To the Editor:
Infections due to drug-resistant organisms are associated with considerable morbidity and mortality.1 While resistant gram-positive infections, such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococcus (VRE), remain a significant threat, the rise of resistant gram-negative infections due to P. aeruginosa, A. baumannii, and carbapenem-resistant enterobacteriaceae (CRE) have led to scenarios where clinicians are forced into treatment with highly toxic, often older, and pharmacologically suboptimal treatment regimens with drugs such as polymyxins or tigecycline.2 Despite the Infectious Diseases Society of America’s (IDSA) 10x20 initiative that promotes the development of 10 novel antimicrobials by 2020, the antimicrobial pipeline remains relatively dry, with only a few agents currently in phase III trials and none of these with a novel mechanism of action.3 These data highlight the need for antimicrobial stewardship efforts aimed at promoting the optimal use of current agents in an effort to limit further development of drug resistance. In addition, antimicrobial use, both appropriate and inappropriate, is the number one driver of Clostridium difficile infection (CDI), which has been shown to be a significant complication of hospitalization, leading to increased length of stay, hospital costs, morbidities, and ultimately mortality.4
In 2007, the IDSA developed and published guidelines and recommendations for antimicrobial stewardship within institutions.5 In this document, the authors recommended 2 core methods for stewardship: prospective audit and feedback (which involves daily monitoring of target antimicrobials and direct feedback with the prescribing team) and formulary restriction and preauthorization (requiring approval, usually by infectious diseases [ID], or predetermined criteria to be met, prior to dispensing an antimicrobial).5 They also recommended additional supplemental methods including education, guidelines and pathway development, intravenous to oral switching, de-escalation, and dose optimization to complement the 2 core methods. The authors suggested that the core stewardship team should consist of an ID physician and an ID-trained pharmacist, with support and collaboration of a clinical microbiologist, information technology, and hospital administration.5
Although these recommendations are commendable, the applicability to a large majority of institutions remains questionable and poorly described. Most institutions may lack both the personnel and the resources to practice stewardship as described in the guidelines. Currently, no national regulatory mandate exists to promote antimicrobial stewardship efforts. This may change in the future as the Center for Medicare & Medicaid Services’ Center for Clinical Standards and Quality recently confirmed plans to propose a condition of participation for antimicrobial stewardship.6 Prior to this, the propagation of antimicrobial stewardship efforts has been undertaken by a number of proactive state-level initiatives. Examples include California Senate Bill 739 (Health and Safety Code §§ 1288.5–1288.9, 2006), which is a state-level regulatory initiative to provide incentives to establish antimicrobial stewardship programs, and a state-sponsored educational program on antimicrobial stewardship in Massachusetts.7 Weston and colleagues reported the impact of a state-sponsored educational program on antimicrobial stewardship in Massachusetts.7 In conjunction with the Michigan Society of Health-System Pharmacists (MSHP), an ID pharmacy networking group was established to explore ways in which to expand antimicrobial stewardship efforts across Michigan. The first endeavor was to determine a baseline understanding of the wants, needs, and capabilities with regard to the stewardship techniques described above of various health care settings in Michigan in order to better direct resources to improve stewardship efforts throughout the state.
A 30-question, electronic survey (SurveyMonkey) was developed to assess antimicrobial stewardship practices and priorities according to suggested core and supplemental strategies from the IDSA and other published antimicrobial stewardship literature. The survey was reviewed and approved by the Wayne State University Institutional Review Board prior to its use. The survey was e-mailed to a current list of pharmacy directors at 179 acute care hospitals in Michigan in February 2014 utilizing MSHP’s contact records. The survey requested the pharmacy director to disseminate the survey to the person within the institution who would be the most appropriate to comment on or respond to the survey questions. The electronic survey contained logic that streamlined the survey based on previous responses. An original 2-week deadline was established and then subsequently extended by an additional week via a follow-up reminder e-mail. Objectives of the survey were to characterize current antimicrobial stewardship practices in health systems in the State of Michigan, identify the perceived stewardship-related needs of Michigan hospitals, and understand how the ID networking group in collaboration with MSHP could fulfill those needs.
All sites that provided electronic consent to participate in the survey and responded to at least one question were included in the analysis. Responses were summarized using descriptive statistics and analyzed by practice setting, institutional size, and stewardship resources. Particular attention was placed on responses from facilities with fewer than 150 beds, because the workgroup felt that these facilities may lack some of the stewardship-related resources found at larger facilities in the state and may benefit most from the collective efforts of the group. Comparisons between responses from institutions with fewer than 150 beds and those with 150 or more beds were analyzed by chi-square tests where appropriate.
Forty-seven institutions (26%) responded to the survey request. Of these, 45% of respondents were from facilities with fewer than 150 licensed beds and 58% of respondents represented facilities with fewer than 15 intensive care unit beds. Seventy-six percent of respondents were from nonteaching facilities and 57% of respondents reported less than 13 full-time equivalent pharmacist positions at their institution. Pharmacy administrators (ie, directors of pharmacy, clinical coordinators, or other pharmacy managers) comprised 74% of responses.
Eighty-three percent (39/47) of respondents reported that they currently have strategies in place to promote antimicrobial stewardship. The 8 institutions who currently lacked stewardship strategies were fewer than 150 beds in size, and 6 of the 8 (75%) reporting indicated further development of their programs was a future goal. A majority of all responders reported that their stewardship programs were multidisciplinary (63%) and less than 2 years old (66%). Pharmacists (100%), ID-trained physicians (80%), infection prevention and control professionals (73%), and clinical microbiologists (70%) were reported as members of respondents’ antimicrobial stewardship teams. Less than half (45%) of facilities with fewer than 150 beds reported that their antimicrobial stewardship team was multidisciplinary in nature.
A larger number of pharmacists without formal ID-training are involved with antimicrobial stewardship programs than those with formalized training. ID-trained clinical pharmacists were reported as team members in 47% of all respondents and in 27% of facilities with fewer than 150 beds. Pharmacy residents and student pharmacists are used as program extenders in 38% of respondent programs. Pharmacists routinely make recommendations regarding antimicrobial dosing (81%), employ pharmacokinetics/pharmacodynamics principles of drug monitoring (93%), regularly review culture results (74%), ensure intravenous to oral medication antimicrobial conversions (77%), and routinely make recommendations regarding choice (74%) and de-escalation (70%) of antimicrobials (Figure 1). Pharmacists in hospitals with fewer than 150 beds were significantly less likely to make interventions related to de-escalation or discontinuation of antimicrobials (11/21 [52%] vs 19/22 [85%]; P = .02) when compared with facilities with 150 beds or more.
Figure 1.
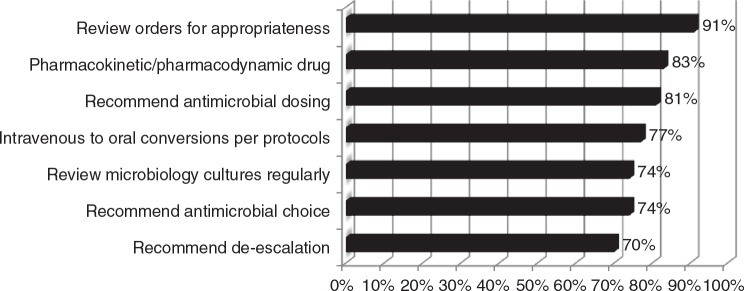
Pharmacist role in antimicrobial prescribing (n = 43).
Improving patient outcomes and decreasing antimicrobial resistance were reported as the most important program goals. Formulary restriction (90%), intravenous to oral conversion (80%), and pharmacist prospective audit and feedback (74%) were the most common strategies utilized. Linezolid and daptomycin (78% and 71%, respectively) were the most commonly reported monitored or restricted antimicrobial agents. Ninety-seven percent of respondents reported the development and availability of institution-specific antibiogram data at their institution at least annually. Continued development of guidelines and protocols to guide antimicrobial use and education programs on antimicrobial stewardship were reported as the most common future initiatives. Guidelines or protocols for the management of patients with pneumonia (community acquired, ventilator, and hospital acquired), perioperative antimicrobials, sepsis, urinary tract infections, and CDI were most commonly available.
Respondents considered a lack of resources, including funding (47%) and resources other than funding (49%), to be barriers to implementing or maintaining the antimicrobial stewardship programs at their institutions. Opposition from physicians (26%), a lack of technology (26%), a lack of expertise needed to implement programs (23%), and lack of hospital administration support (16%) were other reported limitations. Similar barriers were reported when we analyzed the subset of hospitals with fewer than 150 beds.
Standardized order sets (85%), clinical decision support (ie, criteria-based prescribing alerts) through computerized order entry systems (69%), reports generated through the pharmacy order entry system (56%), and educational tools posted on the institution’s intranet sites (44%) were listed as technology resources utilized to support antimicrobial stewardship efforts. Twenty-one percent of respondents reported incorporation of Web-based antimicrobial stewardship software (ie, Theradoc or Sentri7) to support their programs.
In addition to antibiogram review, the impact of antimicrobial stewardship programs are assessed through a number of methods including medication use evaluations to assess compliance with restriction criteria or guideline compliance, antimicrobial utilization comparisons (ie, defined daily doses or days of therapy), readmission rates, patient length of stay, and antimicrobial cost reports.
Interactive education from content experts, shared guidelines/protocol development, and mentoring programs with established practitioners were the most common ways the networking group and MSHP could provide assistance with antimicrobial stewardship initiatives (Figure 2). Novel antimicrobial stewardship practices or practice model discussions, pneumonias, sepsis, perioperative antimicrobial use, and CDI were the most commonly requested services or programming listed as an educational need for review or update.
Figure 2.
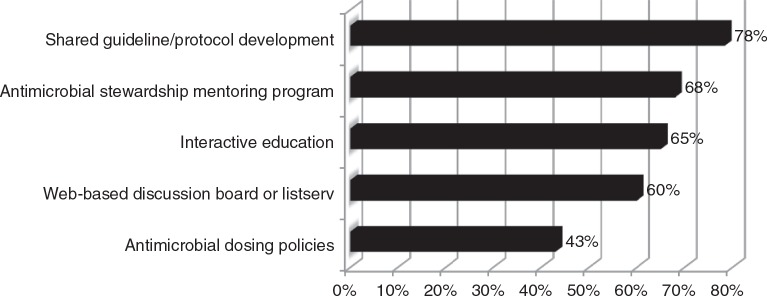
Respondent priorities for state-wide antimicrobial stewardship initiatives (n = 37).
Results of the survey provide a snapshot into the breadth of antimicrobial stewardship practices in Michigan. The large number of facilities reporting the presence of an antimicrobial stewardship program is a positive trend for patients within the state, and Michigan compares favorably when state or national reports are analyzed. Fifty-percent of the 223 general acute care hospitals responding to a survey in California reported the presence of an antimicrobial stewardship program, whereas Pedersen and colleagues in their 2013 American Society of Health-System Pharmacists’ national survey of pharmacy practice in hospital settings reported 63% of hospitals nationwide have an antimicrobial stewardship program.8,9 The prevalence of programs in Michigan is an indicator that the value of antimicrobial stewardship programs is understood by decision makers in Michigan health care facilities responding to the survey. However, this could be impacted by the response rate in the study in comparison to California.
When compared to national results reported by Pedersen and colleagues, antimicrobial stewardship programs in Michigan utilize formulary restriction (90% vs 71%) and clinical decision support systems (69% vs 31%) at higher rates, and a lower percentage evaluate the impact of their stewardship program by following utilization patterns (44% vs 84%) and cost (50% vs 59%) or utilize clinical surveillance software (21% vs 29%).9 This indicates that hospitals in Michigan have successfully implemented many of the recommendations for developing antimicrobial stewardship programs, and further guidance on program evaluation may be beneficial.5
A common theme that emerged from the survey was the need for shared educational opportunities. A future goal of the ID pharmacy networking group is to increase the number of antimicrobial stewardship-related educational opportunities available to pharmacists throughout the state. The plan to accomplish this will include a number of strategies including publication of articles in the MSHP newsletter highlighting individual stewardship programs or unique and novel stewardship practices. Educational programming offered by speakers with stewardship expertise is also planned collaboratively by statewide regional affiliates of MSHP. In order to expand the reach to other health care practitioners, plans are being implemented to reach out to other organizations, including the Michigan Center for Rural Health and the Michigan Department of Community Health, to explore further education and collaboration opportunities.
This survey has highlighted a significant difference in stewardship approaches and resources in small and large hospitals within Michigan. Based on the survey, the ID pharmacy networking group hopes to develop a mentoring program where experts amongst the group can help institutions implement programs where needed and provide education to help existing programs feel more comfortable in making de-escalation and discontinuation recommendations.
Limitations of this study include the potential for nonresponse bias and self-reporting bias. The actual percentage of facilities with antimicrobial stewardship programs and successful implementation of many recommended practices in Michigan may be lower if all facilities responded to the survey query. Engaging practitioners and facilities, particularly in more remote parts of Michigan, remains a goal of the networking group. It is hoped that by targeting efforts locally and distributing educational programming in all areas of the state, antimicrobial stewardship engagement and education can be improved.
Results of the survey help characterize current antimicrobial stewardship practices in Michigan and provide a framework for future efforts to assist clinicians and health systems facilitating antimicrobial stewardship efforts.
Acknowledgments
The authors report no conflicts of interest relevant to this manuscript.
References
- 1.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1–12. [DOI] [PubMed] [Google Scholar]
- 2.Pogue JM, Mann T, Barber KE, Kaye KS. Carbapenem-resistant Acinetobacter baumannii: Epidemiology, surveillance and management. Expert Rev Anti Infect Ther. 2013;11(4):383–393. [DOI] [PubMed] [Google Scholar]
- 3.Boucher HW, Talbot GH, Benjamin DK, Jr, et al. 10 x 20 progress. Development of new drugs active against gram-negative bacilli: An update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(12):1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–455. [DOI] [PubMed] [Google Scholar]
- 5.Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177. [DOI] [PubMed] [Google Scholar]
- 6.McKinney M. Hospitals focus on antibiotic overuse as CMS prepares new mandate. http://www.modernhealthcare.com/article/20141220/MAGAZINE/312209980 Accessed January22, 2015. [PubMed]
- 7.Weston A, Epstein L, Davidson LE, et al. The impact of a Massachusetts state-sponsored educational program on antimicrobial stewardship in acute care hospitals. Infect Control Hosp Epidemiol. 2013;34(4):437–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trivedi KK, Rosenberg J. The state of antimicrobial stewardship programs in California. Infect Control Hosp Epidemiol. 2013;34(4):479–484. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen CA, Schneider PJ, Scheckelhoff DJ. ASHP national survey of pharmacy practice in hospital settings: Prescribing and transcribing – 2013. Am J Health Syst Pharm. 2014;71(11):924–942. [DOI] [PubMed] [Google Scholar]


