nasal discharge or cough were recorded. Similar sampling was also conducted in any horse showing poor performance and/or having clinical signs of respiratory disease outside the planned sampling schedule. Nasal swabs and blood samples were transported at 20°C temperature to the laboratory, serum centrifuged within one day from arrival and stored with nasal swabs at 4°C for one to three days until analysis. An aliquot of serum was stored at −20°C for subsequent analysis for concentration of SAA.
Standardised field exercise test
The SFE was used as an objective measurement of fitness of the horses. All SFEs were conducted on the racetracks at respective TYs and were standardised within each TY. After a standardised warm-up, every horse had its running time recorded over a set distance of 500–2000 m (depending on TY), where the driver used a heart rate (HR) monitor (Polar Equine CS600X, Polar Electro Sverige AB, Bromma, Sweden) to keep the horse working at 210 bpm. This procedure was to ensure that blood lactate accumulation would exceed 4 mmol/l based on earlier studies showing lactate accumulation of 4 mmol/l at a HR of 200 bpm (Persson 1983). While the set HR of SFE likely demanded differing workload from individual horses, every horse was its own control for comparing running times as measures of its fitness.
Poor performance
Performance of the horses was defined in a three-step procedure. First, trainers’ subjective opinions were used to classify performance based on the SFE or the training or race on the same or the previous day. Secondly, performance was defined mathematically where one SFE deviating more than one sd from the individual horse's mean running time of all SFEs, was defined as poor performance. This classification step was only made if the horse had carried out at least four SFEs during the study period. All SFE recordings were then analysed graphically per horse focusing on time trends considering age and experience. A poor performance event based on mathematical criteria could be redefined as normal performance, if an SFE with a slow running time occurred early in the study period, when the horse was young and inexperienced and the graphical evaluation showed a clear trend with improvement during the study period. Three horses were reclassified from poor to normal performance based on this adjustment criterion. The third step combined trainer and SFE data to define poor performance on any criteria.
Laboratory analysis
All laboratory analyses were carried out at SVA. Serum antibodies to equine herpesvirus type 1 (EHV-1) and equine herpesvirus type 4 (EHV-4), equine rhinitis A virus (ERAV) and equine rhinitis B virus (ERBV) were analysed within one to three days after arrival to the laboratory whereas SAA was analysed within two weeks. Remaining serum was kept at −20°C and the swabs were stored at −70°C. Nasal swabs were analysed for the nucleic acid of equine influenza virus (EIV), EHV-1 and EHV-4, ERBV and equine arteritis virus (EAV) using qPCR.
qPCR
Primer and probe sequences for the in-house developed assays targeting EHV-1, EHV-4, ERBV and EIV were designed using the software Beacon Designer (Premier Biosoft), GenBank number for sequences used for the design of oligos: EHV-1: M29234, M19966, L07272; EHV-4: AF030027; ERVB: X96871, EIV: AF001662, AF001664, AF001665, AF001666, AF001667, AF0011668, AF001669, AF001671, AF001672, AF001673. For the detection of EAV, primer and probe sequences as earlier described (Balasuriya and others 2002, Lu and others 2008) were used.
All qPCR assays were performed as follows: 10 µl proteinase k (Sigma, P4850,Sigma Aldrich, St Louis, USA) was added to 90 µl of the Eswab buffer before nucleic acid extraction in a Magnatrix 8000+ robot (NorDiag AB, Hägersten, Sweden) using the extraction kit NorDiag Vet Viral NA (NorDiag AB, Hägersten, Sweden) extraction kit according to the manufacturer's instructions. The nucleic acid was eluted in 70 µl elution buffer of which 2 µl were added to 13 µl of PCR-mix (AgPath-ID One-Step QPCR, Applied Biosystems) for each assay. TaqMan PCR assays targeting the genes described in Table 1 were used. The thermal profile used was: 45°C for 10 minutes, 95°C for 10 minutes followed by (95°C for 15 seconds; 60°C for 45 seconds) ×48. The tests were run in parallel on a 96-well plate. The results from the qPCR were measured as Cq (quantification cycle) values, previously referred to as Ct values, where Cq<40 was considered as a positive result.
TABLE 1:
Primers and probes used in the multiplex qPCR assay
| Virus | Primers and probes | Conc. (nM) |
Sequence 5′-3′ | Reference | Gene size |
|---|---|---|---|---|---|
| EAV | EAV 7.53 F EAV 7.256R EAV 7.92R |
400 400 134 |
GGCGACAGCCTACAAGCTACA CGGCATCTGCAGTGAGTGA 6-FAM-TTGCGGACCCGCA(T)iCTGACCAA |
[14;15] | ORF6-ORF-7 204 bp |
| EHV-1 | EHV-1 Forward EHV-1 Reverse EHV-1 TaqMan |
400 400 134 |
GCGATATTAACTTATGCCTCTGGA GTAGATTCTGTACCGTTTGCGTTA 6-FAM-TAGCTCCAGCCAGAG(T)iACGCCCGC |
This publication | Glycoprotein C 143 bp |
| EHV-4 | EHV-4 Forward EHV-4 Reverse EHV-4 TaqMan |
100 100 134 |
AGCCTACAGCGTGGAACACA CATGTCACCGAGTAGGTAGCG 6-FAM-ACCCTTG(T)iGTTTGACCGCCACCCG |
This publication | ORF2, TK 105 bp |
| ERBV | ERBV Forward ERBV Reverse Rhi TaqMan |
400 400 134 |
GAGGAACCTGACAGTTTTGAGTTG ATCTGTGCAAACAATGAGGAAGC 6-FAM-AAGCC(T)iCAACAGCAGCCTCCAGGT |
This publication | RNA Pol 128 bp |
| EIV | Influenza Forward Influenza Reverse Influenza TaqMan |
100 100 134 |
ATGGACCAGGCAATCATGGATAA GGAAGAGAAGGCAATGGTGAAATT 6-FAM-CGCCAACGAC(T)iGCTCCTTCTTCGG |
This publication | NS1 146 bp |
EAV, equine arteritis virus; EHV, equine herpesvirus; EIV, equine influenza virus; ERBV, equine rhinitis B virus
This table shows the names, mastermix concentrations, sequences and targets for primes and probes used for equine arthritis virus (EAV), EHV-1, EHV-4, ERBV and EIV included within the multiplex qPCR assay that was used in a cohort of Standardbred trotters. (T)i indicates the use of an internal quencher DABCYL.
Virus neutralisation test
Presence of ERAV and ERBV antibodies was demonstrated by virus neutralisation (VN) test (Kriegshauser and others 2005) using ERAV strain 1722/Switzerland and ERBV 5/15/95 Newmarket strain in the assay. Dilutions tested ranged from 1:2 to 1:156250 for ERAV and from 1:2 to 1:256 for ERBV.
Complement fixation test
The presence of antibodies for EHV-1 and EHV-4 was examined by complement fixation (CF) test as earlier described (Thomson and others 1976) with guinea pig complement and erythrocytes from sheep (Rockborn and others 1990). Dilutions tested in this assay ranged from 1:4 to 1:64.
Serum amyloid A
The SAA analyses were performed as earlier described (Hillstrom and others 2010), where a serum concentration of ≤20 mg/l was classified as normal (Jacobsen and Andersen 2007).
Viral activity criteria
Three different criteria were used for defining exposure to infectious disease:
Presence of viral nucleic acids to named viruses in nasal swabs as demonstrated by qPCR assay indicating ongoing viral activity.
A seroconversion defined as a fourfold rise in titres of antibodies between consecutive monthly samples, and interpreted as response to recent viral activity.
A high antibody titre in single or multiple samples, as often used in the equine industry as an indication of recent infection and by extension, a reason for poor performance. Antibodies were considered high according laboratory standards at SVA with titres of 1:8 or higher used for the EHV-1 and EHV-4 CF tests and titres of 1:13975 and 1:90 by VN testing to ERAV and ERBV, respectively.
Statistical analysis
Descriptive statistics on sampling occasions, lab results and performance (SFE and trainers opinion combined) were computed per TY. The number of positive qPCR results and serological samples above the positive threshold are presented as total and according to poor performance (yes/no) assessed in three ways (according to trainer, SFE or either) in separate analyses; in total and per type of sampling occasion (regular v additional). Unconditional associations between poor performance and viral respiratory infection were investigated by logistic regression including only the outcome and the different viral activity criteria evaluated in separate logistic models (one per each specific combination of exposure and outcome), generating crude ORs. As observations were not independent but clustered within horse (up to 13 sample occasions per horse) and TY (n=4), the ses from the unconditional analyses were likely underestimated hence producing overly narrow CIs. Therefore, random effects logistic regression models (one per independent variable, i.e. virus infection parameter) were performed with random effects for horse and TY. The maximum log likelihood estimation of these models was performed using adaptive Gaussian quadrate methods. Results of the additional samples were analysed in similar manners as regularly taken samples.
All analysis was performed using Stata statistical software (Stata, release V.11.2: College Station, Texas, USA: StataCorp LP).
Results
Description of the cohort
Sixty-six horses entered the study and collectively were sampled 672 times during 13 months. At recruitment, the mean age was three years (range 2–8 sd 1.33). Of the 66 horses, 25 were sampled 13 times or more. One horse was excluded from the study on the owner's request and four horses left the study due to moving into breeding or to severe injury. Two horses had change of trainer after the first sampling occasion and were excluded. These horses were replaced by two recent additions to the TY, which were thus included in the study from months 2 and 3, respectively. Another 15 horses left the study prematurely at various time points due to change of trainers after contributing 2–10 occasions. As well, one to three sample occasions were lacking from19 horses due to racing on the sampling day. From the 672 sample occasions, 88 were excluded from further analysis because of injury or disease unrelated to the respiratory tract, and data were therefore missing for those occasions. The results of the authors’ analyses are based on the remaining 584 sampling occasions in 63 horses, of which 560 were regular and 24 were additional sampling occasions. The additional sampling occasions were mostly clustered within one trainer.
Poor performance
The trainers’ opinion was registered on all 584 occasions and results from SFE were available in 327 occasions. The SFE was not performed on 257 occasions due to: altered training schedule due to racing (n=160), poor race track conditions (n=62) or signs of mild respiratory disease (n=35). In total there were 96 (16 per cent) events of poor performance identified at the 584 sampling occasions. Of these 52 events were based on trainers’ opinion and 45 of them on SFE. Of the 52 trainers’ opinion-based events of poor performance 9 also had a SFE conducted, but only one of the SFEs was classified as poor performance.
Laboratory analysis
Results from the laboratory analysis and the performance were subdivided by trainer and listed in Table 2.
TABLE 2:
Laboratory results and performance divided on training yards
| TY 1 | TY 2 | TY 3 | TY 4 | Sum | |
|---|---|---|---|---|---|
| Horses (n) | 36 | 10 | 14 | 3 | 63 |
| Horses excluded (n) | 1 | 0 | 2 | 0 | 3 |
| Horses added (n) | 0 | 0 | 2 | 0 | 2 |
| Sampling occasions* (n) | 314 | 110 | 126 | 34 | 584 |
| Additional sampling occasions† (n) | 20 | 3 | 0 | 1 | 24 |
| Horses with additional sampling occasions (n) | 16 | 3 | 0 | 1 | 20 |
| Sampling occasions (%) max: horse×13 |
67 | 83 | 69 | 100 | – |
| Horses without missing sampling occasions (%) | 0 | 20 | 0 | 100 | – |
| Positive PCR ERBV (n) |
5 | 0 | 0 | 0 | 5 |
| Positive PCR EHV-4 (n) |
0 | 1 | 1 | 0 | 2 |
| Titres above cut-off ERBV (n) | 67 | 23 | 17 | 26 | 133 |
| Titres above cut-off ERAV (n) | 32 | 12 | 27 | 7 | 79 |
| Titres above cut-off EHV-1, EHV-4 (n) | 44 | 18 | 2 | 0 | 64 |
| Fourfold rise titre ERBV (n) |
3 | 3 | 2 | 0 | 8 |
| Fourfold rise titre ERAV (n) |
3 | 0 | 0 | 0 | 3 |
| Fourfold rise titre EHV-1, EHV-4 (n) |
1 | 8 | 1 | 0 | 10 |
| Poor performance, total (n) | 54 | 23 | 15 | 4 | 96‡ |
| Poor performance, SFE (n) | 21 | 8 | 14 | 2 | 45 |
| Poor performance, according to trainer (n) |
33 | 16 | 1 | 2 | 52 |
| SAA above threshold (n) | 7 | 0 | 3 | 0 | 10 |
*The number of sampling occasions that were based on the regular monthly visits
†Additional sampling occasions due to events of poor performance and/or clinical respiratory signs, occurring outside regular sampling occasions
‡The 96 sampling occasions with poor performance included 52 which were based on trainers’ reports and 45 based on SFEs and 1 horse in which trainers’ report and SFE coincided
EHV, equine herpesvirus; ERAV, equine rhinitis A virus; ERBV, equine rhinitis B virus; n, number; SAA, serum amyloid A; SFE, standardised field exercise test; TY, training yard
This table describes the different outcomes and exposures of the horses in a prospective longitudinal study, divided into the four participating TYs.
qPCR
None of the horses were positive for EIV, EHV-1 or EAV on the qPCR, and thus these viruses are not included in Table 2. Five samples from four horses tested positive for ERBV with Cq-values within the range of 33.6–35.9 (one horse was positive twice with one month with negative results in between). None of the qPCR-positive ERBV sampling occasions coincided with seroconversion, and seroconversion did not occur in association with the single qPCR positive sampling occasion for EHV-4 (Cq=28.9).
VN test
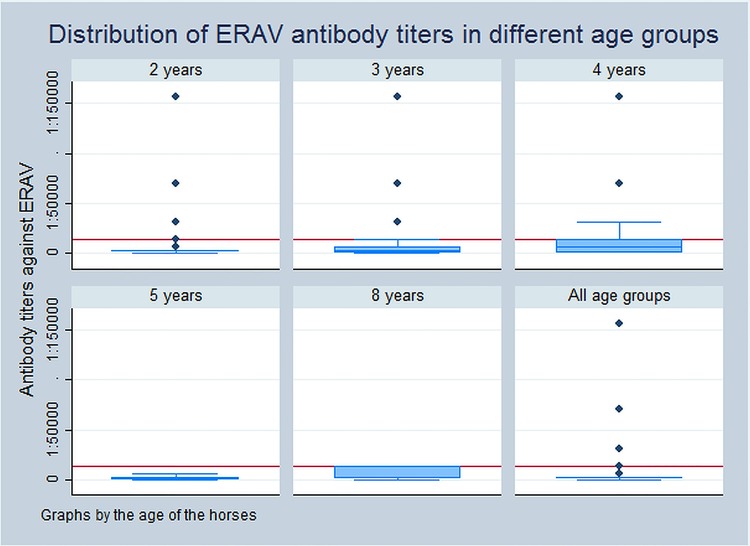
Fig 1 shows the age distribution of the ERAV antibody titres, with measurable but low and stable titres for the majority of individuals, and only two per cent truly seronegative sampling occasions. Thirty-eight of the horses had ERAV antibody titres, that remained under the cut-off during the entire study and the remaining 25 horses had 78 sampling occasions with titres above the cut-off. Three horses from one TY had a fourfold rise in titres between monthly sampling occasions.
FIG 1:
Antibody titres to equine rhinitis A virus (ERAV) distributed in the different age groups of a cohort of Swedish Standardbred trotters
Regarding ERBV 29 horses had titres consistently under the cut-off, and 34 horses had 133 sampling occasions with titres above the cut-off. Eight horses had single events of fourfold rise in titres between monthly sampling occasions. One horse was completely seronegative on a single sampling occasion and had very low titres (1:8–1:16) in remaining samples.
CF test
For the CF analysis of EHV-1 and EHV-4, 64 samples were at the threshold of 1:8 or higher. Of these, 14 had been vaccinated against equine herpesvirus four to eight weeks earlier. There were 10 horses that had paired samples with a fourfold rise in titres of antibodies, eight of which had been vaccinated against EHV-1 and EHV-4 four weeks earlier.
SAA
Of the 584 sample occasions SAA was classified as positive in 10 animals (i.e. SAA >20 mg/l) (4<100 mg/l, 4 130–650 mg/l, 2<900 mg/l).
SAA was elevated in 6 of 96 events of poor performance whereas no elevations were found in sampling occasions when qPCR for virus was positive. SAA was also normal in all samples with high antibody titre for ERAV, and all but one each of the occasions with high titres to ERBV and EHV-1 and EHV-4.
Statistical analysis
Random effects logistic regression did not identify any significant association between poor performances and the activity or presence of different viruses investigated in this study.
Clinical signs versus laboratory analysis
No clinical respiratory signs were observed in qPCR-positive horses (five for ERBV and one for EHV-4) nor in horses with high antibody titres to ERAV, ERBV and EHV-1 and EHV-4. Moreover, none of these horses had changes in concentration of SAA indicative of systemic inflammation.
Poor performance versus laboratory analysis
In Table 3, positive lab results are combined with performance of the horses and divided into three groups: all sampling occasions, regular sampling occasions and additional sampling occasions.
TABLE 3:
Laboratory results and the performance of the horses
| All sampling occasions |
Regular sampling occasions |
Additional sampling occasions | ||||
|---|---|---|---|---|---|---|
| Poor performance (SFE and trainers opinion) |
No | Yes | No | Yes | No | Yes |
| Titres above cut-off ERBV | 112 | 21 | 109 | 17 | 3 | 4 |
| Titres above cut-off ERAV | 68 | 10 | 66 | 9 | 2 | 1 |
| Titres above cut-off EHV-1, EHV-4 | 50 | 14 | 50 | 11 | 0 | 3 |
| Fourfold rise titre ERBV | 7 | 1 | 7 | 1 | 0 | 0 |
| Fourfold rise titre ERAV | 3 | 0 | 3 | 0 | 0 | 0 |
| Fourfold rise titre EHV-1, EHV-4 |
9 | 1 | 9 | 1 | 0 | 0 |
EHV, equine herpesvirus; ERAV, equine rhinitis A virus; ERBV, equine rhinitis B virus; SFE, standardised field exercise test
Estimates of poor performance were based on trainers’ opinion and SFE test, and results from laboratory analysis for viral infections of equine herpesvirus (EHV), ERAV and ERBV, in 63 Standardbred trotters from four high-performing TYs in Sweden. The number of sample occasions in each category is divided into three groups: all samples, the samples taken on regular basis once a month and the extra samples, during which presence of poor performance (Yes/No) was recorded.
The five ERBV sampling occasions positive in qPCR were all from horses from the same trainer. Two of these were from the same horse and detected with two qPCR-negative intervening months. Poor performance (based on SFE) was observed on the second positive occasion. None of the other qPCR-positive samples, including the EHV-4 positive, were associated with episodes of poor performance. Similarly, poor performance was not observed in the three horses with fourfold rise in antibody titres of ERAV.
Discussion
Despite rigorous sampling for viral activity in combination with assessment of several measures of performance in this field study, the authors were unable to detect association between poor performance and subclinical activity of included respiratory viruses by using qPCR assay and available serological methods. However, the limited number of subclinical viral activity detected limits the power of the analysis. Moreover, the paucity of qPCR-positive samples detected in this study suggests a low activity of tested viruses in this population of high-performing horses during the sampling period. Nonetheless, this work clearly identified both respiratory virus shedding occasions and seroconversion in the absence of clinical disease or poor performance.
The low percentage of seronegative samples to ERAV (two per cent) in the present cohort of horses more than two years of age is in agreement with other studies that report a strong positive correlation between age and seroprevalence (Holmes and others 1978, Black and others 2007). Most of the horses had VN antibody titres to ERAV, suggesting that the virus is widespread in the study population. However, the stable titres indicate that active infection was uncommon, and consistent with results from an earlier study that implied high antibody titres to ERAV were maintained long after infection, possibly resulting in lifetime immunity (Burrows 1979). It was possible for the horses to be exposed to viral infections at races or at introduction of new horses at the TYs, however, a rise in titres of antibodies would be expected if a clinical infection would have occurred. In order to evaluate the diagnostic value of single high antibody titre samples when investigating poor performance, the authors analysed all horses individually using the viral activity criteria. However, since one high titre could remain stable without signs of ongoing infection, the diagnostic value of a single serum sample appears to be minimal. In the present study three seroconversions to ERAV at one occasion at one TY without accompanying clinical respiratory signs or poor performance, indicated an active subclinical infection by this virus. Furthermore, the seroconversions without clinical signs observed in already seropositive horses, support other work suggesting that persistent antibodies to ERAV have a preventive effect against clinical disease with re-infection (Diaz-Mendez and others 2014).
For ERBV all but 0.2 per cent of the sampling occasions had VN antibodies, indicating that the virus is widespread. Moreover, the eight seroconversions to ERBV at different occasions occurred without clinical signs and with normal performance indicating subclinical infection. This suggests antibodies to ERBV might have a protective effect against clinical disease after re-infection. Similar to the authors’ findings for ERAV, single high titres to ERBV appear to be of little clinical value to predict recent or ongoing infection.
Despite identifying 10 occasions of SAA elevations that have been shown to occur in EIV (Hulten and others 1999) and other conditions with systemic inflammation (Jacobsen and Andersen 2007), none were associated with either subclinical viral activity or poor performance as it was defined in this study. Furthermore, no correlation between the levels of SAA and the performance could be detected in the serially sampled horses in this study. This contrasts a previous cross-sectional study including 38 endurance horses, where mean values of SAA were higher after workload in the group of 9 horses with poor performance compared with the group of 11 with intermediately performance (Fraipont and others 2011). The difference observed between the studies may be because the mean value of SAA was evaluated and compared, whereas the present study evaluated SAA individually. However, the workloads differed substantially between the endurance horses where exhaustion and metabolic problems can be expected and provide a far stronger inflammatory stimulus for SAA elevation compared with intensive but comparatively short-lived workload of the trotters.
This study used two ways to determine the performance of the horses; one subjective method (trainers opinion) and one objective method (SFE) based on well-established methods using HR and speed to ensure blood lactate exceeded 4 mmol/ml (V4, V200) (Courouce and others 1997; Leleu and others 2005) and then combining them to maximise the cases of poor performance identified. Despite this approach, the poor performance events in this study were few. Unfortunately, it was not possible in this study to identify whether the trainers and the SFE were defining poor performance in a different way, since only nine SFEs were performed of the 52 occasions when poor performance was registered according to the trainer. Out of the 52 occasions when trainers reported poor performance, 15 were taken as additional occasions due to poor performance according to the trainer and therefore SFEs were lacking. Although SFEs and trainers only agreed on poor performance in one of the nine occasions, six of the remaining eight SFEs had among the slowest individual running times (but not slow enough to be classified as poor performance in the SFE). This suggests that the relationship between SFEs and the opinion of the professional trainer deserves further scrutiny. This result also shows the usefulness with complimentary definitions of poor performance in a longitudinal study in TYs.
This study followed high-performing Standardbred trotters in their own environment over a year. The authors were therefore able to study how natural viral infections can appear in the same elite horse over time. The long study period was also a limitation, since the authors could not control if a horse suddenly left the study due to change of trainer, injury or disease. Since the laboratory result, performance and health status were evaluated individually before statistical analysis, loss of observations would not lead to biased results. There are obvious limitations with using trainers’ opinion as a way of determining poor performance since it is very subjective. However, trainers do contact veterinarians when they have the impression of poor performance and the parameter can therefore be of interest to the equine clinician. By sampling horses monthly, there is always a risk of unnoticed infections. In this study the horses were weekly monitored by the same veterinarian, making detection of clinical respiratory disease and the opportunity of extra samples possible. Due its rare occurrence in horses with clinical disease in Sweden, qPCR assay for ERAV was not prioritised in this study. Furthermore, serological analysis against other known viruses such as EIV and EAV was not included, since all horses were vaccinated twice per year against EIV and clinical disease due to EAV is rare in Sweden.
Conclusions
In this study, subclinical viral activity, as indicated by seroconversions, single high antibody titres and/or qPCR positivity to ERAV, ERBV, EHV-1 and EHV-4 were not associated with either quantitative or qualitative episodes of poor performance. Additionally, changes in the acute phase protein SAA were not predictive of either subclinical viral activity or poor performance in these horses. Finally, few to no horses two years or older were completely seronegative to ERAV and ERBV and VN antibody titres to ERBV can remain high for at least one year in the absence of viral shedding or seroconversion.
Acknowledgments
The authors thank Dr Jean-Francois Valarcher at the National Veterinary Institute, Uppsala, Sweden for critical review of the manuscript. The authors also thank Karin Thulin and Anna Lindhe for technical assistance during sampling and are grateful to the trainers who participated with their horses in this study.
Footnotes
Contributors: HB contributed to study execution, data analysis and interpretation and writing of the manuscript. JPe contributed to data analysis and interpretation and writing of the manuscript. JPr contributed to writing of the manuscript and interpretation of data. MI contributed to developing the qPCR technique and preparation of the manuscript. NR was involved in the study design, sample collection and provided contact with the horses and trainers. LTB contributed to study design and sample collection. KS contributed to study design, data analysis and interpretation and writhing of the manuscript. All authors have approved the final version of the manuscript.
Funding: The study was funded by the Swedish-Norwegian Foundation for Equine Research (Grant number H0947319).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: This study was approved by the Swedish Animal Ethical committee. Informed consent was obtained from the trainers.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- Balasuriya U. B. R., Leutenegger C. M., Topol J. B., Mccollum W. H., Timoney P. J., Maclachlan N. J. (2002) Detection of equine arteritis virus by real-time TaqMan (R) reverse transcription-PCR assay. Journal of Virological Methods 101, 21–28 doi:10.1016/S0166-0934(01)00416-5 [DOI] [PubMed] [Google Scholar]
- Black W. D., Wilcox R. S., Stevenson R. A., Hartley C. A., Ficorilli N. P., Gilkerson J. R., Studdert M. J. (2007) Prevalence of serum neutralising antibody to equine rhinitis A virus (ERAV), equine rhinitis B virus 1 (ERBV1) and ERBV2. Veterinary Microbiology 119, 65–71 doi:10.1016/j.vetmic.2006.08.031 [DOI] [PubMed] [Google Scholar]
- Burrows R. (1979) Equine rhinovirus and adenovirus infections. Proceedings of the 24th Annual Convention of the American Association of Equine Practitioners 1978 299–306 [Google Scholar]
- Carman S., Rosendal S., Huber L., Gyles C., Mckee S., Willoughby R. A., Dubovi E., Thorsen J., Lein D. (1997) Infectious agents in acute respiratory disease in horses in Ontario. Journal of Veterinary Diagnostic Investigation 9, 17–23 doi:10.1177/104063879700900104 [DOI] [PubMed] [Google Scholar]
- Courouce A., Chatard J. C., Auvinet B. (1997) Estimation of performance potential of Standardbred trotters from blood lactate concentrations measured in field conditions. Equine Veterinary Journal 29, 365–369 doi:10.1111/j.2042-3306.1997.tb03140.x [DOI] [PubMed] [Google Scholar]
- Daley P., Castriciano S., Chernesky M., Smieja M. (2006) Comparison of flocked and rayon swabs for collection of respiratory epithelial cells from uninfected volunteers and symptomatic patients. Journal of Clinical Microbiology 44, 2265–2267 doi:10.1128/JCM.02055-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Mendez A., Hewson J., Shewen P., Nagy E., Viel L. (2014) Characteristics of respiratory tract disease in horses inoculated with equine rhinitis A virus. American Journal of Veterinary Research 75, 169–178 doi:10.2460/ajvr.75.2.169 [DOI] [PubMed] [Google Scholar]
- Fraipont A., Van Erck E., Ramery E., Richard E., Denoix J. M., Lekeux P., Art T. (2011) Subclinical diseases underlying poor performance in endurance horses: diagnostic methods and predictive tests. Veterinary Record 169, 154 doi:10.1136/vr.d4142 [DOI] [PubMed] [Google Scholar]
- Hillstrom A., Tvedten H., Lilliehook I. (2010) Evaluation of an in-clinic Serum Amyloid A (SAA) assay and assessment of the effects of storage on SAA samples. Acta Veterinaria Scandinavica 52, 8 doi:10.1186/1751-0147-52-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. F., Kemen M. J., Coggins L. (1978) Equine rhinovirus infection—serologic evidence of infection in selected United States horse populations. Equine Infectious Diseases IV 315–319 [Google Scholar]
- Hulten C., Sandgren B., Skioldebrand E., Klingeborn B., Marhaug G., Forsberg M. (1999) The acute phase protein serum amyloid A (SAA) as an inflammatory marker in equine influenza virus infection. Acta Veterinaria Scandinavica 40, 323–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen S., Andersen P. H. (2007) The acute phase protein serum amyloid A (SAA) as a marker of inflammation in horses. Equine Veterinary Education 19, 38–46 doi:10.1111/j.2042-3292.2007.tb00550.x [Google Scholar]
- Kriegshauser G., Deutz A., Kuechler E., Skern T., Lussy H., Nowotny N. (2005) Prevalence of neutralizing antibodies to Equine rhinitis A and B virus in horses and man. Veterinary Microbiology 106, 293–296 doi:10.1016/j.vetmic.2004.12.029 [DOI] [PubMed] [Google Scholar]
- Leleu C., Cotrel C., Courouce-Malblanc A. (2005) Relationships between physiological variables and race performance in French standardbred trotters. Veterinary Record 156, 339–342 doi:10.1136/vr.156.11.339 [DOI] [PubMed] [Google Scholar]
- Lu Z., Branscum A. J., Shuck K. M., Zhang J., Dubovi E. J., Timoney P. J., Balasuriya U. B. R. (2008) Comparison of two real-time reverse transcription polymerase chain reaction assays for the detection of Equine arteritis virus nucleic acid in equine semen and tissue culture fluid. Journal of Veterinary Diagnostic Investigation 20, 147–155 doi:10.1177/104063870802000202 [DOI] [PubMed] [Google Scholar]
- Morris E. A., Seeherman H. J. (1991) Clinical evaluation of poor performance in the race horse-the results of 275 evaluations. Equine Veterinary Journal 23, 169–174 doi:10.1111/j.2042-3306.1991.tb02749.x [DOI] [PubMed] [Google Scholar]
- Mumford J. A., Rossdale P. D. (1980) Virus and its relationship to the poor performance syndrome. Equine Veterinary Journal 12, 3–9 doi:10.1111/j.2042-3306.1980.tb02285.x [DOI] [PubMed] [Google Scholar]
- Persson S. G. B. (1983) Evaluation of exercise tolerance and fitness in the performance horse. Equine exercise physiology. Proceedings of the First International Conference 441–457 [Google Scholar]
- Powell D. G., Burrows R., Spooner P. R., Goodridge D., Thomson G. R., Mumford J. (1978) A study of infectious respiratory disease among horses in Great Britain, 1971–1976. Equine Infectious Diseases IV 451–459 [Google Scholar]
- Pusterla N., Kass P. H., Mapes S., Johnson C., Barnett D. C., Vaala W., Gutierrez C., Mcdaniel R., Whitehead B., Manning J. (2011) Surveillance programme for important equine infectious respiratory pathogens in the USA. Veterinary Record 169, 12 doi:10.1136/vr.d2157 [DOI] [PubMed] [Google Scholar]
- Richard E. A., Fortier G. D., Pitel P.-H., Dupuis M.-C., Valette J.-P., Art T., Denoix J.-M., Lekeux P. M., Van Erck E. (2010) Sub-clinical diseases affecting performance in Standardbred trotters: Diagnostic methods and predictive parameters. Veterinary Journal 184, 282–289 doi:10.1016/j.tvjl.2009.04.016 [DOI] [PubMed] [Google Scholar]
- Rockborn G., Klingeborg B., Junitii N. eds (1990) Diagnostic Virology. Second Part: Guidebook to Procedures. Uppsala, Sweden: Swedish University of Agricultural Sciences. National Veterinary Institute. Swedish International Development Authority [Google Scholar]
- Thomson G. R., Mumford J. A., Campbell J., Griffiths L., Clapham P. (1976) Serological detection of equid herpesvirus 1 infections of the respiratory tract. Equine Veterinary Journal 8, 58–65 doi:10.1111/j.2042-3306.1976.tb03291.x [DOI] [PubMed] [Google Scholar]
- Traub-Dargatz J. L., Salman M. D., Voss J. L. (1991) Medical problems of adult horses, as ranked by equine practitioners. Journal of the American Veterinary Medical Association 198, 1745–1747 [PubMed] [Google Scholar]
- Wilsher S., Allen W. R., Wood J. L. N. (2006) Factors associated with failure of Thoroughbred horses to train and race. Equine Veterinary Journal 38, 113–118 doi:10.2746/042516406776563305 [DOI] [PubMed] [Google Scholar]