Abstract
There is a growing global prevalence of neurodegenerative diseases such as Alzheimer’s disease and dementia. Current treatment for neurodegenerative diseases is limited due to the blood brain barrier’s ability to restrict the entry of therapeutics to the brain. In that context, direct delivery of drugs from nose to brain has gained emerging interest as an important alternative to oral and parenteral routes of administration. Although there are considerable reports showing promising results after intranasal drug delivery in various disease-models and investigatory human clinical trials, there are very few studies showing a detailed pharmacokinetics with regard to the uptake and retention of intranasally delivered material(s) within specific brain regions, which are critical determining factors for dosing conditions and optimal treatment regimen. This investigation compared a time-dependent brain uptake and resident time of various radiolabeled candidate neurotherapeutics after a single bolus intranasal or intraperitoneal administration in mice. Results indicate that the brain uptake of intranasally delivered therapeutic(s) is > 5 times greater than that after intraperitoneal delivery. The peak uptake and resident time of all intranasally delivered test therapeutics for all brain regions is observed to be between 30min-12h, depending upon the distance of brain region from the site of administration, followed by gradual fading of radioactive counts by 24h post intranasal administration. Current study confirms the usefulness of intranasal administration as a non- invasive and efficient means of delivering therapeutics to the brain to treat neurodegenerative diseases including Alzheimer’s disease.
Keywords: Alzheimer’s disease, Nose to Brain delivery, GLP1, Anti-Aβ antibodies, Erythropoietin, Curcumin
Introduction
Growing world population with longer life expectancy has resulted in increased number of “aged” population with a greater prevalence of neurodegenerative diseases (ND) such as Alzheimer’s disease (AD). Currently, there is no cure for ND/AD [1]. The greatest challenge in curing ND/AD is the accessibility and bioavailability of therapeutics to the brain. In that context, intranasal delivery of therapeutics to the central nervous system (CNS) has emerged as a prospective alternative to parenteral routes of administration in treating ND/AD [1–9]. Intranasal delivery bypasses blood brain barrier (BBB) and circumvents systemic extraction of drugs, targeting therapeutics to the brain via olfactory, rostromigratory stream (RMS) and trigeminal pathways [4–13]. PubMed Literature search from late 1990s-to date, indicates that there have been > 200 studies reported thus far showing the utility of intranasal route as an effective means of delivering therapeutics to the CNS, some of which include intranasal delivery of benzodiazepine(s)[14,15], glucocorticoids/steroids/hormones [16–19], neurotrophic growth factors [20–31], vaccine antigens [32,33], Aβ immunogens [34–37], insulin [38–45], insulinomimetics/incretins [46–48], acetyl cholinesterase (AChE) inhibitors (AChEI) [49–58], and other candidate therapeutics [59–64]. Out of all reported intranasal studies, only few have demonstrated delivery of therapeutic antibodies utilizing intranasal route [65–69] including our recently published work [11,70]. Among few studies showing brain transit and pharmacokinetic of intranasally delivered materials [15,56,57,71,72], only one study showed brain-region-specific time-dependent uptake of intranasally delivered materials [46]. This investigation compares brain-region-specific time-dependent uptake of intranasally versus intraperitoneally delivered selected neurotherapeutics in the mouse brain including human recombinant erythropoietin (rhEpo), Curcumin, glucagon-like peptide 1 (GLP1) and anti- Aβ antibodies raised against specific amino acid (aa) epitopes of Aβ peptide.
Materials and Methods
Animals
Three month old mice (C57BL/6J), obtained from Jackson labs, Inc. (Bar Harbor, ME), are used in this study. This study compares the uptake of I-125 labeled test therapeutics in different brain regions of mice at different time points after intranasal (IN) or intraperitoneal (IP) administration. All experiments are approved and authorized by the local Institutional Animal Care and Use Committees at the Jesse Brown VA Medical Center and University of Illinois at Chicago. Animals are divided into nine major groups, analyzed at five different time points after IN and IP administration (N = 4/each time-point/group). Each group is studied and analyzed as an independent experiment and compared for IN vs IP delivery of all test materials at each time point for each group.
Group 1: Mouse non-immune IgG (NG) (Abcam, Cat. #ab37355)
Group 2: N-terminal anti- Aβ IgG2b MOAB-2 antibody raised against recombinant oligomeric Aβ 42 (not specific for Aβ42) (MOAB-2) (Abcam, ab126649)
Group 3: N-terminal anti-Aβ IgG1 antibody (1-17aa Aβ epitopes) (N-anti-Aβ IgG1) (Abcam, ab11132)
Group 4: N-terminal anti-Aβ IgG2a antibody (5-16aa Aβ epitopes) (N-anti-Aβ IgG2a) (Abcam, ab17250)
Group 5: N-terminal anti-Aβ IgG2b antibody (18-22aa Aβ epitopes) (N-anti-Aβ IgG2b) (Covance Research products, SIG-39200)
Group 6: C-terminal anti-Aβ IgG1 antibody (38-43aa ofC-terminus Aβ1-43) (C-anti-Aβ IgG1) (Abcam, ab22258)
Group 7: GLP1 (Alpha Diagnostics International, RP-1506)
Group 8: Curcumin (90% Pure, Cayman Chemical, Item #81025, CAS # 458-37-7)
Group 9: Human recombinant Erythropoietin (rhEpo) (R&D Systems, 287-TC-500)
Treatment
Each group is administered with a single bolus IN (5μg/5μl/ nostril = total dose of 10μg/10μl per mouse) or IP (100μg/100μl per mouse) of I-125 labeled test neurotherapeutics listed above. The radio-labeling is performed at the institutional core facility by technical experts using “Iodobead” kit (Pierce) as per manufacturer’s instructions, which ensures ~90% efficiency of iodination. The samples from different brain regions including olfactory lobes (OL), cerebral cortex (CTX), hippocampus (HP), and cerebellum (CBM) are collected at different time-points (30 min, 4h, 8h, 12h, 24h) following a single bolus IN or IP administration. The brain regions are homogenized in sterile saline (μg brain tissue/μl sterile saline) and 100μl of homogenate equating 100μg of brain tissue is recorded in the gamma scintillation counter. Data are statistically analyzed using GraphPad Prism Program to obtain respective group means with standard deviation (SD), and expressed as Mean ± SD (cpm/100μg) (Figures 1A, 1B, 2A, 2B, 3A, 3B, 4A, 4B). Means are analyzed by 2-tailed t-test to compare the brain-regional uptake of IN vs IP administration. A value of p < 0.05 is considered statistically significant. Means are used to derive the ratio of cerebral uptake after IN administration vs IP administration, and are represented as “fold-increase”. (Table 1)
Figure 1A.
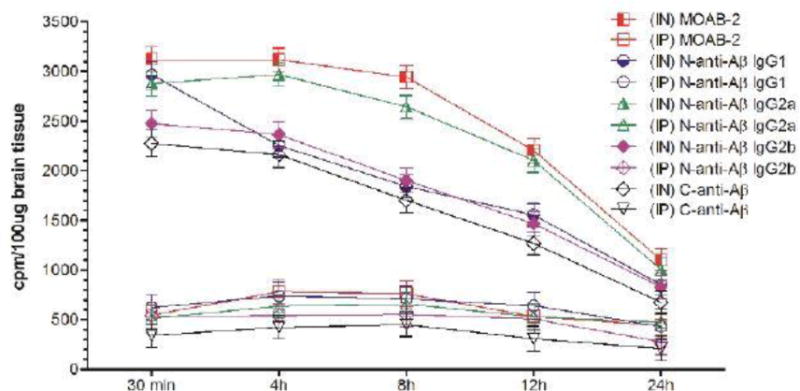
Uptake of I-125 labeled anti-Aß antibodies in the olfactory lobes at different time-points after intranasal (IN) or intraperitoneal (IP) delivery in mice expressed as cpm/100 μg brain tissue and presented as Mean ± SD.
Figure 1B.
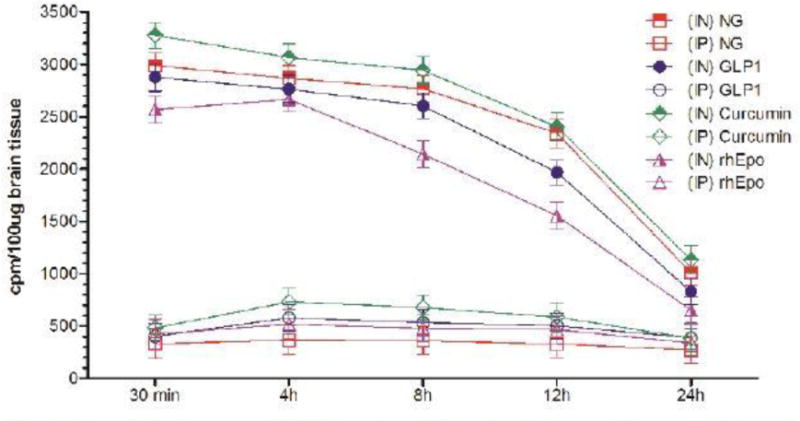
Uptake of I-125 labeled different neurotherapeutics in the olfactory lobes at different time-points after intranasal (IN) or intraperitoneal (IP) delivery in mice expressed as cpm/100 μg brain tissue and presented as Mean ± SD.
Figure 2A.
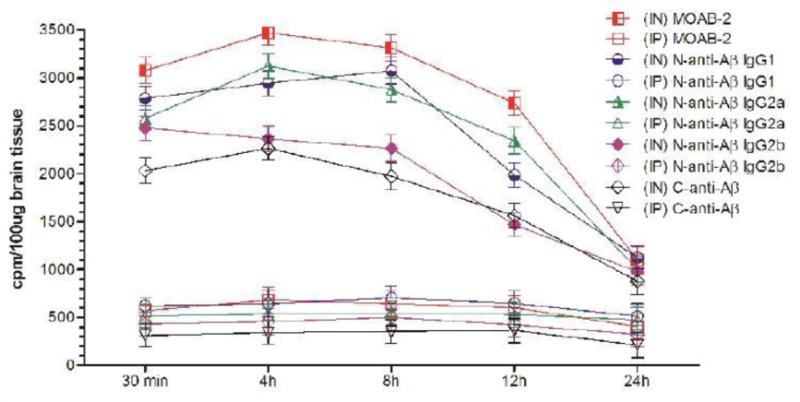
Uptake of I-125 labeled anti-Aß antibodies in the cerebral cortex at different time-points after intranasal (IN) or intraperitoneal (IP) delivery in mice expressed as cpm/100 μg brain tissue and presented as Mean ± SD.
Figure 2B.
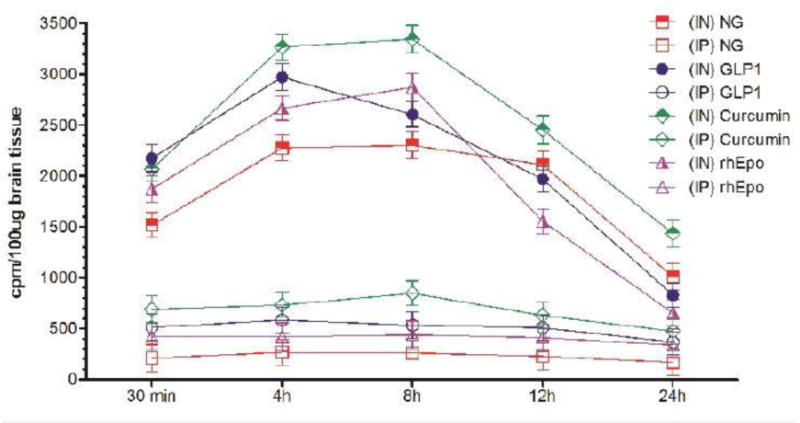
Uptake of I-125 labeled different neurotherapeutics in the cerebral cortex at different time-points after intranasal (IN) or intraperitoneal (IP) delivery in mice expressed as cpm/100 μg brain tissue and presented as Mean ± SD.
Figure 3A.
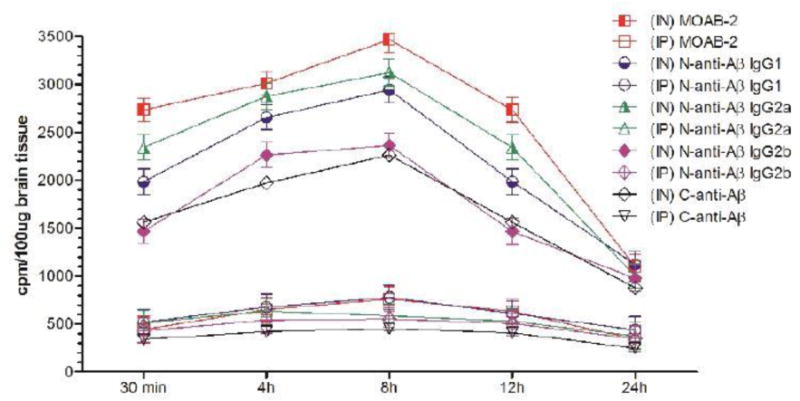
Uptake of I-125 labeled anti-Aß antibodies in the hippocampus at different time-points after intranasal (IN) or intraperitoneal (IP) delivery in mice expressed as cpm/100 μg brain tissue and presented as Mean ± SD.
Figure 3B.
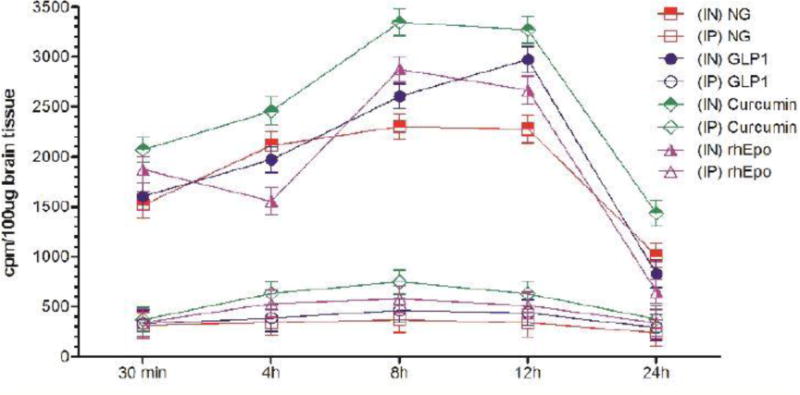
Uptake of I-125 labeled different neurotherapeutics in the hippocampus at different time-points after intranasal (IN) or intraperitoneal (IP) delivery in mice expressed as cpm/100 μg brain tissue and presented as Mean ± SD.
Figure 4A.
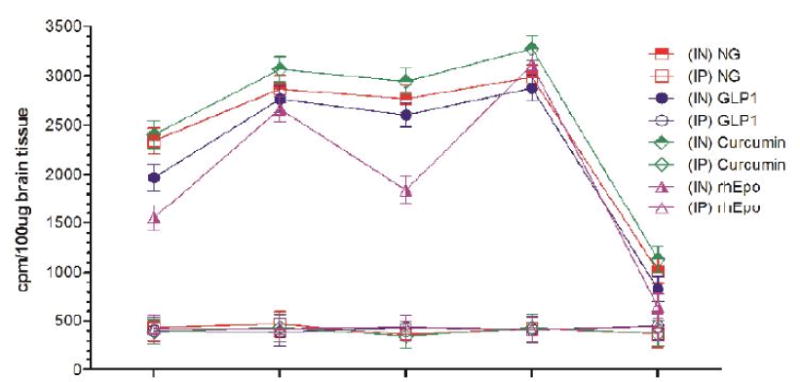
Uptake of I-125 labeled anti-Aß antibodies in the cerebellum at different time-points after intranasal (IN) or intraperitoneal (IP) delivery in mice expressed as cpm/100 μg brain tissue and presented as Mean ± SD.
Figure 4B.
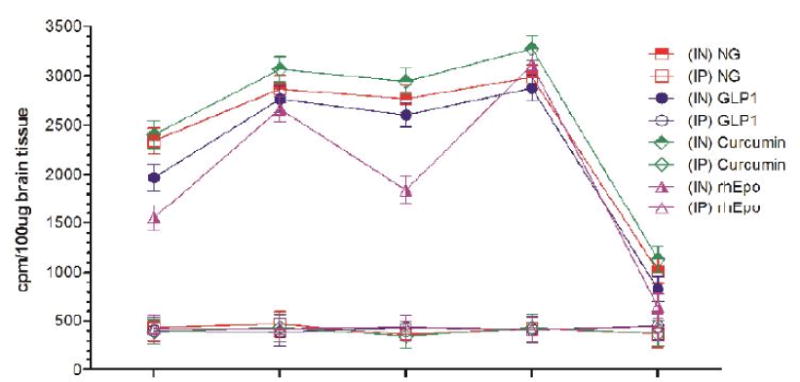
Uptake of I-125 labeled different neurotherapeutics in the cerebellum at different time-points after intranasal (IN) or intraperitoneal (IP) delivery in mice expressed as cpm/100 μg brain tissue and presented as Mean ± SD.
Table 1.
Increased brain uptake of various neurotherapeutics after intranasal (IN) delivery
| Non-Immune Globulin (NG) | N-term MOAB Anti-Aβ antibody | N-term IgG1Anti- Aβ antibody | N-term IgG2a Anti- Aβ antibody | N-term gG2b Anti- Aβ antibody | C-term Anti- Aβ antibody | Recombinant Human Erythropoietin (rhEpo) | Curcumin | Glucagon-Like Peptide 1 (GLP1) | |
|---|---|---|---|---|---|---|---|---|---|
| Olfactory Lobes | |||||||||
| 30 min | 6.99 (↑) | 5.13 (↑) | 5.77 (↑) | 4.59 (↑) | 4.66 (↑) | 5.67 (↑) | 5.08 (↑) | 6.79 (↑) | 6.55 (↑) |
| 4h | 6.81 (↑) | 4.99 (↑) | 3.05 (↑) | 5.68 (↑) | 4.36 (↑) | 4.13 (↑) | 5.33 (↑) | 5.19 (↑) | 5.17 (↑) |
| 8h | 6.67 (↑) | 4.87 (↑) | 2.59 (↑) | 4.02 (↑) | 3.44 (↑) | 3.75 (↑) | 3.48 (↑) | 4.68 (↑) | 4.87 (↑) |
| 12h | 5.12 (↑) | 3.14 (↑) | 2.42 (↑) | 3.98 (↑) | 2.88 (↑) | 3.11 (↑) | 2.34 (↑) | 3.15 (↑) | 3.72 (↑) |
| 24h | 3.66 (↑) | 2.48 (↑) | 1.47 (↑) | 2.11 (↑) | 2.48 (↑) | 2.19 (↑) | 1.32 (↑) | 2.11 (↑) | 2.13 (↑) |
| Cerebral Cortex | |||||||||
| 30 min | 6.18 (↑) | 5.09 (↑) | 4.37 (↑) | 5.01 (↑) | 5.24 (↑) | 6.01 (↑) | 4.44 (↑) | 3.01 (↑) | 3.25 (↑) |
| 4h | 7.22 (↑) | 5.51 (↑) | 4.58 (↑) | 5.85 (↑) | 5.18 (↑) | 6.62 (↑) | 5.36 (↑) | 5.46 (↑) | 5.28 (↑) |
| 8h | 7.81 (↑) | 5.13 (↑) | 4.89 (↑) | 5.26 (↑) | 4.52 (↑) | 5.59 (↑) | 5.74 (↑) | 5.28 (↑) | 5.01 (↑) |
| 12h | 6.32 (↑) | 4.25 (↑) | 3.08 (↑) | 4.13 (↑) | 3.27 (↑) | 4.04 (↑) | 2.78 (↑) | 3.90 (↑) | 3.87 (↑) |
| 24h | 4.01 (↑) | 2.73 (↑) | 2.19 (↑) | 2.11 (↑) | 3.03 (↑) | 2.79 (↑) | 1.32 (↑) | 2.21 (↑) | 2.15 (↑) |
| Hippocampus | |||||||||
| 30 min | 5.86 (↑) | 5.14 (↑) | 4.11 (↑) | 4.25 (↑) | 3.41 (↑) | 3.18 (↑) | 3.94 (↑) | 5.21 (↑) | 3.83 (↑) |
| 4h | 6.19 (↑) | 5.22 (↑) | 4.32 (↑) | 4.54 (↑) | 4.18 (↑) | 3.28 (↑) | 3.37 (↑) | 5.88 (↑) | 4.11 (↑) |
| 8h | 6.41 (↑) | 5.76 (↑) | 4.78 (↑) | 5.32 (↑) | 4.48 (↑) | 4.49 (↑) | 5.22 (↑) | 6.78 (↑) | 4.61 (↑) |
| 12h | 6.71 (↑) | 5.33 (↑) | 3.55 (↑) | 4.43 (↑) | 4.88 (↑) | 3.82 (↑) | 4.96 (↑) | 6.19 (↑) | 5.77 (↑) |
| 24h | 4.25 (↑) | 2.21 (↑) | 2.55 (↑) | 2.66 (↑) | 2.81 (↑) | 2.54 (↑) | 1.92 (↑) | 3.41 (↑) | 2.85 (↑) |
| Cerebellum | |||||||||
| 30 min | 5.42 (↑) | 4.04 (↑) | 2.98 (↑) | 4.08 (↑) | 2.76 (↑) | 3.72 (↑) | 3.48 (↑) | 6.18 (↑) | 4.89 (↑) |
| 4h | 6.67 (↑) | 5.85 (↑) | 4.18 (↑) | 5.16 (↑) | 4.36 (↑) | 5.13 (↑) | 5.21 (↑) | 7.64 (↑) | 6.03 (↑) |
| 8h | 6.14 (↑) | 5.36 (↑) | 4.42 (↑) | 4.93 (↑) | 4.54 (↑) | 4.76 (↑) | 3.57 (↑) | 7.11 (↑) | 4.88 (↑) |
| 12h | 6.76 (↑) | 5.65 (↑) | 5.81 (↑) | 5.64 (↑) | 4.96 (↑) | 5.56 (↑) | 6.36 (↑) | 8.48 (↑) | 5.99 (↑) |
| 24h | 2.75 (↑) | 2.25 (↑) | 1.21 (↑) | 2.03 (↑) | 1.41 (↑) | 1.73 (↑) | 1.48 (↑) | 2.11 (↑) | 1.85 (↑) |
[Values expressed as Ration of Brain-Regional Uptake of Neurotherapeutics after IN vs IP delivery which is expressed as fold-increase (↑)]
[All values for IN delivery were significantly higher than those for IP delivery (p<0.0001, all brain regions, all time points]
(Fold Increase = Brain-Region Uptake after IN Delivery / Brain- Region Uptake after IP Delivery)
Results
Results show that olfactory uptake of neurotherapeutics after IN delivery was ~7 ± 2 times greater than that after IP delivery. The olfactory uptake of all IN delivered neurotherapeutics is observed to peak at 30 min with a total resident time up to 12h which gradually is found to decrease by 24h post IN administration (Table 1, Figures 1A & 1B); while IP delivered neurotherapeutics, exhibit highest uptake in olfactory lobes at 4h post-delivery with gradual decrease by 24h. On the other hand, cortical uptake of IN delivered neurotherapeutics is observed to peak at 4h post-delivery with a total resident time up to 12h which is found to fade by 24h. Cortical uptake of IP delivered neurotherapeutics is observed to peak between 4–8h post-delivery fading by 24h. Cortical uptake of neurotherapeutics after IN delivery is ~6 ± 2 times greater than that after IP delivery (Table 1, Figures 2A & 2B). The hippocampal uptake of IN delivered neurotherapeutics is observed to peak at 8h post-delivery with a total resident time up to 12h declining by 24h. IP delivery exhibits similar trend of hippocampal uptake. Hippocampal uptake of IN delivered neurotherapeutics is ~6 ± 2 times greater than that after IP delivery (Table 1, Figures 3A & 3B). The cerebellar uptake of IN delivered neurotherapeutics is found to peak at 12h rapidly declining by 24h. IP delivery does not exhibit peak cerebellar uptake at any particular time point, rather it is observed to be at the same level at all time-points. Cerebellar uptake of neurotherapeutics after IN delivery is ~5 ± 2 times greater than that after IP delivery (Table 1, Figures 4A & 4B). In summary, current results show significant low uptake of IP delivered materials in all brain regions analyzed. Although the peak uptake of all IN delivered neurotherapeutics in different brain regions is spaced out according to the distance of brain region from the site of administration, all test therapeutics are retained up to 12h in all brain regions. The ratios of IN/IP delivery indicate peak uptake for olfactory lobes at 30 min, 4h for cerebral cortex, 8h for hippocampus and 12h for cerebellum (Table 1). All values for IN delivery are significantly higher than those for IP delivery for all brain regions, all time points (p < 0.0001). Currently observed fading of IN/IP delivered materials by 24h is consistent with previously observed clearance of IN delivered horse radish peroxidase (HRP) labeled anti-Aβ antibodies [11,70].
Discussion
Historically, intranasal drug delivery has been utilized for local treatments such as allergies, etc. Recently, the use of intranasal route as a means of delivering therapeutics to the CNS has gained tremendous interest and momentum [73]. The blood cerebrospinal fluid (CSF) barrier (BCSFB) and BBB protect the CNS by limiting the entry of toxic substances into the CNS, limiting the entry of therapeutics into the CNS [74–76]. In that regard, intranasal route holds a great potential as a non-invasive practical approach of delivering drugs to the CNS that circumvents systemic extraction/ alteration [73]. The major part of the nasal cavity both in human and rodents is covered by respiratory epithelium, across which drug absorption can be efficiently achieved. The unique anatomical and physiological characteristics of nasal mucosa such as the large surface area for drug absorption and close proximity to CNS and CSF [4,77–79] facilitate drug uptake despite minor limitations posed by nasal milieu itself, i.e. exo-/endo-peptidase(s)-mediated degradation of drugs or mucociliary clearance [77,79]. The olfactory epithelium is located just below the cribriform plate separating the nasal cavity from the cranial cavity. The olfactory epithelium (besides olfactory supporting cells and basal cells), contains olfactory sensory bipolar neurons (OSNs) with a single dendritic process bearing non-motile cilia, and with fine non- myelinated axons that connect with neighboring axons forming a bundle surrounded by glial cells penetrating into the cranial cavity through small holes in the cribriform plate which merge with the afferent axons connected to the olfactory tracts of the olfactory bulb [77]. Thus, OSNs congregate to connect with the CNS. Intranasal administration is known to utilize three potential pathways i.e. olfactory, trigeminal and RMS routes to reach CNS [11,80]. In addition, intranasally delivered materials also utilize extracellular diffusion along the open inter-olfactory clefts directly to the olfactory bulb/subarachnoid space/CSF [11,80]. IN route of administration has been exploited to deliver neurotrophic factors [80,81], cytokines [3], neuropeptides [82], and antibodies [11,83]. Despite considerable research in the field of intranasal administration targeted at nose to brain delivery of therapeutics, there are scant studies showing detailed brain-region-specific time-dependent uptake of intranasally delivered therapeutics, which is a critical determining factor for dosing conditions and optimal treatment regimen. In that regard, current study has significantly contributed detailing the entry, uptake and resident- time of intranasally delivered neurotherapeutics in mouse brain in comparison with the intraperitoneally delivered materials reaching the brain. Intranasally delivered therapeutics certainly has advantages not only with regard to efficient delivery but also with regard to their availability as an unaltered material since it bypasses systemic/hepatic extraction. Our observations that the intranasally delivered neurotherapeutics readily reach the brain with a resident time of 12h, provides a new direction for designing CNS-targeting drugs for the treatment of neurological disorders including AD, Parkinson’s disease (PD), traumatic brain injury (TBI), amyotrophic lateral sclerosis (ALS), Stroke and other NDs.
Acknowledgments
This work has been supported in part by the facilities and resources at the Jesse Brown VA Medical Center Chicago, Chicago, IL. The authors acknowledge the support provided by the Westside Institute for Science and Education. The authors also acknowledge the support provided by the Department of Pediatrics, University of Illinois at Chicago, Children’s Hospital of the University of Illinois, Chicago, IL. This work has been supported in part by National Institute of Health (AG039625, NS079614 NBC); and by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation R&D (I0880R, NBC).
References
- 1.Sood S, Jain K, Gowthamarajan K. Intranasal therapeutic strategies for management of Alzheimer’s disease. J Drug Target. 2014;22(4):279–94. doi: 10.3109/1061186X.2013.876644. [DOI] [PubMed] [Google Scholar]
- 2.Banks WA. The CNS as a target for peptides and peptide-based drugs. Expert Opin Drug Deliv. 2006;3(6):707–12. doi: 10.1517/17425247.3.6.707. [DOI] [PubMed] [Google Scholar]
- 3.Hanson LR, Frey WH. Strategies for intranasal delivery of therapeutics for the prevention and treatment of neuroAIDS. J Neuroimmune Pharmacol. 2007;2(1):81–6. doi: 10.1007/s11481-006-9039-x. [DOI] [PubMed] [Google Scholar]
- 4.Hanson LR, Frey WH. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;(Suppl 3):S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malerba F, Paoletti F, Capsoni S, Cattaneo A. Intranasal delivery of therapeutic proteins for neurological diseases. Expert Opin Drug Deliv. 2011;8(10):1277–96. doi: 10.1517/17425247.2011.588204. [DOI] [PubMed] [Google Scholar]
- 6.Tayebati SK, Nwankwo IE, Amenta F. Intranasal drug delivery to the central nervous system: present status and future outlook. Curr Pharm Des. 2013;19(3):510–26. [PubMed] [Google Scholar]
- 7.Landis MS, Boyden T, Pegg S. Nasal-to-CNS drug delivery: where are we now and where are we heading? An industrial perspective. Ther Deliv. 2012;3(2):195–208. doi: 10.4155/tde.11.149. [DOI] [PubMed] [Google Scholar]
- 8.Giordano C, Albani D, Gloria A, Tunesi M, Rodilossi S, Russo T, et al. Nanocomposites for neurodegenerative diseases: hydrogel-nanoparticle combinations for a challenging drug delivery. Int J Artif Organs. 2011;34(12):1115–1127. doi: 10.5301/IJAO.2011.8915. [DOI] [PubMed] [Google Scholar]
- 9.Mittal D, Ali A, Md S, Baboota S, Sahni JK, ali J. Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv. 2014;21(2):75–86. doi: 10.3109/10717544.2013.838713. [DOI] [PubMed] [Google Scholar]
- 10.Scranton RA, Fletcher L, Sprague S, Jimenez DF, Digicaylioglu M. The rostral migratory stream plays a key role in intranasal delivery of drugs into the CNS. PLoS One. 2011;6(4):e18711. doi: 10.1371/journal.pone.0018711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao C, Davis FJ, Chauhan BC, Viola KL, Lacor PN, velasco PT, et al. Brain transit and ameliorative effects of intranasally delivered anti-amyloid-beta oligomer antibody in 5XFAD mice. J Alzheimers Dis. 2013;35(4):777–88. doi: 10.3233/JAD-122419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahadur S, Pathak K. Physicochemical and physiological considerations for efficient nose-to-brain targeting. Expert Opin Drug Deliv. 2012;9(1):19–31. doi: 10.1517/17425247.2012.636801. [DOI] [PubMed] [Google Scholar]
- 13.Rhim T, Lee DY, Lee M. Drug delivery systems for the treatment of ischemic stroke. Pharm Res. 2013;30(10):2429–44. doi: 10.1007/s11095-012-0959-2. [DOI] [PubMed] [Google Scholar]
- 14.Rey E, Treluyer JM, Pons G. Pharmacokinetic optimization of benzodiazepine therapy for acute seizures. Focus on delivery routes. Clin Pharmacokinet. 1999;36:409–24. doi: 10.1007/s11095-012-0959-2. [DOI] [PubMed] [Google Scholar]
- 15.Veldhorst-Janssen NM, Fiddelers AA, van der Kuy PH, Neef C, Marcus MA. A review of the clinical pharmacokinetics of opioids, benzodiazepines, and antimigraine drugs delivered intranasally. Clin Ther. 2009;31(12):2954–87. doi: 10.1016/j.clinthera.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Blaiss MS. Safety considerations of intranasal corticosteroids for the treatment of allergic rhinitis. Allergy Asthma Proc. 2007;28(2):145–52. doi: 10.2500/aap.2007.28.2948. [DOI] [PubMed] [Google Scholar]
- 17.Meltzer EO Intranasal steroids: managing allergic rhinitis and tailoring treatment to patient preference. Allergy Asthma Proc. 2005;26(6):445–51. [PubMed] [Google Scholar]
- 18.Ducharme N, Banks WA, Morley JE, Robinson SM, Niehoff ML, Mattern C, et al. Brain distribution and behavioral effects of progesterone and pregnenolone after intranasal or intravenous administration. Eur J Pharmacol. 2007;641(2–3):128–34. doi: 10.1016/j.ejphar.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banks WA, Morley JE, Niehoff ML, Mattern C. Delivery of testosterone to the brain by intranasal administration: comparison to intravenous testosterone. J Drug Target. 2009;17(2):91–7. doi: 10.1080/10611860802382777. [DOI] [PubMed] [Google Scholar]
- 20.Covaceuszach S, Capsoni S, Ugolini G, Spirito F, Vignone D, Cattaneo A. Development of a non invasive NGF-based therapy for Alzheimer’s disease. Curr Alzheimer Res. 2009;6(2):158–70. doi: 10.2174/156720509787602870. [DOI] [PubMed] [Google Scholar]
- 21.Capsoni S, Covaceuszach S, Ugolini G, Spirito F, Vignone D, Amato G, et al. Delivery of NGF to the brain: intranasal versus ocular administration in anti-NGF transgenic mice. J Alzheimers Dis. 2009;16(2):371–88. doi: 10.3233/JAD-2009-0953. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J, Jiang Y, Xu G, Liu X. Intranasal administration: a potential solution for cross-BBB delivering neurotrophic factors. Histol Histopathol. 2012;27(5):537–48. doi: 10.14670/HH-27.537. [DOI] [PubMed] [Google Scholar]
- 23.Nonaka N, Farr SA, Nakamachi T, Morley JE, Nakamura M, Shioda S, et al. Intranasal administration of PACAP: uptake by brain and regional brain targeting with cyclodextrins. Peptides. 2012;36(2):168–75. doi: 10.1016/j.peptides.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anitua E, Pascual C, Antequera D, Bolos M, Padilla S, orive G, et al. Plasma rich in growth factors (PRGF-Endoret) reduces neuropathologic hallmarks and improves cognitive functions in an Alzheimer’s disease mouse model. Neurobiol Aging. 2014;35(7):1582–95. doi: 10.1016/j.neurobiolaging.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Chen J, Feng C, Shao X, Liu Q, zhang Q, et al. Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer’s disease. Int J Pharm. 2014;461(1–2):192–202. doi: 10.1016/j.ijpharm.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 26.Woodbury ME, Ikezu T. Fibroblast growth factor-2 signaling in neurogenesis and neurodegeneration. J Neuroimmune Pharmacol. 2014;9(2):92–101. doi: 10.1007/s11481-013-9501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lou G, Zhang Q, Xiao F, Xiang Q, Su Z, Zang L, et al. Intranasal administration of TAT-haFGF(??????) attenuates disease progression in a mouse model of Alzheimer’s disease. Neuroscience. 2012;223:225–37. doi: 10.1016/j.neuroscience.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Capsoni S, Marinelli S, Ceci M, Vignone D, Amato G, Malerba F, et al. Intranasal “painless” human Nerve Growth Factor [corrected] slows amyloid neurodegeneration and prevents memory deficits in App X PS1 mice. PLoS One. 2012;7(5):e37555. doi: 10.1371/journal.pone.0037555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanson LR, Fine JM, Hoekman JD, Nguyen TM, Burns RB, Martinez PM, et al. delivery of growth differentiation factor 5 to the central nervous system. Drug Deliv. 2012;19(3):149–54. doi: 10.3109/10717544.2012.657720. [DOI] [PubMed] [Google Scholar]
- 30.Tian L, Guo R, Yue X, Lv Q, Ye X, wang Z, et al. Intranasal administration of nerve growth factor ameliorate beta-amyloid deposition after traumatic brain injury in rats. Brain Res. 2012;1440:47–55. doi: 10.1016/j.brainres.2011.12.059. [DOI] [PubMed] [Google Scholar]
- 31.Feng C, Zhang C, Shao X, Liu Q, Qian Y, Feng L, et al. Enhancement of nose-to-brain delivery of basic fibroblast growth factor for improving rat memory impairments induced by co-injection of beta-amyloid and ibotenic acid into the bilateral hippocampus. Int J Pharm. 2012;423(2):226–34. doi: 10.1016/j.ijpharm.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Zurbriggen R, Metcalfe IC, Gluck R, Viret JF, Moser C. Nonclinical safety evaluation of Escherichia coli heat-labile toxin mucosal adjuvant as a component of a nasal influenza vaccine. Expert Rev Vaccines. 2003;2(2):295–304. doi: 10.1586/14760584.2.2.295. [DOI] [PubMed] [Google Scholar]
- 33.Frenkel D, Maron R, Burt DS, Weiner HL. Nasal vaccination with a proteosome-based adjuvant and glatiramer acetate clears beta-amyloid in a mouse model of Alzheimer disease. J Clin Invest. 2005;115(9):2423–33. doi: 10.1172/JCI23241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maier M, Seabrook TJ, Lazo ND, Jiang L, Das P, Janus C, et al. Short amyloid-beta (Abeta) immunogens reduce cerebral Abeta load and learning deficits in an Alzheimer’s disease mouse model in the absence of an Abeta-specific cellular immune response. J Neurosci. 2006;26(18):4717–28. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HD, Tahara K, Maxwell JA, Lalonde R, Fukuiwa T, Van Kampen KR, et al. Nasal inoculation of an adenovirus vector encoding 11 tandem repeats of Abeta1–6 upregulates IL-10 expression and reduces amyloid load in a Mo/Hu APPswe PS1dE9 mouse model of Alzheimer’s disease. J Gene Med. 2007;9(2):88–98. doi: 10.1002/jgm.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemere CA, Maier M, Peng Y, Jiang L, Seabrook TJ. Novel Abeta immunogens: is shorter better? Curr Alzheimer. 2007;4(4):427–36. doi: 10.2174/156720507781788800. [DOI] [PubMed] [Google Scholar]
- 37.Lemere CA. Developing novel immunogens for a safe and effective Alzheimer’s disease vaccine. Prog Brain Res. 2009;175:83–93. doi: 10.1016/S0079-6123(09)1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Run X, Liang Z, Zhao Y, Dai CL, Iqbal L, et al. Intranasal insulin prevents anesthesia-induced hyperphosphorylation of tau in 3xTg-AD mice. Front Aging Neurosci. 2014;6:100. doi: 10.3389/fnagi.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alagiakrishnan K, Sankaralingam S, Ghosh M, Mereu L, Senior P. Antidiabetic drugs and their potential role in treating mild cognitive impairment and Alzheimer’s disease. Discov Med. 2013;16(90):277–86. [PubMed] [Google Scholar]
- 40.de la Monte SM. Intranasal insulin therapy for cognitive impairment and neurodegeneration: current state of the art. Expert Opin Drug Deliv. 2013;10:1699–1709. doi: 10.1517/17425247.2013.856877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zemva J, Schubert M. The role of neuronal insulin/insulin-like growth factor-1 signaling for the pathogenesis of Alzheimer’s disease: possible therapeutic implications. CNS Neurol Disord Drug Targets. 2013;13(2):322–337. doi: 10.2174/18715273113126660141. [DOI] [PubMed] [Google Scholar]
- 42.Brunner YF, Benedict C, Freiherr J. Targeting the brain through the nose. Effects of intranasally administered insulin. Nervenarzt. 2013;84(8):949–54. doi: 10.1007/s00115-013-3806-8. [DOI] [PubMed] [Google Scholar]
- 43.Freiherr J, Hallschmid M, Frey WH, Brunner YF, Chapman CD, Holscher C, et al. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs. 2013;27(7):505–14. doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Claxton A, Baker LD, Wilkinson CW, Trittschuh EH, Chapman D, Watson GS, et al. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer’s disease. J Alzheimers Dis. 2013;35(4):789–97. doi: 10.3233/JAD-122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhamoon MS, Noble JM, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2009;72(3):292–93. 293–94. doi: 10.1212/01.wnl.0000344246.91081.2c. [DOI] [PubMed] [Google Scholar]
- 46.Banks WA, During MJ, Niehoff ML. Brain uptake of the glucagon-like peptide-1 antagonist exending(9–39) after intranasal administration. J Pharmacol Exp Ther. 2004;309(2):469–75. doi: 10.1124/jpet.103.063222. [DOI] [PubMed] [Google Scholar]
- 47.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9(9):1173–79. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 48.Clark I, Atwood C, Bowen R, Paz-Filho G, Vissel B, Jiao X. Tumor necrosis factor-induced cerebral insulin resistance in Alzheimer’s disease links numerous treatment rationales. Pharmacol Rev. 2012;64:1004–26. doi: 10.1124/pr.112.005850. [DOI] [PubMed] [Google Scholar]
- 49.Jogani VV, Shah PJ, Mishra P, Mishra AK, Misra AR. Nose-to-brain delivery of tacrine. J Pharm Pharmacol. 2009;59(9):1199–205. doi: 10.1211/jpp.59.9.0003. [DOI] [PubMed] [Google Scholar]
- 50.Leonard AK, Sileno AP, Brandt GC, Foerder CA, Quay SC, Costantio HR, et al. In vitro formulation optimization of intranasal galantamine leading to enhanced bioavailability and reduced emetic response in vivo. Int J Pharm. 2007;335(1–2):138–146. doi: 10.1016/j.ijpharm.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Costantino HR, Leonard AK, Brandt G, Johnson PH, Quay Intranasal administration of acetylcholinesterase inhibitors. BMC Neurosci. 2008;9(Suppl 3):S6. doi: 10.1186/1471-2202-9-S3-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arumugam K, Subramanian GS, Mallayasamy SR, Averineni RK, Reddy MS, Udupa N. A study of rivastigmine liposomes for delivery into the brain through intranasal route. Acta Pharm. 2008;58(3):287–97. doi: 10.2478/v10007-008-0014-3. [DOI] [PubMed] [Google Scholar]
- 53.Jogani VV, Shah PJ, Mishra P, Mishra AK, Misra AR. Intranasal mucoadhesive microemulsion of tacrine to improve brain targeting. Alzheimer Dis Assoc Disord. 2008;22(2):116–24. doi: 10.1097/WAD.0b013e318157205b. [DOI] [PubMed] [Google Scholar]
- 54.Bhavna MdS, Ali M, Ali R, Bhatnagar A, Baboota S, Ali J, et al. Donepezil nanosuspension intended for nose to brain targeting: In vitro and in vivo safety evaluation. Int J Biol Macrom. 2014;67:418–25. doi: 10.1016/j.ijbiomac.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 55.Yang ZZ, Zhang YQ, Wang ZZ, Wu K, Lou JN, Qi XR. Enhanced brain distribution and pharmacodynamics of rivastigmine by liposomes following intranasal administration. Int J Pharm. 2013;452(1–2):344–54. doi: 10.1016/j.ijpharm.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Li W, Zhou Y, Zhao N, Hao B, Wang X, Kong P. Pharmacokinetic behavior and efficiency of acetylcholinesterase inhibition in rat brain after intranasal administration of galanthamine hydrobromide loaded flexible liposomes. Environ Toxicol Pharmacol. 2012;34(2):272–9. doi: 10.1016/j.etap.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 57.Fazil M, Md S, Haque S, Kumar M, Baboota S, Ali Je. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur J Pharm Sci. 2012;47(1):6–15. doi: 10.1016/j.ejps.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 58.Yang ZZ, Zhang YQ, Wu K, Wang ZZ, Qi XR. Tissue distribution and pharmacodynamics of rivastigmine after intranasal and intravenous administration in rats. Curr Alzheimer Res. 2012;9(3):315–325. doi: 10.2174/156720512800107528. [DOI] [PubMed] [Google Scholar]
- 59.Wang CY, Wang ZY, Xie JW, Cai JH, Wang T, Xu Y, et al. CD36 Upregulation Mediated by Intranasal LV-NRF2 Treatment Mitigates Hypoxia-Induced Progression of Alzheimer’s-Like Pathogenesis. Antioxid Redox Signal. 2014;21(16):2208–30. doi: 10.1089/ars.2014.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maurice T, Mustafa MH, Desrumaux C, Keller E, Naert G, et al. Intranasal formulation of erythropoietin (EPO) showed potent protective activity against amyloid toxicity in the Aβ????? non-transgenic mouse model of Alzheimer’s disease. J Psychopharmacol. 2013;27(11):1044–57. doi: 10.1177/0269881113494939. [DOI] [PubMed] [Google Scholar]
- 61.Cui X, Cao DY, Wang ZM, Zheng AP. Pharmacodynamics and toxicity of vasoactive intestinal peptide for intranasal administration. Pharmazie. 2013;68(1):69–74. [PubMed] [Google Scholar]
- 62.Guo C, Wang P, Zhong ML, Wang T, Huang XS, et al. Deferoxamine inhibits iron induced hippocampal tau phosphorylation in the Alzheimer transgenic mouse brain. Neurochem Int. 2013;62:165–72. doi: 10.1016/j.neuint.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Fine JM, Baillargeon AM, Renner DB, Hoerster NS, Tokarev J, Colton S, et al. Intranasal deferoxamine improves performance in radial arm water maze, stabilizes HIF-1alpha, and phosphorylates GSK3beta in P301L tau transgenic mice. Exp Brain Res. 2012;219(3):381–90. doi: 10.1007/s00221-012-3101-0. [DOI] [PubMed] [Google Scholar]
- 64.Danielyan L, Klein R, Hanson LR, Buadze M, Schwab M, Gleiter CH. Protective effects of intranasal losartan in the APP/PS1 transgenic mouse model of Alzheimer disease. Rejuvenation Res. 2010;13(2–3):195–201. doi: 10.1089/rej.2009.0944. [DOI] [PubMed] [Google Scholar]
- 65.Kolobov VV, Zakharova IA, Fomina VG, Gorbatov VY, Davydova TV. Effect of antibodies to glutamate on caspase-3 activity in brain structures of rats with experimental Alzheimer’s disease. Bull Exp Biol Med. 2013;154(4):425–427. doi: 10.1007/s10517-013-1967-x. [DOI] [PubMed] [Google Scholar]
- 66.Vetrile LA, Zakharova IA, Kudrin VS, Klodt PM. Effects of antiglutamate antibodies on the development of stress response and neurotransmitter content in the hippocampus and hypothalamus of rats with different behavioral activity. Bull Exp Biol Med. 2013;155(3):318–23. doi: 10.1007/s10517-013-2143-z. [DOI] [PubMed] [Google Scholar]
- 67.Kolobov VV, Davydova TV, Zakharova IA, Gorbatov V, Fomina VG. Repressional effects of the glutamate antibodies on expression of Dffb gene in the brain of rats with experimental Alzheimer’s disease. Mol Biol (Mosk) 2012;46(5):757–65. [PubMed] [Google Scholar]
- 68.Cattepoel S, Hanenberg M, Kulic L, Nitsch RM. Chronic intranasal treatment with an anti-Abeta(30–42) scFv antibody ameliorates amyloid pathology in a transgenic mouse model of Alzheimer’s disease. PLoS One. 2011;6(4):e18296. doi: 10.1371/journal.pone.0018296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gorbatov VY, Trekova NA, Fomina VG, Davydova TV. Antiamnestic effects of antibodies to glutamate in experimental Alzheimer’s disease. Bull Exp Biol Med. 2010;150(1):23–5. doi: 10.1007/s10517-010-1058-1. [DOI] [PubMed] [Google Scholar]
- 70.Chauhan NB, Davis F, Xiao C. Wheat germ agglutinin enhanced cerebral uptake of anti-Abeta antibody after intranasal administration in 5XFAD mice. Vaccine. 2011;29(44):7631–637. doi: 10.1016/j.vaccine.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greenhalgh AD, Ogungbenro K, Rothwell NJ, Galea JP. Translational pharmacokinetics: challenges of an emerging approach to drug development in stroke. Expert Opin Drug Metab Toxicol. 2011;7(6):681–95. doi: 10.1517/17425255.2011.570259. [DOI] [PubMed] [Google Scholar]
- 72.Zheng Z, Tang Y, Lv H, Xu J, Zhao H, Xi Q, et al. Determination of Meserine, a new candidate for Alzheimer’s disease in mice brain by liquid chromatography-tandem mass spectrometry and its application to a pharmacokinetic and tissue distribution study. Anal Bioanal Chem. 2014;406(14):3451–58. doi: 10.1007/s00216-014-7779-7. [DOI] [PubMed] [Google Scholar]
- 73.Peterson A, Bansal A, Hofman F, Chen TC, Zada G. A systematic review of inhaled intranasal therapy for central nervous system neoplasms: an emerging therapeutic option. J Neurooncol. 2014;116(3):437–46. doi: 10.1007/s11060-013-1346-5. [DOI] [PubMed] [Google Scholar]
- 74.Pathan SA, Iqbal Z, Zaidi SM, Talegaonkar S, Vohra D, Jain GK, et al. CNS drug delivery systems: novel approaches. Recent Pat Drug Deliv Formul. 2009;3(1):71–89. doi: 10.2174/187221109787158355. [DOI] [PubMed] [Google Scholar]
- 75.Merkus FW, van den Berg MP. Can nasal drug delivery bypass the blood-brain barrier?: questioning the direct transport theory. Drugs R D. 2007;8(3):133–44. doi: 10.2165/00126839-200708030-00001. [DOI] [PubMed] [Google Scholar]
- 76.Yi X, Manickam DS, Brynskikh A. Kabanov AV Agile delivery of protein therapeutics to CNS. J Control Release. 2014;190C:637–63. doi: 10.1016/j.jconrel.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Illum L Nasal drug delivery–possibilities, problems and solutions. J Control Release. 2003;87:1–3. 187–98. doi: 10.1016/s0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 78.Minn A, Leclerc S, Heydel JM, Minn AL, Denizcot C, Catarelli M, et al. Drug transport into the mammalian brain: the nasal pathway and its specific metabolic barrier. J Drug Target. 2002;10(4):285–96. doi: 10.1080/713714452. [DOI] [PubMed] [Google Scholar]
- 79.Turker S, Onur E, Ozer Y. Nasal route and drug delivery systems. Pharm World Sci. 2004;26(3):137–42. doi: 10.1023/b:phar.0000026823.82950.ff. [DOI] [PubMed] [Google Scholar]
- 80.Thorne RG, Pronk GJ, Padmanabhan V, Frey WH. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–96. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 81.Thorne RG, Frey WH. Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin Pharmacokinet. 2007;40(12):907–46. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- 82.Gao X, Wu B, Zhang Q, Chen J, Zhu J, Zang W, et al. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J Control Release. 2007;121(3):156–67. doi: 10.1016/j.jconrel.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 83.Bourgeois C, Bour JB, Aho LS, Pothier P. Prophylactic administration of a complementarity-determining region derived from a neutralizing monoclonal antibody is effective against respiratory syncytial virus infection in BALB/c mice. J Virol. 1998;72(1):807–10. doi: 10.1128/jvi.72.1.807-810.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


