Abstract
Stair-climbing while doing a concurrent task like talking or holding an object is a common activity of daily living which poses high risk for falls. While biomechanical analyses of overground walking during dual-tasking have been studied extensively, little is known on the biomechanics of stair-climbing while dual-tasking. We sought to determine the impact of performing a concurrent cognitive or motor task during stair-climbing. We hypothesized that a concurrent cognitive task will have a greater impact on stair climbing performance compared to a concurrent motor task and that this impact will be greater on a higher-level step. Ten healthy young adults performed 10 trials of stair-climbing each under four conditions: stair ascending only, stair ascending and performing subtraction of serial sevens from a three-digit number, stair ascending and carrying an empty opaque box and stair ascending, performing subtraction of serial sevens from a random three-digit number and carrying an empty opaque box. Kinematics (lower extremity joint angles and minimum toe clearance) and kinetics (ground reaction forces and joint moments and powers) data were collected. We found that a concurrent cognitive task impacted kinetics but not kinematics of stair-climbing. The effect of dual-tasking during stair ascent also seemed to vary based on the different phases of stair ascent stance and seem to have greater impact as one climbs higher. Overall, the results of the current study suggest that the association between the executive functioning and motor task (like gait) becomes stronger as the level of complexity of the motor task increases.
Keywords: working memory, counting backwards, stair negotiation, stair ascension, multitasking, stepping
1. Introduction
Stair ascent is a common functional and challenging task for several populations. For instance, older adults are at a greater risk for a stairs-related fall due to biomechanical, perception-action or environmental constraints (Startzell et al., 2000) and exhibit altered stair-gait characteristics (Novak and Brouwer, 2011; Reeves et al., 2009; Lee and Chou, 2007; Stacoff et al., 2005; Larsen et al., 2009). Previous researchers have also documented stairs-related difficulties for patients with stroke (Novak and Brouwer, 2012), total hip arthoplasty (Lamontagne et al., 2011), and knee osteoarthritis (Asay et al., 2009). The risk of falls during stair-climbing further increases while performing concurrent tasks like talking and/or carrying an object (Startzell et al., 2000; Ojha et al., 2009; Mudaihat et al., 2010).
There is significant work examining the gait during overground walking using dual-tasking paradigms (Abernethy, 1988; Ebersbach et al., 1995; Sparrow et al., 2002; Beauchet et al., 2005a, b; Siu et al., 2008). During overground walking, an additional cognitive task caused reduction in speed, cadence, and stride length while stride and double support time increased (Al-Yahya et al., 2011; Nadkarni et al., 2010). Cognitive tasks interfering internally (such as counting backwards) have a greater impact on changes in gait than those interfering externally such as reaction time tasks (Al-Yahya et al., 2011). This might be because internal interfering tasks require the use of working memory, which places an additional load on an individual while walking. ‘Working memory’ denotes a system used for storing and manipulating information related to complex cognitive tasks on a temporary basis (Baddeley, 1992). Researchers have also implicated that supraspinal control of gait is associated with decline in working memory in persons with balance impairments (Siu et al., 2008), mild cognitive impairment (Montero-Odasso et al., 2009), multiple sclerosis (Hamilton et al., 2009), in community-dwelling older females (Priest et al., 2008). A majority of these experimental paradigms had participants performing level walking or obstacle clearance but stair-climbing has received scant attention. This is surprising given that stair-climbing is a common activity of daily living.
Biomechanics of stair-climbing (under single-task condition) is quite pervasively studied in healthy young adults. Commonly used parameters include lower-extremity joint angles (McFadyen et al., 1988; Protopapadaki et al., 2007), moments and powers (McFadyen et al., 1988; Costigan et al., 2002; Spanjaard et al., 2008; Vallabhajosula et al., 2012a, b), ground reaction forces (Stacoff et al., 2005), and foot clearance (Hamel et al., 2005). During the stance phase of stair ascent, the hip and the knee joints undergo extension while the ankle joint undergoes plantar flexion (McFadyen et al., 1988). Also, the vertical ground reaction force profile is characterized by two peaks with the second peak greater than the first (McFadyen et al., 1988). Joint moments and powers differ between lower-level and higher-level steps in both sagittal and frontal planes (Vallabhajosula et al., 2012a, b). Also, Ojha et al., (2009) highlighted that older adults required more attentional resources compared to younger adults during stair-climbing while dual-tasking. However, there is lack of research on thorough biomechanical analyses of stair-climbing among healthy young adults under dual-tasking conditions and is warranted given how frequently one climbs stairs performing a concurrent task. Such information has ecological validity as it allows us to understand the factors that could increase the risk of falls during stair-climbing while performing concurrent tasks.
It is speculated that gait is primarily regulated using the cortical inputs to the brain-stem, spinal and cerebellar regions. An additional cognitive task that involves working memory used by prefrontal cortex regions might have different implications than adding an additional motor task that might involve the motor cortex regions. Furthermore, both the concurrent cognitive and motor tasks present different environmental challenges to the person as the secondary motor task like carrying an object might involve reduced/altered visual input and increased use of peripheral resources. Paul et al. (2009) showed that persons with diabetes mellitus walked with a smaller step length and greater double-support time while performing a concurrent motor task compared to performing a concurrent cognitive task, and there was no effect on walking speed, cadence or step time. Using different tasks, O'Shea et al. (2002) showed that there was no difference between the effects of secondary cognitive or motor tasks during gait among persons with Parkinson's disease. While this is useful, stair-climbing is considered a more strenuous motor task compared to gait and the effects of concurrent cognitive and motor tasks during stair-climbing has the potential to highlight the role of supraspinal control of locomotion.
The purpose of the current study was to determine the impact of performing a concurrent cognitive or motor task while stair-climbing. We hypothesized that a concurrent cognitive task will have a greater impact on stair-climbing performance compared to a concurrent motor task. We also hypothesized that this impact will be greater at the higher-level step compared to the lower-level step.
2. Methods
Ten participants (four females, age: 23.9±2.8 years, height: 1.76±0.06 m, mass: 71.3±8.61 kg), signed an informed consent form approved by the local institutional review board. Inclusion criteria: age between 19-35 years, and no history of injuries that could impair gait. Exclusion criteria: presence of any known disorders that may affect gait pattern or ability to ambulate stairs without using handrails.
Kinematic data were collected at 60Hz using eight cameras (Motion Analysis System, Santa Rosa, CA). Kinetic data were collected at 600Hz using two force platforms (AMTI, Watertown, MA) embedded in the lower-level and the higher-level steps, of a four-step instrumented staircase (Fig. 1; see Vallabhajosula et al., 2012a, b for dimensions).
Fig. 1.
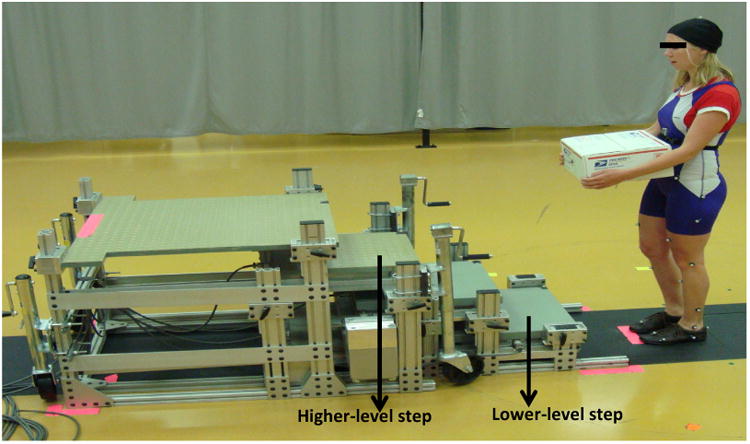
Picture of experimental set-up. Participants started each trial in all the four experimental conditions: ascending the stairs without counting and carrying a box (C1), ascending the stairs while counting backwards and not carrying a box (C2), ascending the stairs while carrying a box and not counting (C3), ascending the stairs while counting backwards and carrying a box (C4); The participants always started from a standing position in front of the staircase; Participants took first step with their dominant leg; Dependent variables were collected for both the lower-level and higher-level steps.
Retro-reflective markers based on the modified Helen Hayes marker set (Davis et al., 1991) were placed on participants wearing tight-fitting suits. Additionally, one marker was placed on the edge of each step. Ten trials were collected in each of the four conditions: (C1) stair ascending only - control, (C2) stair ascending and performing subtraction of serial sevens from a three-digit number - cognitive, (C3) stair ascending and carrying an empty opaque box (12.25″×12.25″×6″; 0.28kg)- motor and (C4) stair ascending, performing subtraction of serial sevens from a random three-digit number and carrying an empty opaque box (12.25″×12.25″×6″; 0.28kg) - combined. Counting backwards by sevens is a commonly used working memory task of sufficient difficulty. The order of the conditions was randomized.
Before data collection began, all participants were tested to be right leg dominant. Dominance was determined by noting which leg the participant preferred to kick a soccer ball. During all the conditions, participants stood with their toes aligned 15cm in front of the lower-level step and looking straight ahead. Upon receiving a visual cue from the experimenter, participants began stair ascent starting with their right leg (onto the lower-level step). For C2 and C4, participants began counting prior to receiving the visual cue. For C3 and C4, participants held the box to their chest. No further instructions were provided. The participants ascended the stairs in a step-over-step manner. They stopped walking and counting once they reached the end of the stairs. The participants practiced till they were confident of performing the task under each condition.
Using a custom-made Matlab (Mathworks Inc., Natick, MA) script, the following dependent variables were calculated for the stance phase of the lower-level and higher-level steps (two consecutive ipsilateral steps; Fig. 1): average speed, two peak vertical forces, loading rate (of the first peak vertical force), minimum force during mid-stance, peak braking and propulsion forces, peak joint angles, range of motion, peak joint moments and powers. The stance phase was defined as the time period between right foot heel-strike to right foot toe-off. Further, minimum toe clearance (MTC) in the anterior and vertical directions was calculated for right foot at both the steps. MTC was defined as the shortest distance between the toe and the edge of the step before the toe crossed the vertical plane of the step. For each condition, an average of 10 trials was used for data analysis.
A 2 (Steps) × 4 (Conditions) repeated measures ANOVA was performed for all the variables using the SPSS software (IBM, Armonk, NY). When significant main effects were found, Bonferroni pairwise comparisons were used to determine the significant differences among the conditions. An α- value of 0.05 was used.
3. Results
Joint angles and ground reaction forces based dependent measures are presented in Table 1. Joint moments, powers, MTC and speed based dependent measures are presented in Table 2. Variables that showed significant step main effects are presented in Table 3. Variables that showed significant condition main effects are presented in Fig. 2. Variables that showed significant interaction are presented in Fig. 3. Outcome measures were normalized by subjects' mass. Figures 4 to 7 show the profiles of the joint angles, ground reaction forces, joint moments and joint powers respectively across all conditions and steps.
Table 1. Mean (SE) of Joint angles and Ground reaction forces-related dependent measures.
| Domain | Dependent measure | Lower-level step | Higher-level step | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C1 | C2 | C3 | C4 | ||
| Joint Angles | Peak ankle plantar flexion (°) | 13.70 (1.55) | 15.86 (1.25) | 14.26 (1.45) | 15.60 (1.32) | 16.10 (1.44) | 16.83(1.37) | 16.59 (1.42) | 16.26 (1.56) |
| Peak ankle dorsiflexion(°) | 11.52 (1.34) | 10.92 (1.21) | 12.37 (1.29) | 11.47 (1.23) | 14.71 (1.04) | 13.51 (1.28) | 14.53 (1.08) | 13.43 (1.11) | |
| Ankle range of motion (°) | 25.22 (1.65) | 26.78 (1.55) | 26.63(1.37) | 27.06(1.77) | 30.81 (1.52) | 30.34 (1.55) | 31.12 (1.46) | 29.69 (1.63) | |
| Peak knee flexion (°) | 53.17 (1.82) | 52.57 (1.63) | 53.33 (1.87) | 53.14 (1.59) | 57.33 (1.40). | 56.47 (1.20) | 57.05 (1.47) | 56.50 (1.19) | |
| Peak knee extension (°) | 8.87 (2.00) | 6.33 (1.55) | 8.95 (1.80) | 6.62 (1.19) | 8.32 (1.44) | 6.59 (1.16) | 8.95 (1.29) | 7.30 (0.98) | |
| Peak knee range of motion | 44.30 (1.25) | 46.24 (1.30) | 44.37 (1.22) | 46.52 (1.48) | 49.01 (1.44) | 49.89 (1.24) | 48.10 (1.29) | 49.20 (1.32) | |
| Peak hip flexion (°) | 54.04 (1.02) | 54.11 (1.09) | 54.35 (0.95 | 52.90.(1.16) | 49.13 (1.27) | 49.78(1.24) | 49.21 (1.13) | 49.63 (1.43) | |
| Peak hip extension (°) | 0.44 (1.32) | 0.43 (1.60) | 0.55 (1.21) | 1.07 (1.54) | 4.60 (1.27) | 4.09 (1.44) | 5.11 (1.11) | 4.26 (1.42) | |
| Hip range of motion (°) | 53.60 (0.92) | 54.55 (1.16) | 54.90 (0.88) | 53.97 (1.19) | 53.73 (1.26) | 53.87 (1.13) | 54.32 (1.08) | 53.88 (1.09) | |
| Ground Reaction Force | First peak vertical force (N/kg) | 1.08 (0.03) | 1.04 (0.01) | 1.09 (0.02) | 1.05 (0.01) | 1.09 (0.02) | 1.04 (0.01) | 1.09 (0.03) | 1.04 (0.01) |
| Second peak vertical force | 1.23 (0.04) | 1.13 (0.04) | 1.23 (0.04) | 1.12 (0.03) | 1.23 (0.04) | 1.15 (0.04) | 1.21 (0.03) | 1.13 (0.03) | |
| Peak vertical force Mid-stance (N/kg) | 0.71 (0.06) | 0.78 (0.04) | 0.72 (0.06) | 0.75 (0.02) | 0.70 (0.02) | 0.75 (0.02) | 0.67 (0.02) | 0.75 (0.02) | |
| Loading rate (N/kg.s) | 5.13 (0.24) | 3.44 (0.28) | 4.88 (0.32) | 3.54 (0.26) | 6.41 (0.22) | 4.81 (0.48) | 6.30 (0.37) | 4.73 (0.48) | |
| Peak propulsion force (N/kg) | 0.06 (0.01) | 0.05 (0.00) | 0.06 (0.01) | 0.05 (0.00) | 0.11 (0.01) | 0.10 (0.01) | 0.10 (0.01) | 0.10 (0.01) | |
| Peak braking force (N/kg) | 0.13 (0.00) | 0.12 (0.01) | 0.13 (0.01) | 0.13 (0.01) | 0.10 (0.01) | 0.10 (0.01) | 0.10 (0.01) | 0.09 (0.01) | |
C1 - ascending the stairs without counting and carrying a box, C2 - ascending the stairs while counting backwards and not carrying a box, C3 - ascending the stairs while carrying a box and not counting, C4 -ascending the stairs while counting backwards and carrying a box
Table 2. Mean (SE) of Joint moments, powers and Minimum toe clearance-related dependent measures.
| Domain | Dependent measure | Lower-level step | Higher-level step | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C1 | C2 | C3 | C4 | ||
| Joint Moments and Powers | Peak ankle dorsiflexion moment (Nm/kg) | 0.04 (0.01) | 0.04 (0.01) | 0.04 (0.01) | 0.04 (0.01) | 0.07 (0.02) | 0.06 (0.01) | 0.06 (0.02) | 0.06 (0.02) |
| Peak ankle plantar flexion moment (Nm/kg) | 1.65 (0.07) | 1.52 (0.07) | 1.64 (0.06) | 1.52 (0.06) | 1.85 (0.08) | 1.71 (0.09) | 1.83 (0.08) | 1.70 (0.07) | |
| Peak knee extensor moment (Nm/kg) | 0.41 (0.04) | 0.46 (0.03) | 0.43 (0.04) | 0.46 (0.03) | 0.50 (0.05) | 0.50 (0.04) | 0.50 (0.05) | 0.50 (0.04) | |
| Peak knee flexor moment (Nm/kg) | 1.07 (0.08) | 1.02 (0.07) | 1.07 (0.09) | 0.99 (0.06) | 0.92 (0.08) | 0.91 (0.06) | 0.93 (0.07) | 0.88 (0.06) | |
| Peak hip extensor moment (Nm/kg) | 0.36 (0 06) | 0.33 (004) | 0.38 (0.05) | 0.35 (0.04) | 0.27 (0.05) | 0.25 (0.05) | 0.27 (0.05) | 0.23 (0.05) | |
| Peak hip flexor moment (Nm/kg) | 0.72 (0.04) | 0.65 (0.04) | 0.71 (0.04) | 0.66 (0.05) | 0.72 (0.05) | 0.66 (0.07) | 0.71 (0.07) | 0.67 (0.07) | |
| Peak ankle power absorbed (W/kg) | 0.37 (0.06) | 0.28 (0.02) | 0.36 (0.03) | 0.31 (0.03) | 0.59 (0.11) | 0.34 (0.06) | 0.54 (0.09) | 0.37 (0.08) | |
| Peak ankle power produced (W/kg) | 3.78 (0.46) | 3.43 (0.38) | 3.83 (0.40) | 3.23 (0.31) | 3.84 (0.46) | 3.62 (0.42) | 3.77 (0.44) | 3.38 (0.39) | |
| Peak knee power produced (W/kg) | 1.70 (0.20) | 1.45 (0.11) | 1.75 (0.16) | 1.52 (0.14) | 1.71 (0.16) | 1.57 (0.13) | 1.73 (0.13) | 1.52 (0.13) | |
| Peak hip power produced (W/kg) | 1.10 (0.07) | 0.88 (0.07) | 1.13 (0.09) | 0.90 (0.09) | 1.20 (0.10) | 0.97 (0.11) | 1.19 (0.10) | 0.95 (0.09) | |
| Peak hip power absorbed (W/kg) | 0.32 (0.12) | 0.26 (0.13) | 0.32 (0.11) | 0.22 (0.09) | 0.26 (0.08) | 0.20 (0.06) | 0.25 (0.07) | 0.22 (0.06) | |
| Minimum Toe Clearance | Anterior (mm) | 22.32 (0.89) | 21.38 (0.90) | 23.98 (1.15) | 21.68 (1.01) | 25.19 (0.96) | 25.36 (1.14) | 24.87 (0.82) | 25.69 (1.10) |
| Vertical (mm) | 10.61 (0.52) | 10.30 (0.43) | 11.00 (0.50) | 9.96 (0.45) | 8.70 (0.25) | 8.30 (0.32) | 8.45 (0.39) | 8.51 (0.32) | |
| Speed | Average speed (mm/s) | 656.91 (33.87) | 523.74 (43.13) | 646.53 (33.47) | 505.22 (35.45) | 617.74 (32.71) | 505.47 (42.47) | 609.33 (26.83) | 483.83 (36.20) |
C1 - ascending the stairs without counting and carrying a box, C2 - ascending the stairs while counting backwards and not carrying a box, C3 - ascending the stairs while carrying a box and not counting, C4 -ascending the stairs while counting backwards and carrying a box
Table 3. Mean (SE) of dependent measures that showed significant step main effect (P < 0.05).
| Domain | Dependent measure | Lower-level step | Higher-level step | P-value |
|---|---|---|---|---|
| Joint Angles | Peak ankle plantar flexion (°) | 14.85 (1.09) | 16.45 (1.21) | 0.016 |
| Peak ankle dorsiflexion (°) | 11.57 (1.05) | 14.05 (1.09) | 0.003 | |
| Ankle range of motion (°) | 26.42 (1.33) | 30.49 (1.23) | < 0.001 | |
| Knee flexion (°) | 53.05 (1.71) | 56.84 (1.29) | < 0.001 | |
| Knee range of motion (°) | 45.36 (1.14) | 49.05 (1.23) | < 0.001 | |
| Hip flexion (°) | 53.85 (1.00) | 49.44 (1.18) | < 0.001 | |
| Hip extension (°) | 0.40 (1.29) | 4.52 (1.25) | 0.001 | |
| Ground Reaction Force | Loading rate (N/kg.s) | 4.25 (0.19) | 5.56 (0.28) | < 0.001 |
| Peak propulsion force (N/kg) | 0.05 (0.00) | 0.10 (0.01) | < 0.001 | |
| Peak braking force (N/kg) | 0.13 (0.01) | 0.09 (0.01) | < 0.001 | |
| Joint Moments and Powers | Peak ankle dorsiflexion moment (Nm/kg) | 0.04 (0.01) | 0.06 (0.01) | 0.030 |
| Peak ankle plantar flexor moment (Nm/kg) | 1.58 (0.06) | 1.77 (0.08) | 0.001 | |
| Peak knee flexor moment (Nm/kg) | 1.03 (0.07) | 0.91 (0.07) | 0.003 | |
| Peak hip extensor moment (Nm/kg) | 0.35 (0.04) | 0.26 (0.05) | 0.010 | |
| Minimum Toe Clearance | Anterior (mm) | 22.34 (0.70) | 25.28 (0.95) | 0.009 |
| Vertical (mm) | 10.47 (0.37) | 8.49 (0.30) | < 0.001 |
Fig. 2.
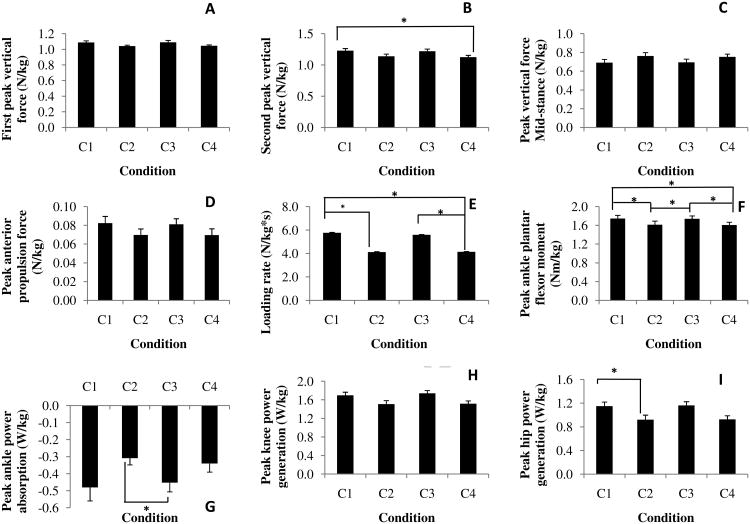
(A-I) Mean (SE) of dependent measures that showed significant condition main effect (P < 0.05); C1 - ascending the stairs without counting and carrying a box, C2 - ascending the stairs while counting backwards and not carrying a box, C3 - ascending the stairs while carrying a box and not counting, C4 - ascending the stairs while counting backwards and carrying a box
Fig. 3.
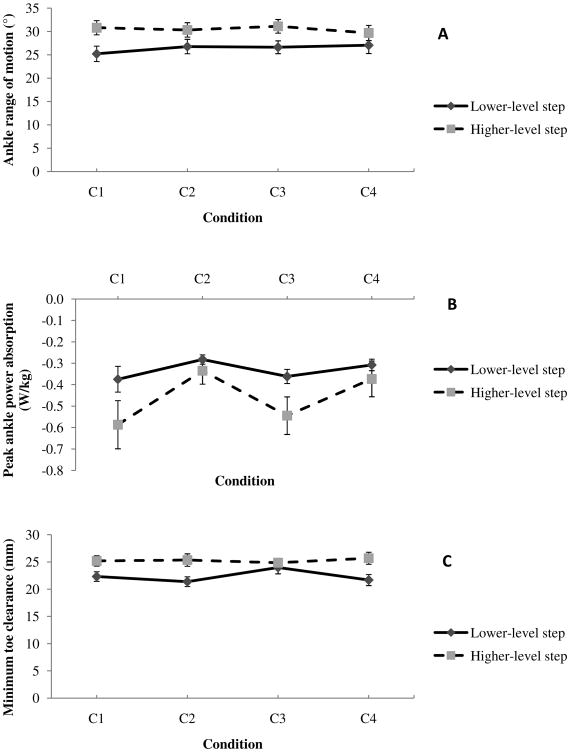
(A-C) Mean (SE) of dependent measures that showed significant interaction (P < 0.05); C1 -ascending the stairs without counting and carrying a box, C2 - ascending the stairs while counting backwards and not carrying a box, C3 - ascending the stairs while carrying a box and not counting, C4 - ascending the stairs while counting backwards and carrying a box
Fig. 4.
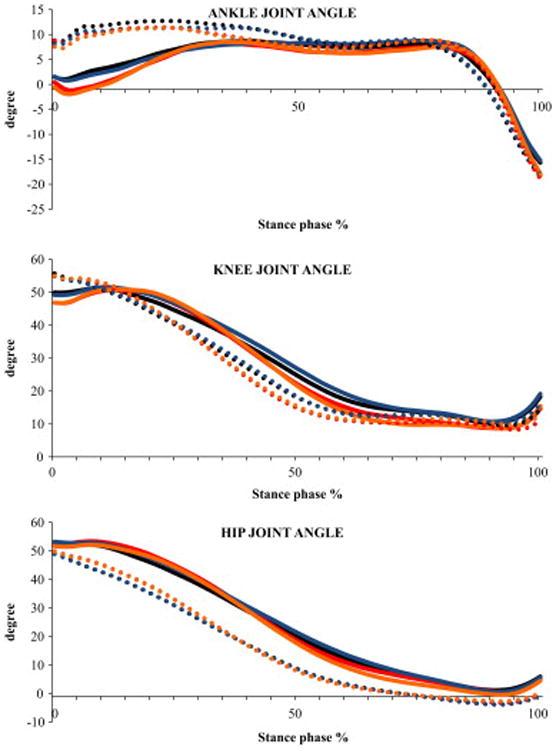
Ensemble averaged profiles of sagittal plane angles of lower-extremity joints during stair ascent for the four conditions (C1 - ascending the stairs without counting and carrying a box, C2 -ascending the stairs while counting backwards and not carrying a box, C3 - ascending the stairs while carrying a box and not counting, C4 - ascending the stairs while counting backwards and carrying a box) and both the lower-level and higher-level steps. These profiles represent an average of all the subjects. Positive and increasing ordinate values represent for ankle: dorsiflexion and for hip and knee: flexion. Solid line represents lower-level step and dotted line represents higher-level step. Black line represents C1, red line represents C2, Blue line represents C3 and Orange line represents C4
 C1
C1
 C2
C2
 C3
C3
 C4
C4
Fig. 7.
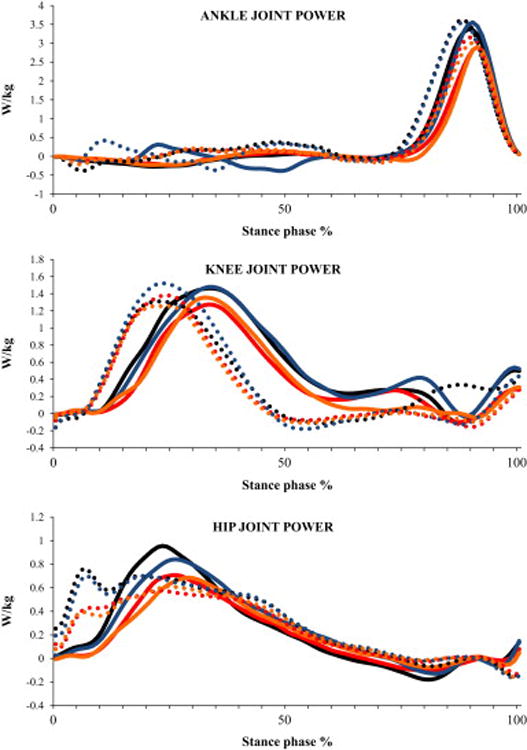
Ensemble averaged profiles of sagittal plane power s of lower-extremity joints during stair ascent normalized to body mass for the four conditions (C1 - ascending the stairs without counting and carrying a box, C2 - ascending the stairs while counting backwards and not carrying a box, C3 -ascending the stairs while carrying a box and not counting, C4 - ascending the stairs while counting backwards and carrying a box) and both the lower-level and higher-level steps. These profiles represent an average of all the subjects. Solid line represents lower-level step and dotted line represents higher-level step. Black line represents C1, red line represents C2, Blue line represents C3 and Orange line represents C4
 C1
C1
 C2
C2
 C3
C3
 C4
C4
3.1. Joint Angles
3.1.1. Ankle
Significant step main effects showed that the participants produced 11% greater peak plantar flexion (P=0.016), 21% greater peak dorsiflexion (P=0.003), 15% greater range of motion (P<0.001) during the higher-level step (Table 3). There were no significant condition main effects. Significant interaction for range of motion (P=0.020) suggested that the value was similar at both the steps during conditions that had a concurrent cognitive loading task (C2 and C4; Fig. 3A).
3.1.2. Knee
Significant step main effects showed that the participants produced 7% greater peak knee flexion (P<0.001), 8% greater range of motion (P<0.001) ascending the higher-level step (Table 3). There were no significant condition main effects and interaction.
3.1.3. Hip
Significant step main effects showed that the participants produced 8% lesser peak flexion angle (P<0.001) at the higher-level step (Table 3). There were no significant condition main effects and interaction.
3.2. Ground reaction forces
Significant step main effects indicated that participants produced 31% greater loading rate (P<0.001), 28% lesser peak braking force (P<0.001), 86% greater peak propulsion force (P<0.001) at the higher-level step (Table 3). Significant condition main effects were produced for the first peak vertical force (P=0.043; Fig. 2A), loading rate (P=0.004, Fig. 2E), vertical force during mid-stance (P=0.034; Fig. 2C), second peak vertical force (P=0.007; Fig. 2B), peak propulsion force (P=0.003; Fig. 2D). Post hoc comparisons showed that participants produced 8% lesser second peak vertical force during C4 compared to C1 condition (P=0.023; Fig. 2B). Also, compared to C1 condition, participants produced 28% lesser loading rate during C2 (P=0.024) and C4 (P=0.017) conditions (Fig. 2E). Participants also produced 26% lesser loading rate during C4 condition compared to C3 condition (P=0.044). There was no significant interaction.
3.3. Joint moments and powers
3.3.1. Ankle
Significant step main effects showed that the participants produced 57% greater peak dorsiflexor moment (P=0.027), 12% greater peak plantar flexor moment (P=0.001) at the higher-level step (Table 3). Significant condition main effects were produced for peak plantar flexor moment (P<0.001; Fig. 2F) and peak power absorption (P=0.017; Fig. 2G). Post hoc tests for peak plantar flexor moment showed that the participants produced 8% lesser moment during C2 and C4 conditions (both P=0.002) compared to the C1 condition. Similarly, participants produced 7% lesser moment during C2 (P=0.002) and C4 (P=0.001) conditions compared to the C3 condition (Fig. 2F). Participants also produced 47% greater peak power absorption during C3 compared to C2 condition (P=0.009; Fig. 2G). Further, significant interaction showed that participants absorbed lesser peak power at the higher-level step compared to the lower-level step during the C4 condition whereas greater power was absorbed at the higher-level step during rest of the conditions (P=0.006; Fig. 3B).
3.3.2. Knee
Significant step main effects showed that the participants produced 12% lesser peak flexor moment (P=0.003) at the higher-level step (Table 3). Significant condition main effects were produced for peak power generation (P=0.037; Fig. 2H). However, post hoc tests did not reveal differences between any two conditions for any of the variables. There was no significant interaction.
3.3.3. Hip
Significant step main effects showed that the participants produced 28% lesser peak extensor moment (P=0.010) at the higher-level step (Table 3). Significant condition main effects were produced for peak power generation (P=0.001). Post hoc tests revealed that peak positive power was 19% lesser during C2 condition compared to C1 condition (P=0.040; Fig. 2I). There was no significant interaction.
3.4. Minimum Toe Clearance
Significant step main effects were observed for MTC anteriorly (P=0.009) and vertically (P<0.001). Post hoc tests showed that from the lower-level step to the higher-level step, the MTC increased by 13% anteriorly and decreased by 19% vertically (Table 3). There were no significant condition main effects. There was a significant interaction for the MTC in the anterior direction (P=0.003). The anterior MTC was smaller during both the cognitive conditions (C2 and C4) at the lower-level step compared to the value during C1 and C3 conditions. However, at the higher-level step, the anterior MTC was similar across all the conditions (Fig. 3C).
When controlled for speed, the dependent measures showed a similar pattern of change. However across all the conditions, the peak ankle dorsiflexion and knee flexion angles became greater when controlled for speed while ascending the lower-level step, indicating that speed played a crucial role in altering the ascent strategy while dual-tasking. With speed as a covariate, differences between conditions remained for peak ankle power absorption and dorsiflexor moment suggesting that other variables were primarily influenced by speed. Participants exhibited similar greater ankle power absorption at both the steps during motor and control conditions compared with the two cognitive conditions. In both the cognitive conditions, less ankle power was absorbed at the higher-level step compared to lower-level step. Also, reduced peak ankle dorsiflexor moment was observed at the higher-level step compared with the lower-level step.
4. Discussion
The present study focused on determining the impact of a concurrent cognitive or motor task on stair-climbing in healthy young adults using comprehensive biomechanical assessment. We hypothesized that impact will be greater when a concurrent cognitive task is performed compared to a concurrent motor task and that the impact will be greater at higher-level steps. Our first hypothesis was true in terms of kinetics parameters (joint moments and power and ground reaction forces) but not kinematics parameters (joint angles and MTC). The second hypothesis was true for most of the parameters.
Condition main effect results indicated that performing a concurrent cognitive task has a greater influence than a concurrent motor task on stair-climbing. The stance phase of stair ascent can be divided into three functional phases: weight-acceptance, pull-up and forward continuance (McFadyen et al., 1988). The influence of a concurrent cognitive task was seen immediately after foot-strike during weight-acceptance phase with ankle dorsiflexion (braking), and knee and hip extension. Lesser values of the corresponding joint powers were seen primarily during the cognitive and combined conditions. Moreover, the peak ankle plantar flexor moment occurs during push-off of the forward continuance phase indicating that during the cognitive and combined conditions, participants produced a lesser moment to lift their foot. During the weight-acceptance and forward continuance phases, greater forces were generated in the control and motor conditions. However, during the pull-up phase, greater forces were generated in the cognitive and combined conditions. Hence, it seems that a concurrent cognitive task alters gait mechanics when the contralateral leg is clearing the intermediate step in its swing phase. Despite these differences, participants seemed to compensate by perhaps slowing down to produce similar joint angles across the conditions. In fact, participants ascended the stairs slowly in the cognitive and combined conditions and at the higher-level step during all the conditions (Table 2).
During the weight-acceptance phase greater peak hip flexion angle, braking force, peak hip extensor moment and decreased peak knee flexion angle, loading rate, and peak dorsiflexor moment at the lower-level step indicate that the participants adopted a safer and slower strategy. This strategy could have been clearing stair-step through greater hip flexion, slower shock absorption through lesser loading rate and greater weight-bearing through greater peak hip extensor moment. Adopting such a strategy may be due to uncertainty associated with ascending stairs while performing an additional task, resulting in a more tentative approach to the lower-level step (three of the four conditions involved dual-tasking). This tentative approach at the lower step might have led to a more optimized stepping strategy at the higher-level step as the participants increased their anterior MTC and decreased their vertical MTC at the higher-level step. During the pull-up phase, greater ankle and knee ranges of motion at the higher-level step compared with lower-level step indicate that participants exerted more effort to create joint extension. Finally, during the forward continuance phase, where ankle generates the maximum energy, greater ankle plantar flexion, peak propulsion force, and peak plantarflexor moment suggest that participants exerted more effort to ascend from the higher-level step. Combined, these results suggest that as one ascends higher, greater effort is needed to clear the next step and this effort increases while dual-tasking.
Step-Condition interaction results for MTC indicated that participants produced lesser values in the anterior direction during cognitive and combined conditions at only the lower-level step. This could happen because of the uncertainty involving the lower-level step while performing a concurrent cognitive task. But once the lower-level step is cleared, the MTC values at the next ipsilateral step became similar due to familiarity with the task requirements to clear a step. Similarly, while ascending the lower-level step, the power absorption at the ankle was similar during braking phase in all the conditions. But while ascending the higher-level step, participants increased the power absorbed at the ankle only in the control and motor conditions. During the control and motor conditions, once the participants completed the lower-level step, due to the familiarity of the task, they were probably able to absorb greater power at the higher-level step. However, in the cognitive and combined conditions, due to the additional cognitive demand, the participants may not have been able to alter their peak ankle power absorption. Overall, the control and motor conditions were similar to each other, but differed from the cognitive and combined conditions.
The mechanics at both the lower-level and higher-level steps could be different due to the starting position prior to stepping onto each step as climbing stairs from a walk compared to from a stand alters the mechanics of stair ascension (Vallabhajosula et al., 2012a, b). Particularly for the lower-level step during single task we found that participants generated greater peak ankle plantar flexor moment, and lesser peak knee flexor moment. In the current study, introducing a secondary task seemed to create similar effects particularly at the ankle and knee joints but not at the hip joint.
The cognitive tasks such as counting backwards are said to challenge one's executive functioning and working memory, thereby activating areas of the frontal lobe like the dorsolateral prefrontal cortex (Al-Yahya et al. 2011; Nadkarni et al., 2010). Previous research has established that gait is not automatic and requires attentional resources from higher-brain centers. Overground walking studies using the dual-tasking paradigm have shown that healthy young adults reduce their gait speed while performing a concurrent cognitive task (Springer et al., 2006) and the speed further decreases for healthy older adults (van Iersel et al., 2007; Verghese et al., 2007). In the current study, a similar reduction in speed was observed for stair-climbing while dual-tasking. Climbing stairs is more challenging than walking overground and could require a greater demand of attentional resources. During stair-climbing, each stair step may be perceived as a new obstacle to clear and hence may require allocation of more cognitive resources. According to the capacity-sharing theory, when two tasks that demand attention are simultaneously performed, the performance on either or both tasks could worsen due to the availability of limited attentional resources (Tombu and Jolicoeur, 2003; Yogev et al., 2008). Results from the current study showed that the participants changed their gait mechanics more during the concurrent cognitive task compared to the concurrent motor task suggesting that the additional motor task probably did not demand as many resources as compared to the corresponding cognitive task. The cross-talk theory suggests that two tasks belonging to the same domain will share the same resources and hence cause lesser interference in the performance of either of the tasks (Schmidt, 1999). This could explain the results of the current study that carrying a box while ascending stairs (primary motor task) had lesser effect. It is possible that a harder concurrent motor task like carrying a glass of water using a tray while climbing stairs can demand more attentional resources. This has to be examined in the future.
Previous researchers have observed that the association of gait to executive functioning becomes stronger with increasing difficulty of the motor task like stepping over an obstacle while walking faster (Ble et al., 2005; Yogev et al., 2008). The results of the current study confirm this observation using stair ascension task. Investigating the role of attention could be important for studying the risk of falls in older adults and patient populations during stair-climbing using dual-tasking approach.
5. Conclusion
Dual-tasking while stair-climbing had a significant impact on kinetics of motion and the impact was greater while performing a concurrent cognitive task. The effect of dual-tasking during stair ascent also seemed to vary based on the stair ascent stance phases and seem to have greater impact as one goes higher. Overall, results suggest that the association between the executive functioning and motor task (like gait) becomes stronger as the level of complexity of the motor task increases.
Fig. 5.
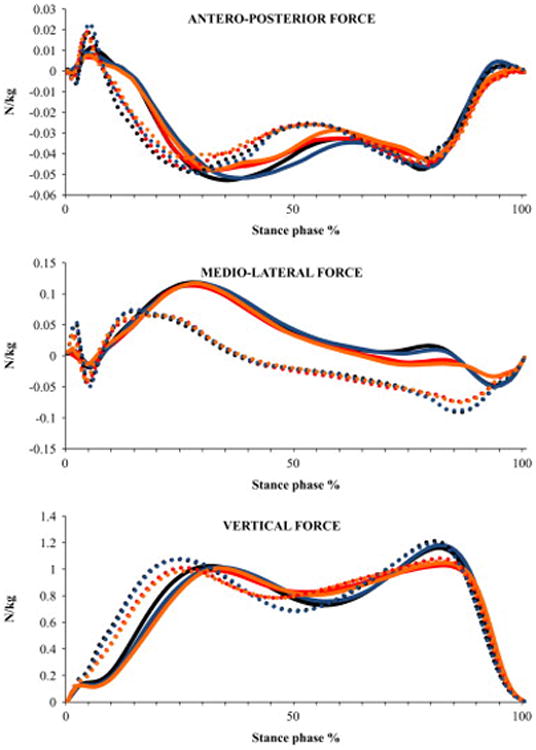
Ensemble averaged profiles of ground reaction force during stair ascent normalized to body mass for the four conditions (C1 - ascending the stairs without counting and carrying a box, C2 -ascending the stairs while counting backwards and not carrying a box, C3 - ascending the stairs while carrying a box and not counting, C4 - ascending the stairs while counting backwards and carrying a box) and both the lower-level and higher-level steps. These profiles represent an average of all the subjects. Solid line represents lower-level step and dotted line represents higher-level step. Black line represents C1, red line represents C2, Blue line represents C3 and Orange line represents C4
 C1
C1
 C2
C2
 C3
C3
 C4
C4
Fig. 6.
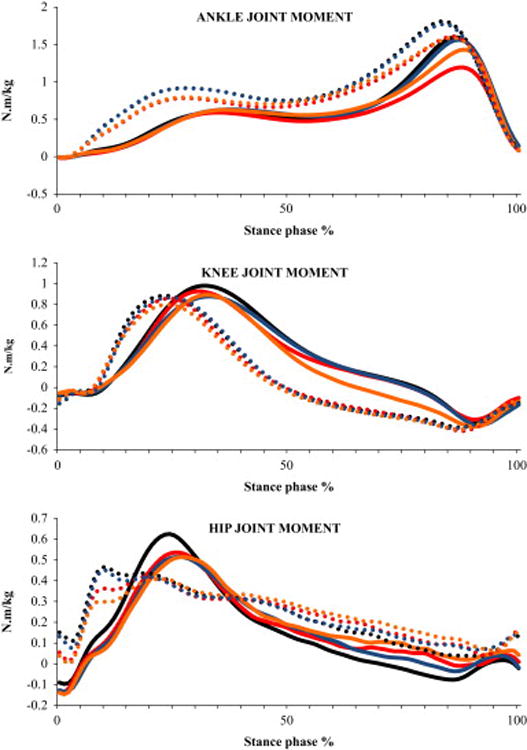
Ensemble averaged profiles of sagittal plane moments of lower-extremity joints during stair ascent normalized to body mass for the four conditions (C1 - ascending the stairs without counting and carrying a box, C2 - ascending the stairs while counting backwards and not carrying a box, C3 - ascending the stairs while carrying a box and not counting, C4 - ascending the stairs while counting backwards and carrying a box) and both the lower-level and higher-level steps. These profiles represent an average of all the subjects. Positive and increasing ordinate values represent for ankle: plantar flexor moment and for hip and knee: extensor moment. Solid line represents lower-level step and dotted line represents higher-level step. Black line represents C1, red line represents C2, Blue line represents C3 and Orange line represents C4
 C1
C1
 C2
C2
 C3
C3
 C4
C4
Acknowledgments
This study was supported by the Biomechanics Research Building. Funding provided by National Institute on Disability and Rehabilitation Research (H133G080023), the National Institute of Health (1RO11AG034995-01A1), and NASA Nebraska Space Grant & EPSCoR (NNX11M06A).
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abernethy B. Dual-task methodology and motor-skills research - some applications and methodological constraints. Journal of Human Movement Studies. 1988;14(3):101–132. [Google Scholar]
- Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J. Cognitive motor interference while walking: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews. 2011;35(3):715–728. doi: 10.1016/j.neubiorev.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Asay JL, Muendermann A, Andriacchi TP. Adaptive patterns of movement during stair climbing in patients with knee osteoarthritis. Journal of Orthopaedic Research. 2009;27(3):325–329. doi: 10.1002/jor.20751. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory - an introduction. International Journal of Psychology. 1992;27(3-4):96–97. [Google Scholar]
- Beauchet O, Aminian K, Gonthier R, Kressig RW. Dual-task-related gait changes in the elderly: Does the type of cognitive task matter? Journal of Motor Behavior. 2005;37(4):259–264. [PubMed] [Google Scholar]
- Beauchet O, Dubost V, Gonthier R, Kressig RW. Dual-task-related gait changes in transitionally frail older adults: The type of the walking-associated cognitive task matters. Gerontology. 2005;51(1):48–52. doi: 10.1159/000081435. [DOI] [PubMed] [Google Scholar]
- Ble A, Volpato S, Zuliani G, Guralnik JM, Bandinelli S, Lauretani F, Bartali B, Maraldi C, Fellin R, Ferrucci L. Executive function correlates with walking speed in older persons: The InCHIANTI study. Journal of the American Geriatrics Society. 2005;53(3):410–415. doi: 10.1111/j.1532-5415.2005.53157.x. [DOI] [PubMed] [Google Scholar]
- Costigan PA, Deluzio KJ, Wyss UP. Knee and hip kinetics during normal stair climbing. Gait & Posture. 2002;16(1):31–37. doi: 10.1016/s0966-6362(01)00201-6. [DOI] [PubMed] [Google Scholar]
- Davis RB, Ounpuu S, Tyburski D, Gage JR. A gait analysis data-collection and reduction technique. Human Movement Science. 1991;10(5):575–587. [Google Scholar]
- Ebersbach G, Dimitrijevic MR, Poewe W. Influence of concurrent tasks on gait - a dual-task approach. Perceptual and Motor Skills. 1995;81(1):107–113. doi: 10.2466/pms.1995.81.1.107. [DOI] [PubMed] [Google Scholar]
- Hamel KA, Okita N, Higginson JS, Cavanagh PR. Foot clearance during stair descent: Effects of age and illumination. Gait & Posture. 2005;21(2):135–140. doi: 10.1016/j.gaitpost.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Hamilton F, Rochester L, Paul L, Rafferty D, O'Leary CP, Evans JJ. Walking and talking: An investigation of cognitive-motor dual tasking in multiple sclerosis. Multiple Sclerosis. 2009;15(10):1215–1227. doi: 10.1177/1352458509106712. [DOI] [PubMed] [Google Scholar]
- Lamontagne M, Beaulieu ML, Beaule PE. Comparison of joint mechanics of both lower limbs of THA patients with healthy participants during stair ascent and descent. Journal of Orthopaedic Research. 2011;29(3):305–311. doi: 10.1002/jor.21248. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Chou LS. Balance control during stair negotiation in older adults. Journal of Biomechanics. 2007;40(11):2530–6. doi: 10.1016/j.jbiomech.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Larsen AH, Sorensen H, Puggaard L, Aagaard P. Biomechanical determinants of maximal stair climbing capacity in healthy elderly women. Scandanavian Journal of Medicine Science & Sports. 2009;19(5):678–686. doi: 10.1111/j.1600-0838.2008.00845.x. [DOI] [PubMed] [Google Scholar]
- McFadyen BJ, Winter DA. An integrated biomechanical analysis of normal stair ascent and descent. Journal of Biomechanics. 1988;21(9):733–744. doi: 10.1016/0021-9290(88)90282-5. [DOI] [PubMed] [Google Scholar]
- Montero-Odasso M, Bergman H, Phillips NA, Wong CH, Sourial N, Chertkow H. Dual-tasking and gait in people with mild cognitive impairment. the effect of working memory. BMC Geriatrics. 2009;9:41–48. doi: 10.1186/1471-2318-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhaidat J, Kerr A, Rafferty D, Skelton DA, Evans JJ. Measuring foot placement and clearance during stair descent. Gait & Posture. 2011;33(3):504–506. doi: 10.1016/j.gaitpost.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Nadkarni NK, Zabjek K, Lee B, McIlroy WE, Black SE. Effect of working memory and spatial attention tasks on gait in healthy young and older adults. Motor Control. 2010;14(2):195–210. doi: 10.1123/mcj.14.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak AC, Brouwer B. Sagittal and frontal lower limb joint moments during stair ascent and descent in young and older adults. Gait & Posture. 2011;33(1):54–60. doi: 10.1016/j.gaitpost.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Novak AC, Brouwer B. Strength and aerobic requirements during stair ambulation in persons with chronic stroke and healthy adults. Archives of Physical Medicine and Rehabilitaion. 2012;93(4):683–689. doi: 10.1016/j.apmr.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Ojha HA, Kern RW, Lin CJ, Winstein CJ. Age affects the attentional demands of stair ambulation: Evidence from a dual-task approach. Physical Therapy. 2009;89(10):1080–1088. doi: 10.2522/ptj.20080187. [DOI] [PubMed] [Google Scholar]
- O'Shea S, Morris ME, Iansek R. Dual task interference during gait in people with parkinson disease: Effects of motor versus cognitive secondary tasks. Physical Therapy. 2002;82(9):888–897. [PubMed] [Google Scholar]
- Paul L, Ellis BM, Leese GP, McFadyen AK, McMurray B. The effect of a cognitive or motor task on gait parameters of diabetic patients, with and without neuropathy. Diabetic Medicine. 2009;26(3):234–239. doi: 10.1111/j.1464-5491.2008.02655.x. [DOI] [PubMed] [Google Scholar]
- Priest AW, Salamon KB, Hollman JH. Age-related differences in dual task walking: A cross sectional study. Journal of Neuroengineering and Rehabilitation. 2008;5:29–36. doi: 10.1186/1743-0003-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopapadaki A, Drechsler WI, Cramp MC, Coutts FJ, Scott OM. Hip, knee, ankle kinematics and kinetics during stair ascent and descent in healthy young individuals. Clinical Biomechanics. 2007;22(2):203–210. doi: 10.1016/j.clinbiomech.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Spanjaard M, Mohagheghi AA, Baltzopoulos V, Maganaris N. Older adults employ alternative strategies to operate within their maximum capabilities when ascending stairs. Journal of Electromyography and Kinesiology. 2009;19(2):E57–E68. doi: 10.1016/j.jelekin.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Schmidt RA, Timothy DL. Motor Control and Learning. Human Kinetics Publishers Inc.; Champaign, Illinois: 1999. [Google Scholar]
- Siu K, Catena RD, Chou L, van Donkelaar P, Woollacott MH. Effects of a secondary task on obstacle avoidance in healthy young adults. Experimental Brain Research. 2008;184(1):115–120. doi: 10.1007/s00221-007-1087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanjaard M, Reeves ND, van Dieen JH, Baltzopoulos V, Maganaris CN. Lower-limb biomechanics during stair descent: Influence of step-height and body mass. Journal of Experimental Biology. 2008;211(9):1368–1375. doi: 10.1242/jeb.014589. [DOI] [PubMed] [Google Scholar]
- Sparrow WA, Bradshaw EJ, Lamoureux E, Tirosh O. Ageing effects on the attention demands of walking. Human Movement Science. 2002;21(5-6):961–972. doi: 10.1016/s0167-9457(02)00154-9. [DOI] [PubMed] [Google Scholar]
- Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: The role of aging, falls, and executive function. Movement Disorders. 2006;21(7):950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- Stacoff A, Diezi C, Luder G, Stüssi E, Kramers-de Quervain IA. Ground reaction forces on stairs: Effects of stair inclination and age. Gait & Posture. 2005;21(1):24–38. doi: 10.1016/j.gaitpost.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Startzell JK, Owens DA, Mulfinger LM, Cavanagh PR. Stair negotiation in older people: A review. Journal of the American Geriatrics Society. 2000;48(5):567–580. doi: 10.1111/j.1532-5415.2000.tb05006.x. [DOI] [PubMed] [Google Scholar]
- Tombu M, Jolicoeur P. A central capacity sharing model of dual-task performance. Journal of Experimental Psychology Human Perception and Performance. 2003;29(1):3–18. doi: 10.1037//0096-1523.29.1.3. [DOI] [PubMed] [Google Scholar]
- Vallabhajosula S, Yentes JM, Momcilovic M, Blanke DJ, Stergiou N. Do lower-extremity joint dynamics change when stair negotiation is initiated with a self-selected comfortable gait speed? Gait & Posture. 2012;35(2):203–208. doi: 10.1016/j.gaitpost.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Vallabhajosula S, Yentes JM, Stergiou N. Frontal joint dynamics when initiating stair ascent from a walk versus a stand. Journal of Biomechanics. 2012;45(3):609–613. doi: 10.1016/j.jbiomech.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iersel MB, Ribbers H, Munneke M, Borm GF, Rikkert MGO. The effect of cognitive dual tasks on balance during walking in physically fit elderly people. Archives of Physical Medicine and Rehabilitation. 2007;88(2):187–191. doi: 10.1016/j.apmr.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Verghese J, Kuslansky G, Holtzer R, Katz M, Xue X, Buschke H, Pahor M. Walking while talking: Effect of task prioritization in the elderly. Archives of Physical Medicine and Rehabilitation. 2007;88(1):50–53. doi: 10.1016/j.apmr.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Movement Disorders. 2008;23(3):329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]