Abstract
22q11.2 deletion syndrome (22q11.2DS) is the most common microdeletion syndrome in humans. Survival to reproductive age and beyond is now the norm. Several manifestations of this syndrome, such as congenital cardiac disease and neuropsychiatric disorders, may increase risk for adverse pregnancy outcomes in the general population. However, there are limited data on reproductive health in 22q11.2DS. We performed a retrospective chart review for 158 adults with 22q11.2DS (75 male, 83 female; mean age 34.3 years) and extracted key variables relevant to pregnancy and reproductive health. We present four illustrative cases as brief vignettes. There were 25 adults (21>age 35 years; 21 female) with a history of one or more pregnancies. Outcomes for women with 22q11.2DS, compared with expectations for the general population, showed a significantly elevated prevalence of small for gestational age liveborn offspring (p<0.001), associated mainly with infants with 22q11.2DS. Stillbirths also showed elevated prevalence (p<0.05). Not all observed adverse events appeared to be attributable to transmission of the 22q11.2 deletion. Recurring issues relevant to reproductive health in 22q11.2DS included the potential impact of maternal morbidities, inadequate social support, unsafe sexual practices, and delayed diagnosis of 22q11.2DS and/or lack of genetic counseling. These preliminary results emphasize the importance of early diagnosis and long term follow-up that could help facilitate genetic counseling for men and women with 22q11.2DS. We propose initial recommendations for pre-conception management, educational strategies, pre-natal planning, and preparation for possible high-risk pregnancy and/or delivery.
Keywords: DiGeorge syndrome, Contraception, Velocardiofacial syndrome, Prenatal testing, Pregnancy complications, Genomic disorder
Introduction
22q11.2 deletion syndrome (22q11.2DS) is the most common microdeletion syndrome in humans (Bassett et al. 2011; Costain et al. 2013; Goodship et al. 1998; Kaminsky et al. 2011). Some features present in infancy or early childhood, including serious congenital heart disease (30–40 % of individuals), palatal anomalies (~40 %), and neonatal hypocalcemia (~10 %) (Bassett et al. 2005, 2011; Cheung et al. 2014a; McDonald-McGinn and Sullivan 2011). Later onset features include intellectual disability (30–40 %), recurrent seizures (~40 %), thyroid disease (~25 %) and psychiatric conditions (collectively >60 %, including schizophrenia in 20–25 %) (Bassett et al. 2005, 2011; Cheung et al. 2014b; Fung et al. 2010; McDonald-McGinn and Sullivan 2011; Schneider et al. 2014). In up to 10 % of individuals newly diagnosed with 22q11.2DS the deletion is found to be inherited (McDonald-McGinn and Sullivan 2011). In the modern era of medical and surgical interventions, most individuals with 22q11.2DS live to reproductive age and beyond (Bassett et al. 2009; McDonald-McGinn and Sullivan 2011). Women with 22q11.2DS who have neither intellectual disability nor schizophrenia have a similar number of offspring as their unaffected sisters (Costain et al. 2011). Many of the features that are prevalent in 22q11.2DS, such as congenital heart disease and schizophrenia, are known to be associated with increased risk for adverse pregnancy and/or birth outcomes in the general population (Hatton et al. 2012; Jablensky et al. 2005; McConnell et al. 2008; Ramos et al. 2012; Vissenberg et al. 2012). The risk of transmission of the 22q11.2 deletion and options for prenatal diagnosis are common elements of genetic counseling for adults with 22q11.2DS (Bassett et al. 2011; Bretelle et al. 2010; McDonald-McGinn and Zackai 2008). However, other issues related to sexual and reproductive health may be under-addressed (Finucane 2010; Taylor et al. 2006).
We hypothesized that relative to population norms, adults with 22q11.2DS would experience elevated rates of spontaneous pregnancy loss, pregnancy and delivery complications, and suboptimal birth parameters in their offspring. Additionally, studies have suggested that some patients with 22q11.2DS may display deficits in judgment and social functioning (Angkustsiri et al. 2014; Bassett et al. 2011; Butcher et al. 2012; Chow et al. 2006; Ho et al. 2012). We therefore anticipated that we might also find evidence of poor reproductive health practices and inadequate social support that could pose barriers to a safe pregnancy and delivery. Using a retrospective chart review design, we conducted an initial investigation of reproductive issues in 22q11.2DS, with the aim of presenting preliminary recommendations for the management of pregnancy in adults with 22q11.2DS.
Methods
Participants
Ascertainment and detailed genotypic and phenotypic characterization of this study cohort are described in previous publications (Bassett et al. 2005, 2008, 2009; Butcher et al. 2012; Costain et al. 2011, 2012; Fung et al. 2008; Karas et al. 2014). Briefly, all were adults (>17.5 years of age) who met clinical criteria for 22q11.2DS and had 22q11.2 deletions confirmed with standard methods (Bassett et al. 2008). All had typical (~2.6 Mb) A to D deletions or nested 22q11.2 deletions. The majority of study participants were initially ascertained through congenital cardiac or psychiatric services. Informed consent was obtained in writing, and the study was approved by local research ethics boards.
Data Collection and Analysis
Data relevant to reproductive health were compiled by the authors (CC, LO) from a comprehensive retrospective chart review, using previously published methods (Bassett et al. 2005; Cheung et al. 2014a; Costain et al. 2011). Interview notes, pregnancy and developmental history assessments, medical records, and/or collateral information provided by family members were available for review. Outcome variables examined included pregnancy outcome (elective termination, miscarriage, stillbirth, or live birth), pregnancy complications (gestational diabetes, placenta abruptio, polyhydramnios, pre-eclampsia, premature rupture of membranes, and preterm labor), method of delivery (vaginal or Caesarean section), and birth parameters (gestational age and birth weight). Any available history of genetic counseling was recorded. Data were not complete for all variables for all subjects (see Tables 1 and 2).
Table 1.
Reproductive practices and relevant social and outcome data for 18 women with 22q11.2DS who had liveborn offspring
| Mode of contraception (ever used)a | n | (%) |
|---|---|---|
| Oral contraceptive pill | 11 | (61.1) |
| Tubal ligation | 6 | (33.3) |
| Uncertain/No data | 5 | (27.8) |
| Characteristics of the 22 male partners who fathered the offspring | n | (%) |
| Physical or emotional abuseb of female partner with 22q11.2DS | 7 | (31.8) |
| Alcohol and/or substance abuseb | 6 | (27.3) |
| History of incarceration | 2 | (9.1) |
| At least one of the above | 13 | (59.1) |
| Marital status at time of offspring’s birth | ||
| Married or common-lawc | 16 | (72.7) |
| Marital status at time of last follow-up | ||
| Married or common-lawc | 8 | (36.4) |
| Offspring living situation (data available for n=36 of 37)d | n | (%) |
| Raised by parent with 22q11.2DS, with or without co-parent | 25 | (69.4) |
| Adopted out of the family | 9 | (25.0) |
| Raised by other parent only | 2 | (5.6) |
Patients may overlap
As documented by a physician involved in the patient’s care
Living together in a conjugal relationship for at least 12 continuous months
Infancy to age 17 years
Table 2.
Pregnancy and delivery/birth characteristics for 37 liveborn offspring of 18 women with 22q11.2DS
| Pregnancy, delivery, and birth outcomesa | 22q11.2 deletion status of the offspring
|
|||||
|---|---|---|---|---|---|---|
| Deletion (n=23) | No deletion (n=14) | |||||
|
| ||||||
| Pregnancy complications (maximum n=37) a | n | (%) | n | (%) | n | (%) |
| Gestational diabetes | 2 | (5.41) | 2 | (8.70) | 0 | 0.00 |
| Placenta abruptio | 1 | (2.70) | 1 | (4.35) | 0 | 0.00 |
| Polyhydramnios | 2 | (5.41) | 2 | (8.70) | 0 | 0.00 |
| Pre-eclampsia | 4 | (10.81) | 3 | (13.04) | 1 | (7.14) |
| Premature rupture of membranes | 2 | (5.41) | 1 | (4.35) | 1 | (7.14) |
| Preterm labor (<37 weeks) b | 6 | (16.22) | 3 | (13.04) | 3 | (21.43) |
| At least one of the above | 11 | (29.73) | 6 | (26.09) | 5 | (35.71) |
| Method of delivery (n=36) a | (data for n=23) | (data for n=13) | ||||
| Vaginal delivery | 25 | (69.44) | 18 | (78.26) | 7 | (53.85) |
| Spontaneous | 13 | (36.11) | 9 | (39.13) | 4 | (30.77) |
| Induced | 5 | (13.89) | 3 | (13.04) | 2 | (15.38) |
| Assisted (forceps or vacuum) | 4 | (11.11) | 3 | (13.04) | 1 | (7.69) |
| Caesarean section | 11 | (30.56) | 5 | (21.74) | 6 | (46.15) |
| Planned | 5 | (13.89) | 2 | (8.70) | 3 | (23.08) |
| Emergency | 6 | (16.67) | 3 | (13.04) | 3 | (23.08) |
| Gestational age (GA) (n=37) a | (data for n=23) | (data for n=14) | ||||
| Term (37–41 weeks GA) | 30 | (81.08) | 18 | (78.26) | 12 | (85.71) |
| Premature (<37 weeks GA)b | 6 | (16.22) | 4 | (17.39) | 2 | (14.29) |
| Postmature (>41 weeks GA) | 1 | (2.70) | 1 | (4.35) | 0 | 0.00 |
| Birth weight for gestational age (n=35) a | (data for n=22) | (data for n=13) | ||||
| Normal (10–90 %ile) | 22 | (62.86) | 11 | (50.00) | 11 | (84.62) |
| Small (<10 %ile) | 12 | (34.29)c | 11 | (50.00)d | 1 | (7.69) |
| Large (>90 %ile) | 1 | (2.86) | 0 | 0.00 | 1 | (7.69) |
Maximum possible sample size=37; numbers for individual features represent minimums, including details about vaginal deliveries
Note: One patient experienced preterm contractions that settled allowing delivery after 37 weeks gestation, and another patient underwent a Caesarean section prior to 37 weeks gestation without preterm labor
Significantly greater proportion than for Canadian norms (p<0.001; see text)
Significantly greater proportion for offspring with a 22q11.2 deletion than for offspring without a 22q11.2 deletion (p=0.01). These included four infants with a 22q11.2 deletion bornSGA <3rd percentile compared to population norms (Kramer et al. 2001). Of the 37 live births, there were five deaths in infancy or early childhood (< age 5 years), involving serious congenital cardiac and/or other major anomalies. Four of these five were born SGA <10th percentile
Fisher’s exact test was used to compare categorical pregnancy outcomes within 22q11.2DS, and binomial tests were used for comparisons with population-based Canadian norms from the median year of birth of study offspring (Fisher et al. 2004; Health Canada 2003; Kramer et al. 2001; Wou et al. 2014). All tests were two-tailed, with statistical significance defined as p<0.05. Recurring themes of potential relevance to medical management or genetic counseling were also identified, including any social determinants of health such as sexual practices, social support, and utilization of health care resources. We then selected four cases for presentation as illustrative vignettes, changing minor details to safeguard anonymity.
Results
There were 158 adults with 22q11.2DS [83 (52.5 %) female; mean age=34.3 (SD=11.8) years] with reproductive data available for study. Of these, 66 (41.8 %) had serious congenital heart disease, 58 (36.7 %) had schizophrenia or schizoaffective disorder, and 75 (47.5 %) had intellectual disability. There was a lifetime prevalence of hypocalcemia in 116 (73.4 %) and hypothyroidism in 39 (24.7 %). In total, 25 adults with 22q11.2DS [15.8 %; 21 female; mean age= 43.7 (SD=11.8) years; n=21>35 years] had a history of one or more pregnancies. Of these, 11 (44.0 %) had serious congenital heart disease including tetralogy of Fallot (n=9), pulmonary atresia with ventricular septal defect (n=1), and sinus of Valsalva aneurysm (n=1). Four (16.0 %) individuals had schizophrenia. The mean IQ of these 25 adults was 73.7 (SD= 7.5; range: 59 to 94); five (20.0 %) had mild intellectual disability. There was a lifetime history of hypocalcemia in 17 (68.0 %) and hypothyroidism in 8 (32.0 %) patients. Consistent with our previous study (Costain et al. 2011), adults with 22q11.2DS who had one or more pregnancies were more likely to be female (p<0.01), less likely to have schizophrenia (p=0.02), and less likely to have intellectual disability (p<0.01).
Twenty-two (88.0 %) of the 25 adults with one or more pregnancies had a liveborn offspring: 18 women with 37 offspring, and 4 men with 8 offspring. All liveborn offspring were from singleton pregnancies except for one set of twins where one twin miscarried. Fifteen (68.2 %) of the 22 parents were diagnosed with 22q11.2DS only after the birth of an affected child. Of the remaining seven individuals, only two pursued prenatal genetic testing during their three total pregnancies (all resulting in live births).
Of the 14 known pregnancies of five partners of four men with 22q11.2DS, there were five miscarriages, one elective termination for nonviable fetus, and eight live births (five with 22q11.2DS). The pregnancy of one offspring with the 22q11.2 deletion was complicated by polyhydramnios and one without the deletion by pre-eclampsia. Three of the offspring were delivered by Caesarean section, and two of the five vaginal births were induced. All were term births except one (unaffected) born premature at 36 weeks. One liveborn offspring with 22q11.2DS was small for gestational age (<10th percentile) (Kramer et al. 2001).
The 58 pregnancies of the 21 women with 22q11.2DS comprised 13 miscarriages, six elective terminations, two stillbirths, and 37 live births. The prevalence of miscarriage (22.4 %) was not significantly different from a general population estimate of 15 % (p=0.17) (Garcia-Enguindanos et al. 2002). As a proportion of live births and terminations, elective terminations were 14.0 %, compared with the Canadian norm (of 22.3 %) (Health Canada 2003). Reasons for terminations included unintended pregnancy, fear of maternal health complications and non-viable fetus; none were on the basis of transmitting the 22q11.2 deletion. As a proportion of live births and stillbirths, stillbirths were 5.4 %, greater than a comparable Canadian norm of 0.41 % (p<0.05) (Wou et al. 2014).
Women with 22q11.2DS with Liveborn Offspring
Eighteen women with 22q11.2DS had at least one liveborn offspring. Table 1 contains data on contraceptive practices and social characteristics. Six (33.3 %) of these mothers subsequently had a tubal ligation, compared with 12 % for comparable Canadian norms (p=0.03) (Fisher et al. 2004). Of the 11 mothers who had ever used oral contraception, three (27.3 %) had noncompliance documented by a health care professional. Of the 22 partners who fathered offspring with these 18 women, 13 (59.1 %) were abusive to the mother, had a history of alcohol or substance abuse, and/or had been incarcerated at least once (Table 1). Most of the mothers with 22q11.2DS were married or in common-law relationships at the time of birth of their offspring; eight (36.4 %) of these relationships were still intact at an average of 16 years later (Table 1). Of the 36 offspring for whom data were available, 11 (30.6 %) were not raised by the mother with 22q11.2DS (Table 1).
Table 2 shows delivery and neonatal characteristics, for the 37 liveborn offspring (median maternal age 27 years, range 18 to 40) of 18 mothers with 22q11.2DS. The prevalence of SGA (defined as gestational age and sex-specific birth weight below the 10th percentile) was significantly elevated over population expectations (34.3 % vs. 10.0 %, p<0.001) (Kramer et al. 2001). Stratifying results by 22q11.2 deletion status revealed a significantly higher prevalence of SGA in those neonates who did, compared to those who did not, inherit the 22q11.2 deletion (50.0 % vs. 7.7 %, p=0.01) (Table 2). The proportion of pregnancies with at least one complication was 29.7 % (Table 2). Rates of Caesarean section deliveries (30.6 %) and prematurity (16.2 %) were not significantly different from those for population-based Canadian norms [17.5 % (p=0.08) and 7.0 % (p= 0.08), respectively] (Health Canada 2003).
Case Vignettes
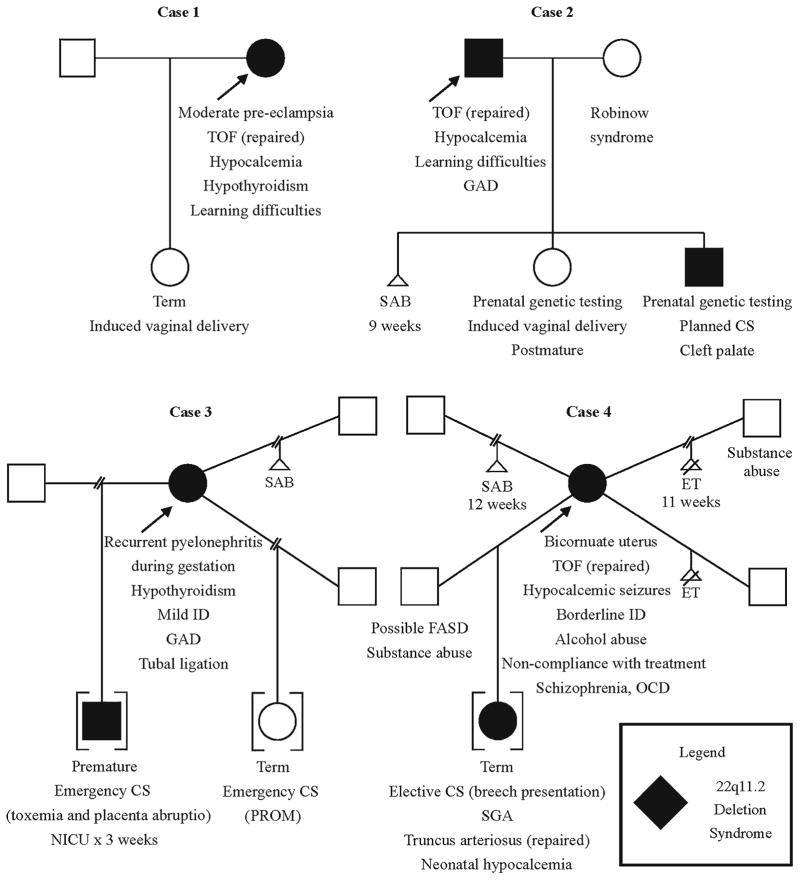
In the course of our detailed chart review, we identified several recurring issues relevant to reproductive health and counseling in 22q11.2DS. These included: (i) a high burden of maternal morbidities including cardiac, endocrine, and neuropsychiatric diseases, and subsequent challenges in managing care during pregnancy and delivery; (ii) unsafe sexual practices leading to inadequate contraception, transmission of sexually transmitted infections (STIs), and sexual victimization; (iii) inadequate social support; and, (iv) delayed diagnosis of 22q11.2DS and/or lack of genetic counseling. We provide four case vignettes to illustrate these key themes and demonstrate the breadth of outcomes observed in our study cohort. Figure 1 includes medical data relevant to each case.
Fig. 1.
Four illustrative cases of parents with 22q11.2 deletion syndrome (22q11.2DS). See text for details. Abbreviations: CS, Caesarean section; ET, elective termination; FASD, fetal alcohol spectrum disorder; GAD, generalized anxiety disorder; ID, intellectual disability; NICU, neonatal intensive care unit; OCD, obsessive-compulsive disorder; PROM, premature rupture of membranes; SAB, spontaneous abortion (miscarriage); SGA, small for gestational age; TOF, tetralogy of Fallot
Patient 1, a fulltime homemaker, was found to have a de novo 22q11.2 deletion at age 17 years. Her features of 22q11.2DS included tetralogy of Fallot (repaired), submucous cleft palate (repaired), hypocalcemia (managed with vitamin D and calcium supplements), and hypothyroidism (managed with replacement therapy).
Social Support
Patient 1 married at 21 years of age. Her husband had a college education and stable employment, and was noted to be a supportive partner.
Reproductive Health and Planning
Patient 1 was on oral contraception before and after her planned pregnancy. Prior to conception, she received genetic counseling with a focus on reproductive decision making, and had a detailed cardiovascular assessment at an obstetric medicine clinic for adults with congenital heart disease. Although she was asymptomatic, she elected to have pulmonary valve replacement surgery for pulmonary regurgitation and poor right ventricular function in preparation for pregnancy.
At age 25 years, the patient began her first and only documented pregnancy. She attended monthly appointments with a local obstetrician and also at a specialized high risk obstetric medicine clinic. Fetal echocardiograms revealed no structural or functional abnormalities. She declined amniocentesis but consented to post-natal genetic testing of her offspring. She was also followed by an endocrinologist and was seen at a high risk anesthesia clinic at 34 weeks gestation.
Medical Morbidities and Pregnancy Complications
At 36 weeks gestation, the patient was diagnosed with moderate pre-eclampsia and microcephaly (<5th percentile) was detected in the fetus. The patient was placed on continuous external fetal surveillance and was given antibiotics for endocarditis prophylaxis. Induced labor the following week was long (35 hours), but she experienced no peri-partum complications. Her daughter was born with mild microcephaly but tested negative for the 22q11.2 deletion and is otherwise in good health.
Patient 2, who worked as a driver, was found to have a de novo 22q11.2 deletion at age 18 years. His features of 22q11.2DS included tetralogy of Fallot (repaired), hypocalcemia (managed with vitamin D and calcium replacement), and generalized anxiety disorder (managed with sertraline).
Social Support
Patient 2 met his wife at age 27 years. She had been diagnosed clinically at 16 months of age with Robinow syndrome (Patton and Afzal 2002). She worked part-time in healthcare, and was noted to be a supportive partner.
Reproductive Health and Planning
The couple met with a medical geneticist and genetic counselor several times prior to their first pregnancy. Records indicate that they were provided with information regarding variable expression of the 22q11.2 deletion with respect to congenital and later-onset features, even between two individuals in the same family.
Medical Morbidities and Pregnancy Complications
The couple’s first pregnancy resulted in a miscarriage. In the second pregnancy, the fetus tested negative for the 22q11.2 deletion on amniocentesis and the mother was induced at 9 days post-term. One year later in a third pregnancy, the fetus tested positive for the 22q11.2 deletion on amniocentesis. Fetal echocardiogram showed no cardiac abnormalities. Since cardiac disease was the major manifestation of the father’s 22q11.2 deletion, the couple reasoned that the child would have a mild lifelong phenotype, even though they were counseled otherwise. The child was born with an overt cleft palate and has developmental delays.
Patient 3 was found to have a de novo 22q11.2 deletion at age 42 years, 11 years after the diagnosis of 22q11.2DS in her first child, who was adopted out at a young age. Her features of 22q11.2DS included hypothyroidism (managed with replacement therapy) and generalized anxiety disorder (managed with citalopram). Her employment history included several jobs in semi-skilled labor, although at time of last contact she was receiving government assistance.
Social Support
Patient 3 left secondary school after her first pregnancy, which resulted in a miscarriage. She lived in group homes during her teenage years and in subsidized housing in adulthood. The patient had a reported history of physical and sexual abuse from relatives and past sexual partners, and was diagnosed with post-traumatic stress disorder and social phobia at age 36 years. Both of her children were monitored by the Children’s Aid Society and were adopted out of the family at a young age.
Reproductive Health and Planning
The patient underwent a tubal ligation after her second child.
Medical Morbidities and Pregnancy Complications
The patient’s first child was born at 34 weeks gestation by emergency Caesarean section due to toxemia and placenta abruptio. The child required oral and gastric suction and extended feeding through a nasogastric tube, and was placed in intensive care for three weeks. The child inherited the 22q11.2 deletion, diagnosed at age 9 years.
The patient’s third pregnancy was complicated by recurrent pyelonephritis that resulted in hospitalizations at 26 and 36 weeks gestation. She was admitted to hospital with Braxton Hicks contractions at 30 weeks gestation. At 38 weeks gestation, her membranes ruptured three days before a planned Caesarean section, necessitating an emergency Caesarean section. Tissue analysis revealed a placental infarct and acute chorioamnionitis. The child was later found not to have inherited the 22q11.2 deletion.
Patient 4 was found to have a de novo 22q11.2 deletion at age 26 years, after the birth of an affected daughter at her second pregnancy. Her features of 22q11.2DS included tetralogy of Fallot (repaired), hypocalcemia leading to seizures, bicornuate uterus, and obsessive-compulsive disorder (OCD), with schizophrenia diagnosed at age 30 years after a long history of behavioral problems. She was prescribed calcium and Vitamin D supplementation, clomipramine, and risperidone, but was frequently noncompliant with treatment. She was often unemployed and supported through government assistance in adulthood.
Social Support
Several of the patient’s sexual partners were described by her as aggressive and abusive. The father of her daughter had a history of alcohol abuse, incarceration, and occasional semi-skilled employment. During their relationship, he physically abused the patient and encouraged her to prostitute herself. Records suggest he might have been previously diagnosed with a fetal alcohol spectrum disorder. The patient’s family was not supportive of her decision to continue with this pregnancy. The child was taken into the care of the Children’s Aid Society at a young age.
Reproductive Health and Planning
Patient 4 had a history of impulsive behavior and poor decision making, including unsafe sexual practices and physical fighting. She was prescribed oral contraception but was frequently noncompliant. After a first pregnancy resulted in a miscarriage at 12 weeks gestation, the patient had a second pregnancy at age 26 years. She discontinued her medication for OCD without consulting a physician due to fears of teratogenicity. She saw a cardiologist at 16 weeks gestation because she was concerned that her heart disease could affect her fetus, but otherwise she did not see an obstetrician or have a pregnancy evaluation until 25 weeks gestation.
After her daughter was born, Patient 4 was pregnant again at ages 30 and 38 years, and elected to terminate both pregnancies. Prior to her last pregnancy, she had expressed a wish to have another child despite repeated warnings from her family and healthcare providers about the dangers a pregnancy would pose to her physical and mental health. She initially believed that the Children’s Aid Society would allow her to keep her child because she felt she was in a stable relationship, even though her partner was unemployed, had a history of substance abuse and a criminal history, and did not share her desire to have a child. The patient ultimately elected to undergo an elective termination due to concern for her own physical well-being after an episode of first trimester bleeding.
Medical Morbidities and Pregnancy Complications
During the pregnancy that resulted in a live birth at age 26 years, the patient had mild pulmonary insufficiency and recurrent seizures in the context of hypocalcemia and noncompliance with treatment. A fetal echocardiogram at 25 weeks gestation revealed truncus arteriosus. The patient underwent an elective Caesarean section at 38 weeks gestation for breech presentation. The infant was SGA (<10th percentile), hypocalcemic, cyanotic, and dyspneic in the immediate post-partum period, requiring digoxin, calcium, and urgent truncus arteriosus repair within days of birth. Post-partum, the patient experienced depression, seizures, and dyspnea.
Discussion
To our knowledge, this is the first study of sexual and reproductive health in adults with 22q11.2DS. For women with 22q11.2DS who had pregnancies, we observed a significantly higher rate of stillbirths, and of SGA in their liveborn offspring, compared with Canadian norms. Findings related to other spontaneous pregnancy loss and pregnancy and birth complications (e.g., polyhydramnios, preterm labor) will require higher powered samples to determine significant differences from population expectations, and whether complications are related to the 22q11.2 deletion in a parent and/or in the fetus. We identified potentially actionable recurring issues relevant to reproductive health in 22q11.2DS. Table 3 summarizes considerations, compiled from multiple sources, for medical geneticists, genetic counselors, obstetricians, or other health care professionals involved in the management of pregnancy related to adults with 22q11.2DS.
Table 3.
Summary of reproductive health issues and suggested management strategies in adults with 22q11.2DS
| Issue | Suggested management strategies to consider |
|---|---|
| Cardiac, endocrine, and neuropsychiatric morbidities of 22q11.2DS |
|
| Social support |
|
| Sexual and reproductive health |
|
| Genetic counseling |
|
Pregnancy, Delivery and Related Issues in 22q11.2DS
Many common manifestations of 22q11.2DS, including intellectual disability and, cardiac, endocrine, and psychiatric disease, can pose risks to the mother and fetus during pregnancy and in the post-partum period (Hatton et al. 2012; Jablensky et al. 2005; McConnell et al. 2008; Negro and Mestman 2011; Parkes et al. 2013; Thorne 2004). Potential in utero effects of maternal morbidities would be expected to be independent of the 22q11.2 deletion status of the fetus. In contrast, the elevated risk of SGA appeared to be driven mainly by fetuses with the 22q11.2 deletion (Table 2). Larger sample sizes would be necessary to determine the relative contributions of transmission of the 22q11.2 deletion and/or possible in utero factors in regard to any increased rates of pregnancy loss and delivery/birth complications.
As in the general population, non-adherence with medical treatment during pregnancy (as seen in the Patient 4 vignette) may occur in adults with 22q11.2DS (Nordeng et al. 2010). Changes in maternal physiology during pregnancy that may exacerbate select medical problems (e.g., schizophrenia, cardiac disease, thyroid disease) and potential teratogenicity of certain medications should prompt reassessment of the treatment plan (Bassett and Chow 2008; Negro and Mestman 2011; Seeman 2013; Thorne 2004).
Co-morbid learning difficulties and/or psychiatric illness, inadequate social support, and low socioeconomic status may delay receipt of appropriate medical care in pregnancy (Butcher et al. 2012; Dunlop et al. 2000; Finucane 2010), as was the case for patients in vignettes 3 and 4. In contrast, Patient 1, who did not have any significant learning or psychiatric disorder and received good social and financial support, was afforded the benefits of pre-conception planning and coordinated pregnancy management.
Social Support and Sexual Health Considerations
Patients with 22q11.2DS may not always receive adequate social support from their spouse and/or family, as seen for Patients 3 and 4. Romantic relationships culminating in pregnancy frequently did not result in stable, long-term unions (Table 1). Assortative mating and related phenomena (e.g., see Patient 2 vignette) may increase risks to the offspring and complicate genetic counseling (Bassett et al. 2011). Additionally, some of the adults with 22q11.2DS followed at our clinic are estranged from their families, as illustrated in the case of Patient 4. This may be due in part to a tension between the legal autonomy and desire for independence in the adult with 22q11.2DS, and the parents’ view of the health risks posed by pregnancy and the adult’s capabilities to care for an offspring (perhaps especially a child with 22q11.2DS) (Butcher et al. 2012; Karas et al. 2014). Inadequate social support may contribute to the involvement of child protection organizations, as for Patients 3 and 4, with adoption proceeding if the parent(s) cannot provide the necessary level of care.
Furthermore, studies have suggested that many patients with 22q11.2DS display weaknesses in social functioning disproportionate to their abilities in other aspects of daily living (Angkustsiri et al. 2014; Bassett et al. 2011; Butcher et al. 2012; Ho et al. 2012). Poor judgment, deficits in social interaction, and intellectual disability may be factors in unsafe sexual practices and increase vulnerability to exploitation (Bassett et al. 2011; Costain et al. 2011). These may also be factors in the rates of tubal ligation and elective terminations observed in our study cohort, perhaps together with inadequate sexual health education and counseling (Servais et al. 2002).
The Case for Early Diagnosis and Effective Genetic Counseling
Many adults with 22q11.2DS are only diagnosed following the birth of a more severely affected offspring (Cirillo et al. 2014; Devriendt et al. 1997; McDonald-McGinn et al. 2001; Oh et al. 2002; Patel et al. 2006). Whether the apparent inter-generational worsening of the 22q11.2DS phenotype in familial cases is solely related to ascertainment and other biases is unclear (Cirillo et al. 2014; Costain et al. 2011). Assortative mating and in utero effects of a maternal 22q11.2 deletion could contribute to a biological basis for progressive worsening of phenotypic severity in some families.
We anticipate that widespread use of chromosomal micro-array analysis and non-invasive prenatal testing, and the resulting increased detection of unexpected 22q11.2 deletions in utero will result in the identification of many more hitherto undiagnosed individuals in need of timely genetic counseling (Costain et al. 2013). As well, guidelines recommending 22q11.2 deletion screening in children with conotruncal heart defects or neonatal hypocalcemia may facilitate early diagnosis and thus genetic counseling for patients with 22q11.2DS (Monteiro et al. 2013); many of the patients in our cohort might have benefitted from such screening. Also, preconception diagnosis of a parent can facilitate timely medical intervention for an affected newborn.
In our study cohort only two patients underwent prenatal genetic testing. Delayed diagnosis, as was the case for Patient 3, precludes opportunities for 22q11.2DS-focused anticipatory care, coordination of services, and pre-conception genetic counseling. This highlights the importance of maintaining a low threshold for testing for 22q11.2DS by the pediatrician, family physician, or other specialists (Greenhalgh et al. 2003; Kapadia and Bassett 2008; Monteiro et al. 2013). Early recognition and treatment of neonatal seizures and hypocalcemia in 22q11.2DS may be associated with fewer severe cognitive outcomes (Cheung et al. 2014a). A recent French study reported that in cases where 22q11.2DS was diagnosed prenatally, parents decided to electively terminate the pregnancy in 68.9 % of cases with a similar rate (57.1 %) when the deletion in the fetus was inherited and the parent was aware of his/her deletion at the time of diagnosis (Besseau-Ayasse et al. 2014). This is considerably higher than the 14.0 % rate of elective terminations in our cohort. Further studies are needed on the impact of early diagnosis and opportunities for prenatal planning.
The variable expression of 22q11.2DS can be a challenging concept to convey in a brief genetic counseling session. Typical genetic counseling may be confounded by co-morbid learning difficulties and/or psychiatric illness, and hence developmentally appropriate strategies, as well as repeated genetic counseling sessions are often needed (Butcher et al. 2012; Finucane 2010).
Study Strengths and Limitations, and Research Recommendations
This study drew upon reproductive health data from the largest single cohort of adults with 22q11.2DS in the world. Nonetheless, we report data for a relatively small number of pregnancies of adults with 22q11.2DS, especially those of partners of men with 22q11.2DS. The available sample size limited the ability to perform comparisons with general population data and to conduct sufficiently powered statistical analyses. The number of univariate tests conducted increased the likelihood of family-wise error. Thus, some of the findings may have achieved statistical significance due to chance. As this was an exploratory study, setting a p value of 0.05 is permissible. Further research is needed, however, to confirm the findings obtained in this study. Given that many of the patients in our clinic were ascertained from psychiatric services, there was a higher proportion of patients with schizophrenia in our study cohort than is documented in other studies of 22q11.2DS. There were incomplete data for many participants due to the retrospective nature of the chart review study design, and miscarriages and pregnancy complications may have been under-reported. We encountered challenges obtaining a comprehensive history and obstetrical care records for many individuals in our study cohort, which to some extent was a consequence of the 22q11.2DS-related issues identified in this report. On the other hand, many adults with 22q11.2DS and positive reproductive outcomes (including unaffected offspring) may never be diagnosed at all and thus would not be included in studies of this kind. The necessary data were not available for us to compare the quality and extent of genetic counseling, contraceptive use, and romantic partner attributes between adults with 22q11.2DS who had and had not conceived at least one pregnancy. Intergenerational comparisons of phenotypes were beyond the scope of this report, and an appropriately powered study accounting for ascertainment and birth cohort effects is a future consideration. Finally, standard recommendations for management of specific maternal morbidities are based on experiences in the general population, and may not be generalizable to a multi-system genomic disorder like 22q11.2DS.
Practice Implications
Managing the care of a patient with 22q11.2DS during pregnancy may involve multiple complexities. Early diagnosis facilitates early genetic counseling, highlighting the importance of awareness of, and a low threshold for testing for, 22q11.2 deletions. Education on sexual health and effective, developmentally-appropriate genetic counseling (Finucane 2010) are recommended to be incorporated into the standard care of a patient with 22q11.2DS, beginning in adolescence or at time of diagnosis (Bassett et al. 2011). An interdisciplinary approach should help to improve the safety and success of pregnancy, labor and delivery, and the post-partum period. The considerations presented here may potentially be extrapolated to other genomic disorders associated with pregnancy and/or birth complications (Lowther et al., 2014).
Conclusions
Outcomes of pregnancies of women with 22q11.2DS showed a significantly higher prevalence of SGA that appeared to be mainly associated with infants with the 22q11.2 deletion. Although stillbirths were elevated over expected rates, this and other findings with respect to spontaneous loss and other pregnancy and birth complications deserve further study using adequately powered samples. Suggested strategies for optimizing reproductive health in 22q11.2DS include the effective management of maternal morbidities pre-conception, tailoring social supports to the patients’ needs, promoting safe sexual practices, and timely, comprehensive, and developmentally appropriate genetic counseling beginning in adolescence. Our data support the importance of early diagnosis of 22q11.2DS, with concomitant opportunities for improved reproductive and other outcomes.
Acknowledgments
The authors thank the adults with 22q11.2DS and their families for their generous contributions to this and related research studies. The authors express gratitude to the students, research assistants, and staff affiliated with the Clinical Genetics Research Program, Clinical Genetics Service, and Toronto Congenital Cardiac Centre for Adults (TCCCA). Special thanks go to Fiona Fu, Lisa Palmer, and Monica Torsan. This work was supported by Canadian Institutes of Health Research (CIHR) grants (MOP-97800 and MOP-89066), MD/PhD studentships from CIHR and the McLaughlin Centre (to Gregory Costain), and an award from the Mach-Gaensslen Foundation administered through the Comprehensive Research Experience for Medical Students (CREMS) summer research program (to Chrystal Chan). Anne S. Bassett holds the Canada Research Chair in Schizophrenia Genetics and Genomic Disorders, and the Dalglish Chair in 22q11.2 Deletion Syndrome.
Footnotes
Conflict of Interest Statements Chrystal Chan, Gregory Costain, Lucas Ogura, Candice K. Silversides, Eva W.C. Chow, and Anne S. Bassett declare that they have no conflict of interest.
Human Studies and Informed Consent All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Animal Studies No animal studies were carried out by the authors for this article.
Contributor Information
Chrystal Chan, Clinical Genetics Research Program, Centre for Addiction and Mental Health, Toronto, ON, Canada, Undergraduate Medical Education, Department of Medicine, University of Toronto, Toronto, ON, Canada.
Gregory Costain, Clinical Genetics Research Program, Centre for Addiction and Mental Health, Toronto, ON, Canada, Undergraduate Medical Education, Department of Medicine, University of Toronto, Toronto, ON, Canada.
Lucas Ogura, Clinical Genetics Research Program, Centre for Addiction and Mental Health, Toronto, ON, Canada.
Candice K. Silversides, Division of Cardiology, Department of Medicine, University Health Network, Toronto, ON, Canada, Toronto Congenital Cardiac Centre for Adults, Toronto General Hospital, Toronto, ON, Canada, Obstetric Medicine Program, Mount Sinai Hospital, Toronto, ON, Canada
Eva W.C. Chow, Clinical Genetics Research Program, Centre for Addiction and Mental Health, Toronto, ON, Canada, Department of Psychiatry, University of Toronto, Toronto, ON, Canada
Anne S. Bassett, Clinical Genetics Research Program, Centre for Addiction and Mental Health, Toronto, ON, Canada, Division of Cardiology, Department of Medicine, University Health Network, Toronto, ON, Canada, Toronto Congenital Cardiac Centre for Adults, Toronto General Hospital, Toronto, ON, Canada, Department of Psychiatry, University of Toronto, Toronto, ON, Canada, Department of Psychiatry, and Toronto General Research Institute, University Health Network, Toronto, ON, Canada, The Dalglish Family Hearts and Minds Clinic for 22q11.2 Deletion Syndrome, Toronto General Hospital, University Health Network, Toronto, ON, Canada
References
- Angkustsiri K, Goodlin-Jones B, Deprey L, Brahmbhatt K, Harris S, Simon T. Social impairments in chromosome 22q11.2 deletion syndrome (22q11.2DS): autism spectrum disorder or a different endophenotype? Journal of Autism and Developmental Disorders. 2014;44(4):739–746. doi: 10.1007/s10803-013-1920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EW. Schizophrenia and 22q11.2 deletion syndrome. Current Psychiatry Reports. 2008;10(2):148–157. doi: 10.1007/s11920-008-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EW, Husted J, Weksberg R, Caluseriu O, Webb GD, et al. Clinical features of 78 adults with 22q11 deletion syndrome. American Journal of Medical Genetics Part A. 2005;138(4):307–313. doi: 10.1002/ajmg.a.30984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Marshall CR, Lionel AC, Chow EW, Scherer SW. Copy number variations and risk for schizophrenia in 22q11.2 deletion syndrome. Human Molecular Genetics. 2008;17(24):4045–4053. doi: 10.1093/hmg/ddn307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EW, Husted J, Hodgkinson KA, Oechslin E, Harris L, et al. Premature death in adults with 22q11.2 deletion syndrome. Journal of Medical Genetics. 2009;46(5):324–330. doi: 10.1136/jmg.2008.063800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, McDonald-McGinn DM, Devriendt K, Digilio MC, Goldenberg P, Habel A, et al. Practical guidelines for managing patients with 22q11.2 deletion syndrome. Journal of Pediatrics. 2011;159(2):332–339. e331. doi: 10.1016/j.jpeds.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besseau-Ayasse J, Violle-Poirsier C, Bazin A, Gruchy N, Moncla A, Girard F, et al. A French collaborative survey of 272 fetuses with 22q11.2 deletion: ultrasound findings, fetal autopsies and pregnancy outcomes. Prenatal Diagnosis. 2014;34(5):424–430. doi: 10.1002/pd.4321. [DOI] [PubMed] [Google Scholar]
- Bretelle F, Beyer L, Pellissier MC, Missirian C, Sigaudy S, Gamerre M, et al. Prenatal and postnatal diagnosis of 22q11.2 deletion syndrome. European Journal of Medical Genetics. 2010;53(6):367–370. doi: 10.1016/j.ejmg.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Butcher N, Chow E, Costain G, Karas D, Ho A, Bassett A. Functional outcomes of adults with 22q11.2 deletion syndrome. Genetics in Medicine. 2012;14(10):836–843. doi: 10.1038/gim.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EN, George SR, Andrade DM, Chow EW, Silversides CK, Bassett AS. Neonatal hypocalcemia, neonatal seizures, and intellectual disability in 22q11.2 deletion syndrome. Genetics in Medicine. 2014a;16(1):40–44. doi: 10.1038/gim.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EN, George SR, Costain GA, Andrade DM, Chow EW, Silversides CK, et al. Prevalence of hypocalcemia and its associated features in 22q11.2 deletion syndrome. Clinical Endocrinology. 2014b;81(2):190–196. doi: 10.1111/cen.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EW, Watson M, Young DA, Bassett AS. Neurocognitive profile in 22q11 deletion syndrome and schizophrenia. Schizophrenia Research. 2006;87(1–3):270–278. doi: 10.1016/j.schres.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo E, Giardino G, Gallo V, Puliafito P, Azzari C, Bacchetta R, et al. Intergenerational and intrafamilial phenotypic variability in 22q11.2 deletion syndrome subjects. BMC Medical Genetics. 2014;15(1):1. doi: 10.1186/1471-2350-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costain G, Chow EW, Silversides CK, Bassett AS. Sex differences in reproductive fitness contribute to preferential maternal transmission of 22q11.2 deletions. Journal of Medical Genetics. 2011;48(12):819–824. doi: 10.1136/jmedgenet-2011-100440. [DOI] [PubMed] [Google Scholar]
- Costain G, Chow EW, Ray PN, Bassett AS. Caregiver and adult patient perspectives on the importance of a diagnosis of 22q11.2 deletion syndrome. Journal of Intellectual Disability Research. 2012;56(6):641–651. doi: 10.1111/j.1365-2788.2011.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costain G, McDonald-McGinn DM, Bassett AS. Prenatal genetic testing with chromosomal microarray analysis identifies major risk variants for schizophrenia and other later-onset disorders. American Journal of Psychiatry. 2013;170(12):1498. doi: 10.1176/appi.ajp.2013.13070880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devriendt K, Moerman P, Van Schoubroeck D, Vandenberghe K, Fryns JP. Chromosome 22q11 deletion presenting as the Potter sequence. Journal of Medical Genetics. 1997;34(5):423–425. doi: 10.1136/jmg.34.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop S, Coyte PC, McIsaac W. Socio-economic status and the utilisation of physicians’ services: results from the Canadian national population health survey. Social Science and Medicine. 2000;51(1):123–133. doi: 10.1016/s0277-9536(99)00424-4. [DOI] [PubMed] [Google Scholar]
- Fantasia HC, Fontenot HB. The sexual safety of adolescents. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2011;40(2):217–224. doi: 10.1111/j.1552-6909.2011.01217.x. [DOI] [PubMed] [Google Scholar]
- Finucane B. Genetic counseling for women with intellectual disabilities. In: LeRoy BS, Veach PM, Bartels DM, editors. Genetic Counseling Practice: Advanced Concepts and Skills. Hoboken: Wiley-Blackwell; 2010. [Google Scholar]
- Fisher W, Boroditsky R, Morris B. The 2002 Canadian contraception study: part 2. Journal of Obstetrics and Gynaecology Canada. 2004;26(7):646–656. doi: 10.1016/s1701-2163(16)30612-0. [DOI] [PubMed] [Google Scholar]
- Fung WL, Chow EW, Webb GD, Gatzoulis MA, Bassett AS. Extracardiac features predicting 22q11.2 deletion syndrome in adult congenital heart disease. International Journal of Cardiology. 2008;131(1):51–58. doi: 10.1016/j.ijcard.2007.08.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung WL, McEvilly R, Fong J, Silversides C, Chow E, Bassett A. Elevated prevalence of generalized anxiety disorder in adults with 22q11.2 deletion syndrome. American Journal of Psychiatry. 2010;167(8):998. doi: 10.1176/appi.ajp.2010.09101463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Enguindanos A, Calle ME, Valero J, Luna S, Dominquez-Rojas V. Risk factors in miscarriage: a review. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2002;102(2):111–119. doi: 10.1016/s0301-2115(01)00613-3. [DOI] [PubMed] [Google Scholar]
- Goodship J, Cross I, LiLing J, Wren C. A population study of chromosome 22q11 deletions in infancy. Archives of Disease in Childhood. 1998;79(4):348–351. doi: 10.1136/adc.79.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh KL, Aligianis IA, Bromilow G, Cox H, Hill C, Stait Y, et al. 22q11 deletion: a multisystem disorder requiring multidisciplinary input. Archives of Disease in Childhood. 2003;88(6):523–524. doi: 10.1136/adc.88.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton R, Colman JM, Sermer M, Grewal J, Silversides CK. Cardiac risks and management of complications in pregnant women with congenital heart disease. Future Cardiology. 2012;8(2):315–327. doi: 10.2217/fca.12.18. [DOI] [PubMed] [Google Scholar]
- Health Canada. Canadian Perinatal Health Report, 2003. Ottawa: Minister of Public Works and Government Services Canada; 2003. [Google Scholar]
- Ho JS, Radoeva PD, Jalbrzikowski M, Chow C, Hopkins J, Tran WC, et al. Deficits in mental state attributions in individuals with 22q11.2 deletion syndrome (velo-cardio-facial syndrome) Autism Research : Official Journal of the International Society for Autism Research. 2012;5(6):407–418. doi: 10.1002/aur.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablensky AV, Morgan V, Zubrick SR, Bower C, Yellachich LA. Pregnancy, delivery, and neonatal complications in a population cohort of women with schizophrenia and major affective disorders. American Journal of Psychiatry. 2005;162(1):79–91. doi: 10.1176/appi.ajp.162.1.79. [DOI] [PubMed] [Google Scholar]
- Kaminsky EB, Kaul V, Paschall J, Church DM, Bunke B, Kunig D, et al. An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genetics in Medicine. 2011;13(9):777–784. doi: 10.1097/GIM.0b013e31822c79f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia RK, Bassett AS. Recognizing a common genetic syndrome: 22q11.2 deletion syndrome. Canadian Medical Association Journal. 2008;178(4):391–393. doi: 10.1503/cmaj.071300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas DJ, Costain G, Chow EW, Bassett AS. Perceived burden and neuropsychiatric morbidities in adults with 22q11.2 deletion syndrome. Journal of Intellectual Disability Research. 2014;58(2):198–210. doi: 10.1111/j.1365-2788.2012.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham C, Harris S, Grzybowski S. Evidence-based pre-natal care: part I. General prenatal care and counseling issues. American Family Physician. 2005;71(7):1307–1316. [PubMed] [Google Scholar]
- Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108(2):E35. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- Lowther C, Costain G, Stavropoulos DJ, Melvin R, Silversides CK, Andrade DM, et al. Delineating the 15q13.3 microdeletion phenotype: a case series and comprehensive review of the literature. Genet Med. 2014 doi: 10.1038/gim.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell D, Mayes R, Llewellyn G. Women with intellectual disability at risk of adverse pregnancy and birth outcomes. Journal of Intellectual Disability Research. 2008;52(Pt 6):529–535. doi: 10.1111/j.1365-2788.2008.01061.x. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn D, Sullivan K. Chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) Medicine. 2011;90(1):1–18. doi: 10.1097/MD.0b013e3182060469. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Zackai EH. Genetic counseling for the 22q11.2 deletion. Developmental Disabilities Research Reviews. 2008;14(1):69–74. doi: 10.1002/ddrr.10. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Tonnesen MK, Laufer-Cahana A, Finucane B, Driscoll DA, Emanuel BS, et al. Phenotype of the 22q11.2 deletion in individuals identified through an affected relative: cast a wide FISHing net! Genetics in Medicine. 2001;3(1):23–29. doi: 10.1097/00125817-200101000-00006. [DOI] [PubMed] [Google Scholar]
- Monteiro FP, Vieira TP, Sgardioli IC, Molck MC, Damiano AP, Souza J, et al. Defining new guidelines for screening the 22q11.2 deletion based on a clinical and dysmorphologic evaluation of 194 individuals and review of the literature. European Journal of Pediatrics. 2013;172(7):927–945. doi: 10.1007/s00431-013-1964-0. [DOI] [PubMed] [Google Scholar]
- Moos MK, Dunlop AL, Jack BW, Nelson L, Coonrod DV, Long R, et al. Healthier women, healthier reproductive outcomes: recommendations for the routine care of all women of reproductive age. American Journal of Obstetrics and Gynecology. 2008;199(6 Suppl 2):S280–S289. doi: 10.1016/j.ajog.2008.08.060. [DOI] [PubMed] [Google Scholar]
- Negro R, Mestman JH. Thyroid disease in pregnancy. Best Practice & Research Clinical Endocrinology & Metabolism. 2011;25(6):927–943. doi: 10.1016/j.beem.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Nordeng H, Ystrom E, Einarson A. Perception of risk regarding the use of medications and other exposures during pregnancy. European Journal of Clinical Pharmacology. 2010;66(2):207–214. doi: 10.1007/s00228-009-0744-2. [DOI] [PubMed] [Google Scholar]
- Oh DC, Min JY, Lee MH, Kim YM, Park SY, Won HS, et al. Prenatal diagnosis of tetralogy of Fallot associated with chromosome 22q11 deletion. Journal of Korean Medical Science. 2002;17(1):125–128. doi: 10.3346/jkms.2002.17.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes I, Schenker JG, Shufaro Y. Parathyroid and calcium metabolism disorders during pregnancy. Gynecological Endocrinology. 2013;29(6):515–519. doi: 10.3109/09513590.2012.754880. [DOI] [PubMed] [Google Scholar]
- Patel ZM, Gawde HM, Khatkhatay MI. 22q11 microdeletion studies in the heart tissue of an abortus involving a familial form of congenital heart disease. Journal of Clinical Laboratory Analysis. 2006;20(4):160–163. doi: 10.1002/jcla.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton MA, Afzal AR. Robinow syndrome. Journal of Medical Genetics. 2002;39(5):305–310. doi: 10.1136/jmg.39.5.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos GA, Chopra IJ, Bales SR. Endocrine disorders in pregnancy. In: Di Saia PJ, Chaudhuri G, Guidice LC, Moore TR, Porto M, Smith LH, editors. Women’s Health Review: A Clinical Update in Obstetrics-Gynecology. Philadelphia: Elsevier Saunders; 2012. pp. 226–234. [Google Scholar]
- Rasiah SV, Publicover M, Ewer AK, Khan KS, Kilby MD, Zamora J. A systematic review of the accuracy of first-trimester ultrasound examination for detecting major congenital heart disease. Ultrasound in Obstetrics and Gynecology. 2006;28(1):110–116. doi: 10.1002/uog.2803. [DOI] [PubMed] [Google Scholar]
- Schneider M, Debbané M, Bassett AS, Chow EWC, Fung WLA, Van den Bree MBM, et al. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. American Journal of Psychiatry. 2014;171(6):627–639. doi: 10.1176/appi.ajp.2013.13070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman MV. Clinical interventions for women with schizophrenia: pregnancy. Acta Psychiatrica Scandinavica. 2013;127(1):12–22. doi: 10.1111/j.1600-0447.2012.01897.x. [DOI] [PubMed] [Google Scholar]
- Servais L, Jacques D, Leach R, Conod L, Hoyois P, Dan B, et al. Contraception of women with intellectual disability: prevalence and determinants. Journal of Intellectual Disability Research. 2002;46(Pt 2):108–119. doi: 10.1046/j.1365-2788.2002.00360.x. [DOI] [PubMed] [Google Scholar]
- Taylor MR, Edwards JG, Ku L. Lost in transition: challenges in the expanding field of adult genetics. American Journal of Medical Genetics Part C, Seminars in Medical Genetics. 2006;142C(4):294–303. doi: 10.1002/ajmg.c.30105. [DOI] [PubMed] [Google Scholar]
- Thorne SA. Pregnancy in heart disease. Heart. 2004;90(4):450–456. doi: 10.1136/hrt.2003.027888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissenberg R, van den Boogaard E, van Wely M, Van der Post JA, Fliers E, Bisschop PH, et al. Treatment of thyroid disorders before conception and in early pregnancy: a systematic review. Human Reproduction Update. 2012;18(4):360–373. doi: 10.1093/humupd/dms007. [DOI] [PubMed] [Google Scholar]
- Wou K, Ouellet MP, Chen MF, Brown RN. Comparison of the aetiology of stillbirth over five decades in a single centre: a retrospective study. BMJ Open. 2014;4(6):e004635. doi: 10.1136/bmjopen-2013-004635. [DOI] [PMC free article] [PubMed] [Google Scholar]