Abstract
Background
Aortic valve stenosis is common in the elderly, with a prevalence of nearly 3% in patients aged 75 years or older. Despite the fact that sleep-related breathing disorders (SRBD) are thought to be associated with cardiac disease, little is known about their prevalence in this patient cohort. The purpose of this study was to evaluate the prevalence of SRBD in older patients with aortic valve stenosis admitted for transcatheter aortic valve implantation.
Methods
Forty-eight consecutive patients (mean age 81±6 years; 37.5% male) with symptomatic aortic valve stenosis and considered for transcatheter aortic valve replacement were screened for SRBD. Sleep studies were performed by in-hospital unattended cardiorespiratory polygraphy measuring nasal air flow, chest and abdominal efforts, as well as oxygen saturation and body position. The patients were divided in subgroups dependent on the documented apnea–hypopnea index (AHI; no SRBD was defined as an AHI of <5 events/hour; mild SRBD as AHI 5–15 events/hour, and moderate to severe SRBD as AHI ≥15 events/hour).
Results
Thirty-seven patients (77%) had SRBD defined as an AHI of ≥5 events/hour. Eleven patients had an unremarkable investigation, with AHI <5 events/hour (mean 3.0±1.3 events/hour). Among patients with sleep apnea, 19 patients had mild SRBD, with an AHI of 5–15 events/hour (mean 9.9±3.4 events/hour) and 18 patients had moderate to severe SRBD (mean 26.6±11.3 events/hour). Mainly, obstructive apneas were found. Subgroups were not different regarding EuroSCORE (European System for Cardiac Operative Risk Evaluation) or aortic valve area. Also, no correlations were found between AHI and the additive or logistic EuroSCORE or aortic valve area. Significant correlations were found for AHI and N-terminal of the prohormone brain natriuretic peptide (r=0.53; P=0.003) and for AHI and glomerular filtration rate (r=−0.39; P=0.007).
Conclusion
SRBD is common in elderly patients with symptomatic aortic valve stenosis admitted for transcatheter aortic valve replacement. Interestingly, this finding is not reflected by the currently used risk scores. Further randomized studies are needed to evaluate the clinical significance of concomitant SRBD in the management of severe aortic stenosis.
Keywords: aortic valve stenosis, sleep-related breathing disorders, transcatheter aortic valve replacement
Introduction
Sleep-related breathing disorders (SRBD), mainly obstructive and central sleep apnea, are characterized by repetitive hypopnea/apnea leading to intermittent periods of hypoxia and arousals. Sleep fragmentation causes daytime sleepiness and has been shown to be associated with an increased risk of cognitive impairment in elderly women.1 The prevalence of SRBD increases with age, ranging from approximately 5% to more than 50% in the elderly.2 However, the clinical significance of these findings and their relevance for perioperative or peri-interventional care are still under discussion.3
Aortic valve disease is common in elderly patients, with a prevalence of critical stenosis of nearly 3% in patients aged 75 years or older.4,5 Once symptoms like dyspnea, angina pectoris, or syncope occur, the average survival without valve replacement is 50% at 2 years and 20% at 5 years.6 Management of symptomatic aortic valve stenosis remains primarily surgical, although transcatheter aortic valve replacement (TAVR) is becoming an accepted alternative to surgical aortic valve replacement for patients at high operative risk. Since SRBD are associated with increased operative risk and more postoperative complications,7,8 we examined the prevalence of SRBD in elderly patients admitted for a TAVR procedure, hypothesizing that prevalence of SRBD might be reflected by the use of established risk scores.
Patients and methods
Patients
Forty-eight consecutive patients (mean 81±6 years) with symptomatic aortic valve stenosis who were admitted to the Department for Cardiology, Pneumology and Angiology, Heinrich Heine University Düsseldorf, Germany for TAVR were included in the study. All subjects were screened by clinical history, physical examination, lung function tests, and routine chemical analysis. Perioperative risk was estimated by calculation of the EuroSCORE (European System for Cardiac Operative Risk Evaluation) as well as logistic EuroSCORE.9,10 Pre-interventional studies included right and left heart catheterization and echocardiography for quantification of aortic valve stenosis. These examinations were done as a matter of clinical routine with respect to planned TAVR. This study was undertaken in accordance with the Declaration of Helsinki of 1975 and all subjects were informed about the objectives and methods of the research. The patients gave written informed consent to all of the diagnostic procedures and the use of the data. Ethical approval was not sought for this study as all of the examinations were done as a part of clinical routine.
Determination of severity of aortic valve stenosis
The severity of aortic valve stenosis was estimated by right and left heart catheterization as well as by transthoracic and transesophageal echocardiography. Right heart catheterization was performed for measurement of cardiac output as well as assessment of pulmonary hypertension. Left heart catheterization including coronary angiography was performed for measurement of the peak to peak gradient between the left ventricle and aorta as well as the mean gradient. Aortic valve area was calculated using the Gorlin formula.11 Transthoracic and transesophageal echocardiography was used to quantify aortic valve stenosis by planimetry or by application of the continuity equation.
Screening for SRBD
Sleep studies were performed by in-hospital unattended cardiorespiratory polygraphy (Apnoe-Screen, Viasys, Germany). With the Apnoe-Screen, nasal air flow, chest and abdominal efforts, oxygen saturation, and body position are documented continuously. The automated analysis was reviewed and corrected manually. According to the recommendations of the American Academy of Sleep Medicine, patients with an apnea–hypopnea index (AHI) of 5–14 events/hour were considered to have mild sleep apnea. Sleep apnea was classified as moderate to severe if the AHI was ≥15 events/hour. If thoracic and abdominal inspiration efforts were documented, sleep apnea was considered to be obstructive (OSA), otherwise central (CSA). If both central and obstructive events were observed, OSA or CSA was diagnosed according to the prevalence of either event (>50% of the events).
Statistical analysis
Statistical analyses were performed using PASW software (SPSS Inc, Chicago, IL, USA). The data are presented as the mean ± standard deviation. For comparing differences between the three study groups, analysis of variance was performed. Univariate correlations were calculated using Pearson’s coefficient (r). P-values ≤0.05 were accepted as statistically significant.
Results
Presence of SRBD in patients with severe aortic stenosis
Forty-eight patients with severe aortic stenosis were analyzed (Table 1). The mean age of the study group was 81±6 years; 37.5% of patients were male. Thirty-seven patients (77%) had SRBD, defined as an AHI of ≥5 events/hour. Eleven patients had an unremarkable investigation with AHI of <5 events/hour (mean 3.0±1.3 events/hour). Among patients with SRBD, 19 had mild SRBD with an AHI of 5–15 events/hour (mean 9.9±3.4 events/hour) and 18 had a moderate to severe SRBD with an AHI of ≥15 events/hour (26.6±11.3 events/hour; Table 1). Obstructive apneas were more common (obstructive apnea index 1.8±1.2 versus 8.1±3.8 versus 21.1±13.4 events/hour), while the number of central apneas was surprisingly low (central apnea index 0.7±1.2 versus 0.3±0.9 versus 1.4±2.4 events/hour). There was only one patient (2%) with central sleep apnea. Mean minimum oxygen saturation (89%±2% versus 89%±3% versus 88%±5%; P=0.60) and time below oxygen saturation of 90% (6.8±7.0 versus 30.8±70.5 versus 33.8±28.3 minutes; P=0.37) were not different between the subgroups (Table 1).
Table 1.
Baseline characteristics of the study groups
| No SRBD (AHI ≥5 events/hour) |
Mild SRBD (AHI 5–15 events/hour) |
Moderate to severe SRBD (AHI >15 events/hour) |
P-value | |
|---|---|---|---|---|
| n | 11 | 19 | 18 | |
| Age (years) | 80±5 | 82±4 | 81±8 | 0.51 |
| Body mass index (kg/m2) | 25±3 | 26±6 | 26±5 | 0.85 |
| CAD (%) | 82 | 63 | 79 | |
| Atrial fibrillation (%) | 36 | 39 | 47 | |
| Glomerular filtration rate (mL/min) | 67±28 | 60±20 | 55±19 | 0.34 |
| Systolic blood pressure (mmHg) | 156±20 | 143±28 | 147±21 | 0.38 |
| Diastolic blood pressure (mmHg) | 74±15 | 66±8 | 66±13 | 0.21 |
| Mean arterial pressure (mmHg) | 101±16 | 91±13 | 93±14 | 0.23 |
| NTproBNP (pg/mL) | 986±862 | 2,337±2,758 | 4,270±4,421 | 0.10 |
| FEV1 (L) | 1.77±0.80 | 1.48±0.55 | 1.64±0.55 | 0.46 |
| FEV1 predicted (%) | 79±25 | 75±20 | 81±27 | 0.79 |
| AHI (per hour) | 3±1 | 10±3 | 27±11 | |
| Central apnea index (per hour) | 0.7±1.2 | 0.3±0.9 | 1.4±2.4 | |
| Obstructive apnea index (per hour) | 1.8±1.2 | 8.1±3.8 | 21.1±13.4 | |
| Mean minimal oxygen saturation (%) | 89±2 | 89±3 | 88±5 | 0.60 |
| Time below 90% (minutes) | 6.8±7.0 | 30.8±70.5 | 33.8±28.3 | 0.37 |
Abbreviations: AHI, apnea–hypopnea index; SRBD, sleep-related breathing disorders; CAD, coronary artery disease; NTproBNP, n-terminal of the prohormone brain natriuretic peptide; FEV1, forced expiratory volume after 1 second.
Perioperative risk
Additive (11%±1% versus 11%±3% versus 10%±2%; P=0.62) or logarithmic EuroSCORE (23%±11% versus 27%±18% versus 23%±13%; P=0.65) did not differ significantly between patients without SRBD and those with mild or moderate to severe SRBD (Figure 1).
Figure 1.
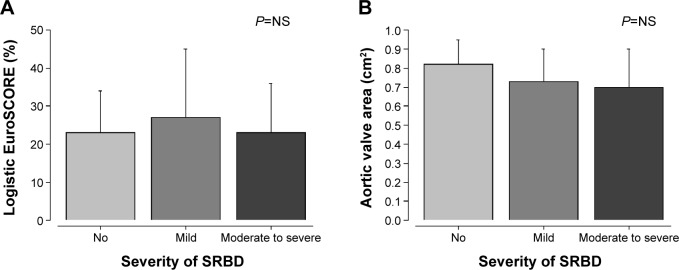
Comparison of logistic EuroSCORE and aortic valve area between study groups.
Notes: (A) The logistic EuroSCORE was comparable between the study groups, which were stratified by the severity of sleep apnea. (B) The aortic valve area was comparable between the study groups. NS = not significant at P>0.05.
Abbreviations: EuroSCORE, European System for Cardiac Operative Risk Evaluation; SRBD, sleep-related breathing disorders.
Hemodynamic measurements
Systolic (156±20 versus 143±28 versus 147±21 mmHg; P=0.38), diastolic (74±15 versus 66±8 versus 66±13 mmHg; P=0.21), or mean arterial pressure (101±16 versus 91±13 versus 93±14 mmHg; P=0.23) were comparable between the three subgroups (Table 1). Also, cardiac function as characterized by ejection fraction (57%±5% versus 51%±8% versus 52%±10%; P=0.22) and cardiac index (5.0±0.9 versus 4.5±1.1 versus 4.5±1.3 L/min*m2; P=0.26) were not different between patients without SRBD and those with mild or moderate to severe SRBD (Table 2).
Table 2.
Preoperative echocardiographic and hemodynamic data
| No SRBD (AHI ≥5 events/hour) |
Mild SRBD (AHI 5–15 events/hour) |
Moderate to severe SRBD (AHI >15 events/hour) |
P-value | |
|---|---|---|---|---|
| Ejection fraction (%) | 57±5 | 51±8 | 52±10 | 0.22 |
| AVA echocardiography (cm2) | 0.82±0.13 | 0.73±0.17 | 0.70±0.20 | 0.34 |
| dPmax (mmHg) | 72±13 | 70±27 | 70±16 | 0.95 |
| dPmean (mmHg) | 39±12 | 41±15 | 40±13 | 0.97 |
| AVA/catheter (cm2) | 0.73±0.23 | 0.64±0.21 | 0.71±0.25 | 0.59 |
| Systolic PA pressure (mmHg) | 35±21 | 51±17 | 50±14 | 0.13 |
| Mean PA pressure (mmHg) | 25±6 | 31±10 | 31±9 | 0.33 |
| PCWP (mmHg) | 17±5 | 21±7 | 21±8 | 0.60 |
| LVEDP (mmHg) | 24±9 | 23±8 | 23±7 | 0.94 |
| Cardiac index (L/min*m2) | 2.87±0.52 | 2.55±0.43 | 2.56±0.63 | 0.26 |
Abbreviations: AHI, apnea–hypopnea index; AVA, aortic valve area; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; LVEDP, left ventricular end diastolic pressure; SRBD, sleep-related breathing disorders; dPmax, maximal systolic pressure gradient between left ventricle and aorta ascendens; dpmean, mean systolic pressure gradient between left ventricle and aorta ascendens.
Severity of aortic valve stenosis
The aortic valve area determined by echocardiography (0.8±0.1 versus 0.7±0.2 versus 0.7±0.2 cm2; P=0.34) and by cardiac catheterization (0.7±0.2 versus 0.6±0.2 versus 0.7±0.3 cm2; P=0.59) were not significantly different between the subgroups (Table 2).
Determinants of apnea–hypopnea index
Correlations between AHI and laboratory, echocardiographic, and invasive measurements were calculated to estimate determinants of AHI (Table 3). Surprisingly, AHI did not show a significant correlation with body mass index. No correlations were found between AHI and blood pressure, ejection fraction, or cardiac index. Further, no correlation was found between AHI and aortic valve area. AHI did not show significant correlations with the additive or logistic EuroSCORE. Significant correlations were found for AHI and N-terminal of the prohormone brain natriuretic peptide (NTproBNP; r=0.53; P=0.003; Figure 2A) and for AHI and glomerular filtration rate (r=−0.39; P=0.007; Figure 2B).
Table 3.
Univariate correlations between the apnea-hypopnea index, the mean minimal oxygen saturation and clinical characteristics or measurements
| AHI
|
Mean minimal SO2
|
|||
|---|---|---|---|---|
| r | P-value | r | P-value | |
| Age | −0.07 | 0.66 | −0.1 | 0.56 |
| Body mass index | −0.03 | 0.82 | 0.18 | 0.29 |
| Glomerular filtration rate | −0.39 | 0.007 | −0.22 | 0.31 |
| Mean arterial pressure | −0.2 | 0.2 | 0.26 | 0.14 |
| ES log | −0.09 | 0.53 | −0.08 | 0.63 |
| NTproBNP | 0.53 | 0.003 | −0.22 | 0.31 |
| Ejection fraction | −0.09 | 0.55 | 0.06 | 0.74 |
| AVA/echocardiography | −0.15 | 0.31 | 0.04 | 0.82 |
| dPmax | −0.19 | 0.22 | 0.05 | 0.82 |
| dPmean | −0.16 | 0.28 | −0.01 | 0.1 |
| Systolic PA pressure | 0.13 | 0.45 | 0.17 | 0.38 |
| Cardiac index | 0.05 | 0.75 | 0.18 | 0.3 |
Notes: Data in bold indicates statistical significance.
Abbreviations: AHI, apnea–hypopnea index; AVA, aortic valve area; ES log, logarithmic EuroSCORE; PA, pulmonary artery; EuroSCORE, European System for Cardiac Operative Risk Evaluation; NTproBNP, N-terminal of the prohormone brain natriuretic peptide; SO2, arterial oxygen saturation; dPmax, maximal systolic pressure gradient between left ventricle and aorta ascendens; dpmean, mean systolic pressure gradient between left ventricle and aorta ascendens.
Figure 2.
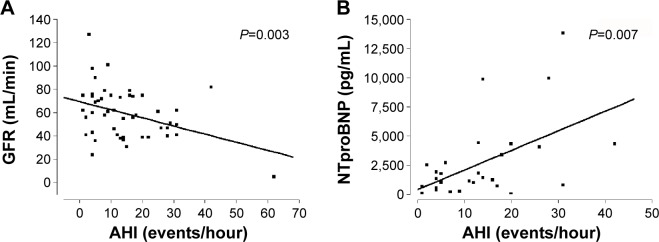
Determinants of the AHI.
Notes: (A) The AHI showed a significant inverse correlation with GFR. (B) A correlation was found for AHI and NTproBNP.
Abbreviations: NTproBNP, N-terminal of the prohormone brain natriuretic peptide; AHI, apnea–hypopnea index; GFR, glomerular filtration rate.
Discussion
In this cohort study, we found a high prevalence of SRBD, predominantly obstructive apneas, in elderly patients with symptomatic severe aortic stenosis admitted for a TAVR procedure. Further, SRBD were not reflected by score-calculated perioperative risk (EuroSCORE, logistic EuroSCORE). However, since SRBD might contribute to perioperative risk and influence perioperative management in these patients, it seems reasonable to screen elderly patients with aortic stenosis.
SRBD are known to be associated with heart disease, eg, coronary artery disease, heart failure, and atrial fibrillation,12–14 but less is known about their prevalence in valve disease. Similar to our findings, Prinz et al reported a prevalence of 71% of SRBD with a median AHI of 23 events/hour in severe aortic valve stenosis.15 In contrast with their study, in which OSA and CSA were distributed nearly half and half, we found a much higher prevalence of OSA, and CSA in only one patient (2%). Dimitriadis et al found SRBD in nearly all patients.16 Nevertheless, it should be kept in mind that all studies dealing with SRBD in aortic valve disease use screening devices to detect SRBD, not polysomnography, which is still the gold standard in diagnosis of SRBD. Therefore, there might be bias and misclassification of respiratory events.
It is still unclear if there is a causal relationship between SRBD and aortic valve stenosis or if it is just an epiphenomenon. In this regard, two studies report that there is a significant impact of TAVR on severity of CSA but not of OSA.16,17 This implicates a causal role of aortic valve stenosis in the pathogenesis of SRBD, at least for central forms. There are several mechanisms that might explain the pathophysiologic links between aortic valve stenosis and SRBD, ie, respiratory control instability (circulatory delay causing periodic drive to breath) due to diastolic and/or diastolic myocardial dysfunction; intrathoracic pressure swings that lead to increased left ventricular afterload (this will be detrimental in aortic valve stenosis); and upper airway instability.18
The relevance of hemodynamic determinants remains unresolved. We and Prinz et al did not find correlations with hemodynamic determinants such as aortic valve area, cardiac index, or ejection fraction, which might be explained by the size of the cohort.15 However, we found a significant correlation between the AHI and the NTproBNP concentration, which points toward an impact of the severity of heart failure for SRBD. Concerning heart failure, the presence of diastolic dysfunction related to severe aortic stenosis may be considered as a potentially relevant characteristic. With regard to the study reported by Linhart et al17 it may be that aortic valve replacement leads to reduction of left ventricular afterload, which might have positive effects on diastolic and systolic heart failure and therefore results in fewer respiratory events.
There is growing evidence that sleep apnea contributes to perioperative risk.19 Patients with SRBD undergoing surgery have adverse outcomes due to higher reintubation rates, hypercapnia, myocardial injury, arrhythmia, unplanned transfer to intensive care and longer hospital stays.19,20 Several mechanisms are likely:21 administration of anesthetics might induce pharyngeal collapse and abolish arousal from sleep, depression of ventilatory response to carbon dioxide, and alteration of sleep architecture and sleep deprivation in the postoperative period. These sleep disturbances seem to be related to the invasiveness of the surgical protocol.22
To our knowledge, this is the first study focusing on scoring systems in patients with SRBD and aortic valve disease. These scores are calculated on the basis of risk factors contributing to cardiac surgical mortality, eg, age, body mass index, hypertension, diabetes mellitus, and chronic renal failure.9,10 Since some of these factors are known to be strongly associated with SRBD, we hypothesized that the more severe the sleep apnea, the higher the Euro-SCORE would be. Surprisingly, there was no difference with respect to EuroSCORE between patients without and with mild or moderate to severe sleep apnea. However, it should be considered that the EuroSCORE and logistic EuroSCORE were established to determine perioperative risk more than 15 years ago, and in the meantime there have been continuous developments in operative and interventional techniques. Therefore, it is under discussion whether the EuroSCORE is still the gold standard.23 Nevertheless, the EuroSCORE is still used in clinical practice although the comorbidities of the individual patient should be considered in order to calculate operative risk.23 Because of the high prevalence of severe aortic valve stenosis in elderly patients, recognition of SRBD is meaningful.
Limitations of the study
Given that this study did not include a control group without aortic valve stenosis, the impact of aortic valve stenosis on SRBD is speculative. Nevertheless, SRBD had a very high prevalence and might be of clinical importance with regard to peri-interventional complications. Further on, detection of SRBD using screening methods might be associated with bias in calculation of the severity of SRBD and differentiation between central and obstructive forms. Still, this is common practice, and these methods are often used in clinical research projects for practical reasons. It should be emphasized that valid classification of respiratory events (OSA, CSA, Cheyne–Stokes breathing) as well as initiation of therapy with positive airway pressure is based on the results of polysomnography.
Conclusion
SRBD are very common in elderly patients with symptomatic aortic valve stenosis admitted for TAVR. This finding, which is thought to be associated with higher perioperative risk, is not reflected by the currently used risk scores. Further randomized studies are needed to evaluate outcomes in elderly patients with severe aortic stenosis and concomitant SRBD after TAVR. Also, it would be of interest to investigate whether pre-interventional treatment of sleep apnea has any impact on peri-interventional morbidity or mortality.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooke J, Ancoli-Israel S. Sleep-related breathing disorders in the elderly. Prog Respir Res. 2006;35:215–223. [Google Scholar]
- 3.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 4.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 5.Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–1225. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 6.Ross J, Braunwald E. Aortic stenosis. Circulation. 1968;38(Suppl 1):61–67. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- 7.Liao P, Yegneswaran B, Vairavanathan S, Zilberman P, Chung F. Postoperative complications in patients with obstructive sleep apnea: a retrospective matched cohort study. Can J Anaesth. 2009;56:819–828. doi: 10.1007/s12630-009-9190-y. [DOI] [PubMed] [Google Scholar]
- 8.Adesanya AO, Lee W, Greilich NB, Joshi GP. Perioperative management of obstructive sleep apnea. Chest. 2010;138:1489–1498. doi: 10.1378/chest.10-1108. [DOI] [PubMed] [Google Scholar]
- 9.Roques F, Michel N, de Vincentiis G, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19,030 patients. Eur J Cardiothorac Surg. 1999;15:816–823. doi: 10.1016/s1010-7940(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 10.Roques F, Michel P, Goldstone A, Nashef S. The logistic EuroSCORE. Eur Heart J. 2002;24:1–2. doi: 10.1016/s0195-668x(02)00799-6. [DOI] [PubMed] [Google Scholar]
- 11.Gorlin R, Gorlin S. Hydraulic formula for calculations of the area of stenotic mitral valve, other cardiac valve and central circulatory shunt. Am Heart J. 1951;41:291. doi: 10.1016/0002-8703(51)90002-6. [DOI] [PubMed] [Google Scholar]
- 12.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 13.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 14.Arzt M, Hetzenecker A, Steiner S, Buchner S. Sleep disordered breathing and coronary artery disease. Can J Cardiol. 2015;31:909–917. doi: 10.1016/j.cjca.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 15.Prinz C, Bitter T, Oldenburg O, Faber L, Horstkotte D, Piper C. Sleep apnoea in severe aortic stenosis. Postgrad Med J. 2011;87:458–462. doi: 10.1136/pgmj.2010.112052. [DOI] [PubMed] [Google Scholar]
- 16.Dimitriadis Z, Wiemer M, Scholtz W, et al. Sleep-disordered breathing in patients undergoing transfemoral aortic valve implantation for severe aortic stenosis. Clin Res Cardiol. 2013;102:895–903. doi: 10.1007/s00392-013-0603-0. [DOI] [PubMed] [Google Scholar]
- 17.Linhart M, Pabst S, Fustera R, et al. Transcatheter valve implantation improves central sleep apnea in severe aortic stenosis. Eurointervention. 2013;9:923–928. doi: 10.4244/EIJV9I8A155. [DOI] [PubMed] [Google Scholar]
- 18.Naughton MT, Sanner BM. Cardiovascular complication of sleep related breathing disorders. In: Randerath AJ, Sanner BH, Somers VK, editors. Sleep Apnea. Progress in Respiratory Research. Basel: Karger; 2006. [Google Scholar]
- 19.Vasu T, Grewal R, Doghramji K. Obstructive sleep apnea syndrome and perioperative complications. a systematic review of the literature. J Clin Sleep Med. 2012;8:199–207. doi: 10.5664/jcsm.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaw R, Chung F, Pasupuleti V, Mehta J, Gay PC, Hernandez AV. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth. 2012;109:897–906. doi: 10.1093/bja/aes308. [DOI] [PubMed] [Google Scholar]
- 21.Den Herder C, Schmeck J, Appelboom DJ, de Vries N. Risks of general anaesthesia in people with obstructive sleep apnoea. BMJ. 2004;329:955–959. doi: 10.1136/bmj.329.7472.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franklin KA, Anttila H, Axelsson S, et al. Effects and side-effects of surgery for snoring and obstructive sleep apnea: a systematic review. Sleep. 2009;32:27. [PMC free article] [PubMed] [Google Scholar]
- 23.Bode C, Kelm M. EuroSCORE: still gold standard or less? Clin Res Cardiol. 2009;98:353–354. doi: 10.1007/s00392-009-0022-4. [DOI] [PubMed] [Google Scholar]


