Abstract
Four decades ago, it was observed that stimulation of T cells induces rapid changes in cellular cholesterol that are required before proliferation can commence. Investigators returning to this phenomenon have finally revealed its molecular underpinnings. Cholesterol trafficking and its dysregulation are now also recognized to strongly influence dendritic cell function, T cell polarization, and antibody responses. In this review, the state of the literature is reviewed on how cholesterol and its trafficking regulate the cells of the adaptive immune response and in vivo disease phenotypes of dysregulated adaptive immunity, including allergy, asthma, and autoimmune disease. Emerging evidence supporting a potential role for statins and other lipid-targeted therapies in the treatment of these diseases is presented. Just as vascular biologists have embraced immunity in the pathogenesis and treatment of atherosclerosis, so should basic and clinical immunologists in allergy, pulmonology, and other disciplines seek to encompass a basic understanding of lipid science.
Keywords: Cholesterol, Lipoprotein, T Cell, Dendritic Cell, Asthma, Statins
Introduction
Recent literature has begun to define the central importance of cholesterol homeostasis to the programming, initiation, and resolution of the adaptive immune response. Whereas lipid trafficking may have formerly been considered a topic reserved for vascular biologists and endocrinologists, the rising tide of dyslipidemia and increased use of cholesterol-targeted therapeutics mandate an improved understanding of cholesterol in immunity. In the present review, after opening with a brief overview of cholesterol homeostasis and lipid raft microdomains, recent literature is discussed that has identified roles for cholesterol and the proteins that regulate its intra- and extracellular trafficking, in the biology of the major cells that interact in the adaptive immune response – T cells, B cells, and dendritic cells (DCs). Next, the impact of sterols on allergic sensitization and on diseases of adaptive immunity, including atopic asthma and autoimmune disease, is discussed. Last, potential therapeutic implications are addressed, in particular, emerging roles for 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR) inhibitors (statins) in the treatment of diseases of the adaptive immune response.
A Brief Overview of Cholesterol Trafficking and Lipid Rafts
As a central determinant of membrane fluidity and development and a precursor to steroid hormones, cholesterol is vital to health. Dysregulation of cholesterol levels has been associated with disease in virtually every organ system. Here, key points on cellular cholesterol and its regulation are discussed. For a comprehensive overview of the biochemistry and intra- and extracellular trafficking of cholesterol, the reader is referred to recent scholarly reviews [1, 2].
Nucleated cells accumulate cholesterol via de novo synthesis from acetyl CoA (the mevalonate pathway) and internalization of lipoprotein-associated cholesterol via either the low density lipoprotein receptor (LDLR) or scavenger receptors (SRs). Cellular cholesterol is regulated via the antagonistic actions of two families of transcription factors: i) the Liver × Receptors (LXRα and -β), which induce expression of genes that promote cellular cholesterol efflux (e.g., ATP Binding Cassette [ABC] transporters A1 and G1) and degrade LDLR; and ii) the Sterol Response Element Binding Proteins (SREBPs), which induce genes that promote cholesterol accumulation (e.g., LDLR, and enzymes of the mevalonate pathway such as HMGCR). LXRs and SREBPs are coordinately controlled via cholesterol and oxysterols (enzymatically oxidized cholesterol, e.g., 25-OH-cholesterol [25HC]). Oxysterols activate LXRs and (along with cholesterol) reciprocally suppress SREBPs. Conversely, during cellular sterol deficit, LXRs are inactive, whereas SREBPs become activated.
Extracellularly, cholesterol is carried through the organism on lipoprotein particles, the cellular docking of which is determined by apolipoproteins (apo). The liver, in response to dietary cholesterol and other inputs, synthesizes triglyceride-rich very low density lipoprotein (VLDL) and exports it into the plasma. VLDL is either internalized by cells via interactions of apoE with VLDLR, or processed by lipases ultimately into LDL, which serves as the major vehicle for cholesterol delivery to peripheral cells via interactions of its apoB component with LDLR. In turn, the liver and intestines also produce and release HDL, which, in large part due to its component apoA-I, serves as the major plasma acceptor and return vehicle for ABCA1/ABCG1-effluxed cellular cholesterol. Plasma HDL cholesterol is cleared by the liver via hepatic SR-BI, and then processed into bile acids for receptor-dependent export into the biliary tract, and thereby, into the feces. This completes the disposal pathway of ‘reverse cholesterol transport’. Increased peripheral cholesterol delivery (LDL excess) and defective reverse cholesterol transport (deficits of HDL, apoA-I, LXR, ABCA1, or ABCG1) both lead to peripheral cell cholesterol overload, the classical example of which is the macrophage ‘foam cell’ observed in atherosclerotic lesions. During dyslipidemia, bioactive oxysterols are also increased both in circulating LDL (i.e., oxidized LDL [oxLDL]) and in vascular lesions.
In normal cells, the majority of cholesterol resides in the plasma membrane, much of it within cholesterol-enriched membrane microdomains called lipid rafts. Rafts are thought to serve as platforms that organize signaling by many receptors and accessory proteins, including the B cell receptor (BCR), T cell receptor (TCR), major histocompatibility class (MHC) II, high-affinity IgE receptor (FcεRI), and Toll like Receptors (TLRs)[3]. The localization of proteins to rafts and their signaling interactions are critically sensitive to raft cholesterol content. Raft cholesterol, in turn, is regulated by intracellular cholesterol trafficking. Extracellular cholesterol acceptors such as apoA-I, HDL, and β-cyclodextrins reduce raft cholesterol and attenuate raft-dependent signaling, whereas raft cholesterol overload due to deficient efflux (e.g., ABCG1 deletion) or enhanced loading (e.g., diet-induced hypercholesterolemia) enhances raft-dependent signaling [3]. In this manner, as discussed below, cholesterol trafficking in health and disease ‘tunes’ the immune response by regulating signaling by immune cells.
Reciprocal Effects Between Hypercholesterolemia and Adaptive Immune Cells
Arguing against cholesterol homeostasis and immunity being purely extrinsic and incidental bedfellows, recent literature has revealed bidirectional crosstalk between the two at the systems level. Deletion of either conventional DCs or T regulatory cells (Tregs) induces a significant increase in plasma cholesterol, and in vivo expansion of DCs reduces plasma cholesterol, in particular the pro-atherogenic VLDL- and LDL-cholesterol (LDL-C) fractions [4, 5]. Although the precise mechanism remains unclear, Tregs appear to play a role in promoting clearance of VLDL particles [5]. Further suggesting a fundamental role for T cells in cholesterol homeostasis, T cell-specific overexpression of the tumor necrosis factor superfamily member LIGHT induces hypercholesterolemia, likely through direct interactions with lymphotoxin β receptor on hepatocytes that downregulate the VLDL-metabolizing enzyme, hepatic lipase [6]. In addition, it has been shown that genetic deficiency of mast cells reduces serum cholesterol and triglyceride in mice [7].
Conversely, severe hypercholesterolemia has been shown to skew T cell programming, in particular promoting a T helper (Th)1-to-Th2 switch. Thus, hypercholesterolemic mice produce strong Th2-type antibody responses (increased IgG1 and IgE, decreased IgG2a) to both endogenous and exogenous antigens, in conjunction with increased IL-4 and reduced interferon (IFN)-γ in splenocytes [8, 9]. Th2 skewing during hypercholesterolemia may in part derive from oxLDL-induced attenuation of pro-inflammatory (Th1-promoting) cytokine production by CD8α- DCs [10]. Other reports have noted that hypercholesterolemia leads to sustained increases in Tregs in the spleen [11] and increased expression of IFN-γ in CD8+CD28+ T cells in lymph nodes draining the aortic root [12]. Taken together, these reports indicate reciprocal crosstalk between cholesterol and adaptive immune programming, suggesting that an improved understanding of the underlying cellular mechanisms may potentially reveal novel sites for therapeutic intervention.
Sterol Regulation of Cells of the Adaptive Immune Response
T cells
Decades ago, it was noted that mitogenesis of human T cells with either phytohemagglutinin or immobilized anti-CD3 antibody induces robust HMGCR-dependent cholesterol synthesis that precedes, and is required for, DNA synthesis and cell cycle progression [13, 14]. It was presumed that this requirement for cholesterol derives in large part from the need for rapid biogenesis of new cell membranes during cell division. Recently, investigators returning to this phenomenon of T cell cholesterol synthesis have shown that it arises from coordinated shifts in LXR and SREBP activity driven by changes in intracellular oxysterol availability that occur during T cell activation (Figure 1).
Figure 1. Cholesterol and its metabolism program T cell-APC interactions.
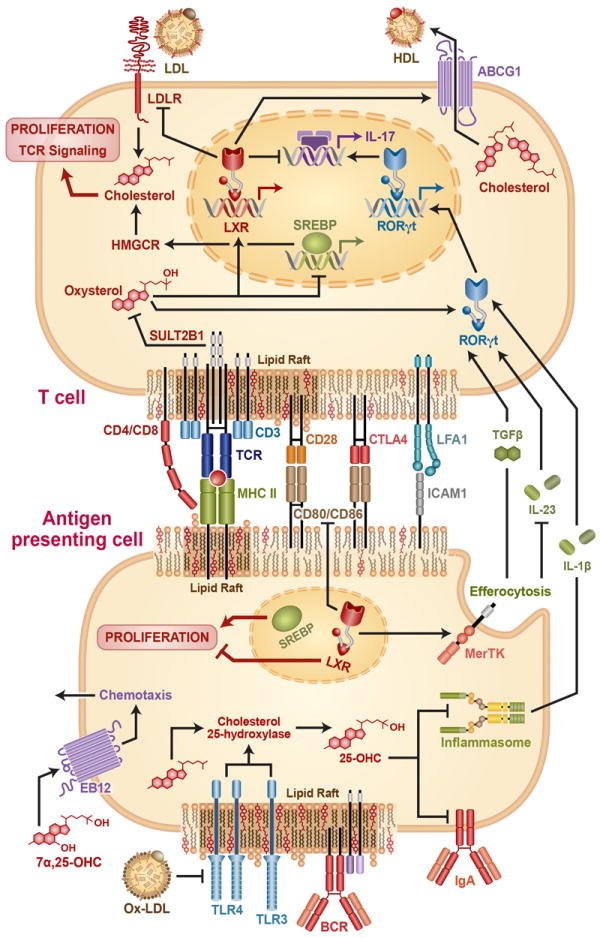
The figure shows a T cell and a representative antigen presenting cell (APC) in contact at an immunological synapse (IS). Some synaptic proteins, as well as Toll like Receptors (TLRs) and the B Cell Receptor (BCR) either reside in or are recruited to cholesterol-enriched lipid rafts upon activation. Their signaling is thereby regulated by raft cholesterol levels. TCR activation leads to inhibitory sulfation of oxysterols via SULT2B1, repressing Liver × Receptor (LXR) and de-repressing sterol response element binding proteins (SREBPs), with consequent effects on expression of ATP Binding Cassette G1 (ABCG1) – a transporter that effluxes cholesterol to high density lipoprotein (HDL); HMGCR – the rate-limiting enzyme in cholesterol synthesis; and low density lipoprotein receptor (LDLR). Cellular cholesterol promotes TCR-induced signaling and cell proliferation. Some oxysterols also directly activate RORγt, the master regulator of T helper 17 differentiation, or induce chemotaxis of APCs via the G-protein coupled receptor Ebi2. In APCs, TLR activation induces synthesis of the oxysterol 25-OH-cholesterol (25HC) via upregulating the enzyme Ch25h. In turn, 25HC represses IgA class switching and inflammasome-dependent generation of IL-1β. LXR-dependent upregulation of Mertk in APCs also promotes efferocytosis (phagocytic clearance of apoptotic cells), which promotes release of TGFβ and suppresses release of IL-23, two cytokines which, along with IL-1β, impact T cell differentiation. Oxidized LDL (oxLDL) also suppresses activation of TLR4, with indirect cytokine-mediated effects upon T cell differentiation.
In a groundbreaking report, Bensinger and colleagues showed that T cell activation triggers simultaneous suppression of LXRβ and de-repression of SREBPs (favoring net cholesterol accumulation) in part via induction of the oxysterol-metabolizing enzyme SULT2B1 [15]. Rapid SREBP activation is required for upregulation of enzymes of the cholesterol synthesis pathway and for cholesterol accumulation and endoplasmic reticulum membrane expansion; SREBP-deficient CD8+ T cells display defective proliferation and deficient clonal expansion during viral infection [16]. Upregulation of cholesterol synthesis in Tregs is also required for proliferation and suppressive function, and is itself dependent upon mechanistic target of rapamycin complex 1 (mTORC1)[17]. In resting T cells, LXRβ activity suppresses proliferation in an ABCG1-dependent manner; thus, Lxrb-/- mice display increased steady state as well antigen-driven T cell expansion, whereas LXR agonists suppress cell cycle progression and proliferation in wild type but not Abcg1-/- cells [15]. Suggesting that ABCG1 serves as a cell cycle checkpoint by limiting cellular cholesterol, Abcg1-/- CD4+ T cells [18], T cells from Apoai-/- mice with deficiency of HDL (the major inducer/acceptor of ABCG1-dependent cholesterol efflux)[19], and cholesterol-loaded wild type T cells [18] have all been shown to be hyperproliferative. Others have shown that LXR activation also interferes with IL-2-induced cell cycle progression of T cells due to inhibited phosphorylation of retinoblastoma protein and downregulation of cyclin B [20].
Recently, LXR has been shown to regulate not only proliferation but also polarization of T cells. LXR deficiency promotes, and LXR overexpression suppresses Th17 differentiation. This occurs through binding of Srebp-1 (an LXR target gene) to the E-box element on the Il17 promoter, thereby inhibiting aryl hydrocarbon receptor-dependent Il17 transcription [21]. Another group has recently shown that T0901317, a widely used synthetic LXR agonist, is also an inverse agonist for the master transcription factors of Th17 differentiation, RAR-related orphan receptor (ROR) -γt and -α [22], and that this rather than LXR activation is the mechanism by which it suppresses Th17 differentiation and function [23]. Additional T cell-targeting actions recently attributed to T0901317 include inhibition of chemokine-induced migration [24] and attenuation of Th1 cytokine production [25].
It has recently also been shown that naturally occurring oxysterols impact Th17 polarization through LXR-independent mechanisms. For example, several non-LXR agonist oxysterols including 7β,27-dihydroxycholesterol were recently reported to be agonists for RORγt [26]. Exogenous treatment of murine and human CD4+ T cells with these oxysterols enhances Th17 differentiation. Moreover, suggesting that endogenous sterol metabolism may program CD4+ T cells, RORγt-active oxysterols are preferentially produced in Th17 cells compared to Th1 cells, whereas mice deficient in CYP27A1, a key enzyme that oxidizes cholesterol into RORγt-active oxysterols, have Th17 cell deficiency comparable to that of RORγt knockout mice [26]. A recent report has also implicated mevalonate pathway intermediates that accumulate in activated T cells, desmosterol in particular, as endogenous RORγt agonists that are required for Th17 differentiation [27]. By contrast, it was recently reported that 25HC indirectly suppresses Th17 differentiation through inhibiting inflammasome-dependent IL-1β production by macrophages through an LXR-independent mechanism; thus, mice deficient in Ch25h, the enzyme that synthesizes 25HC from cholesterol, have increased splenic Th17 cells [28].
In addition to supporting cell cycle/proliferation in T cells, cholesterol promotes TCR signaling through formation of lipid raft microdomains in the plasma membrane. Although controversy persists over the nature and size of raft microdomains, studies using a variety of techniques continue to support the existence of cholesterol-sensitive membrane microdomains [3]. Several reports have suggested that in resting T cells TCR and CD3 reside outside of rafts, but that they translocate to aggregated rafts upon ligation, where they encounter Lck and are phosphorylated [29]. The TCRβ chain may itself bind to cholesterol in membranes, thereby causing TCR dimerization, nanoclustering, and increased antigen avidity [30]. An additional potential implication of raft-localization of the TCR is that raft clustering may regulate pathway activity. Interestingly, crosslinking of the raft-specific ganglioside GM1 using its ligand, cholera toxin B subunit, induces TCR-dependent signaling and provides efficient co-stimulation, suggesting that ‘passive’ raft aggregation in T cells may be sufficient to activate the TCR [31, 32]. In support of the possibility that native co-stimulatory proteins may also remodel rafts, CD28 engagement induces clustering of kinase-rich rafts at the site of TCR engagements, enhancing and sustaining tyrosine phosphorylation events [32].
A growing number of reports have confirmed that TCR signaling is sensitive to cholesterol. Cholesterol-loading and ABCG1 deletion in CD4+ T cells both increase lipid rafts and TCR-dependent signaling, including Zap70 and ERK phosphorylation [18, 33]. Administration of squalene, a precursor of cholesterol, to mice also increases membrane cholesterol and GM1 in CD4+ T cells, and promotes raft-localization of IL-2Rα, IL-4Rα, and IL-12βR2, as well as Th1 skewing after TCR stimulation [34]. Interestingly, endogenous cell type-dependent differences in cholesterol handling may possibly underlie developmental differences in the T cell lineage. Thus, gammadelta T cells display higher cholesterol, lipid raft content, and TCR-dependent signaling than αβ T cells, and all of this is reversible by cholesterol depletion [35]. The impact of cholesterol trafficking may vary by cell type as Abcg1-/- invariant NKT cells display reduced membrane lipid raft content, impaired proliferation, and altered cytokine production in response to CD3/CD28 stimulation [36].
B cells
B cells carry out key roles as amplifiers and effectors of the adaptive immune response. Like DCs, they present antigen to T cells on MHC II molecules via an ‘immunological synapse.’ B cells also produce and release antigen-specific immunoglobulin (Ig) that modifies the function of other immune cells through its Fc domain (e.g., activation of mast cells by IgE via FcεRI). Interestingly, analogous roles for cholesterol have recently been identified in B cells to those observed in T cells.
Human peripheral B cells express both LXRα and LXRβ [37]. Parallel to the case in T cells, LXR suppresses BCR-driven mitogenesis; this endogenous checkpoint is released by BCR stimulation, at which time reciprocal LXR suppression and SREBP activation occur [15]. LXR activation also reduces anti-CD40 and IL-4-mediated IgE secretion by B cells in vitro and reduces allergen-specific IgE in ovalbumin (OVA)-sensitized mice in vivo [37]. On the other hand, LXR agonists increase serum IgM in mice, and this may arise from their direct induction of macrophage IL-5 through binding to a LXR response element in the IL-5 promoter [38].
Important LXR-independent roles have also been identified for oxysterols in B cell biology. 25HC, an oxysterol secreted by macrophages and DCs in response to TLR activation [39], suppresses IgA class switching in B cells by reducing IL-2-mediated cell proliferation and repressing induction of activation-induced cytidine deaminase; thus, Ch25h-null mice have increased serum and airspace IgA [40]. Further processing of 25HC by Cyp7b1 produces 7α,25-diOH-cholesterol (7α,25-OHC), an oxysterol recently shown to chemoattract several immune cell types, including B cells, via the G-protein coupled receptor Epstein-Barr virus-induced gene 2 (Ebi2). Interestingly, both Ebi2-null and Ch25h-null mice fail to position activated B cells within the spleen to the outer follicle and mount a reduced plasma cell response after immune challenge [41, 42].
Several reports have now shown that lipid rafts regulate signaling by the BCR and also play important roles in antigen trafficking in B cells [43, 44]. In resting mature B cells, the BCR appears to be excluded from rafts, whereas BCR crosslinking by antigen induces its recruitment to rafts in a cholesterol-dependent manner, where it interacts with Lyn and other signaling proteins [43]. Interestingly, the relationship of the BCR to rafts appears to be developmentally regulated. Thus, transitional immature B cells have significantly lower membrane cholesterol than mature B cells, impairing aggregation-induced translocation of their BCR into cholesterol-enriched membrane microdomains as well as downstream signaling; remarkably, cholesterol loading the cells corrects BCR translocation and signaling to the level seen in mature B cells [45]. This suggests that BCR biology may be developmentally modified by changes in cholesterol handling that occur during B cell maturation in the spleen.
Rafts regulate the full course of antigen trafficking through the B cell, ranging from initial BCR-mediated uptake, to intracellular trafficking through MHC II-loading compartments, to presentation to T cells at the immunological synapse [44]. MHC II molecules are constitutively present in B cell rafts, and raft disruption by cholesterol-targeting agents (nystatin, cyclodextrin) dramatically compromises antigen presentation at limiting concentrations of antigen [46]. B cell rafts also deliver specific MHC II-peptide complexes to the immunological synapse, and are required for T:B cell conjugate formation and for T cell activation at low densities of class II-peptide complexes [47].
Dendritic Cells
DCs bridge innate and adaptive immunity, in particular through internalizing and processing antigens and then migrating to lymph nodes, where they serve as antigen-presenting cells (APCs) that activate and program T cells. As with B and T cells, a growing array of roles for cholesterol trafficking continue to be identified in DC biology (Figure 1).
Several reports indicate that cholesterol, oxysterols, and proteins that regulate sterol traffic impact both the differentiation and maturation of DCs. Oxysterols, 27HC in particular, promotes the differentiation of monocytic cells into phenotypically mature DC-like cells, upregulating multiple signature cell-surface molecules (MHC II, CCR7, CD40, CD80) and inducing DC-like morphology [48]. Some of these effects are shared by oxLDL [49]. LXR may possibly mediate some of these changes as LXR activation enhances myeloid DC differentiation, perhaps through inhibition of STAT3 phosphorylation [50], and also upregulates CCR7 [51]. In human DCs, LXR activation also induces maturation markers (CD80, CD86) and augments pro-inflammatory cytokine production and capacity to promote T cell proliferation [52], although some reports suggest that LXR activation interferes with LPS-induced maturation of human DCs [53]. Conversely, apoA-I inhibits DC differentiation (surface marker upregulation, endocytic activity) and ability to activate T cells, possibly through induction of prostaglandin E2 and IL-10 [54]. HDL treatment also inhibits the ability of DCs to activate T cells after TLR stimulation; interestingly, this effect appears to derive at least in part from the phospholipid fraction of HDL [55].
Sterol homeostasis and its dysregulation also affect DC migration in vivo. Hypercholesterolemic mice have impaired migration of DCs to lymph nodes associated with reduced contact hypersensitivity and delayed-type hypersensitivity responses; this appears to derive from an increase in platelet activating factor (PAF) or PAF-like like activity in oxLDL, and is reversed by treatment with HDL [56]. LXR also plays a key role in CCR7-dependent egress of monocyte-derived cells from vascular lesions during atherosclerosis regression [51]. Finally, parallel to its chemotactic role in splenic B cell positioning mentioned above, 7α,25-OHC plays a key role in EBI2-dependent maintenance and localization of CD4+ DCs in marginal zone bridging channels of the spleen [57].
Similar to the case in B cells, MHC II localization to lipid rafts in DCs has been shown to be functionally important. The majority of newly generated MHC II-peptide complexes in DCs cluster in cholesterol-dependent microdomains on the DC cell surface; cholesterol depletion disrupts these clusters as well as the APC function of DCs, especially in the setting of limiting concentrations of antigen [58]. Interestingly, structural studies indicate that cholesterol itself binds to the transmembrane domain of MHC II, altering its conformation in a manner that may allosterically modulate the MHC II peptide groove and promote T cell activation [59]. MHC II-peptide complex targeting to cholesterol-dependent microdomains in the DC appears to be required for Th1 programming [60], and raft clustering at the synaptic interface with T cells appears particularly important [61]. Of note, the cholesterol-dependence of MHC II clustering may have physiological and therapeutic implications. Cholesterol depletion of antigen-loaded DCs with HDL or apoA-I reduces their capacity to activate T cells in a manner that is reversed by cholesterol repletion [62]. Statins also reduce MHC II display by DCs through disrupting cholesterol-dependent membrane microdomains [63].
Putting it All Together: Sterol Regulation of Adaptive Immunity in Disease
Allergic Sensitization
While several reports in rodent models have suggested that hypercholesterolemia promotes Th2 immunity [8, 9], the relationship of cholesterol to atopy in humans appears to be complex. Of interest, ex vivo treatment of mononuclear cells from latex-allergic atopic dermatitis patients with cholesterol enhances production of latex-specific IgE, IL-4, IL-10, and IL-13 in a dose-dependent fashion [64]. At a population level, however, varying relationships have been reported between serum cholesterol measures and allergic sensitization in cross-sectional studies. In a study of >1000 Chinese twins, low HDL-C and high LDL-C were associated with allergic sensitization as assessed by skin prick testing [65]. In a general population of 11 year old children, total cholesterol (TC) and LDL-C levels were also significantly positively associated with higher IgE and a higher rate of allergic sensitization [66]. On the other hand, in a longitudinal study that tracked 200 newborns prospectively up to age 20 years, those adolescents who developed allergic symptoms, skin test positivity, and elevated IgE had lower TC levels in infancy and childhood than non-atopic subjects [67]. Genetic background may be an important source of variation, as we have noted marked inter-racial differences between serum cholesterol and allergen-specific IgE in the U.S. population [68].
Atopic Asthma
Asthma imposes a tremendous public health burden. The majority of cases of asthma are associated with atopy, and it is thought that Th2 and Th17 immunity contribute to disease. Recent findings in human populations and animal models suggest an important role for cholesterol in asthma pathogenesis.
Reminiscent of past reports that diet-induced hypercholesterolemia increases airway eosinophils and Th2 cytokines in the murine OVA sensitization/challenge model of asthma [69], several investigators have recently found that high serum cholesterol is associated with asthma in humans. In a recent study of ~500 Taiwanese children, serum TC and LDL-C were significantly higher in asthmatic than non-asthmatic subjects [70]. Other investigators have found the relationship of serum cholesterol to asthma to vary by density subclass of LDL [71]. Conversely, some evidence suggests a potentially ‘protective’ role for serum HDL in asthma. Thus, low HDL-C was independently associated with wheezing in a study of >85,000 Spanish workers [72], and with reduced expiratory airflows in a study of >18,000 U.S. adults [73]. In adolescent subjects, asthma was also found to be associated with low HDL-C, particularly in those with a drop in HDL-C between childhood and adolescence [74]. That said, a wide and divergent range of relationships has been reported between different cholesterol measures and asthma [75, 76], suggesting that differences in study design and/or in unmeasured covariates may be responsible. Asthma may also conceivably impact lipoprotein metabolism. Indeed, asthma is associated with reduced airspace cholesterol [77] and apoA-I [78], increased plasma oxLDL [79] and lung levels of oxidized lipids and anti-oxLDL antibodies [80], and impaired anti-inflammatory and anti-oxidant function of HDL [79, 80].
Mice targeted for cholesterol transport genes known to be expressed in the lung have recently provided new mechanistic insights into the connection between cholesterol and asthma. Mice deleted for ABCG1, the primary effector for HDL-induced cholesterol efflux, display augmented Th17-dependent airway neutrophilia in the OVA asthma model [81]. Consistent with this, mice deficient in HDL due to deletion of apoA-I have increased peripheral airway resistance, airway inflammation, and lung oxidative stress compared with wild type counterparts in the naïve state [82], and increased G-CSF-dependent airway neutrophilia after OVA sensitization and challenge [78]. On the other hand, mice engineered to have elevated HDL-C through inactivation of endothelial lipase have lower airway inflammation, Th2 cytokines, and airway hyperresponsiveness (AHR) than controls [83]. Suggesting a role for VLDL in asthma, VLDLR-null mice also display increased eosinophilia and Th2 cytokines in the house dust mite (HDM) asthma model, a finding that derives from cell-intrinsic alterations in DC function [84]. Finally, both LDLR-null and apoE-null mice have increased airway remodeling in the HDM model, suggesting a protective apoE-LDLR pathway in asthma [85]. Implying that variations in apoE function in humans may influence asthma, mice engineered to express the three major human APOE alleles (ε2, ε3, ε4) display significant differences in HDM-induced airway disease [86].
Extending upon the findings in apo-deficient mice and borrowing from the cardiovascular literature, investigators have recently tested the potential therapeutic efficacy of synthetic apolipoprotein mimetic peptides in asthma models. ApoA-I mimetic peptides decrease airway eosinophilia, lung oxidative stress, and IgE in the OVA model [80], and reduce airway inflammation, Th2 and Th17 cytokines, AHR, and airway remodeling in the HDM model [87]. An apoE mimetic peptide was also recently shown to suppress AHR/remodeling, Th2 and Th17 cytokines, and eosinophilia in the HDM model through an LDLR-dependent mechanism [85]. Also on the therapeutic frontier, studies suggest a potential therapeutic role for synthetic LXR agonists in asthma models [88], consistent with their reported anti-inflammatory efficacy in contact dermatitis models [89]. In the section on statins below, we discuss emerging roles for statins in asthma at further length.
Autoimmune Disease
Autoimmune diseases encompass a large number of discrete as well as overlapping syndromes in which dysregulated innate and adaptive immunity conspire to cause inappropriate host tissue damage. Recently, cholesterol regulatory pathways have been shown to intersect the pathogenesis of autoimmunity in the areas of apoptotic cell clearance and lipid raft composition, suggesting potential new strategies for therapeutic intervention.
Efferocytosis (phagocytic clearance of apoptotic cells) is executed by the TAM (Tyro3, Axl, Mer) receptor kinases and their ligands (Gas6, Protein S) as well as by additional phagocyte receptor-apoptotic cell ligand pairs. Defective efferocytosis is thought to play a central role in autoimmunity by delaying autoantigen clearance [90, 91]. Recently, a key role for LXR was identified in efferocytosis and prevention of autoimmunity. A-Gonzalez and colleagues demonstrated that the phagocyte receptor for apoptotic cells, Mer, is a direct LXR target gene, and that apoptotic cell engulfment induces phagocyte expression of Mer via sterol-dependent activation of LXR [92]. Consequently, LXR-null macrophages display defective efferocytosis and LXR-null mice have increased apoptotic bodies in multiple tissues and develop autoantibodies and autoimmune glomerulonephritis [92]. Supporting a potential role for LXR in treatment of autoimmune disease, synthetic LXR agonists have now been shown to reduce lymphadenopathy and glomerular antibody deposition in B6lpr/lpr mice [92], clinical scores in collagen-induced arthritis [93], clinical scores and Th17-associated inflammation in experimental autoimmune encephalomyelitis [94, 95], and Th1- and Th17-associated inflammation in experimental autoimmune uveitis [96].
Additional LXR targets and other genes involved in reverse cholesterol transport have been implicated in autoimmunity, potentially by acting as bridging ligands or receptors for efferocytosis. ApoE promotes efferocytosis, and apoE-null mice have increased apoptotic bodies in several tissues associated with low-level inflammation [97]. SR-BI has also been suggested to play a role in efferocytosis [98], and SR-BI-null mice spontaneously develop splenomegaly, increased autoantibodies, and autoimmune glomerulonephritis [99]. ABCA1 has been suggested to mediate efferocytosis [100], and recently mimetic peptides for its ligand apoA-I have been shown to reduce lymphadenopathy, autoantibody titers, glomerulonephritis, and proteinuria in a murine model of systemic lupus erythematosus (SLE)[101]. Consistent with a role for endogenous apoA-I in suppression of autoimmunity, apoA-I-deficient mice on an LDLR-null background display lymphadenopathy, splenomegaly, increased T cell activation, and autoantibodies [19].
Complex, reciprocal interactions have been identified between serum lipoproteins and autoimmunity. HDL, the major lipoprotein carrier for apoA-I, has been known for many years to suppress lymphocyte proliferation [102]; abnormal HDL from gene-targeted mice is deficient in this function [99]. Interestingly, several reports have documented reduced and/or dysfunctional HDL in patients with autoimmune disease [103, 104]. Defective HDL may arise as a consequence of systemic inflammation [105]; whether it reciprocally de-represses autoimmune inflammation is an interesting question worthy of study. An additional indirect feedback mechanism between serum lipoproteins and autoimmunity involves anti-oxLDL autoantibodies, which have been noted to increase in parallel with serum oxLDL during dyslipidemia and, interestingly, to cross-react with apoptotic cells [106]. Anti-oxLDL antibodies modulate efferocytosis by macrophages and dendritic cells, suppress inflammation, and inhibit collagen-induced arthritis [106, 107].
Several reports have recently shown that the hyperactivated B cells and T cells in SLE patients may arise from lipid raft abnormalities. B cells from SLE patients have reduced raft levels of lyn kinase, a negative regulator of BCR signaling, likely due to increased c-Cbl-dependent ubiquitination [108]. T cell rafts from SLE patients have multiple abnormalities, including elevations in cholesterol, gangliosides, activated Syk kinase, and CD45, and a reduction in Lck [109-111]. Moreover, SLE T cells display increased calcium signaling in response to raft cross-linking and relative resistance to cholesterol reduction with cyclodextrins, suggesting their hypersensitive phenotype stems from increased cholesterol-dependent rafts [110]. Supporting a potential role for statins in molecular therapy of SLE rafts, atorvastatin restores raft Lck and normalizes raft-associated TCR signaling in T cells from SLE patients [111]. Statins may also theoretically indirectly rectify SLE rafts by normalizing dyslipidemia, given that the increased glycosphingolipid synthesis and trafficking to rafts in SLE T cells is reportedly driven by LXR activation in response to dyslipidemic serum [112]. Finally, suggesting an additional exciting frontier for raft therapy in SLE, NB-DNJ, a glycosphingolipid synthesis inhibitor approved for clinical use in Gaucher disease, partially normalizes rafts and TCR signaling in SLE T cells [112].
Statins as Potential Therapeutics in Diseases of Adaptive Immunity
Effects of Statins on T Cell Activation and Polarization
Statins inhibit HMGCR, the rate-limiting enzyme of the mevalonate pathway, and are widely prescribed for hypercholesterolemia. In the last several years, studies have elucidated a wide range of immunomodulatory actions of statins on multiple cell types. In addition to reducing cellular/serum cholesterol, statins reduce the cellular pool of mevalonate-derived isoprenoids (geranylgeranyl [GG]-PP, farnesyl [F]-PP), lipids which, through protein post-translational modification (‘prenylation’), serve important biologic roles in immune and other cells by promoting membrane-localization of key signaling proteins (e.g., Rho, Ras, Rab). Some statins also antagonize LFA-1-dependent cell adhesion of immune cells through mevalonate-independent mechanisms [113]. In most cases, it is difficult to know the physiological relevance of studies of statins, especially those conducted in rodents, wherein dosing on a body weight basis is generally far higher than that used in humans, and drug and lipid metabolism also differ. While a comprehensive review of the impact of statins on immunity is outside the scope of the present review, key findings relating to T cell activation/polarization are discussed below.
Statins attenuate T cell activation in part by impairing antigen presentation. In nonprofessional APCs including endothelial cells, monocytes, and microglia, statins reduce IFNγ-inducible upregulation of MHC II through inhibited transcription [114]. Additional actions on DCs and B cells include inhibition of co-stimulatory molecule (CD40, CD86) and chemokine receptor (CCR7) expression, antigen uptake, and cytokine expression [115]. Reduction of antigen uptake and processing by DCs arises from blocked prenylation of Rho and Rab GTPases [116]. In vivo, statins may also suppress T cell activation by reducing T cell numbers in the spleen and lymph nodes, an effect that has been ascribed to impaired homing [117].
Interestingly, several studies indicate that statins bias away from Th1 and Th17 and towards Th2 polarization. Statin-pretreated DCs promote Th2 polarization of CD4+ T cells by upregulation of the lectin Ym1 [118]. In addition, direct pro-Th2 effects of statins upon CD4+ T cells have been identified. Atorvastatin inhibits T cell proliferation and activation and favors Th2 differentiation by decreasing prenylation of RhoA and Ras in T cells, thereby compromising TCR activation of mitogen-activated protein kinases and the pro-Th1 transcription factor c-fos [119]. Consistent with isoprenoid depletion as the mechanism, Th2 skewing is observed in vitro and in vivo and occurs without a discernible change in serum or T cell raft cholesterol [114, 119, 120]. In vivo treatment of immunized mice with statins reduces Th1 responses upon antigen restimulation [121]. Reduced ex vivo proliferation of T cells from statin-treated patients has also been observed [122], although not all studies have confirmed effects on Th1/Th2 balance in humans [123] and important differences among statin formulations have been noted [124]. Statins also inhibit Th17 polarization through effects on both APCs and T cells. Simvastatin inhibits expression of Th17-promoting cytokines (IL-1β, IL-6, IL-23, IL-21) by APCs through induction of suppressor of cytokine secretion (SOCS)1, SOCS3, and SOCS7 [125, 126], and directly suppresses T cell IL-17 by inhibiting expression of RORγt [125]. Finally, statins also enhance induction of Tregs by blocking geranylgeranylation, potentially through a mechanism involving demethylation of the Foxp3 promoter [127], and augment the capacity of steroids to increase the Treg/Th17 ratio of CD4+ T cells [128], although another report indicates that statins reduce Treg proliferation and suppressive function [17].
Statins in Asthma
Their reported pro-Th2 effects notwithstanding, statins have many actions that might be predicted to ameliorate asthma pathogenesis, including inhibition of antigen presentation, and inhibition of proliferation, homing, and Th17 polarization of CD4+ T cells. In addition, statins inhibit eosinophil adhesion [129], IgE-dependent release of histamine by mast cells [130], matrix-remodeling responses of airway smooth muscle cells and fibroblasts to TGFβ1 [131, 132], and proliferation and antigen-induced hypercontractility of airway smooth muscle cells [133, 134]. Multiple reports have in fact now confirmed that several statins, delivered through a variety of routes, reduce disease measures in murine models of asthma. Pravastatin and simvastatin administered intraperitoneally reduce airway leukocytes, Th2 cytokines, and OVA-specific IgE in the OVA/alum model [69, 135, 136]. Interestingly, metabolic rescue of mice by in vivo co-treatment with mevalonic acid, the product of HMGCR, reverses the reduction in airway leukocytes but not Th2 cytokines by simvastatin [137]. Statins inhibit antigen presentation by CD11c+ lung cells and IL-17 production by thoracic lymph node cells [138]. Efficacy is also observed with intragastric delivery of statins, and in both C57BL/6 and Balb/C mice [69, 136]. Simvastatin, pravastatin, and atorvastatin also reduce AHR [137, 139], possibly by inhibiting RhoA-dependent signaling [140]. Reduction of AHR by simvastatin is not blocked by in vivo co-treatment of mice with mevalonic acid [137], whereas its inhibition of airway goblet cell hyperplasia is [141]. A recent report interestingly indicates that simvastatin delivered to mice by aerosol inhalation also potently reduces OVA/alum-induced airway cellular inflammation, Th2 cytokines, and AHR, and achieves much higher lung deposition and potency in the face of reduced serum concentrations compared with systemic administration [140].
Several reports of statins in human asthma have now appeared, including both observational studies (Table 1) and prospective, randomized treatment trials (Table 2). Although some minor improvements in intermediate endpoints have been noted in some trials, results have been mixed, and few trials have found meaningful effects on hard clinical outcomes. A recent systematic review of randomized controlled trials and observational studies of statins in asthma found no consistent statistically significant improvements in clinical or airway function outcomes [142], and a meta-analysis of randomized controlled trials found no significant improvement in spirometry [143]. That said, the trials to date have been relatively small and short-term, have not tested all statins on the market, and have been underpowered to properly test asthma subphenotypes (e.g., obese asthmatic, non-atopic asthmatic). Inhaled statin formulations have not yet been developed for clinical use, and remain untested. Finally, a recent report has identified a polymorphism in HLA-G that modifies statin benefit in asthma [144]. Thus, several research gaps remain and further results are anticipated.
Table 1.
Observational studies of statins in clinical asthma.
| Design | N | Patients | Duration | Effect of statin therapy | |
|---|---|---|---|---|---|
|
| |||||
| Lokhandwala et al. [152] | Propensity-matched retrospective cohort analysis of Medicaid data | 1437 | Asthma | 1 year | OR 0.55 (95% CI, 0.37-0.84) for asthma-related hospitalizations and/or ER visits; OR 0.48 (95% CI, 0.28-0.82) for asthma-related ER visits |
| Zeki et al. [153] | Retrospective cross-sectional study | 165 | Severe asthma | 1 year (statin use, median) | Better asthma symptom control (increase in mean ACT symptom score by 2.2 ± 0.94 points; p=0.02). |
| No differences in lung function, corticosteroid or rescue BD use, or eosinophilia | |||||
| Huang et al. [154] | Population-based analysis of Taiwan National Health Insurance Database | 11808 | Asthma | 4.66 ± 2.32 years | HR, 0.82 (95% CI, 0.71-.095; p=0.006) for asthma hospitalization |
| Ostroukhova et al. [155] | Retrospective cohort study | 50 | Atopic Asthma | 2 years (3, 6, 12, and 24 month followup) | 3-5% median worsening of FEV1; at 6 months, more patients needed increased maintenance medication (p=0.005), used albuterol more often (p<.001); had more nocturnal awakening (p<.001), and were seen more frequently in office for acute asthma (p=0.003). |
| Tse et al. [156] | Propensity score-matched cohort study | 16696 | Asthma | 36 months | OR 0.64 (95% CI, 0.53-77; p<0.0001) for asthma-related ER visits;OR 0.90 (95% CI, 0.81-0.99; p=0.04) for ≥2 oral corticosteroid dispensings. No significant difference in asthma-related hospitalizations |
| Tse et al. [157] | Observational study using Medco Health Solutions admin. database | 6652 | Asthmatics with recent exacerbation | 1 year followup | Among ICS users, statins had OR 0.77 (95% CI 0.64-0.94; p=0.008) for asthma-related ER visits, but no odds reduction for asthma-related hospitalizations. No significant associations among non-ICS users. |
Abbreviations: ACQ, asthma control questionnaire; ACT, asthma control test; AHR, airway hyperresponsiveness; AQLQ, asthma quality of life questionnaire; BD, bronchodilator; CI, confidence interval; d/c, discontinuation; ER, emergency room; FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroid; NO, nitric oxide; OR, odds ratio; PEF, peak expiratory flow.
Table 2.
Prospective, randomized, placebo-controlled trials of statins in asthma.
| Design | N | Patients | Duration | Effect of statin therapy | |
|---|---|---|---|---|---|
|
| |||||
| Braganza et al. [158] | Double-blind: Atorvastatin 40mg/d vs. placebo (4 wks); then ICS added to both groups (4 wks) | 71 | Smokers with mild-to-moderate asthma | 8 weeks | No improvement in morning PEF. |
| Improvement in asthma AQLQ score (0.52, 95% CI, 0.17-0.87; p=0.005). | |||||
| No change in induced sputum cells | |||||
| Maneechotesuwan et al. [159] | Double-blind: Simvastatin 10mg/d | 50 | Mild asthmatics on low-dose ICS | 10 weeks (8 wk treatment) | Significant reduction in sputum eosinophil percentage |
| Menzies et al. [160] | Cross-over trial: Simvastatin vs. placebo after d/c of anti-inflammatory medications | 16 | Mild-to-moderate asthma on ICS | 1 month | No significant change in exhaled NO, AHR, or eosinophilia |
| Moini et al. [161] | Atorvastatin 80 mg/d | 62 | Mild-to-moderate asthma | 8 weeks | No significant difference in ACT score, spirometry, or blood eosinophil count |
| Hothersall et al. [162] | Double-blind crossover trial: Atorvastatin 80 mg/d | 54 | Atopic asthmatics on ICS | 24 weeks (2 × 8 wk treatments) | No significant difference in mean morning PEF, spirometry, or AQLQ score. Significant reduction in sputum macrophages and leukotriene B4. |
| Cowan et al. [163] | Crossover trial: Simvastatin 40 mg/d, simultaneous ICS reduction until loss of control, then ICS increase until control | 43 | Asthmatics on ICS | ≥3 months treatment | No significant difference in minimal ICS dose required for control. |
| In patients who stopped ICS, ACQ was lower (p=0.037), FEV1 higher (p<0.01), and sputum eosinophils lower (p=0.033) on simvastatin. | |||||
Abbreviations: ACQ, asthma control questionnaire; ACT, asthma control test; AHR, airway hyperresponsiveness; AQLQ, asthma quality of life questionnaire; BD, bronchodilator; CI, confidence interval; d/c, discontinuation; ER, emergency room; FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroid; NO, nitric oxide; OR, odds ratio; PEF, peak expiratory flow.
Statins in Autoimmune Disease
Several features of statins, most notably their suppression of Th1 and Th17 polarization and T cell proliferation, suggest that they may be efficacious in the therapy of autoimmune disease. Multiple reports have now in fact confirmed that statins improve clinical and inflammatory measures in experimental models of autoimmune disease, as comprehensively summarized by Greenwood and colleagues [145]. This includes rodent models of experimental autoimmune myocarditis, experimental autoimmune encephalomyelitis (the animal model for multiple sclerosis), experimental arthritis, experimental autoimmune uveoretinitis, and experimental SLE, although some studies have found no significant therapeutic effect in individual models [145].
Of interest, some early clinical studies have also suggested clinical efficacy of statins in human autoimmune disease. In a randomized, double-blind, placebo-controlled trial of 116 patients with rheumatoid arthritis with a 6-month followup, patients treated with 40 mg atorvastatin daily in conjunction with their existing regimen had a statistically significant albeit modest improvement in disease activity score [146]. Recently, a meta-analysis of 15 studies involving 992 patients of statins in rheumatoid arthritis confirmed consistent, significant improvements in clinical scores and inflammatory measures [147]. In addition, in a multicenter, open-label, single-arm study of 30 patients with relapsing-remitting multiple sclerosis, 80 mg simvastatin daily was associated with a 44% reduction in the number of gadolinium-enhancing lesions on brain MRI compared with pretreatment scans [148]. Similarly, an observational trial of 7 patients with relapsing-remitting multiple sclerosis reported that lovastatin was associated with a reduction in the number in enhancing brain lesions [149]. On the other hand, mixed results have been reported so far as to whether statins enhance the clinical efficacy of IFNβ therapy in multiple sclerosis [115].
In sum, several studies have suggested promise for statins in autoimmune disease, even if only as adjunctive agents, but larger, prospective trials will be required to resolve the issue. Importantly, accelerated atherosclerosis is observed in autoimmune diseases, including rheumatoid arthritis and SLE [150, 151], suggesting that there may be a compelling cardiovascular indication for statins in autoimmune disease patients. Whether or not statins will impose unacceptable increases in risk for myositis and/or hepatic injury in autoimmune disease patients is an important question that will, however, require further clinical experience to answer.
Conclusions
A growing basic, translational, and now clinical literature is providing compelling evidence that cholesterol metabolism fundamentally impacts the adaptive immune system, and that there may be exciting opportunities for repurposing of lipid-targeted therapeutics to immunological disease. Just as the cardiovascular sciences have embraced molecular immunology in the pathogenesis and treatment of atherosclerosis, allergists, pulmonologists, and rheumatologists among other clinicians must now consider the potential role of cholesterol and dyslipidemia in clinical immunology. It appears almost certain that in coming years some of the emerging insights, research tools, and/or therapeutics in lipid science and vascular biology will prove of real and perhaps transformative value to scientists and clinicians of the adaptive immune response.
Acknowledgments
The author thanks Dr. Sue Edelstein for assistance with figure production. This work was supported by the National Institutes of Health, National Institute of Environmental Health Sciences (Z01 ES102005).
References
Papers of particular interest, published recently, have been highlighted as:
-
•
Of importance
-
••
Of major importance
- 1.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nature reviews Molecular cell biology. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 2.Marcel YL, Ouimet M, Wang MD. Regulation of cholesterol efflux from macrophages. Current opinion in lipidology. 2008;19:455–461. doi: 10.1097/MOL.0b013e32830f4a1d. [DOI] [PubMed] [Google Scholar]
- 3.Fessler MB, Parks JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. Journal of immunology. 2011;187:1529–1535. doi: 10.4049/jimmunol.1100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Pirault J, Deswaerte V, Ginhoux F, Miller ER, Witztum JL, Chapman MJ, Lesnik P. Conventional dendritic cells at the crossroads between immunity and cholesterol homeostasis in atherosclerosis. Circulation. 2009;119:2367–2375. doi: 10.1161/CIRCULATIONAHA.108.807537. [DOI] [PubMed] [Google Scholar]
- 5.Klingenberg R, Gerdes N, Badeau RM, Gistera A, Strodthoff D, Ketelhuth DF, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, Zoller S, Lohmann C, Luscher TF, Jauhiainen M, Sparwasser T, Hansson GK. Depletion of foxp3+ regulatory t cells promotes hypercholesterolemia and atherosclerosis. The Journal of clinical investigation. 2013;123:1323–1334. doi: 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo JC, Wang Y, Tumanov AV, Bamji M, Yao Z, Reardon CA, Getz GS, Fu YX. Lymphotoxin beta receptor-dependent control of lipid homeostasis. Science. 2007;316:285–288. doi: 10.1126/science.1137221. [DOI] [PubMed] [Google Scholar]
- 7.Heikkila HM, Trosien J, Metso J, Jauhiainen M, Pentikainen MO, Kovanen PT, Lindstedt KA. Mast cells promote atherosclerosis by inducing both an atherogenic lipid profile and vascular inflammation. Journal of cellular biochemistry. 2010;109:615–623. doi: 10.1002/jcb.22443. [DOI] [PubMed] [Google Scholar]
- 8.Robertson AK, Zhou X, Strandvik B, Hansson GK. Severe hypercholesterolaemia leads to strong th2 responses to an exogenous antigen. Scandinavian journal of immunology. 2004;59:285–293. doi: 10.1111/j.0300-9475.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Paulsson G, Stemme S, Hansson GK. Hypercholesterolemia is associated with a t helper (th) 1/th2 switch of the autoimmune response in atherosclerotic apo e-knockout mice. The Journal of clinical investigation. 1998;101:1717–1725. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shamshiev AT, Ampenberger F, Ernst B, Rohrer L, Marsland BJ, Kopf M. Dyslipidemia inhibits toll-like receptor-induced activation of cd8alpha-negative dendritic cells and protective th1 type immunity. The Journal of experimental medicine. 2007;204:441–452. doi: 10.1084/jem.20061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maganto-Garcia E, Tarrio ML, Grabie N, Bu DX, Lichtman AH. Dynamic changes in regulatory t cells are linked to levels of diet-induced hypercholesterolemia. Circulation. 2011;124:185–195. doi: 10.1161/CIRCULATIONAHA.110.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolbus D, Ramos OH, Berg KE, Persson J, Wigren M, Bjorkbacka H, Fredrikson GN, Nilsson J. Cd8+ t cell activation predominate early immune responses to hypercholesterolemia in apoe(-)(/)(-) mice. BMC immunology. 2010;11:58. doi: 10.1186/1471-2172-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakrabarti R, Engleman EG. Interrelationships between mevalonate metabolism and the mitogenic signaling pathway in t lymphocyte proliferation. The Journal of biological chemistry. 1991;266:12216–12222. [PubMed] [Google Scholar]
- 14.Chen HW, Heiniger HJ, Kandutsch AA. Relationship between sterol synthesis and DNA synthesis in phytohemagglutinin-stimulated mouse lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:1950–1954. doi: 10.1073/pnas.72.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. Lxr signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Kidani Y, Elsaesser H, Hock MB, Vergnes L, Williams KJ, Argus JP, Marbois BN, Komisopoulou E, Wilson EB, Osborne TF, Graeber TG, Reue K, Brooks DG, Bensinger SJ. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector t cells and adaptive immunity. Nature immunology. 2013;14:489–499. doi: 10.1038/ni.2570. The authors demonstrate a critical role for SREBP in blasting of CD8+ T cells as well as clonal expansion of T cells during viral infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. Mtorc1 couples immune signals and metabolic programming to establish t(reg)-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong AJ, Gebre AK, Parks JS, Hedrick CC. Atp-binding cassette transporter g1 negatively regulates thymocyte and peripheral lymphocyte proliferation. Journal of immunology. 2010;184:173–183. doi: 10.4049/jimmunol.0902372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilhelm AJ, Zabalawi M, Grayson JM, Weant AE, Major AS, Owen J, Bharadwaj M, Walzem R, Chan L, Oka K, Thomas MJ, Sorci-Thomas MG. Apolipoprotein a-i and its role in lymphocyte cholesterol homeostasis and autoimmunity. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:843–849. doi: 10.1161/ATVBAHA.108.183442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geyeregger R, Shehata M, Zeyda M, Kiefer FW, Stuhlmeier KM, Porpaczy E, Zlabinger GJ, Jager U, Stulnig TM. Liver × receptors interfere with cytokine-induced proliferation and cell survival in normal and leukemic lymphocytes. Journal of leukocyte biology. 2009;86:1039–1048. doi: 10.1189/jlb.1008663. [DOI] [PubMed] [Google Scholar]
- 21.Cui G, Qin X, Wu L, Zhang Y, Sheng X, Yu Q, Sheng H, Xi B, Zhang JZ, Zang YQ. Liver × receptor (lxr) mediates negative regulation of mouse and human th17 differentiation. The Journal of clinical investigation. 2011;121:658–670. doi: 10.1172/JCI42974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, Busby SA, Garcia-Ordonez RD, Burris TP, Griffin PR. The benzenesulfoamide t0901317 [n-(2,2,2-trifluoroethyl)-n-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethy l]phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-alpha/gamma inverse agonist. Molecular pharmacology. 2010;77:228–236. doi: 10.1124/mol.109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solt LA, Kamenecka TM, Burris TP. Lxr-mediated inhibition of cd4+ t helper cells. PloS one. 2012;7:e46615. doi: 10.1371/journal.pone.0046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walcher D, Vasic D, Heinz P, Bach H, Durst R, Hausauer A, Hombach V, Marx N. Lxr activation inhibits chemokine-induced cd4-positive lymphocyte migration. Basic research in cardiology. 2010;105:487–494. doi: 10.1007/s00395-010-0092-5. [DOI] [PubMed] [Google Scholar]
- 25.Walcher D, Kummel A, Kehrle B, Bach H, Grub M, Durst R, Hombach V, Marx N. Lxr activation reduces proinflammatory cytokine expression in human cd4-positive lymphocytes. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1022–1028. doi: 10.1161/01.ATV.0000210278.67076.8f. [DOI] [PubMed] [Google Scholar]
- 26••.Soroosh P, Wu J, Xue X, Song J, Sutton SW, Sablad M, Yu J, Nelen MI, Liu X, Castro G, Luna R, Crawford S, Banie H, Dandridge RA, Deng X, Bittner A, Kuei C, Tootoonchi M, Rozenkrants N, Herman K, Gao J, Yang XV, Sachen K, Ngo K, Fung-Leung WP, Nguyen S, de Leon-Tabaldo A, Blevitt J, Zhang Y, Cummings MD, Rao T, Mani NS, Liu C, McKinnon M, Milla ME, Fourie AM, Sun S. Oxysterols are agonist ligands of rorgammat and drive th17 cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:12163–12168. doi: 10.1073/pnas.1322807111. The authors identify several naturally occurring oxysterols as RORγt agonists and show that they promote Th17 polarization of T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Hu X, Wang Y, Hao L, Liu X, Lesch CA, Sanchez BM, Wendling JM, Morgan RW, Aicher TD, Carter LL, Toogood PL, Glick GD. Sterol metabolism controls t17 differentiation by generating endogenous rorgamma agonists. Nature chemical biology. 2015;11(2):141–7. doi: 10.1038/nchembio.1714. The authors report that during Th17 differentiation coordinated changes in cholesterol metabolism lead to accumulation of desmosterol, which functions as a potent endogenous RORγt agonist. [DOI] [PubMed] [Google Scholar]
- 28••.Reboldi A, Dang EV, McDonald JG, Liang G, Russell DW, Cyster JG. Inflammation. 25-hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type i interferon. Science. 2014;345:679–684. doi: 10.1126/science.1254790. The authors report that 25-hydroxycholesterol suppresses production of IL-1 family cytokines by broading inhibiting inflammasomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He HT, Lellouch A, Marguet D. Lipid rafts and the initiation of t cell receptor signaling. Seminars in immunology. 2005;17:23–33. doi: 10.1016/j.smim.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Molnar E, Swamy M, Holzer M, Beck-Garcia K, Worch R, Thiele C, Guigas G, Boye K, Luescher IF, Schwille P, Schubert R, Schamel WW. Cholesterol and sphingomyelin drive ligand-independent t-cell antigen receptor nanoclustering. The Journal of biological chemistry. 2012;287:42664–42674. doi: 10.1074/jbc.M112.386045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the t cell antigen receptor. The Journal of cell biology. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 33.Chyu KY, Lio WM, Dimayuga PC, Zhou J, Zhao X, Yano J, Trinidad P, Honjo T, Cercek B, Shah PK. Cholesterol lowering modulates t cell function in vivo and in vitro. PloS one. 2014;9:e92095. doi: 10.1371/journal.pone.0092095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Surls J, Nazarov-Stoica C, Kehl M, Olsen C, Casares S, Brumeanu TD. Increased membrane cholesterol in lymphocytes diverts t-cells toward an inflammatory response. PloS one. 2012;7:e38733. doi: 10.1371/journal.pone.0038733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng HY, Wu R, Gebre AK, Hanna RN, Smith DJ, Parks JS, Ley K, Hedrick CC. Increased cholesterol content in gammadelta (gammadelta) t lymphocytes differentially regulates their activation. PloS one. 2013;8:e63746. doi: 10.1371/journal.pone.0063746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sag D, Wingender G, Nowyhed H, Wu R, Gebre AK, Parks JS, Kronenberg M, Hedrick CC. Atp-binding cassette transporter g1 intrinsically regulates invariant nkt cell development. Journal of immunology. 2012;189:5129–5138. doi: 10.4049/jimmunol.1201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heine G, Dahten A, Hilt K, Ernst D, Milovanovic M, Hartmann B, Worm M. Liver × receptors control ige expression in b cells. Journal of immunology. 2009;182:5276–5282. doi: 10.4049/jimmunol.0801804. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Duan Y, Kang Y, Yang X, Jiang M, Zhang L, Li G, Yin Z, Hu W, Dong P, Li X, Hajjar DP, Han J. Activation of liver × receptor induces macrophage interleukin-5 expression. The Journal of biological chemistry. 2012;287:43340–43350. doi: 10.1074/jbc.M112.403394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park K, Scott AL. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type i interferons. Journal of leukocyte biology. 2010;88:1081–1087. doi: 10.1189/jlb.0610318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauman DR, Bitmansour AD, McDonald JG, Thompson BM, Liang G, Russell DW. 25-hydroxycholesterol secreted by macrophages in response to toll-like receptor activation suppresses immunoglobulin a production. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16764–16769. doi: 10.1073/pnas.0909142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hannedouche S, Zhang J, Yi T, Shen W, Nguyen D, Pereira JP, Guerini D, Baumgarten BU, Roggo S, Wen B, Knochenmuss R, Noel S, Gessier F, Kelly LM, Vanek M, Laurent S, Preuss I, Miault C, Christen I, Karuna R, Li W, Koo DI, Suply T, Schmedt C, Peters EC, Falchetto R, Katopodis A, Spanka C, Roy MO, Detheux M, Chen YA, Schultz PG, Cho CY, Seuwen K, Cyster JG, Sailer AW. Oxysterols direct immune cell migration via ebi2. Nature. 2011;475:524–527. doi: 10.1038/nature10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu C, Yang XV, Wu J, Kuei C, Mani NS, Zhang L, Yu J, Sutton SW, Qin N, Banie H, Karlsson L, Sun S, Lovenberg TW. Oxysterols direct b-cell migration through ebi2. Nature. 2011;475:519–523. doi: 10.1038/nature10226. [DOI] [PubMed] [Google Scholar]
- 43.Pierce SK. Lipid rafts and b-cell activation. Nature reviews Immunology. 2002;2:96–105. doi: 10.1038/nri726. [DOI] [PubMed] [Google Scholar]
- 44.Gupta N, DeFranco AL. Lipid rafts and b cell signaling. Seminars in cell & developmental biology. 2007;18:616–626. doi: 10.1016/j.semcdb.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karnell FG, Brezski RJ, King LB, Silverman MA, Monroe JG. Membrane cholesterol content accounts for developmental differences in surface b cell receptor compartmentalization and signaling. The Journal of biological chemistry. 2005;280:25621–25628. doi: 10.1074/jbc.M503162200. [DOI] [PubMed] [Google Scholar]
- 46.Anderson HA, Hiltbold EM, Roche PA. Concentration of mhc class ii molecules in lipid rafts facilitates antigen presentation. Nature immunology. 2000;1:156–162. doi: 10.1038/77842. [DOI] [PubMed] [Google Scholar]
- 47.Hiltbold EM, Poloso NJ, Roche PA. Mhc class ii-peptide complexes and apc lipid rafts accumulate at the immunological synapse. Journal of immunology. 2003;170:1329–1338. doi: 10.4049/jimmunol.170.3.1329. [DOI] [PubMed] [Google Scholar]
- 48.Son Y, Kim SM, Lee SA, Eo SK, Kim K. Oxysterols induce transition of monocytic cells to phenotypically mature dendritic cell-like cells. Biochemical and biophysical research communications. 2013;438:161–168. doi: 10.1016/j.bbrc.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 49.Perrin-Cocon L, Coutant F, Agaugue S, Deforges S, Andre P, Lotteau V. Oxidized low-density lipoprotein promotes mature dendritic cell transition from differentiating monocyte. Journal of immunology. 2001;167:3785–3791. doi: 10.4049/jimmunol.167.7.3785. [DOI] [PubMed] [Google Scholar]
- 50.Zhong L, Yang Q, Xie W, Zhou J. Liver × receptor regulates mouse gm-csf-derived dendritic cell differentiation in vitro. Molecular immunology. 2014;60:32–43. doi: 10.1016/j.molimm.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Feig JE, Pineda-Torra I, Sanson M, Bradley MN, Vengrenyuk Y, Bogunovic D, Gautier EL, Rubinstein D, Hong C, Liu J, Wu C, van Rooijen N, Bhardwaj N, Garabedian M, Tontonoz P, Fisher EA. Lxr promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. The Journal of clinical investigation. 2010;120:4415–4424. doi: 10.1172/JCI38911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torocsik D, Barath M, Benko S, Szeles L, Dezso B, Poliska S, Hegyi Z, Homolya L, Szatmari I, Lanyi A, Nagy L. Activation of liver × receptor sensitizes human dendritic cells to inflammatory stimuli. Journal of immunology. 2010;184:5456–5465. doi: 10.4049/jimmunol.0902399. [DOI] [PubMed] [Google Scholar]
- 53.Geyeregger R, Zeyda M, Bauer W, Kriehuber E, Saemann MD, Zlabinger GJ, Maurer D, Stulnig TM. Liver × receptors regulate dendritic cell phenotype and function through blocked induction of the actin-bundling protein fascin. Blood. 2007;109:4288–4295. doi: 10.1182/blood-2006-08-043422. [DOI] [PubMed] [Google Scholar]
- 54.Kim KD, Lim HY, Lee HG, Yoon DY, Choe YK, Choi I, Paik SG, Kim YS, Yang Y, Lim JS. Apolipoprotein a-i induces il-10 and pge2 production in human monocytes and inhibits dendritic cell differentiation and maturation. Biochemical and biophysical research communications. 2005;338:1126–1136. doi: 10.1016/j.bbrc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 55.Perrin-Cocon L, Diaz O, Carreras M, Dollet S, Guironnet-Paquet A, Andre P, Lotteau V. High-density lipoprotein phospholipids interfere with dendritic cell th1 functional maturation. Immunobiology. 2012;217:91–99. doi: 10.1016/j.imbio.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 56.Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA, Randolph GJ. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Yi T, Cyster JG. Ebi2-mediated bridging channel positioning supports splenic dendritic cell homeostasis and particulate antigen capture. eLife. 2013;2:e00757. doi: 10.7554/eLife.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bosch B, Heipertz EL, Drake JR, Roche PA. Major histocompatibility complex (mhc) class ii-peptide complexes arrive at the plasma membrane in cholesterol-rich microclusters. The Journal of biological chemistry. 2013;288:13236–13242. doi: 10.1074/jbc.M112.442640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roy K, Ghosh M, Pal TK, Chakrabarti S, Roy S. Cholesterol lowering drug may influence cellular immune response by altering mhc ii function. Journal of lipid research. 2013;54:3106–3115. doi: 10.1194/jlr.M041954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buatois V, Baillet M, Becart S, Mooney N, Leserman L, Machy P. Mhc class ii-peptide complexes in dendritic cell lipid microdomains initiate the cd4 th1 phenotype. Journal of immunology. 2003;171:5812–5819. doi: 10.4049/jimmunol.171.11.5812. [DOI] [PubMed] [Google Scholar]
- 61.Eren E, Yates J, Cwynarski K, Preston S, Dong R, Germain C, Lechler R, Huby R, Ritter M, Lombardi G. Location of major histocompatibility complex class ii molecules in rafts on dendritic cells enhances the efficiency of t-cell activation and proliferation. Scandinavian journal of immunology. 2006;63:7–16. doi: 10.1111/j.1365-3083.2006.01700.x. [DOI] [PubMed] [Google Scholar]
- 62.Wang SH, Yuan SG, Peng DQ, Zhao SP. Hdl and apoa-i inhibit antigen presentation-mediated t cell activation by disrupting lipid rafts in antigen presenting cells. Atherosclerosis. 2012;225:105–114. doi: 10.1016/j.atherosclerosis.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 63.Kuipers HF, Biesta PJ, Groothuis TA, Neefjes JJ, Mommaas AM, van den Elsen PJ. Statins affect cell-surface expression of major histocompatibility complex class ii molecules by disrupting cholesterol-containing microdomains. Human immunology. 2005;66:653–665. doi: 10.1016/j.humimm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 64.Kimata H. Cholesterol selectively enhances in vitro latex-specific ige production in atopic dermatitis patients with latex allergy. Life sciences. 2005;76:1527–1532. doi: 10.1016/j.lfs.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 65.Ouyang F, Kumar R, Pongracic J, Story RE, Liu X, Wang B, Xing H, Liu X, Li Z, Zhang W, Fang Y, Zhang S, Xu X, Wang X. Adiposity, serum lipid levels, and allergic sensitization in chinese men and women. The Journal of allergy and clinical immunology. 2009;123:940–948 e910. doi: 10.1016/j.jaci.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kusunoki T, Morimoto T, Sakuma M, Mukaida K, Yasumi T, Nishikomori R, Fujii T, Heike T. Total and low-density lipoprotein cholesterol levels are associated with atopy in schoolchildren. The Journal of pediatrics. 2011;158:334–336. doi: 10.1016/j.jpeds.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 67.Pesonen M, Ranki A, Siimes MA, Kallio MJ. Serum cholesterol level in infancy is inversely associated with subsequent allergy in children and adolescents. A 20-year follow-up study. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2008;38:178–184. doi: 10.1111/j.1365-2222.2007.02875.x. [DOI] [PubMed] [Google Scholar]
- 68.Fessler MB, Jaramillo R, Crockett PW, Zeldin DC. Relationship of serum cholesterol levels to atopy in the us population. Allergy. 2010;65:859–864. doi: 10.1111/j.1398-9995.2009.02287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yeh YF, Huang SL. Enhancing effect of dietary cholesterol and inhibitory effect of pravastatin on allergic pulmonary inflammation. Journal of biomedical science. 2004;11:599–606. doi: 10.1007/BF02256124. [DOI] [PubMed] [Google Scholar]
- 70.Chen YC, Tung KY, Tsai CH, Su MW, Wang PC, Chen CH, Lee YL. Lipid profiles in children with and without asthma: Interaction of asthma and obesity on hyperlipidemia. Diabetes & metabolic syndrome. 2013;7:20–25. doi: 10.1016/j.dsx.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 71.Scichilone N, Rizzo M, Benfante A, Catania R, Giglio RV, Nikolic D, Montalto G, Bellia V. Serum low density lipoprotein subclasses in asthma. Respiratory medicine. 2013;107:1866–1872. doi: 10.1016/j.rmed.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 72.Fenger RV, Gonzalez-Quintela A, Linneberg A, Husemoen LL, Thuesen BH, Aadahl M, Vidal C, Skaaby T, Sainz JC, Calvo E. The relationship of serum triglycerides, serum hdl, and obesity to the risk of wheezing in 85,555 adults. Respiratory medicine. 2013;107:816–824. doi: 10.1016/j.rmed.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 73.Cirillo DJ, Agrawal Y, Cassano PA. Lipids and pulmonary function in the third national health and nutrition examination survey. American journal of epidemiology. 2002;155:842–848. doi: 10.1093/aje/155.9.842. [DOI] [PubMed] [Google Scholar]
- 74.Yiallouros PK, Savva SC, Kolokotroni O, Dima K, Zerva A, Kouis P, Bousquet J, Middleton N. Asthma: The role of low high-density-lipoprotein cholesterol in childhood and adolescence. International archives of allergy and immunology. 2014;165:91–99. doi: 10.1159/000368405. [DOI] [PubMed] [Google Scholar]
- 75.Fessler MB. Next stop for hdl: The lung. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2012;42:340–342. doi: 10.1111/j.1365-2222.2011.03942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fessler MB, Massing MW, Spruell B, Jaramillo R, Draper DW, Madenspacher JH, Arbes SJ, Calatroni A, Zeldin DC. Novel relationship of serum cholesterol with asthma and wheeze in the united states. The Journal of allergy and clinical immunology. 2009;124:967–974. e961–915. doi: 10.1016/j.jaci.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ho WE, Xu YJ, Xu F, Cheng C, Peh HY, Tannenbaum SR, Wong WS, Ong CN. Metabolomics reveals altered metabolic pathways in experimental asthma. American journal of respiratory cell and molecular biology. 2013;48:204–211. doi: 10.1165/rcmb.2012-0246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dai C, Yao X, Keeran KJ, Zywicke GJ, Qu X, Yu ZX, Dagur PK, McCoy JP, Remaley AT, Levine SJ. Apolipoprotein a-i attenuates ovalbumin-induced neutrophilic airway inflammation via a granulocyte colony-stimulating factor-dependent mechanism. American journal of respiratory cell and molecular biology. 2012;47:186–195. doi: 10.1165/rcmb.2011-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ekmekci OB, Donma O, Ekmekci H, Yildirim N, Uysal O, Sardogan E, Demirel H, Demir T. Plasma paraoxonase activities, lipoprotein oxidation, and trace element interaction in asthmatic patients. Biological trace element research. 2006;111:41–52. doi: 10.1385/bter:111:1:41. [DOI] [PubMed] [Google Scholar]
- 80.Nandedkar SD, Weihrauch D, Xu H, Shi Y, Feroah T, Hutchins W, Rickaby DA, Duzgunes N, Hillery CA, Konduri KS, Pritchard KA., Jr D-4f, an apoa-1 mimetic, decreases airway hyperresponsiveness, inflammation, and oxidative stress in a murine model of asthma. Journal of lipid research. 2011;52:499–508. doi: 10.1194/jlr.M012724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Draper DW, Gowdy KM, Madenspacher JH, Wilson RH, Whitehead GS, Nakano H, Pandiri AR, Foley JF, Remaley AT, Cook DN, Fessler MB. Atp binding cassette transporter g1 deletion induces il-17-dependent dysregulation of pulmonary adaptive immunity. Journal of immunology. 2012;188:5327–5336. doi: 10.4049/jimmunol.1101605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang W, Xu H, Shi Y, Nandedkar S, Zhang H, Gao H, Feroah T, Weihrauch D, Schulte ML, Jones DW, Jarzembowski J, Sorci-Thomas M, Pritchard KA., Jr Genetic deletion of apolipoprotein a-i increases airway hyperresponsiveness, inflammation, and collagen deposition in the lung. Journal of lipid research. 2010;51:2560–2570. doi: 10.1194/jlr.M004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Otera H, Ishida T, Nishiuma T, Kobayashi K, Kotani Y, Yasuda T, Kundu RK, Quertermous T, Hirata K, Nishimura Y. Targeted inactivation of endothelial lipase attenuates lung allergic inflammation through raising plasma hdl level and inhibiting eosinophil infiltration. American journal of physiology Lung cellular and molecular physiology. 2009;296:L594–602. doi: 10.1152/ajplung.90530.2008. [DOI] [PubMed] [Google Scholar]
- 84.Fredriksson K, Mishra A, Lam JK, Mushaben EM, Cuento RA, Meyer KS, Yao X, Keeran KJ, Nugent GZ, Qu X, Yu ZX, Yang Y, Raghavachari N, Dagur PK, McCoy JP, Levine SJ. The very low density lipoprotein receptor attenuates house dust mite-induced airway inflammation by suppressing dendritic cell-mediated adaptive immune responses. Journal of immunology. 2014;192:4497–4509. doi: 10.4049/jimmunol.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yao X, Fredriksson K, Yu ZX, Xu X, Raghavachari N, Keeran KJ, Zywicke GJ, Kwak M, Amar MJ, Remaley AT, Levine SJ. Apolipoprotein e negatively regulates house dust mite-induced asthma via a low-density lipoprotein receptor-mediated pathway. American journal of respiratory and critical care medicine. 2010;182:1228–1238. doi: 10.1164/rccm.201002-0308OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yao X, Dai C, Fredriksson K, Lam J, Gao M, Keeran KJ, Nugent GZ, Qu X, Yu ZX, Jeffries N, Lin J, Kaler M, Shamburek R, Costello R, Csako G, Dahl M, Nordestgaard BG, Remaley AT, Levine SJ. Human apolipoprotein e genotypes differentially modify house dust mite-induced airway disease in mice. American journal of physiology Lung cellular and molecular physiology. 2012;302:L206–215. doi: 10.1152/ajplung.00110.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yao X, Dai C, Fredriksson K, Dagur PK, McCoy JP, Qu X, Yu ZX, Keeran KJ, Zywicke GJ, Amar MJ, Remaley AT, Levine SJ. 5a, an apolipoprotein a-i mimetic peptide, attenuates the induction of house dust mite-induced asthma. Journal of immunology. 2011;186:576–583. doi: 10.4049/jimmunol.1001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi Y, Xu X, Tan Y, Mao S, Fang S, Gu W. A liver-×-receptor ligand, t0901317, attenuates ige production and airway remodeling in chronic asthma model of mice. PloS one. 2014;9:e92668. doi: 10.1371/journal.pone.0092668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fowler AJ, Sheu MY, Schmuth M, Kao J, Fluhr JW, Rhein L, Collins JL, Willson TM, Mangelsdorf DJ, Elias PM, Feingold KR. Liver × receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: Liver-×-receptor-specific inhibition of inflammation and primary cytokine production. The Journal of investigative dermatology. 2003;120:246–255. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- 90.Munoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nature reviews Rheumatology. 2010;6:280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 91.Rothlin CV, Lemke G. Tam receptor signaling and autoimmune disease. Current opinion in immunology. 2010;22:740–746. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.AG N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Diaz M, Gallardo G, de Galarreta CR, Salazar J, Lopez F, Edwards P, Parks J, Andujar M, Tontonoz P, Castrillo A. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor lxr. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chintalacharuvu SR, Sandusky GE, Burris TP, Burmer GC, Nagpal S. Liver × receptor is a therapeutic target in collagen-induced arthritis. Arthritis and rheumatism. 2007;56:1365–1367. doi: 10.1002/art.22528. [DOI] [PubMed] [Google Scholar]
- 94.Hindinger C, Hinton DR, Kirwin SJ, Atkinson RD, Burnett ME, Bergmann CC, Stohlman SA. Liver × receptor activation decreases the severity of experimental autoimmune encephalomyelitis. Journal of neuroscience research. 2006;84:1225–1234. doi: 10.1002/jnr.21038. [DOI] [PubMed] [Google Scholar]
- 95.Xu J, Wagoner G, Douglas JC, Drew PD. Liver × receptor agonist regulation of th17 lymphocyte function in autoimmunity. Journal of leukocyte biology. 2009;86:401–409. doi: 10.1189/jlb.1008600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang H, Zheng S, Qiu Y, Yang Y, Wang C, Yang P, Li Q, Lei B. Activation of liver × receptor alleviates ocular inflammation in experimental autoimmune uveitis. Investigative ophthalmology & visual science. 2014;55:2795–2804. doi: 10.1167/iovs.13-13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grainger DJ, Reckless J, McKilligin E. Apolipoprotein e modulates clearance of apoptotic bodies in vitro and in vivo, resulting in a systemic proinflammatory state in apolipoprotein e-deficient mice. Journal of immunology. 2004;173:6366–6375. doi: 10.4049/jimmunol.173.10.6366. [DOI] [PubMed] [Google Scholar]
- 98.Murao K, Terpstra V, Green SR, Kondratenko N, Steinberg D, Quehenberger O. Characterization of cla-1, a human homologue of rodent scavenger receptor bi, as a receptor for high density lipoprotein and apoptotic thymocytes. The Journal of biological chemistry. 1997;272:17551–17557. doi: 10.1074/jbc.272.28.17551. [DOI] [PubMed] [Google Scholar]
- 99.Feng H, Guo L, Wang D, Gao H, Hou G, Zheng Z, Ai J, Foreman O, Daugherty A, Li XA. Deficiency of scavenger receptor bi leads to impaired lymphocyte homeostasis and autoimmune disorders in mice. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2543–2551. doi: 10.1161/ATVBAHA.111.234716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hamon Y, Chambenoit O, Chimini G. Abca1 and the engulfment of apoptotic cells. Biochimica et biophysica acta. 2002;1585:64–71. doi: 10.1016/s1388-1981(02)00325-6. [DOI] [PubMed] [Google Scholar]
- 101.Woo JM, Lin Z, Navab M, Van Dyck C, Trejo-Lopez Y, Woo KM, Li H, Castellani LW, Wang X, Iikuni N, Rullo OJ, Wu H, La Cava A, Fogelman AM, Lusis AJ, Tsao BP. Treatment with apolipoprotein a-1 mimetic peptide reduces lupus-like manifestations in a murine lupus model of accelerated atherosclerosis. Arthritis research & therapy. 2010;12:R93. doi: 10.1186/ar3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morse JH, Witte LD, Goodman DS. Inhibition of lymphocyte proliferation stimulated by lectins and allogeneic cells by normal plasma lipoproteins. The Journal of experimental medicine. 1977;146:1791–1803. doi: 10.1084/jem.146.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Charles-Schoeman C, Watanabe J, Lee YY, Furst DE, Amjadi S, Elashoff D, Park G, McMahon M, Paulus HE, Fogelman AM, Reddy ST. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis and rheumatism. 2009;60:2870–2879. doi: 10.1002/art.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Svenungsson E, Gunnarsson I, Fei GZ, Lundberg IE, Klareskog L, Frostegard J. Elevated triglycerides and low levels of high-density lipoprotein as markers of disease activity in association with up-regulation of the tumor necrosis factor alpha/tumor necrosis factor receptor system in systemic lupus erythematosus. Arthritis and rheumatism. 2003;48:2533–2540. doi: 10.1002/art.11264. [DOI] [PubMed] [Google Scholar]
- 105.Azzam KM, Fessler MB. Crosstalk between reverse cholesterol transport and innate immunity. Trends in endocrinology and metabolism: TEM. 2012;23:169–178. doi: 10.1016/j.tem.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Y, Park YB, Patel E, Silverman GJ. Igm antibodies to apoptosis-associated determinants recruit c1q and enhance dendritic cell phagocytosis of apoptotic cells. Journal of immunology. 2009;182:6031–6043. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen Y, Khanna S, Goodyear CS, Park YB, Raz E, Thiel S, Gronwall C, Vas J, Boyle DL, Corr M, Kono DH, Silverman GJ. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses tlr responses and inhibits inflammatory arthritis. Journal of immunology. 2009;183:1346–1359. doi: 10.4049/jimmunol.0900948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Flores-Borja F, Kabouridis PS, Jury EC, Isenberg DA, Mageed RA. Decreased lyn expression and translocation to lipid raft signaling domains in b lymphocytes from patients with systemic lupus erythematosus. Arthritis and rheumatism. 2005;52:3955–3965. doi: 10.1002/art.21416. [DOI] [PubMed] [Google Scholar]
- 109.Jury EC, Flores-Borja F, Kabouridis PS. Lipid rafts in t cell signalling and disease. Seminars in cell & developmental biology. 2007;18:608–615. doi: 10.1016/j.semcdb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krishnan S, Nambiar MP, Warke VG, Fisher CU, Mitchell J, Delaney N, Tsokos GC. Alterations in lipid raft composition and dynamics contribute to abnormal t cell responses in systemic lupus erythematosus. Journal of immunology. 2004;172:7821–7831. doi: 10.4049/jimmunol.172.12.7821. [DOI] [PubMed] [Google Scholar]
- 111.Jury EC, Isenberg DA, Mauri C, Ehrenstein MR. Atorvastatin restores lck expression and lipid raft-associated signaling in t cells from patients with systemic lupus erythematosus. Journal of immunology. 2006;177:7416–7422. doi: 10.4049/jimmunol.177.10.7416. [DOI] [PubMed] [Google Scholar]
- 112.McDonald G, Deepak S, Miguel L, Hall CJ, Isenberg DA, Magee AI, Butters T, Jury EC. Normalizing glycosphingolipids restores function in cd4+ t cells from lupus patients. The Journal of clinical investigation. 2014;124:712–724. doi: 10.1172/JCI69571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nature medicine. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 114.Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, Bravo M, Mitchell DJ, Sobel RA, Steinman L, Zamvil SS. The hmg-coa reductase inhibitor, atorvastatin, promotes a th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 115.Ulivieri C, Baldari CT. Statins: From cholesterol-lowering drugs to novel immunomodulators for the treatment of th17-mediated autoimmune diseases. Pharmacological research : the official journal of the Italian Pharmacological Society. 2014;88:41–52. doi: 10.1016/j.phrs.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 116.Ghittoni R, Napolitani G, Benati D, Ulivieri C, Patrussi L, Laghi Pasini F, Lanzavecchia A, Baldari CT. Simvastatin inhibits the mhc class ii pathway of antigen presentation by impairing ras superfamily gtpases. European journal of immunology. 2006;36:2885–2893. doi: 10.1002/eji.200636567. [DOI] [PubMed] [Google Scholar]
- 117.Ulivieri C, Fanigliulo D, Benati D, Pasini FL, Baldari CT. Simvastatin impairs humoral and cell-mediated immunity in mice by inhibiting lymphocyte homing, t-cell activation and antigen cross-presentation. European journal of immunology. 2008;38:2832–2844. doi: 10.1002/eji.200838278. [DOI] [PubMed] [Google Scholar]
- 118.Arora M, Chen L, Paglia M, Gallagher I, Allen JE, Vyas YM, Ray A, Ray P. Simvastatin promotes th2-type responses through the induction of the chitinase family member ym1 in dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7777–7782. doi: 10.1073/pnas.0508492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dunn SE, Youssef S, Goldstein MJ, Prod’homme T, Weber MS, Zamvil SS, Steinman L. Isoprenoids determine th1/th2 fate in pathogenic t cells, providing a mechanism of modulation of autoimmunity by atorvastatin. The Journal of experimental medicine. 2006;203:401–412. doi: 10.1084/jem.20051129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Blank N, Schiller M, Krienke S, Busse F, Schatz B, Ho AD, Kalden JR, Lorenz HM. Atorvastatin inhibits t cell activation through 3-hydroxy-3-methylglutaryl coenzyme a reductase without decreasing cholesterol synthesis. Journal of immunology. 2007;179:3613–3621. doi: 10.4049/jimmunol.179.6.3613. [DOI] [PubMed] [Google Scholar]
- 121.Hakamada-Taguchi R, Uehara Y, Kuribayashi K, Numabe A, Saito K, Negoro H, Fujita T, Toyo-oka T, Kato T. Inhibition of hydroxymethylglutaryl-coenzyme a reductase reduces th1 development and promotes th2 development. Circulation research. 2003;93:948–956. doi: 10.1161/01.RES.0000101298.76864.14. [DOI] [PubMed] [Google Scholar]
- 122.Hillyard DZ, Cameron AJ, McDonald KJ, Thomson J, MacIntyre A, Shiels PG, Panarelli M, Jardine AG. Simvastatin inhibits lymphocyte function in normal subjects and patients with cardiovascular disease. Atherosclerosis. 2004;175:305–313. doi: 10.1016/j.atherosclerosis.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 123.Coward W, Chow SC. Effect of atorvastatin on th1 and th2 cytokine secreting cells during t cell activation and differentiation. Atherosclerosis. 2006;186:302–309. doi: 10.1016/j.atherosclerosis.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 124.Fehr T, Kahlert C, Fierz W, Joller-Jemelka HI, Riesen WF, Rickli H, Wuthrich RP, Ammann P. Statin-induced immunomodulatory effects on human t cells in vivo. Atherosclerosis. 2004;175:83–90. doi: 10.1016/j.atherosclerosis.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 125.Zhang X, Jin J, Peng X, Ramgolam VS, Markovic-Plese S. Simvastatin inhibits il-17 secretion by targeting multiple il-17-regulatory cytokines and by inhibiting the expression of il-17 transcription factor rorc in cd4+ lymphocytes. Journal of immunology. 2008;180:6988–6996. doi: 10.4049/jimmunol.180.10.6988. [DOI] [PubMed] [Google Scholar]
- 126.Zhang X, Tao Y, Wang J, Garcia-Mata R, Markovic-Plese S. Simvastatin inhibits secretion of th17-polarizing cytokines and antigen presentation by dcs in patients with relapsing remitting multiple sclerosis. European journal of immunology. 2013;43:281–289. doi: 10.1002/eji.201242566. [DOI] [PubMed] [Google Scholar]
- 127.Kim YC, Kim KK, Shevach EM. Simvastatin induces foxp3+ t regulatory cells by modulation of transforming growth factor-beta signal transduction. Immunology. 2010;130:484–493. doi: 10.1111/j.1365-2567.2010.03269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maneechotesuwan K, Kasetsinsombat K, Wamanuttajinda V, Wongkajornsilp A, Barnes PJ. Statins enhance the effects of corticosteroids on the balance between regulatory t cells and th17 cells. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2013;43:212–222. doi: 10.1111/cea.12067. [DOI] [PubMed] [Google Scholar]
- 129.Robinson AJ, Kashanin D, O’Dowd F, Fitzgerald K, Williams V, Walsh GM. Fluvastatin and lovastatin inhibit granulocyte macrophage-colony stimulating factor-stimulated human eosinophil adhesion to inter-cellular adhesion molecule-1 under flow conditions. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2009;39:1866–1874. doi: 10.1111/j.1365-2222.2009.03334.x. [DOI] [PubMed] [Google Scholar]
- 130.Krauth MT, Majlesi Y, Sonneck K, Samorapoompichit P, Ghannadan M, Hauswirth AW, Baghestanian M, Schernthaner GH, Worda C, Muller MR, Sperr WR, Valent P. Effects of various statins on cytokine-dependent growth and ige-dependent release of histamine in human mast cells. Allergy. 2006;61:281–288. doi: 10.1111/j.1398-9995.2006.00997.x. [DOI] [PubMed] [Google Scholar]
- 131.Schaafsma D, Dueck G, Ghavami S, Kroeker A, Mutawe MM, Hauff K, Xu FY, McNeill KD, Unruh H, Hatch GM, Halayko AJ. The mevalonate cascade as a target to suppress extracellular matrix synthesis by human airway smooth muscle. American journal of respiratory cell and molecular biology. 2011;44:394–403. doi: 10.1165/rcmb.2010-0052OC. [DOI] [PubMed] [Google Scholar]
- 132.Schaafsma D, McNeill KD, Mutawe MM, Ghavami S, Unruh H, Jacques E, Laviolette M, Chakir J, Halayko AJ. Simvastatin inhibits tgfbeta1-induced fibronectin in human airway fibroblasts. Respiratory research. 2011;12:113. doi: 10.1186/1465-9921-12-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Takeda N, Kondo M, Ito S, Ito Y, Shimokata K, Kume H. Role of rhoa inactivation in reduced cell proliferation of human airway smooth muscle by simvastatin. American journal of respiratory cell and molecular biology. 2006;35:722–729. doi: 10.1165/rcmb.2006-0034OC. [DOI] [PubMed] [Google Scholar]
- 134.Chiba Y, Sato S, Misawa M. Inhibition of antigen-induced bronchial smooth muscle hyperresponsiveness by lovastatin in mice. Journal of smooth muscle research = Nihon Heikatsukin Gakkai kikanshi. 2008;44:123–128. doi: 10.1540/jsmr.44.123. [DOI] [PubMed] [Google Scholar]
- 135.Kim DY, Ryu SY, Lim JE, Lee YS, Ro JY. Anti-inflammatory mechanism of simvastatin in mouse allergic asthma model. European journal of pharmacology. 2007;557:76–86. doi: 10.1016/j.ejphar.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 136.McKay A, Leung BP, McInnes IB, Thomson NC, Liew FY. A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. Journal of immunology. 2004;172:2903–2908. doi: 10.4049/jimmunol.172.5.2903. [DOI] [PubMed] [Google Scholar]
- 137.Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin inhibits airway hyperreactivity: Implications for the mevalonate pathway and beyond. American journal of respiratory and critical care medicine. 2009;180:731–740. doi: 10.1164/rccm.200901-0018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Imamura M, Okunishi K, Ohtsu H, Nakagome K, Harada H, Tanaka R, Yamamoto K, Dohi M. Pravastatin attenuates allergic airway inflammation by suppressing antigen sensitisation, interleukin 17 production and antigen presentation in the lung. Thorax. 2009;64:44–49. doi: 10.1136/thx.2007.094540. [DOI] [PubMed] [Google Scholar]
- 139.Huang CF, Peng HJ, Wu CC, Lo WT, Shih YL, Wu TC. Effect of oral administration with pravastatin and atorvastatin on airway hyperresponsiveness and allergic reactions in asthmatic mice. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2013;110:11–17. doi: 10.1016/j.anai.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 140.Xu L, Dong XW, Shen LL, Li FF, Jiang JX, Cao R, Yao HY, Shen HJ, Sun Y, Xie QM. Simvastatin delivery via inhalation attenuates airway inflammation in a murine model of asthma. International immunopharmacology. 2012;12:556–564. doi: 10.1016/j.intimp.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 141.Zeki AA, Bratt JM, Rabowsky M, Last JA, Kenyon NJ. Simvastatin inhibits goblet cell hyperplasia and lung arginase in a mouse model of allergic asthma: A novel treatment for airway remodeling? Translational research : the journal of laboratory and clinical medicine. 2010;156:335–349. doi: 10.1016/j.trsl.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Silva D, Couto M, Delgado L, Moreira A. A systematic review of statin efficacy in asthma. The Journal of asthma : official journal of the Association for the Care of Asthma. 2012;49:885–894. doi: 10.3109/02770903.2012.721433. [DOI] [PubMed] [Google Scholar]
- 143.Si XB, Zhang S, Huo LY, Dai WL, Wang HL. Statin therapy does not improve lung function in asthma: A meta-analysis of randomized controlled trials. The Journal of international medical research. 2013;41:276–283. doi: 10.1177/0300060513477005. [DOI] [PubMed] [Google Scholar]
- 144.Naidoo D, Wu AC, Brilliant MH, Denny J, Ingram C, Kitchner TE, Linneman JG, McGeachie MJ, Roden DM, Shaffer CM, Shah A, Weeke P, Weiss ST, Xu H, Medina MW. A polymorphism in hla-g modifies statin benefit in asthma. The pharmacogenomics journal. 2014 doi: 10.1038/tpj.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: From protein prenylation to immunomodulation. Nature reviews Immunology. 2006;6:358–370. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, Capell HA, Sattar N. Trial of atorvastatin in rheumatoid arthritis (tara): Double-blind, randomised placebo-controlled trial. Lancet. 2004;363:2015–2021. doi: 10.1016/S0140-6736(04)16449-0. [DOI] [PubMed] [Google Scholar]
- 147.Lv S, Liu Y, Zou Z, Li F, Zhao S, Shi R, Bian R, Tian H. The impact of statins therapy on disease activity and inflammatory factor in patients with rheumatoid arthritis: A meta-analysis. Clinical and experimental rheumatology. 2014 [PubMed] [Google Scholar]
- 148.Vollmer T, Key L, Durkalski V, Tyor W, Corboy J, Markovic-Plese S, Preiningerova J, Rizzo M, Singh I. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 2004;363:1607–1608. doi: 10.1016/S0140-6736(04)16205-3. [DOI] [PubMed] [Google Scholar]
- 149.Sena A, Pedrosa R, Graca Morais M. Therapeutic potential of lovastatin in multiple sclerosis. Journal of neurology. 2003;250:754–755. doi: 10.1007/s00415-003-1070-8. [DOI] [PubMed] [Google Scholar]
- 150.Gkaliagkousi E, Gavriilaki E, Doumas M, Petidis K, Aslanidis S, Stella D. Cardiovascular risk in rheumatoid arthritis: Pathogenesis, diagnosis, and management. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2012;18:422–430. doi: 10.1097/RHU.0b013e31827846b1. [DOI] [PubMed] [Google Scholar]
- 151.Tu H, Li Q, Xiang S, Jiang H, Mao Y, Shou Z, Chen J. Dual effects of statins therapy in systemic lupus erythematosus and sle-related atherosclerosis: The potential role for regulatory t cells. Atherosclerosis. 2012;222:29–33. doi: 10.1016/j.atherosclerosis.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 152.Lokhandwala T, West-Strum D, Banahan BF, Bentley JP, Yang Y. Do statins improve outcomes in patients with asthma on inhaled corticosteroid therapy? A retrospective cohort analysis. BMJ open. 2012;2 doi: 10.1136/bmjopen-2012-001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zeki AA, Oldham J, Wilson M, Fortenko O, Goyal V, Last M, Last A, Patel A, Last JA, Kenyon NJ. Statin use and asthma control in patients with severe asthma. BMJ open. 2013;3 doi: 10.1136/bmjopen-2013-003314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang CC, Chan WL, Chen YC, Chen TJ, Chou KT, Lin SJ, Chen JW, Leu HB. Statin use in patients with asthma: A nationwide population-based study. European journal of clinical investigation. 2011;41:507–512. doi: 10.1111/j.1365-2362.2010.02434.x. [DOI] [PubMed] [Google Scholar]
- 155.Ostroukhova M, Kouides RW, Friedman E. The effect of statin therapy on allergic patients with asthma. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2009;103:463–468. doi: 10.1016/S1081-1206(10)60261-X. [DOI] [PubMed] [Google Scholar]
- 156.Tse SM, Li L, Butler MG, Fung V, Kharbanda EO, Larkin EK, Vollmer WM, Miroshnik I, Rusinak D, Weiss ST, Lieu T, Wu AC. Statin exposure is associated with decreased asthma-related emergency department visits and oral corticosteroid use. American journal of respiratory and critical care medicine. 2013;188:1076–1082. doi: 10.1164/rccm.201306-1017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tse SM, Charland SL, Stanek E, Herrera V, Goldfarb S, Litonjua AA, Weiss ST, Wu AC. Statin use in asthmatics on inhaled corticosteroids is associated with decreased risk of emergency department visits. Current medical research and opinion. 2014;30:685–693. doi: 10.1185/03007995.2013.865599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Braganza G, Chaudhuri R, McSharry C, Weir CJ, Donnelly I, Jolly L, Lafferty J, Lloyd SM, Spears M, Mair F, Thomson NC. Effects of short-term treatment with atorvastatin in smokers with asthma--a randomized controlled trial. BMC pulmonary medicine. 2011;11:16. doi: 10.1186/1471-2466-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2, 3-dioxygenase. The Journal of allergy and clinical immunology. 2010;126:754–762 e751. doi: 10.1016/j.jaci.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 160.Menzies D, Nair A, Meldrum KT, Fleming D, Barnes M, Lipworth BJ. Simvastatin does not exhibit therapeutic anti-inflammatory effects in asthma. The Journal of allergy and clinical immunology. 2007;119:328–335. doi: 10.1016/j.jaci.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 161.Moini A, Azimi G, Farivar A. Evaluation of atorvastatin for the treatment of patients with asthma: A double-blind randomized clinical trial. Allergy, asthma & immunology research. 2012;4:290–294. doi: 10.4168/aair.2012.4.5.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hothersall EJ, Chaudhuri R, McSharry C, Donnelly I, Lafferty J, McMahon AD, Weir CJ, Meiklejohn J, Sattar N, McInnes I, Wood S, Thomson NC. Effects of atorvastatin added to inhaled corticosteroids on lung function and sputum cell counts in atopic asthma. Thorax. 2008;63:1070–1075. doi: 10.1136/thx.2008.100198. [DOI] [PubMed] [Google Scholar]
- 163.Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Simvastatin in the treatment of asthma: Lack of steroid-sparing effect. Thorax. 2010;65:891–896. doi: 10.1136/thx.2010.138990. [DOI] [PubMed] [Google Scholar]


