Abstract
It well appreciated that the endocannabinoid system can regulate immune responses via the cannabinoid receptor 2 (CB2), which is primarily expressed by cells of the hematopoietic system. The endocannabinoid system is composed of receptors, ligands and enzymes controlling the synthesis and degradation of endocannabinoids. Along with endocannabinoids, both plant-derived and synthetic cannabinoids have been shown to bind to and signal through CB2 via G proteins leading to both inhibitory and stimulatory signals depending on the biological process. Because no cannabinoid ligand has been identified that only binds to CB2, the generation of mice deficient in CB2 has greatly expanded our knowledge of how CB2 contributes to immune cell development and function in health and disease. In regards to humans, genetic studies have associated CB2 with a variety of human diseases. Here, we review the endocannabinoid system with an emphasis on CB2 and its role in the immune system.
Keywords: Cannabinoid receptor 2, endocannabinoid, immune regulatory, knockout, polymorphism
1. Introduction
The endocannabinoid system is evolutionarily conserved from plants to mammals and consists of the cannabinoid receptors, endocannabinoids and their synthesizing and metabolizing enzymes. While is has been known for centuries that plant-derived cannabinoids produced by the Cannabis sativa plant could modulate neurological and immunological functions it was in the mid 1960s to the early 1970s that Gaoni and Mechoulam first isolated cannabinoids from marijuana including the identification of (−) Δ9-tetrahydrocannabinol (THC), the psychoactive component of marijuana [1–3]. It took until 1992 for the first cannabinoid receptor to be identified and cloned [4]. Cannabinoid receptors are members of the G-protein coupled receptor (GPCR) family. Endogenous ligands for cannabinoid receptors are eicosanoids capable of binding to and activating cannabinoid receptors and are highly lipophilic with low water solubility. The best-studied cannabinoid receptors are cannabinoid receptor 1 (CB1) and CB2. CB1 is expressed by a variety of cell types including high expression in the brain and low expression levels in the hematopoietic system. The consensus in the field is that CB2 is primarily expressed by cells of the immune system and is either not expressed or at very low levels by non-hematopoietic cells in the brain. CB1 and CB2 have high sequence identity and can signal through G-proteins of the Gi/o type. The best-studied endocannabinoids are ararachidonate-based lipids and include N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoyl glycerol (2AG). Anandamide is degraded by fatty acid amide hydrolase (FAAH) into arachidonic acid and ethanolamine. 2-AG is degraded by monoacylglycerol lipase (MAGL) into arachidonic acid and glycerol. The endocannabinoid system has been shown to play a role in a wide variety of neuronal and immunological processes including: memory, neurogenesis, appetite, metabolism, stress/anxiety, analgesia, thermoregulation sleep and immune cell function. Current research support the concept that CB1 largely modulates neurological functions while CB2 is immune modulatory. This review will focus on the biology and function of CB2.
2. Cannabinoid receptor 2
While a number of ligands for endogenous, plant-derived and synthetic cannabinoids have been described only two have been well characterized. It was known that a GPCR was expressed in the brain and neural cell lines that was responsive to psychoactive cannabinoids, such as THC, and inhibited adenylate cyclase activity. This receptor, now termed CB1 [5], was first cloned from a rat brain library [4]. Shortly thereafter, the cloning of a human cannabinoid receptor cDNA was reported [6]. CB1 is now known to be responsible for the psychoactive effects of cannabinoids and is highly expressed in the central nervous system (CNS), as well as by many other tissues including the immune system [4, 5, 7–9]. Three years later, a second cannabinoid receptor (CB2) was identified from a cDNA library generated from the HL60 human promyelocytic leukemina cell line and was also shown to be expressed in splenic monocytes/macrophages [5]. CB2 is located on chromosome 4 in mice and 1p36 in humans and the gene has a simple structure containing a single coding exon. CB2 is a member of the GPCR family of proteins and structurally is comprised of a single polypeptide chain that contains seven transmembrane α-helices, with an extracellular glycosylated N-terminus and an intracellular C-terminus (Figure 1) [10]. Human CB2 and CB1 have 44% amino acid identity, indicating that they did not diverge recently [5]. CB2 is even less divergent between species. Sequence identity is 82% between human and mouse, 81% between human and rat and 90% between mouse and rat [5, 11, 12].
Figure 1. A schematic representation of the CB2 receptor.
CB2 consists of an extracellular N-terminus, 2 extracellular loops (EC1-3), 7 transmembrane domains (TM1-7), 3 intracellular loops (IC1-3) and an intracellular C-terminal domain, which is involved in signal transduction. The arrows indicate the protein segment that is deleted in Cnr2tmZim mice.
2.1. CB2 expression in hematopoietic cells
Additional studies measuring CNR2 mRNA revealed that it is expressed by all hematopoietic cells, but expression levels were shown to vary among immune cell populations in both their resting and activation states. In humans, CNR2 expression levels among the immune cell populations is as follows: B cells > NK cells > macrophages > polymorphonuclear cells > CD8 T cells > CD4 T cells [8, 9]. However, relatively few studies have evaluated differences in protein expression. This has been facilitated by the development of anti-human antibodies that can distinguish between active and inactive CB2. The phosphorylation status of CB2 can be measured using an polyclonal antibody specific to the COOH-terminal that can discriminate between the active phosphorylated and inactive non-phosphorylated CB2 isoforms at serine 352 [13]. It was shown that after receptor engagement with an agonist ligand, CB2 was phosphorylated at serine 352 internalized and desensitized and that the commonly used CB2 inverse agonist SR 144528 was able to reverse this process by inducing CB2 dephosphorylation at serine 352 and its increased expression [13]. When the expression pattern of inactive CB2 (C-terminal antibody) was investigated in human lymph nodes, spleen and tonsil B cell expression of CB2 was limited to the marginal zone and the mantel zone of the secondary follicles, and in the primary follicles [14–16]. In contrast, the N-terminal anti-CB2 polyclonal antibody that presumably binds active CB2 stained the germinal centers of the secondary follicles [14]. Further examination using these antibodies showed that expression of CB2 was generally absent on T-lymphocytes in reactive, non-malignant human lymphoid tissues. However, as suggested by the authors, CB2 expression by T cells may be below the level of detection by immunohistochemical methods [15]. In addition, the N-terminal, but not C-terminal, CB2 antibody was found to recognize CD23+ follicular dendritic cells and a small subpopulation of CD68+ macrophages [15]. These cumulative studies indicate that CB2 is only constitutively active on specific immune cell populations and that the majority of immune cells express inactive CB2.
2.2. CB2 expression in the central nervous system (CNS)
The question of whether CB2 is expressed by neurons in the brain is controversial and much of the data has been generated in mice. Some studies have reported that CB2 is not detectable on neurons under normal circumstances in concordance with the lack of Cnr2 message in the brain measured by Northern blotting and in situ hybridization [5]. However, using the more sensitive technique real-time PCR, we were able to detect low levels of Cnr2 message in the CNS [17]. However, we attributed this expression to CNS resident hematopoietic lineage microglial cells and not neurons [17]. Interestingly, other studies have reported abundant neuronal expression. It is generally thought that these apparent discrepancies are due to poorly validated CB2 antibodies and immunohistochemical procedures as suggested by a recent study demonstrating methodological problems that were encountered using three different commercial CB2 antibodies [18]. While each of the antibodies bound to various regions of the brain, they did not specifically recognize CB2 as demonstrated by similar staining patterns in mice lacking CB2 [18, 19]. Using the same CB2-deficient mice, we have also encountered non-specific staining with commercially purchased CB2 antibodies (unpublished observations). Thus a clear determination of whether specific neurons in the healthy or diseased state do or can express CB2 awaits the generation of more specific reagents.
3. Endocannabinoids
The identification of endogenous compounds that bind to cannabinoid receptors gave rise to the concept of the endocannabinoid system [20, 21]. Endocannabinoids are biologically active fatty acid ligands with anandamide and 2AG being the best studied. Additional polyunsaturated ethanolamines including 2-arachidonyl glyceryl ether (noladine ether), virodhamine and N-arachidonoyl dopamine that bind cannabinoid receptors have also been described [22–24]. Of these, noladine ether is of interest because it has been shown to be a full agonist at CB2 [25]. AEA, which is formed from N-arachidonoyl phosphatidylethanolamine by multiple pathways, was the first endogenous ligand reported [20, 26]. AEA is a partial agonist at CB1 [27–30] and is a weak partial agonist at CB2 [31, 32]. 2AG was isolated from the canine gut and brain [21, 33] and acts as a full agonist toward CB2 [31] and has been reported to lack both receptor efficacy and affinity in humans [30]. 2-AG is considered the primary endogenous ligand for CB2 [34]. Apart from their binding to CB1 and CB2, endocannabinoids are thought to bind to other receptors. For example, AEA may activate the vanilloid receptor type 1 [35–37]. Both AEA and 2-AG have been reported to bind to peroxisome proliferator-activated receptors [38–40]. Furthermore, AEA and 2-AG have been implicated in several pathological processes by activating the orphan receptor GPR55, which has been shown to function as a novel cannabinoid receptor and may be a target for the treatment of inflammation and pain [41–43].
3.1. Endocannabinoid synthesis and degradation
Endocannabinoids are made and released on demand following the appropriate stimuli and while their lipophilic nature allows them to diffuse through the cell membrane, it is thought that a putative transporter termed the AEA membrane transporter (AMT) facilitates their uptake into cells [44]. The AMT seems to facilitate both the release and re-uptake of endocannabinoids [44]. However, to date, the AMT has not been cloned. Complex enzymatic cascades regulate endocannabinoid production and inactivation (Figure 2) [44]. To date five hydrolytic enzymes have been identified. NAPE-selective phospholipase D (NAPE-PLD) catalyzes synthesis of AEA, and the fatty acid amide hydrolase (FAAH) catalyzes its degradation into arachidonate and ethanolamine [44–51]. The other three are attributed to the synthesis and degradation of 2-AG. The sn-1-selective diacylglycerol lipases (DAGL) α and β are thought to catalyze the formation of 2-AG [44, 52], and monoacylglycerol lipase (MAGL) degrades 2-AG into arachidonate and glycerol [44, 53, 54]. Thus even though endocannabinoids are going through a continues generation and degradation cycle, detectable levels of both AEA and 2-AG have been measured in many mammalian biological fluids and tissues; including brain, plasma, adipose tissue, gut, bone marrow and spleen. The endocannabinoid system consisting of cannabinoid receptors, endocannabinoids and the enzymes of their anabolic and catabolic pathways is highly conserved among species and has modulating activity on proteins and nuclear factors involved in cell proliferation, differentiation and survival [44, 55–57]. A transient increment seems to be an adaptive reaction to restore cell homeostasis when it is perturbed. For instance, high AEA concentrations were detected in placenta, umbilical vein and plasma from the maternal circulation [58]. However, in some chronic conditions, the alteration of the endocannabinoid systems seems to contribute to the progress of neurodegenerative disorders [59]. Elevated levels of AEA and 2AG have been also reported in several tumors compared to the respective healthy tissue [60]. However, an increase in endocannabinoids doesn’t always occur during disease. Using a mouse model of multiple sclerosis (MS), experimental autoimmune encephalomyelitis (EAE) that is associated with inflammation, demyelination and neuronal damage, we found no difference in either 2-AG or arachidonic acid as disease progressed [17]. For the treatment of human disease, all components of the endocannabinoid system have been considered as pharamacological targets.
Figure 2. A schematic representation of the endocannabinoid system.
Endocannabinoids such as anadamide and 2-AG, synthesized locally by neighboring cells, can bind to cell surface cannabinoid receptors including CB1 and CB2, initiating intracellular signaling cascades (I). The availability of endocannabinoids is regulated at the level fo synthesis and degradation. Endocannabinoids can diffuse throught the cell membrane (II) or can be transported inside the cell by putative transporter proteins (III), where they are hydrolyzed (IV). FAAH is the primary enzyme that degrades anandamide, while MGL hydrolyzes 2-AG.
4. Phytocannabinoids
Cannabis or marijuana is the most frequently used elicit drug in the world and its use is particularly popular among young adolescents [61]. This is of potential concern since epidemiological studies demonstrated that heavy use during adolescence is particularly detrimental with respect to long-term damage on cognition and mental health [61, 62]. Other cannabis effects in humans include disruption of short-term memory, enhanced body awareness in coordination, sleepiness and hypothermia, mood alterations with euphoria or dysphoria according to prior experience of the user, mood state at the time of onset and dose and route of administration [61]. However, marijuana use for medicinal purposes has been used for thousands of years, which has been used for controlling pain, convulsion, inflammation and asthma [63–65].
4.1. Cannabinoids produced by Cannabis sativa
The cannabis plant has two main subspecies, Cannabis indica and Cannabis sativa differing in physical characteristics. While indica-dominant strains are short plants with dark green leaves, sativa-dominant strains are taller with thin leaves of a pale green color. Cannabis sativa is a complex plant containing about ~421 chemical compounds, of which more than 60 are cannabinoid compounds [66, 67]. Currently, the term ‘cannabinoid’ refers to more than 100 terpenophenols derived from Cannabis sativa that directly or indirectly interact with cannabinoid receptors [65]. While THC was the first psychoactive component of cannabis that was identified [2], cannabidiol (CBD), Δ8-THC and cannabinol are also major constituents of the sativa plant and bind to cannabinoid receptors. Although all four cannabinoids have similar chemical structures, their pharmacological effects can be very different. The most researched compounds are THC and CBD. It is quite established that the detrimental effects of cannabis on mental health are primarily attributable to the main psychoactive ingredient, THC. THC is similar to AEA in its affinity to CB1 where it acts as a partial agonist, although with less efficacy than AEA [68]. THC is also considered a partial agonist at CB2, but has even lower efficacy than for CB1 [68]. In contrast, CBD is a potent antagonist of CB1 and CB2 receptor agonists [68, 69]. However, it also has been reported to function as an inverse agonist at CB1 and CB2 [68].
4.2. Therapeutic potential of cannabinoids
Over recent years intensive study on the molecular mechanisms of action of Cannabis sativa derivatives has been carried out. For example, clinical studies have shown that some effects of cannabinoids may be therapeutically useful. While cannabis use is controversial, its medicinal use within the United States as steadily gained acceptance and is currently being used for the treatment of a variety of disorders including cancer, HIV/AIDS and autoimmunity. General affects include treatment of pain, nausea and vomiting and emesis [61]. Recently, the states of Colorado and Washington legalized marijuana for recreational use. Currently Nabilone, a structural analogue of THC, was recently approved by the Food and Drug administration (FDA) to treat chemotherapy-induced nausea and vomiting [70]. THC and its synthetic analogue Dronabinol (Marinol®) are used as appetite stimulators in AIDS patients [71]. Sativex constituted of THC and CBD is not currently approved in the United States, but is undergoing Phase III clinical trials for the treatment of pain in cancer patients and for the treatment of pain and spasticity associated with MS [72, 73]. However, Sativex is approved for use in Canada and many European countries [72, 74]. There are at least five additional drugs based on cannabis that have either completed or undergoing clinical trials in various countries [72]. Nonetheless, the usefulness of THC-based therapeutic drugs or of synthetic compounds with similar pharmacological action is greatly hampered by their psychoactive effects and by their abuse potential. In addition, the blocking of neuronal pathways modulated by CB1 has proven difficult as evidenced by CB1 inverse agonists developed for obesity that were either taken off the market or ceased further development due to side effects including depression, anxiety and suicidal thoughts [72, 75].
The second major compound of the plant, CBD, is of particular interest since is not psychoactive and does not affect locomotor function, body temperature or memory on its own. However, it was suggested that higher doses of CBD enhanced the pharmacological effects of THC via a CB1-dependent mechanism [76]. CBD exhibits much lower affinity than THC for CB1 and CB2 and is thought to enhance endocannabinoid efficacy by inhibiting AEA inactivation [32, 69, 77, 78]. Furthermore, both animal and human studies suggest anxiolytic properties of CBD. In a recent double-blind study carried out on patients suffering from generalized social anxiety disorder, CBD was shown to significantly reduce anxiety and its effect was related to its action on limbic and paralimbic areas as shown by single photon emission computed tomography [79]. CBD has also been proposed to have antipsychotic effects and is considered a potential antipsychotic drug, particularly due to its relatively low side-effect profile [80]. Furthermore, it is also being developed as a possible therapeutic for several other conditions, including diabetes, ischemia and cancer [67]. Thus the future looks bright for both the development and acceptance of drugs either extracted from Cannabis sativa or based on the structure of its various unique cannabinoids.
5. Synthetic cannabinoids
The intensive research on the structure and activity of phytocannabinoids and endocannabinoids has lead to the development of synthetic compounds with high potency and selectivity for cannabinoid receptors. These synthetic cannabinoids have opened new avenues for targeting of the endocannabinoid system using selective cannabinoid receptor ligands with improved pharmacotherapeutic potential and limited toxicity. These new molecules consist of agonists and antagonists that bind directly to cannabinoid receptors and to agents able to enhance the synthesis or reduce the reuptake and the degradation of endocannabinoids. Classified according to their chemical structures, cannabinoid receptor agonists can be distinguished into classical cannabinoids, non-classical cannabinoids, aminoalkylindoles and eicosanoids [81]. Specific cannabinoids discussed are shown in Table 1. Classical cannabinoids include tricyclic dibenzopyran derivatives, and this group includes compounds occurring naturally in the plant Cannabis sativa or synthetic analogues of these compounds like 11-hydroxy-Δ8-THC-dimethylheptyl (HU-210), a synthetic compound that shows the highest potency at the CB1 [81–83]. The non-classical group is represented by bicyclic and tricyclic analogs of THC that lack a dihydropyran ring. The most representative member of this group is CP-55940, a potent and full agonist at both the CB1 and CB2 [81, 84, 85]. Members of the aminoalkylindole group are cannabinoid receptor agonists that have structures markedly different from those of both classical and non-classical compounds. Aminoalkylindoles are non-cannabinoid molecules endowed with cannabimimetic activity [81, 86]. The most representative compound is R-(+)-WIN-55,212-2, which behaves as a complete agonist at both the CB1 and CB2, although in some studies is was shown to possess slightly higher CB2 than CB1 affinity [81]. The most representative members of the eicosanoid group are the endocannabinoids AEA and 2AG. Based on the structure of AEA, minor chemical changes have led to the development of synthetic CB1-selective agonists, of which R(+)- methanandamide and arachidonyl-2’ chloroethylamiden (ACEA) are the most representative [81, 87]. Structural changes in THC structure led to the synthesis of selective CB2 agonists, those most frequently used as pharmacological tools are HU-308 [88], a non-classical cannabinoid; JWH-133, a classical cannabinoid; and JWH-015 and AM124, aminoalkylindoles [81, 89]. New series of compounds have been recently described including diarylether sulfonylesters [90], pyrroles with neuro-protective properties [91], naphthyridin and quinolin derivatives [92, 93], which possess immune-modulatory properties [94, 95].
Table 1.
CB1 and CB2 agonist properties of cannabinoids
| Cannabinoids | CB1 | CB2 | Type | Cannabinoid Group |
|---|---|---|---|---|
| THC | Partial agonist | Partial agonist | natural | Classical |
| HU-210 | Full agonist | Full agonist | synthetic | Classical |
| CP-55940 | Full agonist | Full agonist | synthetic | Non-classical |
| R-(+)-WIN55,212–2 | Full agonist | Full agonist | natural | Aminoalkylindole |
| AEA | Partial agonist | Partial agonist | natural | Eicosanoid |
| 2-AG | Full agonist | Full agonist | natural | Eicosanoid |
| R(+)-methanandamide | Partial agonist | Partial agonist | synthetic | Eicosanoid |
| ACEA | Partial agonist | Partial agonist | synthetic | Eicosanoid |
| HU-308 | Weak CB1 binding | Selective agonist | synthetic | Non-classical |
| JWH-133 | Weak CB1 binding | Selective agonist | synthetic | Classical |
| JWH-015 | Weak CB1 binding | Selective agonist | synthetic | Aminoalkylindole |
| AM124 | Weak CB1 binding | Selective agonist | synthetic | Aminoalkylindole |
The development of cannabinoid receptor compounds with antagonist activity occurred soon after the discovery of CB2. The most widely studied and utilized are SR 144528 and AM630 [96, 97]. These cannabinoids are both more potent at blocking CB2 than CB1 activation, possess much higher affinity for CB2 than for CB1 and are able to block agonist-induced CB2 activation in a competitive manner [81, 89]. Both SR 144528 and AM630 are classified as inverse agonists since when administered alone they were shown to produce inverse cannabimimetic effects in CB2 receptor-expressing tissues [96, 98]. However, the binding and signaling mechanisms driving their inverse agonist properties have not been adequately investigated.
6. Endocannabinoid induced CB2 signaling
CB2 is usually coupled to a pertussis toxin-sensitive Gi/Goα protein that triggers a canonical signaling pathway leading to inhibition of adenylyl cyclase activity and reduced cAMP levels [99–103]. The lack of cAMP accumulation attenuates activation of protein kinase A (PKA). Since activated PKA phosphorylates the cAMP response element-binding protein, which is a transcription factor regulating a variety of genes that promote survival, proliferation and differentiation of immune cells; the net result is immune inhibition [104]. The endocannabinoids 2-AG and noladin ether at CB2 were found to inhibit adenylyl cyclase activity, with noladin ether being more potent [25, 33, 102]. CB2 signaling through Gβγ subunits also occurs and is coupled to the mitogen-activated protein kinase (MAPK) pathway [100, 102, 105]. MAPK signaling plays important roles in cell migration, cytokine production, proliferation and apoptosis [106, 107]. There are three major MAPK that have been found to be regulated by CB2, which are extracellular signal-regulated protein kinases (ERK), p38 MAPK and c-Jun NH2-terminal kinases (JNK) [103]. For the MAPK-ERK pathway, 2-AG was found to be more potent than noladine either at activating this pathway [102]. In addition, anandamide was shown to attenuate the production of IL-12 and IL-23 in microglial cells, which are CNS-resident myeloid cells, induced by LPS/IFN-γ in a manner partially dependent upon CB2 [108]. Anandamide was shown to act through ERK1/2 and JNK, but not p38 [108]. Furthermore, anandamide enhanced LPS/IFN-γ-induced IL-10 production in microglial cells through the same MAPK signaling pathways and was also shown to inhibit NF- κB activation by abrogating phosphorylation of IκBα thereby inhibiting its degradation preventing the translocation of NF-κB to the nucleus [109]. These signaling events were also shown to be partially dependent upon CB2 [109]. In addition, anandamide was shown to induce a transient rise in Ca2+ in calf pulmonary endothelial cells due to activation of protein lipase C and release of intracellular Ca2+ stores in a CB2- depenent manner [110]. Similarly, 2-AG was shown to induce a transient release of intracellular Ca2+ in CHO cells in a CB2-dependent manner [102]. Whether a similar pathway exists in immune cells is not known. Thus CB2 engagement and signaling via endocannabinoids can both inhibit and promote immune cell functions depending upon the signaling pathway engaged.
7. Cnr2−/− mice
Because both synthetic and endogenous cannabinoids exhibit some binding affinity to both CB1 and CB2, as well as other putative cannabinoid receptors, differentiating specific signaling and biological specific effects of each receptor has been difficult. This has somewhat been possible with the use of antagonists and inverse agonists at CB1 and CB2. But even these cannabinoids bind to both CB1 and CB2 with some affinity [103]. Thus the generation of mice deficient in CB1 or CB2 has greatly benefited the cannabinoid field in terms of definitively attributing biological functions to each receptor.
7.1. Characterization of the Cnr2tmZim mouse deficient in CB2
The first Cnr2−/− mouse (Cnr2tmZim) was generated by Dr. Nancy Buckley in the laboratory of Dr. Andreas Zimmer [19]. Using Cnr2−/− mice is now the gold standard for determining CB2-dependent activities and the Cnr2−/− mouse has been used in a multitude of published studies due to the generosity of Drs. Buckely and Zimmer. The strategy to knockout the Cnr2 gene was by replacement of part of the gene with a neomycin resistance targeting cassette eliminating part of the intracellular loop 3, transmembrane domains 6 and 7 and the carboxy terminus (Figure 1). Ligand receptor binding studies were used to confirm a loss in CB2 receptor function [19]. A single functional assay demonstrated that THC was unable to attenuate the production of IL-2 by a T cell hybridoma in the presence of peritoneal macrophages [19]. In contrast, CB2 deficiency had no affect on THC-induced hypothermia and catalepsy. These studies confirmed a role for CB2 in suppressing immune cell function, but not in inducing CNS-specific effects of THC. A caveat in using these mice is that they still harbor the Cnr2 promoter and have an intact N terminus, which has been reported to generate a truncated CB2 receptor message [19, 111, 112]. In addition, this may lead to the production of a dysfunctional protein that may functionally influence other signaling pathways in addition to CB2 [112]. In addition, Cnr2tmZim mice were generated as chimeras of 129 (H-2b) and C57BL/6 (H-2b) mice and are thus in linkage disequilibrium with the gene silencing cassette [19, 112]. Thus even extensive backcrossing cannot totally eliminate strain specific genetic affects that could contribute to experimental outcomes. For this reason, control WT mice need to be generated from the same breeders as the Cnr2tmZim mice. Thus, while Cnr2tmZim mice have been an important tool for deciphering CB2-specific functions, especially in disease models, data need to be interpreted with caution keeping in mind the possibility that a truncated Cnr2 mRNA and/or protein could be modulating other unknown signaling pathways.
7.2. Characterization of the Cnr2tm1Dgen mouse deficient in CB2
A second Cnr2-deficient mouse has been generated by Deltagen (Cnr2tm1Dgen), which contains a 391 base pair deletion in the N terminus [112, 113]. Cnr2tm1Dgen mice are commercially available from The Jackson Laboratory (Bar Harbor, ME), which report that the mice they sell were generated on a mixed C57BL/6j;C57BL/6N background. A recent report compared phenotypic and genomic differences between the two C57BL strains [114]. How the strain of mouse affects genetic susceptibility to disease is unknown, but there is precedence for this possibility. One such example is in the animal of MS, EAE, where we reported that when the Cnr2tmZim mice were crossed to B10.PL-Hu2 H2-T18a/(73NS)SnJ (B10.PL) mice (H-2u) they exhibited a much more severe EAE disease course as compared to controls following immunization with the myelin basic protein (MBP) immunodominant peptide Ac1–11 [115]. However, EAE in the Cnr2tmZim mice (H-2b) induced by immunization with the myelin oligodendrocyte glycoprotein (MOG) immunodominat peptide MOG35–55 in our animal colony disease was only mildly more severe as compared to WT (Fig. 3). However, a greater difference in EAE severity between WT and Cnr2tmZim littermates, with the knockout mice being more severe, was reported [116]. In contrast, using a similar MOG35–55 EAE protocol, the WT littermates of Cnr2tm1Dgen mice were poorly susceptible to EAE induction while the CB2 knockout littermates exhibited disease virtually identical to what we observed [112]. One way to interpret this data is that the genetic background of the Cnr2tm1Dgen mouse inhibited EAE induction in the WT mice. Since C57BL/6 mice are fully susceptible to EAE, it suggests that some genetic element in the C57BL/6N background is inhibiting EAE. B10.PL mice are congenic mice on the C57BL/10Sn strain with the MHC locus from PL/J mice and their current generation as reported by The Jackson Laboratory is n8f114 (as of 01/14/13). PL/J mice are susceptible to EAE [117]. Thus the PL MHC locus in B10.PL mice is very likely controlling susceptibility to EAE induction. When the Cnr2tm1Dgen mice were bred to Biozzi ABH mice, there was no difference in EAE severity between the WT and Cnr2tmZim mice, with both strains exhibiting severe disease [112]. When EAE is induced by active immunization, naïve T cells are primed in vitro to the self-antigen and a CB2-deficiency could alter this process. To examine this possibility, we induced EAE in B10.PL Cnr2tmZim and WT mice by adoptive transfer using WT encephalitogentic T cells generated in vitro and found no difference in disease severity [115, 118, 119]. Our data suggests that CB2 does not alter T cell priming and that differences in EAE susceptibility and severity are likely genetic or environmental.
Figure 3. EAE disease severity is not altered in Cnr2tmZim mice.
EAE was induced in Cnr2tmZim mice by immunization with 300 µg MOG35–55 emulsified in complete Freund’s adjuvant containing 4 mg/ml Mycobacterium tuberculosis (Chondrex, Redmond, WA) at two sites on the back. Pertussis toxin (200 ng) (List biological Laboratories, Campbell, CA) was given i.p. on days 0 and 2 post-immunization. Clinical symptons of EAE were scored daily as follows: 0, no disease; 1, limp tail; 1.5, hind limb ataxia; 2, hind limb paresis; 2.5; partial hind limb paralysis; 3, total hind limb paralysis; 4, hind and fore limb paralysis; and 5, death. Data shown are the daily average of eight WT and nine Cnr2tmZim mice.
7.3. Comparison of Cnr2tmZim and Cnr2tm1Dgen mice
We know of only one study that directly compared Cnr2tmZim and Cnr2tm1Dgen mice within the same laboratory setting. A difference between the two Cnr2−/− mice was reported following treatment with benzamide, which stimulates GPR55. Benzamide was shown to inhibit electrically induced contraction in a vas deferens assay in WT, but not in GPR55-deificient mice. Benzamide was also able to inhibit contractions in Cnr1−/− and Cnr2tm1Dgen mice, but in Cnr2tmZim mice its effects were attenuated [112]. The reason for this difference is not known, but suggests that a dysfunctional CB2 protein produced in the Cnr2tmZim mice may interfere with GPR55 signaling [112]. Cross-talk between CB2 and GPR55 signaling pathways has been reported in human neutrophils whereby GPR55 activation augmented their migration towards 2-AG, while abrogating neutrophil degranulation and formation of reactive oxygen species (ROS) [120]. Thus some results obtained in Cnr2tmZim mice may be influenced by alterations in GPR55 signaling. In summary, while the generation of Cnr2−/− mice has greatly advanced our knowledge of CB2 signaling and roles in diseases, the results obtained may reflect differences in genetic background and/or unknown effects on other signaling pathways.
7.4. Immunological phenotype of Cnr2−/− mice
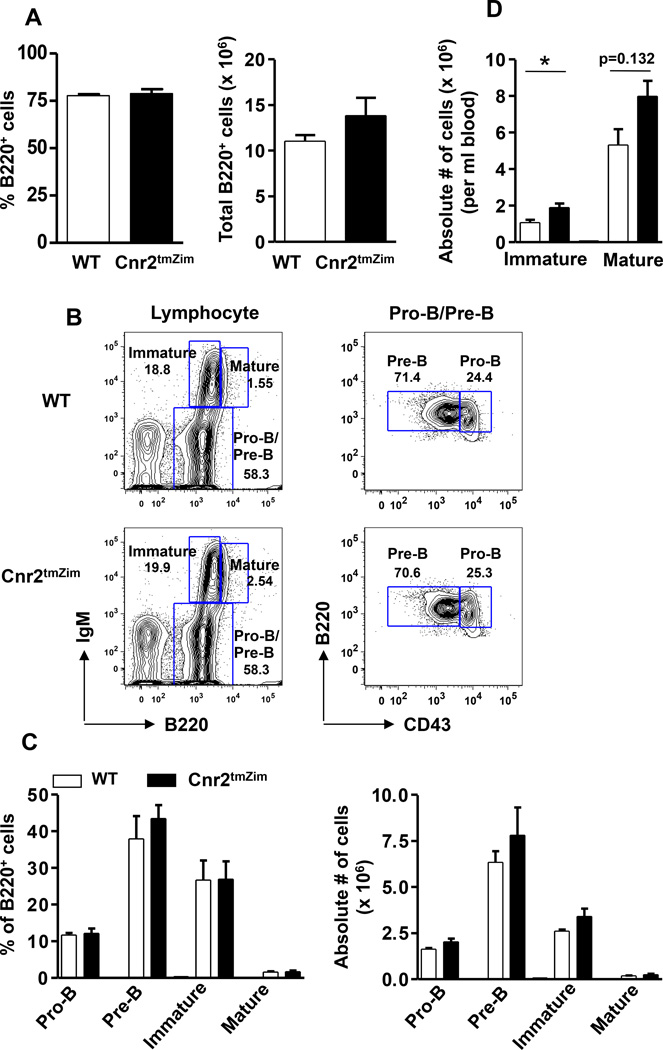
Whenever a global knockout is generated with the capacity to alter immune function, it must be thoroughly phenotyped to determine whether any developmental alterations have occurred. This has been done for the Cnr2tmZim mouse, which was phenotyped for changes in immune cell populations in the bone marrow, thymus, spleen and lymph nodes. In the first such report, it was reported that Cnr2tmZim mice had a reduction in splenic marginal zone (MZ) B cells, B1a B cells, splenic memory T cells, and intestinal natural killer (NK) cells and NKT cells [121]. In this study, Cnr2tmZim mice were compared to heterozygote littermate controls and the data were provided as percentages [121]. Because heterozygotes can show intermediate effects and percentages can be misleading since a change in one population can alter the percentage in another making it look like there is a change in the cell population number when there is not, we independently phenotyped Cnr2tmZim. For this analysis, we compared Cnr2tmZim mice to homozygous WT mice generated by crossing Cnr2tmZim heterozygotes and determined both the percentage and absolute number of immune cells. As already reported, in the spleen, we found that there was no change in the percentage or absolute number of B cells in the transitional (T) 1 and T2 stages in Cnr2tmZim mice [122]. Also in the spleen, we previously reported that while the percentage of follicular B cells was not different, there was a significant decrease in their absolute number in the Cnr2tmZim mice [122]. As for the MZ lineage, there was no change in the percentage of T2-MZ precursors, but their absolute number was significantly decreased [122]. We also found that both the percentage and absolute number of MZ B cells was decreased in Cnr2tmZim mice [122]. A reduction in the absolute number of MZ B cells was also reported in Cnr2tm1Dgen mice [123]. Because macrophages are important for MZ B cell development and retention [124, 125], we phenotyped the MARCO+ MZ residing macrophages thought to facilitate the retention of MZ B cells and found no difference in either their percentage or absolute number in the Cnr2tmZim mice (Fig. 4A). In addition, the structure of the B cell follicle was not altered in Cnr2tmZim mice as shown by the ring of MARCO+ macrophages around the B220hi B cell follicle (Fig. 4B). In the lymph node, we found no difference in the percentage or absolute number of B cells between the two groups of mice (Fig. 5A). In the peritoneal cavity, we also found no alteration in the percentage of total B cells (B220+), B2 or B1 B cells (Fig. 5B). When we subdivided B1 cells into B1a and B1b cells, we found no difference in their percentage or absolute number between wild-typoe and Cnr2tmZim mice (Fig. 5C). This is in contrast to the report by Ziring and colleagues, which reported a decrease in the B1a B cells in the peritoneal cavity using a similar gating strategy [121]. In the bone marrow (BM), we found no difference in the percentage or absolute number of total B cells (B220+) (Fig. 6A). Using a gating strategy to identify B cell progenitors (Fig. 6B), we also found no difference in the percentage or absolute number of pro-B, Pre-B, immature or mature B cell subpopulations (Fig. 6C). This finding is consistent with a study by the Cyster laboratory [126]. However, within the sinusoids, the Cyster laboratory found a significant decrease in the number of immature B cells [126]. Furthermore, immature B cells were found to express higher levels of Cnr2 message, which translated into increased migration towards 2-AG as compared to pro- and pre-B cells [126]. In addition, a reduction in the percentage of B cells expressing the Igλ chain was observed in both the peripheral blood and spleen, suggesting that CB2 plays a role in setting the balance of the Igλ and Igκ B cell repertoire [126]. The conclusions from this study were that CB2 is required for the efficient lodgment in the BM sinusoids. The consequence of a decrease in lodgment in the BM was increased numbers of immature, but not mature, B cells in the blood [126], a finding that we independently confirmed (Fig. 6D). We did not specifically phenotype NK cells, NKT cells or memory lymphocyte populations.
Figure 4. Analysis of marginal zone macrophages from Cnr2tmZim mice.
(A) Splenocytes from WT (open bar) and Cnr2tmZim (closed bar) mice were stained and analyzed for the percentage (left panel) and absolute number (right panel) of MARCO+ cells by flow cytometry. Data shown are the mean ± SEM of two independent experiments, each with three mice per group. (B) Seven µm frozen spleen sections from WT and Cnr2tmZim mice were stained with anti-B220 (red) and anti-MARCO (green) to indicate B cell follicles (B220hi, arrowheads) and MZ macrophages (MARCO+, thick arrows) residing in the MZ compartment outside the white pulp. Magnification is 100×. Data is representative of two independent experiments (n=2).
Figure 5. CB2 is dispensable for the development/maintenance of lymph node and peritoneal cavity B cell subsets.
(A) Inguinal lymph node cells were stained with anti-B220, and analyzed by flow cytometry. The absolute cell numbers were determined by counting total LN cells with trypan blue. The percentage (left panel) and absolute numbers (right panel) of B220+ cells from WT (open bar) and Cnr2tmZim (closed bar) mice are indicated. Data are the mean ± SEM from 2 independent experiments (n = 6 mice/group). (B, C) Peritoneal cells were isolated from WT (open bar) and Cnr2tmZim (closed bar) mice and stained for B220, CD23, CD11b, and CD5 to identify total B (B220+), B2 (B220+CD23+), B1 (B220+CD23−CD11b+), B1a (B220+CD23−CD5+CD11b+) and B1b (B220+CD23−CD5CD11b+) cells. The percentage of total B and B2 and B1 cells among the B220+ population is shown (B). (C) The cumulative percentage (left panel) and absolute number (right panel) of B1a and B1b cells is shown. (B, C) Data are the mean ± SEM from 2 independent experiments (n=6 mice/group).
Figure 6. CB2 is dispensable for the early B cell development in the BM and in its absence immature B cells are more prevalent in the peripheral blood.
(A) BM cells from WT (open bar) and Cnr2tmZim (closed bar) mice were isolated from the left femur and tibia, stained with anti-B220, and analyzed by flow cytometry. For the determination of cellularity, RBC-depleted BM cells were counted with Turk’s solution. The percentage (left panel) and absolute number (right panel) of total B cells from WT and CB2−/− mice are indicated. Data are the mean ± SEM from 2 independent experiments with 3 mice per group. (B, C) BM cells from WT and Cnr2tmZim mice were stained with monoclonal antibodies specific to B220, IgM, and CD43 to identify Pro B (B220+IgM−CD43+), Pre B (B220+IgM−CD43−), immature (B220intIgM+), and mature (B220hiIgM+) B cells by flow cytometry. (B) Representative contour plots show relative frequencies of Pro B/Pre B, immature and mature B cells (left panel), and pro B and Pre B cells separately (right panel) from WT (top panel) and Cnr2tmZim (bottom panel) mice. Numbers on the plots represent the percentage of cells in the corresponding gate. (C) The cumulative percentages (left panel) and absolute numbers (right panel) of the indicated B cell subsets from WT (open bar) and Cnr2tmZim (closed bar) mice are shown. Data are the mean ± SEM from 2 independent experiments with 3 mice per group. (D) Peripheral blood from WT (open bar) and Cnr2tmZim (closed bar) mice was stained with monoclonal antibodies against B220 and CD93 to identify mature/recirculating (B220+CD93−) and immature (B220+CD93+) and B cells. For the determination of absolute cell numbers, blood counts were performed using a Heska ABC animal cell counter. Data are the mean ± SEM from 2 independent experiments with 3 mice per group. *p<0.05.
In studies to determine the mechanism leading to lower numbers of marginal zone B cells in CB2-deficient mice, we, and others found that it was due to a reduction in the homing and retention of marginal zone B cells [122, 123]. The net result of a reduction in marginal zone B cells was reduced resting serum titers of IgM and impaired T-independent immune responses [122, 123]. In a follow-up study, we determined that T-dependent B cell immune responses were not similarly impaired [127]. However, the absolute number of both germinal center B cells and memory B cells were reduced, but this reduction did not result in lower levels of antigen-specific IgG1 [127]. Thus overall, while CB2 is widely expressed within the immune system, CB2 deficiency affects those B cell subpopulations that require CB2 for either homing and/or retention the most, highlighting an important role for CB2 in migration. This knowledge is essential for the interpretation of studies examining disease processes involving B cell responses.
8. Role for CB2 in disease
A far greater number of papers have been published implicating a role for CB2 in a variety of disease models than can be reviewed here. Thus we have focused on articles germane to the theme of neuroendocrine regulation of immunity. This review is also restricted to those studies conducted in both WT and Cnr2−/− mice to confirm a role for CB2. Selective synthetic agonists, inverse agonists and antagonists for CB2 have some binding affinity to other cannabinoid receptors and they have been reported to have off target effects, especially if used at high doses. Thus, while the demonstration that a particular cannabinoid has efficacy in disease is important, when used alone they are insufficient evidence for a definitive role for CB2.
8.1. Role of CB2 in EAE
As discussed above, CB2 has been shown to play a role in EAE susceptibility and severity depending on the background of the mice and the specific CB2 knockout used. When a global knockout is used to study disease it is not possible to determine the immune and non-immune cells on which CB2 is exerting regulation. To examine this question, we induced EAE in Cnr2tmZim mice by adoptive transfer with WT MBP-specific encephalitogenic T cells. We found that EAE disease onset and severity in the WT and Cnr2tmZim mice was essentially identical [115]. When EAE was induced by Cnr2tmZim encephalitogenic T cells, EAE was very severe and was associated with increased numbers of encephalitogenic T cells in the CNS, but not in total mononuclear cells or myeloid cells [115]. These cumulative data indicate that CB2 exerts it regulation in EAE at the level of the T cell and not CNS resident cells or other immune cells. In this same study, we showed that the CB2 selective agonist JWH-133 suppressed CD4 T cell proliferation and cytokine production in a CB2-dependent manner [115]. In regards to CNS resident immune cells, we were the first to clearly demonstrate the expression of CB2 on microglial cells and showed that its expression was upregulated following their activation during EAE and in vitro with the cytokines GM-CSF and IFN-γ [17]. While many CB2 agonists have been shown to attenuate EAE severity, the only study that validated this finding using CB2-deficient mice showed that the highly selective CB2 agonist Gp1a reduced the severity of EAE [128]. While the EAE disease scores were not shown for Gp1a treated mice with EAE, data was described (not shown) indicating that Gp1a treatment in Cnr2tm1Dgen did not reduce the production of IFN-γ and IL-17 by MOG35–55 re-stimulated splenocytes as it did in WT mice [128]. CNS parameters in the Cnr2tm1Dgen mice treated and not treated with Gp1a were not described. Nevertheless, these data suggest that Gp1a exerts its negative regulation through CB2 via suppressing T cell effector function, which is consistent with our previous findings [115].
8.2. Role of CB2 in stroke
Another CNS disorder in which the role of CB2 has been widely studied is ischemic stroke, which is caused by a prolonged blockage in arterial blood flow to the brain that leads to a residual tissue infarction [129]. The first study to our knowledge that utilized CB2-deficient mice in a cerebral ischemic/reperfusion injury model (MCAO) found that Cnr2tm1Dgen mice had a significantly larger cerebral infarction area and worse neurological function as compared to WT mice (not littermates) [130]. In this same study, the CB2 agonist 0–1966 was shown to reduce the effects of MCAO including leukocyte/endothelial cell interactions, adhesion molecule expression and BBB breakdown [130]. However, 0–1966 was only effective if given prior to the ischemic event or 1 hour later and it was no longer effective when administered five hours later [130]. However, whether 0–1966 specifically functioned through CB2 was not tested [130]. In a second study, Murikinati and colleagues showed that pretreatment with the CB2 agonist JWH-133 also reduced infarct volume, which was shown to be dependent upon CB2 by the use of Cnr2tmZim mice [131]. The generation of BM-derived chimeras indicated that the CB2-dependent effects were mediated through hematopoietic cells with additional studies indicating that the mechanism was due to the inhibition of neutrophil migration into the CNS [131]. Interestingly, JWH-133 did not alter expression of TNF-α, IL-1β or CXCL2 in the ischemic brain [131]. This later finding is important because TNF-α can induce the expression of CXCL2 in the CNS, which is a chemokine important for neutrophil recruitment into tissues [131–133]. Using migration assays, it was found that JWH-133 inhibited CXCL2 activation of MAP kinase p38, which was shown to be required for neutrophil migration towards CXCL2, but not fMLP [131]. Using Cnr2tmZim mice the entire process was shown to be dependent upon CB2 [131]. In a third study, Cnr2tmZim mice bred onto the Swiss background were used to also demonstrate that JWH-133 reduced infarct size and the neurological severity score in a CB2-depdendent manner [134]. One important experimental outcome difference between this study and that by Murikinati, et al, was that there was no difference in infarct size and the neurological severity score between WT and Cnr2tmZim mice. In addition, JWH-133 was found to reduce the expression of a number of cytokines and did not lead to a reduction in neutrophils that were recruited into the ischemic [134]. From these three studies, the data clearly indicate that CB2 agonists, in a CB2-dependent manner, when given prior to or shortly after the induction of MCAO can reduce ischemia neurological injury. This scenario would limit the ability to use CB2 agonists for stroke patients because the drugs would have to be administered very quickly after the onset of the ischemia event. Less clear is whether endogenous cannabinoids play a protective role.
9. CB2 polymorphisms in human disease
It was reported that Cnr2tmZim mice have a decrease in bone mass that resembles human osteoporosis [135]. This same article showed that CB2 is expressed by osteoblasts, osteocytes and osteoclasts and that the Cnr2−/− phenotype is associated with increased activity of the bone-resorbing osteoclasts and the bone-forming osteoblasts and a reduction in the number of diaphyseal osteoblast precursors [135]. The CB2 agonist HU-308, in a CB2-dependent manner, partially reversed the effects of CB2 deficiency, the mechanism of which was suggested to be the inhibition of osteoclast precursor proliferation and receptor activation in BM-derived osteoblasts/stromal cells [135]. This same group explored whether CB2 was associated with human osteoporosis and found a strong association with a single nucleotide polymorphism (SNP) (rs2501431) within the CNR2 gene [136]. This SNP is a single nonsynonymous polymorphism that occurs in amino acids 188–189 that creates an AA→GG polymorphism resulting in a change of amino acid at position 63 from glutamine to arginine (Q63R) [136–138]. In autoimmune patients, Sipe and colleagues found that Caucasian patients had a statistically higher frequency of the GG allele (62%) as compared to control subjects (54%) [137]. This study only used Caucasian subjects leaving the question of whether CNR2 polymorphisms are equally represented in other ethnic groups. This question was at least partially answered by a study that also found an association of the Q63R polymorphism with bone mineral density in Japanese men and women [139]. These data indicate that CNR2 could possibly play an important role in loss of immune regulation in autoimmune diseases. Further support for this possibility comes from two studies examining the association of the Q63R polymorphism in the autoimmune disease immune thrombocytopenic purpura. Studies conducted in Egypt and Italy found an association between Q63R and thrombocytopenia in children [140, 141]. Also immunologically related is an association between Q63R and more severe inflammation and hepatocellular necrosis in patients with chronic hepatitis C infection [142]. In addition, Q63R was associated with inflammation, but not steatosis or fibrosis that occurs during non-alcoholic fatter liver disease [143]. The CNR2 Q63R polymorphism was also associated with eating disorders, which is presumably a non-immunologic disorder [144]. At least two studies have found that Q63R is not associated with myocardial infarction and cardiovascular risk factors and bipolar disorder in humans [130, 145].
9.1. Functional significance of the Q63R CNR2 polymorphism
The Q63R polymorphism is predicted to be functionally significant because of its location near the nadir of the first intracellular CNR2 signaling loop [146]. Indeed, its was shown by Sipe and colleagues that cannabinoid inhibition of antigen-nonspecific T cell proliferation was reduced in humans carrying the homozygous GG/GG polymorphism as compared to AA/AA [137]. This difference in Q63R function was further illustrated in in vitro studies whereby human CB2 was mutated to express the Q63R polymorphism [138]. The polymorphic receptor bound cannabinoid ligands, including AEA and 2-AG, with similar affinity [138]. While the polymorphic receptor was able to signal there was a reduction in the maximal response when 2-AG inhibition of forskolin-stimulated cAMP accumulation was measured [138]. However, the constitutive activity of the Q63R receptor to forskolin simulation was not different from WT [138]. Thus the Q63R polymorphism seems to exert reduced activity after ligand binding presumably dampening its ability to inhibit immunological responses.
10. Concluding remarks
While much has been learned about the endocannabinoid system since the cloning of the first cannabinoid receptor, the ability to manipulate and harness the system for a variety of therapeutic purposes is still beyond or grasp. This is due to a number of reasons, but the nature of the endocannabinoid system as a tuner modulating many types of neurological and immunological responses and not as an on or off switch makes its targeting difficult. In addition, due to the sequence and structural similarities between the cannabinoid receptors it has not been possible to identify natural or generate synthetic cannabinoids that will bind and signal at only one cannabinoid receptor. In addition, cannabinoid ligands can exhibit complicated pharmacology being a full agonist, partial agonist or even an inverse agonist at different cannabinoid receptors. Such a drug would be difficult to assess biologically in a human. Even compounds first thought to be antagonists upon further study turned out to be inverse agonists. The identification of additional cannabinoid receptors and endogenous ligands in addition to the best studied CB1, CB2, anandamide and 2-AG is serving to make the endocannabinoid system even more difficult to target. The generation of mice deficient in specific components of the endocannabinoid system has greatly advanced our understanding of how they modulate various biological processes. Still poorly understood are the factors and conditions that regulate CB2 expression, and the downstream signaling cascades induced by cannabinoids leading to alterations in cell function are incomplete. In addition, the lipophilic nature of cannabinoids allows them to enter cells and exhibit off target effects that are independent of CB2. Finally, cross-talk between CB2 and other GPCR is known to occur, but the molecular and cellular basis for the interactions and the extent to which they occur are not well understood. Thus there is still much to be learned regarding the mechanisms whereby CB2 modulates immune cell function in both health and disease.
Acknowledgements
These studies were supported in part by NIH grants R01 NS046662 (BND), R01 AI069358 (BND), National Multiple Sclerosis Society Research Grants 4432-A-5 and PP1434 (BND), BloodCenter Research Foundation (BND) and Associazione Italiana Sclerosi Mutipla (AMM, MB, BND).
References
- 1.Mechoulam R, Gaoni Y, Hashish IV. The isolation and structure of cannabinolic cannabidiolic and cannabigerolic acids. Tetrahedron. 1965;21:1223–1229. doi: 10.1016/0040-4020(65)80064-3. [DOI] [PubMed] [Google Scholar]
- 2.Gaoni Y, Mechoulam R. Isolaton, structure, and partial synthesis of an active constituent of Hashish. J Amer Chem Soc. 1964;86:1646–1647. [Google Scholar]
- 3.Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. Journal of the American Chemical Society. 1971;93:217–224. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 5.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 6.Gerard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerard C, Mollereau C, Vassart G, Parmentier M. Nucleotide sequence of a human cannabinoid receptor cDNA. Nucleic Acids Res. 1990;18:7142. doi: 10.1093/nar/18.23.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouaboula M, Rinaldi M, Carayon P, Carillon C, Delpech B, Shire D, et al. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem. 1993;214:173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- 9.Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 10.Cabral GA, Griffin-Thomas L. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert reviews in molecular medicine. 2009;11:3. doi: 10.1017/S1462399409000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shire D, Calandra B, Rinaldi-Carmona M, Oustric D, Pessegue B, Bonnin-Cabanne O, et al. Molecular cloning, expression and function of the murine CB2 peripheral cannabinoid receptor. Biochim Biophys Acta. 1996;1307:132–136. doi: 10.1016/0167-4781(96)00047-4. [DOI] [PubMed] [Google Scholar]
- 12.Griffin G, Tao Q, Abood ME. Cloning and pharmacological characterization of the rat CB(2) cannabinoid receptor. J Pharmacol Exp Ther. 2000;292:886–894. [PubMed] [Google Scholar]
- 13.Bouaboula M, Dussossoy D, Casellas P. Regulation of peripheral cannabinoid receptor CB2 phosphorylation by the inverse agonist SR 144528 Implications for receptor biological responses. J Biol Chem. 1999;274:20397–20405. doi: 10.1074/jbc.274.29.20397. [DOI] [PubMed] [Google Scholar]
- 14.Rayman N, Lam KH, Laman JD, Simons PJ, Lowenberg B, Sonneveld P, et al. Distinct expression profiles of the peripheral cannabinoid receptor in lymphoid tissues depending on receptor activation status. J Immunol. 2004;172:2111–2117. doi: 10.4049/jimmunol.172.4.2111. [DOI] [PubMed] [Google Scholar]
- 15.Rayman N, Lam KH, Van Leeuwen J, Mulder AH, Budel LM, Lowenberg B, et al. The expression of the peripheral cannabinoid receptor on cells of the immune system and non-Hodgkin's lymphomas. Leukemia & lymphoma. 2007;48:1389–1399. doi: 10.1080/10428190701377030. [DOI] [PubMed] [Google Scholar]
- 16.Carayon P, Marchand J, Dussossoy D, Derocq JM, Jbilo O, Bord A, et al. Modulation and functional involvement of CB2 peripheral cannabinoid receptors during B-cell differentiation. Blood. 1998;92:3605–3615. [PubMed] [Google Scholar]
- 17.Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- 18.Baek JH, Darlington CL, Smith PF, Ashton JC. Antibody testing for brain immunohistochemistry: brain immunolabeling for the cannabinoid CB(2) receptor. J Neurosci Methods. 2013;216:87–95. doi: 10.1016/j.jneumeth.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- 20.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 21.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 22.Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, et al. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci U S A. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci U S A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- 25.Shoemaker JL, Joseph BK, Ruckle MB, Mayeux PR, Prather PL. The endocannabinoid noladin ether acts as a full agonist at human CB2 cannabinoid receptors. J Pharmacol Exp Ther. 2005;314:868–875. doi: 10.1124/jpet.105.085282. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Ueda N. Biology of endocannabinoid synthesis system. Prostaglandins & other lipid mediators. 2009;89:112–119. doi: 10.1016/j.prostaglandins.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Glass M, Northup JK. Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol Pharmacol. 1999;56:1362–1369. doi: 10.1124/mol.56.6.1362. [DOI] [PubMed] [Google Scholar]
- 28.Burkey TH, Quock RM, Consroe P, Ehlert FJ, Hosohata Y, Roeske WR, et al. Relative efficacies of cannabinoid CB1 receptor agonists in the mouse brain. Eur J Pharmacol. 1997;336:295–298. doi: 10.1016/s0014-2999(97)01255-7. [DOI] [PubMed] [Google Scholar]
- 29.Mackie K, Devane WA, Hille B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol Pharmacol. 1993;44:498–503. [PubMed] [Google Scholar]
- 30.Steffens M, Zentner J, Honegger J, Feuerstein TJ. Binding affinity and agonist activity of putative endogenous cannabinoids at the human neocortical CB1 receptor. Biochem Pharmacol. 2005;69:169–178. doi: 10.1016/j.bcp.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 31.Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol. 2000;57:1045–1050. [PubMed] [Google Scholar]
- 32.Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- 33.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 34.Sugiura T, Waku K. Cannabinoid receptors and their endogenous ligands. J Biochem. 2002;132:7–12. doi: 10.1093/oxfordjournals.jbchem.a003200. [DOI] [PubMed] [Google Scholar]
- 35.Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Petrocellis L, Davis JB, Di Marzo V. Palmitoylethanolamide enhances anandamide stimulation of human vanilloid VR1 receptors. FEBS Lett. 2001;506:253–256. doi: 10.1016/s0014-5793(01)02934-9. [DOI] [PubMed] [Google Scholar]
- 37.Ross RA, Gibson TM, Brockie HC, Leslie M, Pashmi G, Craib SJ, et al. Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br J Pharmacol. 2001;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517:174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 39.Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, et al. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 40.Rockwell CE, Snider NT, Thompson JT, Vanden Heuvel JP, Kaminski NE. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor gamma independently of cannabinoid receptors 1 and 2. Mol Pharmacol. 2006;70:101–111. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]
- 41.Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends in pharmacological sciences. 2006;27:1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Staton PC, Hatcher JP, Walker DJ, Morrison AD, Shapland EM, Hughes JP, et al. The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain. 2008;139:225–236. doi: 10.1016/j.pain.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Bisogno T, Ligresti A, Di Marzo V. The endocannabinoid signalling system: biochemical aspects. Pharmacol Biochem Behav. 2005;81:224–238. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 45.Hansen HS, Lauritzen L, Moesgaard B, Strand AM, Hansen HH. Formation of N-acyl-phosphatidylethanolamines and N-acetylethanolamines: proposed role in neurotoxicity. Biochem Pharmacol. 1998;55:719–725. doi: 10.1016/s0006-2952(97)00396-1. [DOI] [PubMed] [Google Scholar]
- 46.Schmid HH, Berdyshev EV. Cannabinoid receptor-inactive N-acylethanolamines and other fatty acid amides: metabolism and function. Prostaglandins Leukot Essent Fatty Acids. 2002;66:363–376. doi: 10.1054/plef.2001.0348. [DOI] [PubMed] [Google Scholar]
- 47.Schmid HH, Schmid PC, Natarajan V. N-acylated glycerophospholipids and their derivatives. Progress in lipid research. 1990;29:1–43. doi: 10.1016/0163-7827(90)90004-5. [DOI] [PubMed] [Google Scholar]
- 48.Schmid HH, Schmid PC, Natarajan V. The N-acylation-phosphodiesterase pathway and cell signaling. Chem Phys Lipids. 1996;80:133–142. doi: 10.1016/0009-3084(96)02554-6. [DOI] [PubMed] [Google Scholar]
- 49.Schmid PC, Reddy PV, Natarajan V, Schmid HH. Metabolism of N-acylethanolamine phospholipids by a mammalian phosphodiesterase of the phospholipase D type. J Biol Chem. 1983;258:9302–9306. [PubMed] [Google Scholar]
- 50.Bisogno T, De Petrocellis L, Di Marzo V. Fatty acid amide hydrolase, an enzyme with many bioactive substrates Possible therapeutic implications. Current pharmaceutical design. 2002;8:533–547. doi: 10.2174/1381612023395655. [DOI] [PubMed] [Google Scholar]
- 51.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 52.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Marzo V, Bisogno T, De Petrocellis L, Melck D, Orlando P, Wagner JA, et al. Biosynthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in circulating and tumoral macrophages. Eur J Biochem. 1999;264:258–267. doi: 10.1046/j.1432-1327.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- 54.Goparaju SK, Ueda N, Taniguchi K, Yamamoto S. Enzymes of porcine brain hydrolyzing 2-arachidonoylglycerol, an endogenous ligand of cannabinoid receptors. Biochem Pharmacol. 1999;57:417–423. doi: 10.1016/s0006-2952(98)00314-1. [DOI] [PubMed] [Google Scholar]
- 55.McPartland JM, Norris RW, Kilpatrick CW. Coevolution between cannabinoid receptors and endocannabinoid ligands. Gene. 2007;397:126–135. doi: 10.1016/j.gene.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 56.Bifulco M, Di Marzo V. Targeting the endocannabinoid system in cancer therapy: a call for further research. Nat Med. 2002;8:547–550. doi: 10.1038/nm0602-547. [DOI] [PubMed] [Google Scholar]
- 57.Bifulco M, Laezza C, Pisanti S, Gazzerro P. Cannabinoids and cancer: pros and cons of an antitumour strategy. Br J Pharmacol. 2006;148:123–135. doi: 10.1038/sj.bjp.0706632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marczylo TH, Lam PM, Amoako AA, Konje JC. Anandamide levels in human female reproductive tissues: solid-phase extraction and measurement by ultraperformance liquid chromatography tandem mass spectrometry. Analytical biochemistry. 2010;400:155–162. doi: 10.1016/j.ab.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 59.Romero J, Orgado JM. Cannabinoids and neurodegenerative diseases. CNS & neurological disorders drug targets. 2009;8:440–450. doi: 10.2174/187152709789824589. [DOI] [PubMed] [Google Scholar]
- 60.Malfitano AM, Ciaglia E, Gangemi G, Gazzerro P, Laezza C, Bifulco M. Update on the endocannabinoid system as an anticancer target. Expert opinion on therapeutic targets. 2011;15:297–308. doi: 10.1517/14728222.2011.553606. [DOI] [PubMed] [Google Scholar]
- 61.Greydanus DE, Hawver EK, Greydanus MM, Merrick J. Marijuana: Current Concepts. Frontiers in public health. 2013;1:42. doi: 10.3389/fpubh.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.CB2a. http://www.drugabuse.gov/publications/drugfacts/marijuana.
- 63.Salzet M, Breton C, Bisogno T, Di Marzo V. Comparative biology of the endocannabinoid system possible role in the immune response. Eur J Biochem. 2000;267:4917–4927. doi: 10.1046/j.1432-1327.2000.01550.x. [DOI] [PubMed] [Google Scholar]
- 64.Berdyshev EV. Cannabinoid receptors and the regulation of immune response. Chem Phys Lipids. 2000;108:169–190. doi: 10.1016/s0009-3084(00)00195-x. [DOI] [PubMed] [Google Scholar]
- 65.Appendino G, Chianese G, Taglialatela-Scafati O. Cannabinoids: occurrence and medicinal chemistry. Curr Med Chem. 2011;18:1085–1099. doi: 10.2174/092986711794940888. [DOI] [PubMed] [Google Scholar]
- 66.Dewey WL. Cannabinoid pharmacology. Pharmacological reviews. 1986;38:151–178. [PubMed] [Google Scholar]
- 67.Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends in pharmacological sciences. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ware MA, Daeninck P, Maida V. A review of nabilone in the treatment of chemotherapy-induced nausea and vomiting. Therapeutics and clinical risk management. 2008;4:99–107. doi: 10.2147/tcrm.s1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dejesus E, Rodwick BM, Bowers D, Cohen CJ, Pearce D. Use of Dronabinol Improves Appetite and Reverses Weight Loss in HIV/AIDS-Infected Patients. J Int Assoc Physicians AIDS Care (Chic) 2007;6:95–100. doi: 10.1177/1545109707300157. [DOI] [PubMed] [Google Scholar]
- 72.CB2b. http://medicalmarijuana.procon.org/view.resource.php?resourceID=000883.
- 73.Russo EB, Guy GW, Robson PJ. Cannabis, pain, and sleep: lessons from therapeutic clinical trials of Sativex, a cannabis-based medicine. Chemistry & biodiversity. 2007;4:1729–1743. doi: 10.1002/cbdv.200790150. [DOI] [PubMed] [Google Scholar]
- 74.Pryce G, Baker D. Emerging properties of cannabinoid medicines in management of multiple sclerosis. Trends Neurosci. 2005;28:272–276. doi: 10.1016/j.tins.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 75.CB2c. Anti-obesity drug use suspended. BBC News. 2008 Retrieved 4 March 2010. [Google Scholar]
- 76.Hayakawa K, Mishima K, Hazekawa M, Sano K, Irie K, Orito K, et al. Cannabidiol potentiates pharmacological effects of Delta(9)-tetrahydrocannabinol via CB(1) receptor-dependent mechanism. Brain Res. 2008;1188:157–164. doi: 10.1016/j.brainres.2007.09.090. [DOI] [PubMed] [Google Scholar]
- 77.Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- 78.Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318:1375–1387. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- 79.Crippa JA, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25:121–130. doi: 10.1177/0269881110379283. [DOI] [PubMed] [Google Scholar]
- 80.Zuardi AW, Morais SL, Guimaraes FS, Mechoulam R. Antipsychotic effect of cannabidiol. The Journal of clinical psychiatry. 1995;56:485–486. [PubMed] [Google Scholar]
- 81.Pertwee RG. Cannabinoid Receptor Ligands. Tocris Bioscience Scientific Review Seris. http://www.tocris.com:1-15.
- 82.Howlett AC, Champion TM, Wilken GH, Mechoulam R. Stereochemical effects of 11-OH-delta 8-tetrahydrocannabinol-dimethylheptyl to inhibit adenylate cyclase and bind to the cannabinoid receptor. Neuropharmacology. 1990;29:161–165. doi: 10.1016/0028-3908(90)90056-w. [DOI] [PubMed] [Google Scholar]
- 83.Mechoulam R, Feigenbaum JJ, Lander N, Segal M, Jarbe TU, Hiltunen AJ, et al. Enantiomeric cannabinoids: stereospecificity of psychotropic activity. Experientia. 1988;44:762–764. doi: 10.1007/BF01959156. [DOI] [PubMed] [Google Scholar]
- 84.Fouda HG, Lukaszewicz J, Luther EW. Selected ion monitoring analysis of CP-55,940, a cannabinoid derived analgetic agent. Biomedical & environmental mass spectrometry. 1987;14:599–602. doi: 10.1002/bms.1200141104. [DOI] [PubMed] [Google Scholar]
- 85.Thomas BF, Gilliam AF, Burch DF, Roche MJ, Seltzman HH. Comparative receptor binding analyses of cannabinoid agonists and antagonists. J Pharmacol Exp Ther. 1998;285:285–292. [PubMed] [Google Scholar]
- 86.Pacheco M, Childers SR, Arnold R, Casiano F, Ward SJ. Aminoalkylindoles: actions on specific G-protein-linked receptors. J Pharmacol Exp Ther. 1991;257:170–183. [PubMed] [Google Scholar]
- 87.Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, et al. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J Pharmacol Exp Ther. 1999;289:1427–1433. [PubMed] [Google Scholar]
- 88.Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc Natl Acad Sci U S A. 1999;96:14228–14233. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Howlett AC. The cannabinoid receptors. Prostaglandins & other lipid mediators. 2002;68–69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- 90.Mauler F, Mittendorf J, Horvath E, De Vry J. Characterization of the diarylether sulfonylester (−)-(R)-3-(2-hydroxymethylindanyl-4-oxy)phenyl-4,4,4-trifluoro-1-sulfonate (BAY 38–7271) as a potent cannabinoid receptor agonist with neuroprotective properties. J Pharmacol Exp Ther. 2002;302:359–368. doi: 10.1124/jpet.302.1.359. [DOI] [PubMed] [Google Scholar]
- 91.Tarzia G, Duranti A, Tontini A, Spadoni G, Mor M, Rivara S, et al. Synthesis and structure-activity relationships of a series of pyrrole cannabinoid receptor agonists. Bioorg Med Chem. 2003;11:3965–3973. doi: 10.1016/s0968-0896(03)00413-9. [DOI] [PubMed] [Google Scholar]
- 92.Manera C, Benetti V, Castelli MP, Cavallini T, Lazzarotti S, Pibiri F, et al. Design, synthesis, and biological evaluation of new 1,8-naphthyridin-4(1H)-on-3-carboxamide and quinolin-4(1H)-on-3-carboxamide derivatives as CB2 selective agonists. Journal of medicinal chemistry. 2006;49:5947–5957. doi: 10.1021/jm0603466. [DOI] [PubMed] [Google Scholar]
- 93.Manera C, Saccomanni G, Adinolfi B, Benetti V, Ligresti A, Cascio MG, et al. Rational design, synthesis, and pharmacological properties of new 1,8-naphthyridin-2(1H)-on-3-carboxamide derivatives as highly selective cannabinoid-2 receptor agonists. Journal of medicinal chemistry. 2009;52:3644–3651. doi: 10.1021/jm801563d. [DOI] [PubMed] [Google Scholar]
- 94.Malfitano AM, Laezza C, D'Alessandro A, Procaccini C, Saccomanni G, Tuccinardi T, et al. Effects on immune cells of a new 1,8-naphthyridin-2-one derivative and its analogues as selective CB2 agonists: implications in multiple sclerosis. PLoS One. 2013;8:62511. doi: 10.1371/journal.pone.0062511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Malfitano AM, Laezza C, Saccomanni G, Tuccinardi T, Manera C, Martinelli A, et al. Immune-modulation and properties of absorption and blood brain barrier permeability of 1,8-naphthyridine derivatives. J Neuroimmune Pharmacol. 2013;8:1077–1086. doi: 10.1007/s11481-013-9494-0. [DOI] [PubMed] [Google Scholar]
- 96.Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, et al. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- 98.Bouaboula M, Desnoyer N, Carayon P, Combes T, Casellas P. Gi protein modulation induced by a selective inverse agonist for the peripheral cannabinoid receptor CB2: implication for intracellular signalization cross-regulation. Mol Pharmacol. 1999;55:473–480. [PubMed] [Google Scholar]
- 99.Bayewitch M, Avidor-Reiss T, Levy R, Barg J, Mechoulam R, Vogel Z. The peripheral cannabinoid receptor: adenylate cyclase inhibition and G protein coupling. FEBS Lett. 1995;375:143–147. doi: 10.1016/0014-5793(95)01207-u. [DOI] [PubMed] [Google Scholar]
- 100.Kobayashi Y, Arai S, Waku K, Sugiura T. Activation by 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, of p42/44 mitogen-activated protein kinase in HL-60 cells. J Biochem. 2001;129:665–669. doi: 10.1093/oxfordjournals.jbchem.a002904. [DOI] [PubMed] [Google Scholar]
- 101.Slipetz DM, O'Neill GP, Favreau L, Dufresne C, Gallant M, Gareau Y, et al. Activation of the human peripheral cannabinoid receptor results in inhibition of adenylyl cyclase. Mol Pharmacol. 1995;48:352–361. [PubMed] [Google Scholar]
- 102.Shoemaker JL, Ruckle MB, Mayeux PR, Prather PL. Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J Pharmacol Exp Ther. 2005;315:828–838. doi: 10.1124/jpet.105.089474. [DOI] [PubMed] [Google Scholar]
- 103.Basu S, Dittel BN. Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulaton in health and disease. Immunol Res. 2011 doi: 10.1007/s12026-011-8210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaminski NE. Immune regulation by cannabinoid compounds through the inhibition of the cyclic AMP signaling cascade and altered gene expression. Biochem Pharmacol. 1996;52:1133–1140. doi: 10.1016/0006-2952(96)00480-7. [DOI] [PubMed] [Google Scholar]
- 105.Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrie B, Rinaldi-Carmona M, et al. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur J Biochem. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- 106.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 107.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Correa F, Docagne F, Mestre L, Clemente D, Hernangomez M, Loria F, et al. A role for CB2 receptors in anandamide signalling pathways involved in the regulation of IL-12 and IL-23 in microglial cells. Biochem Pharmacol. 2009;77:86–100. doi: 10.1016/j.bcp.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 109.Correa F, Hernangomez M, Mestre L, Loria F, Spagnolo A, Docagne F, et al. Anandamide enhances IL-10 production in activated microglia by targeting CB(2) receptors: roles of ERK1/2, JNK, and NF-kappaB. Glia. 2010;58:135–147. doi: 10.1002/glia.20907. [DOI] [PubMed] [Google Scholar]
- 110.Zoratti C, Kipmen-Korgun D, Osibow K, Malli R, Graier WF. Anandamide initiates Ca(2+) signaling via CB2 receptor linked to phospholipase C in calf pulmonary endothelial cells. Br J Pharmacol. 2003;140:1351–1362. doi: 10.1038/sj.bjp.0705529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Onaivi ES. Commentary: Functional Neuronal CB2 Cannabinoid Receptors in the CNS. Current neuropharmacology. 2011;9:205–208. doi: 10.2174/157015911795017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sisay S, Pryce G, Jackson SJ, Tanner C, Ross RA, Michael GJ, et al. Genetic background can result in a marked or minimal effect of gene knockout (GPR55 and CB2 receptor) in experimental autoimmune encephalomyelitis models of multiple sclerosis. PLoS One. 2013;8:76907. doi: 10.1371/journal.pone.0076907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 114.Simon MM, Greenaway S, White JK, Fuchs H, Gailus-Durner V, Wells S, et al. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013;14:82. doi: 10.1186/gb-2013-14-7-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB(1) on neurons and CB(2) on autoreactive T cells. Nat Med. 2007;13:492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- 116.Palazuelos J, Davoust N, Julien B, Hatterer E, Aguado T, Mechoulam R, et al. The CB(2) cannabinoid receptor controls myeloid progenitor trafficking: involvement in the pathogenesis of an animal model of multiple sclerosis. J Biol Chem. 2008;283:13320–13329. doi: 10.1074/jbc.M707960200. [DOI] [PubMed] [Google Scholar]
- 117.Fritz RB, Chou CH, McFarlin DE. Induction of experimental allergic encephalomyelitis in PL/J and (SJL/J × PL/J)F1 mice by myelin basic protein and its peptides: localization of a second encephalitogenic determinant. J Immunol. 1983;130:191–194. [PubMed] [Google Scholar]
- 118.Dittel BN. Direct suppression of autoreactive lymphocytes in the central nervous system via the CB2 receptor. Br J Pharmacol. 2008;153:271–276. doi: 10.1038/sj.bjp.0707493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dittel BN, Merchant RM, Janeway CA., Jr Evidence for Fas-dependent and Fas-independent mechanisms in the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol. 1999;162:6392–6400. [PubMed] [Google Scholar]
- 120.Balenga NA, Aflaki E, Kargl J, Platzer W, Schroder R, Blattermann S, et al. GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils. Cell research. 2011;21:1452–1469. doi: 10.1038/cr.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ziring D, Wei B, Velazquez P, Schrage M, Buckley NE, Braun J. Formation of B and T cell subsets require the cannabinoid receptor CB2. Immunogenetics. 2006;58:714–725. doi: 10.1007/s00251-006-0138-x. [DOI] [PubMed] [Google Scholar]
- 122.Basu S, Ray A, Dittel BN. Cannabinoid receptor 2 is critical for the homing and retention of marginal zone B lineage cells and for efficient T-independent immune responses. J Immunol. 2011;187:5720–5732. doi: 10.4049/jimmunol.1102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Muppidi JR, Arnon TI, Bronevetsky Y, Veerapen N, Tanaka M, Besra GS, et al. Cannabinoid receptor 2 positions and retains marginal zone B cells within the splenic marginal zone. J Exp Med. 2011;208:1941–1948. doi: 10.1084/jem.20111083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karlsson MC, Guinamard R, Bolland S, Sankala M, Steinman RM, Ravetch JV. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J Exp Med. 2003;198:333–340. doi: 10.1084/jem.20030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Elomaa O, Kangas M, Sahlberg C, Tuukkanen J, Sormunen R, Liakka A, et al. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell. 1995;80:603–609. doi: 10.1016/0092-8674(95)90514-6. [DOI] [PubMed] [Google Scholar]
- 126.Pereira JP, An J, Xu Y, Huang Y, Cyster JG. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat Immunol. 2009;10:403–411. doi: 10.1038/ni.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Basu S, Ray A, Dittel BN. Cannabinoid Receptor 2 (CB2) Plays a Role in the Generation of Germinal Center and Memory B Cells, but Not in the Production of Antigen-Specific IgG and gM, in Response to T-dependent Antigens. PLoS One. 2013;8:67587. doi: 10.1371/journal.pone.0067587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kong W, Li H, Tuma RF, Ganea D. Selective CB2 receptor activation ameliorates EAE by reducing Th17 differentiation and immune cell accumulation in the CNS. Cell Immunol. 2014;287:1–17. doi: 10.1016/j.cellimm.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Capettini LS, Savergnini SQ, da Silva RF, Stergiopulos N, Santos RA, Mach F, et al. Update on the role of cannabinoid receptors after ischemic stroke. Mediators of inflammation. 2012;2012:824093. doi: 10.1155/2012/824093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang M, Adler MW, Abood ME, Ganea D, Jallo J, Tuma RF. CB2 receptor activation attenuates microcirculatory dysfunction during cerebral ischemic/reperfusion injury. Microvascular research. 2009;78:86–94. doi: 10.1016/j.mvr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murikinati S, Juttler E, Keinert T, Ridder DA, Muhammad S, Waibler Z, et al. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. 2010;24:788–798. doi: 10.1096/fj.09-141275. [DOI] [PubMed] [Google Scholar]
- 132.Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, et al. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32:414–425. doi: 10.1016/j.immuni.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yan Y, Ding X, Li K, Ciric B, Wu S, Xu H, et al. CNS-specific therapy for ongoing EAE by silencing IL-17 pathway in astrocytes. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:1338–1348. doi: 10.1038/mt.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zarruk JG, Fernandez-Lopez D, Garcia-Yebenes I, Garcia-Gutierrez MS, Vivancos J, Nombela F, et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke; a journal of cerebral circulation. 2012;43:211–219. doi: 10.1161/STROKEAHA.111.631044. [DOI] [PubMed] [Google Scholar]
- 135.Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci U S A. 2006;103:696–701. doi: 10.1073/pnas.0504187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Karsak M, Cohen-Solal M, Freudenberg J, Ostertag A, Morieux C, Kornak U, et al. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum Mol Genet. 2005;14:3389–3396. doi: 10.1093/hmg/ddi370. [DOI] [PubMed] [Google Scholar]
- 137.Sipe JC, Arbour N, Gerber A, Beutler E. Reduced endocannabinoid immune modulation by a common cannabinoid 2 (CB2) receptor gene polymorphism: possible risk for autoimmune disorders. J Leukoc Biol. 2005;78:231–238. doi: 10.1189/jlb.0205111. [DOI] [PubMed] [Google Scholar]
- 138.Carrasquer A, Nebane NM, Williams WM, Song ZH. Functional consequences of nonsynonymous single nucleotide polymorphisms in the CB2 cannabinoid receptor. Pharmacogenetics and genomics. 2010;20:157–166. doi: 10.1097/FPC.0b013e3283367c6b. [DOI] [PubMed] [Google Scholar]
- 139.Yamada Y, Ando F, Shimokata H. Association of candidate gene polymorphisms with bone mineral density in community-dwelling Japanese women and men. International journal of molecular medicine. 2007;19:791–801. [PubMed] [Google Scholar]
- 140.Mahmoud Gouda H, Mohamed Kamel NR. Cannabinoid CB2 receptor gene (CNR2) polymorphism is associated with chronic childhood immune thrombocytopenia in Egypt. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2013;24:247–251. doi: 10.1097/MBC.0b013e32835aba1d. [DOI] [PubMed] [Google Scholar]
- 141.Rossi F, Mancusi S, Bellini G, Roberti D, Punzo F, Vetrella S, et al. CNR2 functional variant (Q63R) influences childhood immune thrombocytopenic purpura. Haematologica. 2011;96:1883–1885. doi: 10.3324/haematol.2011.045732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Coppola N, Zampino R, Bellini G, Macera M, Marrone A, Pisaturo M, et al. Association between a polymorphism in cannabinoid receptor 2 and severe necroinflammation in patients with chronic hepatitis C. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:334–340. doi: 10.1016/j.cgh.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 143.Rossi F, Bellini G, Alisi A, Alterio A, Maione S, Perrone L, et al. Cannabinoid receptor type 2 functional variant influences liver damage in children with non-alcoholic fatty liver disease. PLoS One. 2012;7:42259. doi: 10.1371/journal.pone.0042259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ishiguro H, Carpio O, Horiuchi Y, Shu A, Higuchi S, Schanz N, et al. A nonsynonymous polymorphism in cannabinoid CB2 receptor gene is associated with eating disorders in humans and food intake is modified in mice by its ligands. Synapse. 64:92–96. doi: 10.1002/syn.20714. [DOI] [PubMed] [Google Scholar]
- 145.Reinhard W, Stark K, Neureuther K, Sedlacek K, Fischer M, Baessler A, et al. Common polymorphisms in the cannabinoid CB2 receptor gene (CNR2) are not associated with myocardial infarction and cardiovascular risk factors. International journal of molecular medicine. 2008;22:165–174. [PubMed] [Google Scholar]
- 146.Xie XQ, Chen JZ, Billings EM. 3D structural model of the G-protein-coupled cannabinoid CB2 receptor. Proteins. 2003;53:307–319. doi: 10.1002/prot.10511. [DOI] [PubMed] [Google Scholar]