Summary
Generation of different CD4 T cell responses to commensal and pathogenic bacteria is crucial for maintaining healthy gut environment, but the associated cellular mechanisms are poorly understood. Dendritic cells (DCs) and macrophages (Mfs) integrate microbial signals and direct adaptive immunity. Although the role of DCs in initiating T cell responses is well appreciated, how Mfs contribute to the generation of CD4 T cell responses to intestinal microbes is unclear. Th17 cells are critical for mucosal immune protection and at steady state are induced by commensal bacteria, such as segmented filamentous bacteria (SFB). Here, we examined the roles of mucosal DCs and Mfs in Th17 induction by SFB in vivo. We show that Mfs, and not conventional CD103+ DCs, are essential for generation of SFB-specific Th17 responses. Thus, Mfs drive mucosal T cell responses to certain commensal bacteria.
Introduction
How the mucosal immune system integrates signals from vastly diverse types of antigenic stimuli, such as food antigens, invasive pathogens, and various commensal bacteria in order to maintain a healthy gut without compromising protective immunity remains of critical interest. The intestinal lamina propria (LP) contains a dense network of mononuclear phagocytes (MNPs), which play an essential role in inducing specific immunity or maintaining tolerance.
LP MNPs consist of conventional dendritic cells (DCs), most of which express the integrin CD103, and CX3CR1+ intestinal macrophages (Mfs) (Bogunovic et al., 2012; Farache et al., 2013). LP DCs develop from DC-restricted precursors and require the cytokine Flt3L (Bogunovic et al., 2009; Varol et al., 2009). Although originally classified as DCs, CX3CR1+ Mfs are distinguished by expression of the macrophage-specific markers CD64 and F4/80, derive from CCR2+Ly6Chi blood monocytes at steady state, and depend on CSF-1 for their development (Bain et al., 2013; Bogunovic et al., 2009; Tamoutounour et al., 2012; Varol et al., 2009; Zigmond and Jung, 2013). A distinguishing feature of intestinal Mfs, compared to other tissue Mfs (e.g., peritoneal macrophages, alveolar macrophages, microglia), is that they express high levels of MHCII, suggesting that, similarly to DCs, they may actively participate in priming, activation, or maintenance of mucosal effector CD4 T cell responses.
The differential functional roles of LP MNP subsets in intestinal T cell homeostasis are a topic of intense investigation, but remain poorly defined, especially in vivo. LP DCs are professional antigen presenting cells that migrate to the mesenteric lymph nodes (MLN), where they prime effector CD4 T cells to intestinal antigens and imbue them with gut-homing capabilities (Bogunovic et al., 2012; Farache et al., 2013; Grainger et al., 2014). In contrast, LP Mfs are generally confined to the mucosa and control local intestinal immune responses via production of IL-10 during the steady state, or via production of pro-inflammatory cytokines during active immune responses or inflammation (Cerovic et al., 2014; Murai et al., 2009; Rivollier et al., 2012; Schreiber et al., 2013).
The role of MNP subsets in LP Th17 cell induction in vivo is unclear. Previous studies have proposed either LP DCs or Mfs as mediators of Th17 cell induction (Atarashi et al., 2008; Denning et al., 2011; Scott et al., 2014). However, this was largely based on assessing the ability of isolated MNP subsets to skew T cell differentiation in vitro and their differential roles under physiological conditions are not clear. The small intestinal LP contains a distinct DC subset, termed double-positive DCs (DP DCs), because of the co-expression of CD103 and CD11b. Deficiencies in DP DC generation result in partial decrease of Th17 cells in vivo (Lewis et al., 2011; Persson et al., 2013; Schlitzer et al., 2013; Welty et al., 2013). Therefore, DP DCs are considered essential for Th17 cell responses in vivo, although whether these cells are required for commensal-induced Th17 responses has not been investigated. We previously showed that at steady state resident mucosa-associated bacteria, called segmented filamentous bacteria (SFB), induce LP Th17 cells (Ivanov et al., 2009). We also showed that most SFB-induced Th17 cells are SFB-specific and that presentation of SFB antigens for Th17 cell induction requires MHCII expression by CD11c+ LP MNPs (Goto et al., 2014). Here, we took advantage of this tractable system to investigate the contribution of individual CD11c+ MNP subsets to commensal induction of LP Th17 cells in vivo. We found that CD103+ DCs are dispensable for induction of antigen-specific Th17 cell responses following SFB colonization. In contrast, intestinal Mfs were essential for this process. Nongenotoxic depletion of intestinal Mfs prior to SFB colonization resulted not only in a loss of Th17 cell induction, but in a loss of SFB-specific T cells in the LP, suggesting that LP Mfs are required for acquisition and presentation of SFB antigens. These results demonstrate a crucial role for intestinal Mfs in mediating antigen-specific Th17 cell responses to mucosa-associated commensals.
Results
We recently showed that commensal Th17 cell induction is mediated by the antigen-presenting function of CD11c+MHCII+ MNPs in the small intestinal (SI) LP (Goto et al., 2014). To characterize the role of different MNP subsets in this process we examined Th17 cell induction by SFB following genetic ablation. Four major CD11c+MHCII+ MNP subsets were followed throughout this study using the gating strategies in Figure 1A and S1A. Conventional CD103+ LP DCs consist of gut-specific CD103+CD11b+ DCs (DP DCs) controlled by the transcription factors Notch2 and IRF4 (Lewis et al., 2011; Persson et al., 2013; Schlitzer et al., 2013) and CD103+CD11b− DCs (CD103 SP DCs) that require BATF3 and IRF8 for their development (Edelson et al., 2010). The remaining CD103−CD11b+ MNPs express the chemokine receptor CX3CR1 and consist predominantly of intestinal Mfs, which were identified based on expression of CD64 and F4/80 (Tamoutounour et al., 2012), and a smaller population of CD64−F4/80− MNPs, that express intermediate levels of the monocyte/Mf marker CX3CR1, but also express the DC markers CD24 and CD26 (Figure S1B). Although CD103−CD11b+CD64−CD24+ cells may represent a phenotypically and developmentally heterogeneous population (Cerovic et al., 2013; Denning et al., 2011; Scott et al., 2014), we refer to them here as CD11b single positive DCs (CD11b SP DCs).
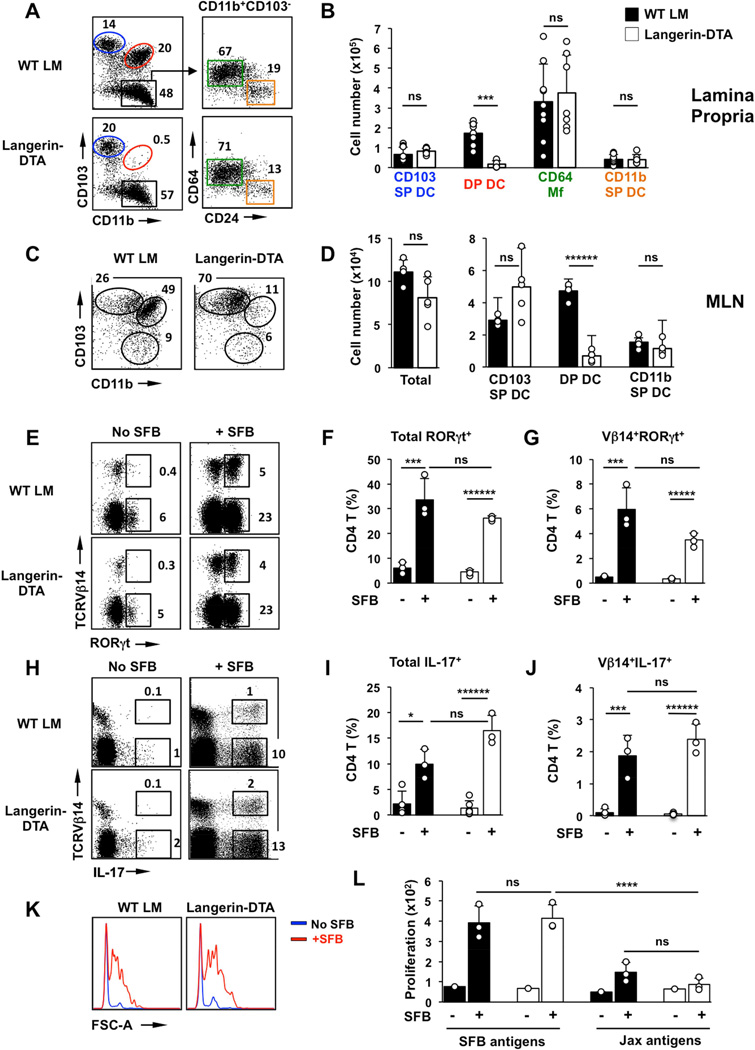
Figure 1. CD103+CD11b+ (DP) DCs are dispensable for commensal Th17 cell induction.
(A,B) CD11c+MHCII+ MNP subsets in SI LP of Langerin-DTA mice and control wildtype littermates (WT LM). (A left) FACS plots gated on CD11c+MHCII+ cells. A (right) Distribution of CD64 Mfs and CD11b SP DCs within the CD103−CD11b+ gate. Numbers represent percentage of cells in the corresponding gate. (B) Total number of cells in individual MNP subsets as defined in (A). (C,D) Total cell numbers and cell numbers in MNP subsets in the migratory DC fraction of mesenteric lymph nodes (MLN). Plots in (C) are gated on Lin−CD11cloMHCIIhi migratory DC. (E–G) Induction of RORγt+ Th17 cells by SFB in small intestinal (SI) LP. Plots gated on TCRβ+CD4+ cells. (H–J) Induction of IL-17+ Th17 cells by SFB in SI LP. Plots gated on TCRb+CD4+ cells. (K,L) Response of purified SI LP CD4 T cells to SFB antigens or control bacterial antigens (Jax antigens) prepared as described in Methods. Open circles in bar graphs in all panels represent data from individual animals. Data from three experiments with similar results.
DP DCs are dispensable for Th17 cell induction
DP DCs have been shown to promote Th17 cell differentiation in vitro (Denning et al., 2011). In addition, we and others have shown a decrease in LP Th17 cell numbers in mice with genetic deficiency of DP DCs, suggesting a role for this MNP subset in vivo (Lewis et al., 2011; Persson et al., 2013; Schlitzer et al., 2013; Welty et al., 2013). However, the specific role of DP DCs in microbiota-mediated induction of Th17 cells has not been examined. To this end, we colonized DP DC-deficient mice and wildtype (WT) littermates, with SFB and examined Th17 cell induction and induction of SFB-specific CD4 T cells in the SI LP.
Langerin-DTA mice (Kaplan et al., 2005) express diphtheria toxin (DT) under transcriptional control of the human Langerin promoter resulting in selective ablation of epidermal Langerhans cells as well as DP DCs in the SI LP (Figure 1A,B, Table S1 and (Welty et al., 2013)). Migratory DP DCs were also absent in MLN of Langerin-DTA mice (Figure 1C,D). Colonization of WT littermates with SFB led to induction of RORγt+ and IL-17+ (Th17) CD4 T cells in the SI LP (Figure 1E–J). In addition, SFB colonization resulted in induction of SFB-specific CD4 T cells as demonstrated by the enrichment of Vβ14+ Th17 cells (Goto et al., 2014; Yang et al., 2014) (Figure 1G,J) and by the response of purified SI LP CD4 T cells to SFB antigens in vitro (Figure 1K,L). When Langerin-DTA mice were colonized with SFB, Th17 cells in the LP expanded similarly to those in WT littermates (Figure 1E–J). Moreover, significant induction of SFB-specific Vβ14+ Th17 cells and response of LP CD4 T cells to SFB antigens in vitro were evident (Figure 1J–L). These results demonstrate that DP DCs are dispensable for both T cell priming and Th17 cell differentiation following SFB colonization.
We obtained similar results using another model of DP DC depletion. DP DC development depends on Notch2 and conditional deletion of Notch2 in CD11c+ cells leads to significant loss of DP DCs (Lewis et al., 2011). Similarly to Langerin-DTA mice, loss of DP DCs in CD11c-Cre/Notch2-flox mice did not affect Th17 cell induction by SFB (Figure S2).
CD103 DCs are dispensable for Th17 cell induction by SFB
CD103 SP DCs are capable of migrating to the MLN, share a developmental pathway with CD8α+ splenic DC, and are proficient in cross-presentation (Cerovic et al., 2013; Cerovic et al., 2015; Edelson et al., 2010; Ginhoux et al., 2009). Whether they play a non-redundant role in commensal CD4 Th17 cell responses is not known. To address their role in SFB-induced Th17 cell differentiation, we colonized SFB-negative BATF3-deficient mice and heterozygous littermates with SFB and compared Th17 cell induction and induction of SFB-specific CD4 T cells (Figure S3). As previously reported (Edelson et al., 2010), BATF3-deficient mice lacked CD103 SP DCs in LP and MLN (Figure S3A–D). Nevertheless, Th17 cell induction after SFB colonization was unaffected in these animals. Similarly, induction of SFB-specific CD4 T cells and response to SFB antigens were similar to littermate controls (Figure S3E–M). Therefore, CD103 SP DCs are not required for commensal-induced Th17 cell priming and differentiation.
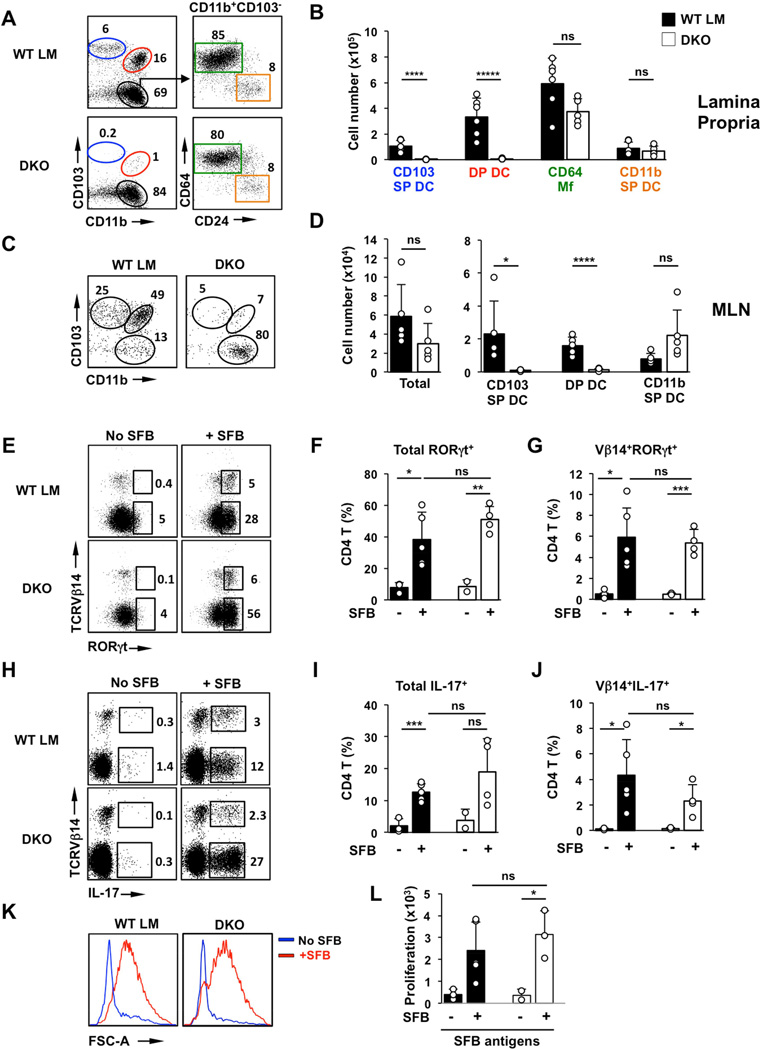
The two subsets of CD103+ DCs represent the main conventional DC subsets in the LP and have both been shown to migrate to MLN and prime CD4 T cell responses (Bogunovic et al., 2009; Cerovic et al., 2013; Schulz et al., 2009; Varol et al., 2009). To account for potential redundant functions of these subsets in Th17 responses to SFB, we crossed Langerin-DTA mice and BATF3-deficient mice (Figure 2). The resulting double-knockout (DKO) mice lacked all CD103 DC subsets in both SI LP and MLN (Figure 2A–D and Table S1). Despite the absence of virtually all CD103 DCs, colonization of DKO and littermate control mice with SFB led to a similar induction of RORγt+ and IL-17+ CD4 T cells in the SI LP (Figure 2E–J). In addition, there was a significant induction of Vβ14+RORγt+ and Vβ14+IL-17+ SFB-specific CD4 T cells in the DKO small intestine (Figure 2E,G,H,J), and isolated SI LP CD4 T cells from DKO mice responded to SFB antigens in in vitro proliferation assays similarly to WT CD4 T cells (Figure 2K,L). We generated another model of CD103 DC deficiency by crossing BATF3-deficient mice and CD11c-Cre/Notch2-flox mice. These animals also lacked both CD103+ DC subsets and showed normal Th17 cell responses to SFB (data not shown). These results demonstrate that LP CD103 DCs are dispensable for priming of SFB-specific CD4 T cells and Th17 cell induction in response to SFB.
Figure 2. CD103+ DCs are dispensable for commensal Th17 cell induction.
(A,B) CD11c+MHCII+ MNP subsets in SI LP of Langerin-DTA X Batf3−/− (DKO) mice and control littermates. (A left) FACS plots gated on CD11c+MHCII+ cells. Numbers represent percentage of cells in the corresponding gate. (B) total number of cells in individual MNP subsets. (C,D) Total cell numbers and cell numbers in MNP subsets in the migratory DC fraction of mesenteric lympn nodes (MLN). (E–G) Induction of RORγt+ Th17 cells by SFB in small intestinal (SI) LP. Plots gated on TCRβ+CD4+ cells. (H–J) Induction of IL-17+ Th17 cells by SFB in SI LP. Plots gated on TCRβ+CD4+ cells. (K) Response to SFB antigens of purified SI LP CD4 T cells from DKO and control littermates. SI LP CD4 T cells were isolated before (No SFB) and after (+SFB) colonization with SFB and incubated with CD11c+ splenic DCs for 72 hours in the presence of SFB antigens. (L) Proliferation of purified SI LP CD4 T cells in response to SFB antigens. LP CD4 T cells were isolated and cultured as in K in the presence of SFB antigens as described in Methods. Open circles in bar graphs in all panels represent data from individual animals. Data from three experiments with similar results.
Conventional DCs are dispensable for commensal Th17 cell induction at steady state
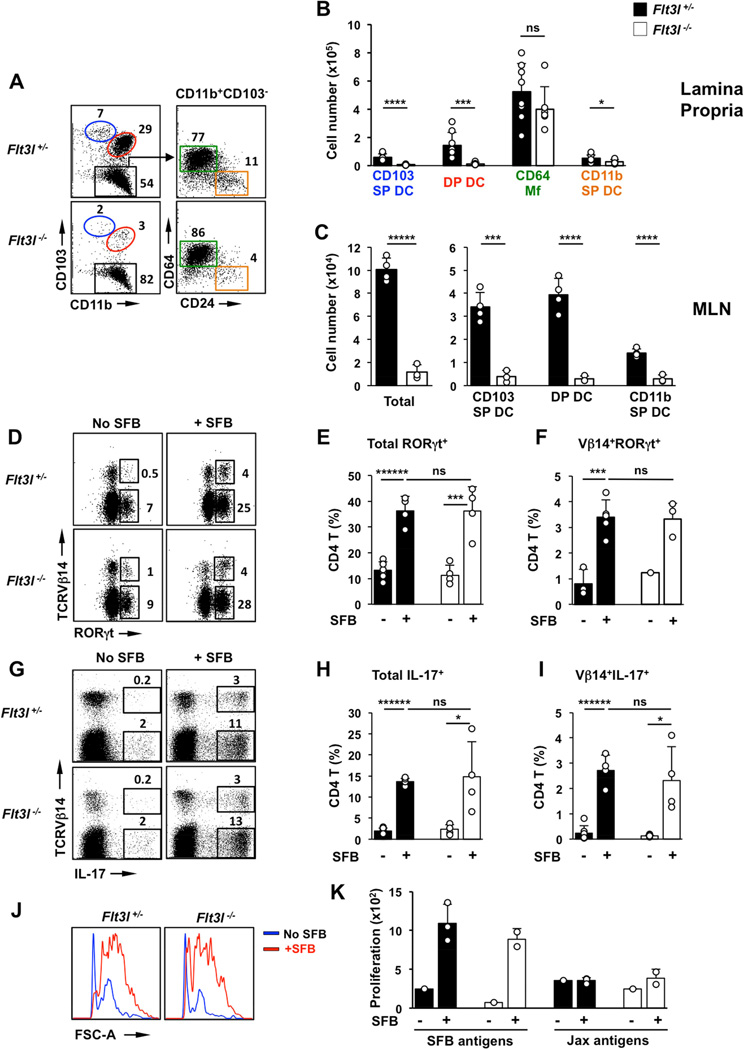
Conventional intestinal DCs depend on the DC-specific growth factor Flt3L (Bogunovic et al., 2009; Koscso et al., 2015; Scott et al., 2014). To determine if conventional DCs in general play a role in generation of SFB-induced Th17 cells, we examined Th17 cell induction in Flt3L-deficient mice. Similarly to CD103+ DCs, CD11b+CD103− DCs have been shown to derive from pre-DC precursors, be dependent on Flt3L and are, significantly decreased in Flt3L-deficient mice (Scott et al., 2014). We established SFB-negative Flt3L-deficient mice and compared Th17 cell induction following SFB colonization. CD103+ DC were almost absent from the SI LP in these animals (>90% reduction, compared to heterozygous littermates). Flt3L-deficient mice also had significantly diminished CD11b SP DCs, in agreement with previous studies (Scott et al., 2014) (Figure 3A,B and Table S1). All subsets of migratory DCs were also severely reduced in MLN (Figure 3C). In contrast, the total number of CD64+ Mfs in the SI LP was similar between control littermates and Flt3L-deficient mice (Figure 3A,B). Surprisingly, despite the severe defect in DC numbers, as well as possible defects in lymphocyte development in Flt3L-deficient animals, SFB still induced normal levels of Th17 cells (Figure 3D–I). In addition, priming and generation of SFB-specific CD4 T cells was virtually unperturbed, as was the generation of antigen-specific Th17 cells (Figure 3I–K).
Figure 3. Conventional DCs are dispensable for commensal Th17 cell induction.
(A,B) CD11c+MHCII+ MNP subsets in SI LP of Flt3l−/− mice and control heterozygous littermates (Flt3l+/−). (A left) FACS plots gated on CD11c+MHCII+ cells. Numbers represent percentage of cells in the corresponding gate. (B) total number of cells in individual MNP subsets. (C) Total cell numbers and cell numbers in MNP subsets in the migratory DC fraction of mesenteric lymph nodes (MLN). (D–F) Induction of RORγt+ Th17 cells by SFB in SI LP. Plots gated on TCRβ+CD4+ cells. (G–I) Induction of IL-17+ Th17 cells by SFB in SI LP. Plots gated on TCRβ+CD4+ cells. (J,K) Response of purified SI LP CD4 T cells to SFB antigens and antigens isolated from SFB-negative feces (Jax antigens). CD4 T cells were isolated from the SI LP of SFB-colonized Flt3l+/− or Flt3l−/− mice and assessed for antigen reactivity as described in Methods. Open circles in bar graphs represent data from individual animals. Data combined from two experiments with similar results.
Based on the combined data in Figures 1–3, we conclude that conventional gut CD103+ DCs and Flt3L-dependent CD103− DCs are not required for the acquisition and presentation of SFB antigens, priming of SFB-specific T cells and induction of Th17 cells in the SI LP.
Nongenotoxic depletion of intestinal monocyte-derived cells prevents SFB-specific Th17 cell responses
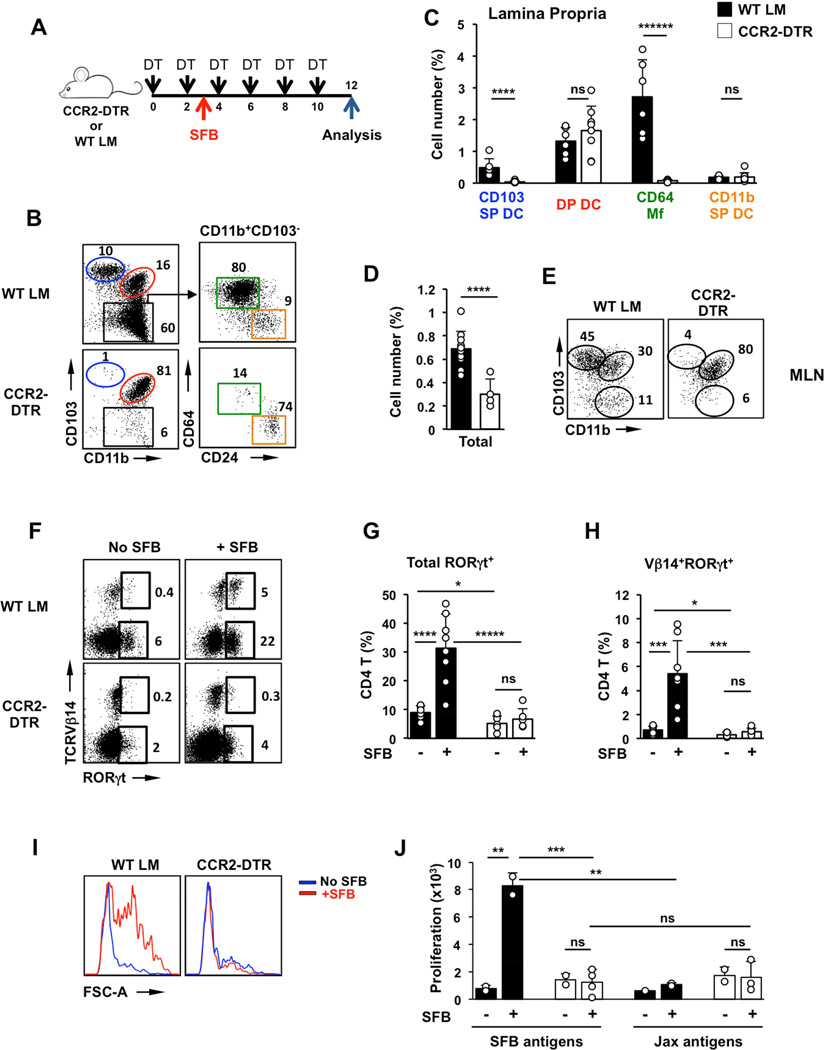
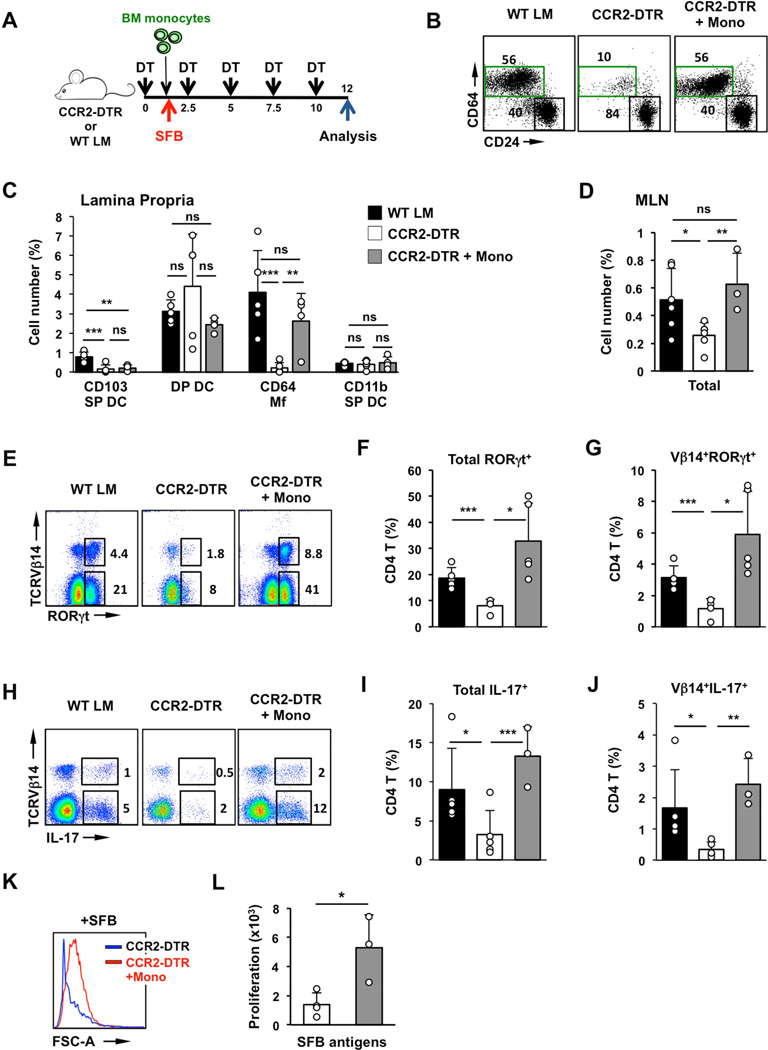
To directly examine the role of intestinal Mfs we utilized a transient depletion system. Although only a fraction of LP Mfs express high levels of CCR2 (Figure S6A), steady state intestinal Mfs are derived from CCR2+ blood monocytes (Bain et al., 2013; Bogunovic et al., 2009; Varol et al., 2009) and can be depleted in CCR2-DTR mice following diphtheria toxin (DT) treatment (Hohl et al., 2009; Kinnebrew et al., 2012). A single DT injection led to a near complete ablation of intestinal Mfs beginning at 24h and lasting until at least 72 hours post treatment (Figure S4D). Depletion of Mfs could be maintained with DT injections every 2 days for at least 12 days (Figure 4A). DT treatment did not affect CD103 DP DCs, which were still present in the LP and in the migratory DC population in MLN in treated CCR2-DTR mice (Figure 4B–E). In addition, few LP CD4 T cells and SFB-induced Th17 cells expressed CCR2 and DT treatment did not affect Th17 cell numbers or the presence of SFB-specific Th17 cells in SFB-positive CCR2-DTR mice (Figure S4A–C). To assess the role of monocyte-derived Mfs, SFB-negative CCR2-DTR mice and littermate controls were treated with DT every 48 hours for 10 days. The mice were colonized with SFB on Day 2 and Th17 induction was analyzed 8 days later (Figure 4A). SFB colonization was similar between the two groups (Figure S4E). SFB induced high levels of Th17 cells in control animals with induction of Vβ14+ SFB-specific Th17 cells and proliferation of SI LP CD4 T cells in response to SFB antigens (Figure 4F–J and Figure S4G–I). In contrast, SFB colonization did not lead to Th17 cell induction in DT treated CCR2-DTR mice (Figure 4F,G and Figure S4G,H). Moreover, SI LP CD4 T cells from CCR2-DTR mice depleted of Mfs did not respond to SFB antigens in vitro and did not contain Vβ14+ SFB-specific Th17 cells (Figure 4F,H,I,J and Figure S4G,I). These results suggest that monocyte-derived cells are required for induction of SFB-specific Th17 cell responses.
Figure 4. Intestinal Mfs are required for mucosal Th17 cell induction.
(A) Experimental design. CCR2-DTR mice and WT littermates were treated with DT every 48h as described in Methods. SFB colonization occurred on Day 3 and Th17 cells were examined on Day 12. (B,C) CD11c+MHCII+ MNP subsets in SI LP of CCR2-DTR mice and control littermates (WT LM) treated with DT for 12 days. (B left) FACS plots gated on CD11c+MHCII+ cells. Numbers represent percentage of cells in the corresponding gate. (C) Number of cells in individual MNP subsets represented as percentage of total live SI LP cells (gate R1 in Figure S1B). (D,E) Cell numbers represented as percentage of total live single cells (D) and MNP subsets in the migratory DC fraction of mesenteric lymph nodes (MLN). Plots in (E) are gated on Lin−CD11cloMHCIIhi migratory DCs. (F–H) Induction of RORγt+ Th17 cells by SFB in SI LP. Plots gated on TCRβ+CD4+ cells. (I) Response to SFB antigens of purified SI LP CD4 T cells from DT-treated CCR2-DTR and control littermates. (J) Proliferation of purified SI LP CD4 T cells. LP CD4 T cells were isolated and cultured as in (I) in the presence of SFB antigens or control bacterial antigens (Jax antigens) as described in Methods. Open circles in bar graphs represent data from individual animals. Data combined from two out of three experiments with similar results.
Transfer of exogenous monocytes rescues defects in Th17 cell induction following Mf depletion
DT treatment in CCR2-DTR mice resulted in depletion of all CCR2 monocyte-derived subsets. However, we found that prolonged treatment also affected certain DC subsets. Prolonged DT treatment led to a loss of CD103 SP DCs (Figure 4B,C). In addition, DT treatment led to depletion of a subset of CD11b SP DCs that express CCR2 (Scott et al., 2014) (Figure S6A). However, as shown earlier, CD103 SP DCs and Flt3L-dependent CCR2+ CD11b SP DCs (Scott et al., 2014) are dispensable for SFB-mediated Th17 cell induction (Figure 3 and S3). Prolonged DT treatment also resulted in a decrease in total migratory DCs in the MLN, although the numbers of MLN CD103+CD11b+ DCs (DP DCs) were normal (Figure 4D,E and S4F).
To better investigate whether the defect in Th17 cell induction is due to the lack of monocyte-derived cells, and to further exclude the possibility that DT treatment affects CD4 T cells or other non-monocyte derived populations, we performed gain-of-function experiments. We isolated Lin−Ly6C+CCR2+ monocytes to high purity from bone marrow (BM) of CD45.1 C57BL/6 congenic mice. Lineage markers included CD3, B220, NK1.1, CD11c, and c-Kit, to eliminate dendritic cell progenitors and hematopoietic stem cells (Samstein et al., 2013). CD45.2 CCR2-DTR mice were treated with DT every 60 hours to maintain depletion of endogenous monocytes. After the initial DT injection, one group of CCR2-DTR mice received 5–10 × 106 CD45.1+ BM monocytes. Control mice received DT, but did not receive any recipient cells. Following the monocyte graft, mice were colonized with SFB and Th17 cell induction was assessed 10 days later (Figure 5A). In agreement with previous studies (Varol et al., 2009), transferred monocytes exclusively reconstituted the CD64 Mf compartment and donor-derived CD45.1 cells were not detectable in any of the other MNP subsets, neither in SI LP nor in MLN (Figure 5B,C and Figure S5C,D). Similarly to previous experiments, SFB colonization did not induce SFB-specific Th17 cells in control CCR2-DTR mice without transfer. In contrast, transfer of monocytes and recovery of the LP Mf population, resulted in recovery of SFB-specific Th17 cell responses, including the presence of CD4+RORγt+ cells, CD4+IL-17+ cells in the LP, and response of LP CD4 T cells to SFB antigens in vitro (Figure 5E–L). Interestingly, monocyte transfer also led to partial increase in endogenous CD45.2+ (host-derived) DCs, especially in the migratory DC fraction of MLN (Figure 5D and S5A,B), although it did not significantly increase the number of CD103 SP DCs, underscoring the fact that this subset is dispensable for Th17 cell induction. These results demonstrate that monocyte-derived LP Mfs are essential for initiation and maintenance of SFB-specific Th17 cell responses, possibly with help from migratory DCs.
Figure 5. Exogenous monocytes recover Th17 cell induction in Mf-depleted mice.
(A) Experimental design. DT-treated CCR2-DTR mice were reconstituted on Day 1.5 with 5–10 × 106 Lin−Ly6Chi monocytes purified from bone marrow of CD45.1 congenic mice. (B) Reconstitution of CD64 Mfs in SI LP by transfer of BM monocytes (+Mono). (C) SI LP MNP subsets represented as percentage of total live single cells (D) Cell number of migratory DCs in MLN represented as percentage of total live single cells. (E–G) Induction of RORγt+ Th17 cells by SFB in SI LP. Plots gated on TCRβ+CD4+ cells. (H–J) Induction of IL-17+ Th17 cells by SFB in SI LP. Plots gated on TCRβ+CD4+ cells. (K,L) Response of purified SI LP CD4 T cells to SFB antigens. Open circles represent data from individual animals. Data combined from four independent experiments.
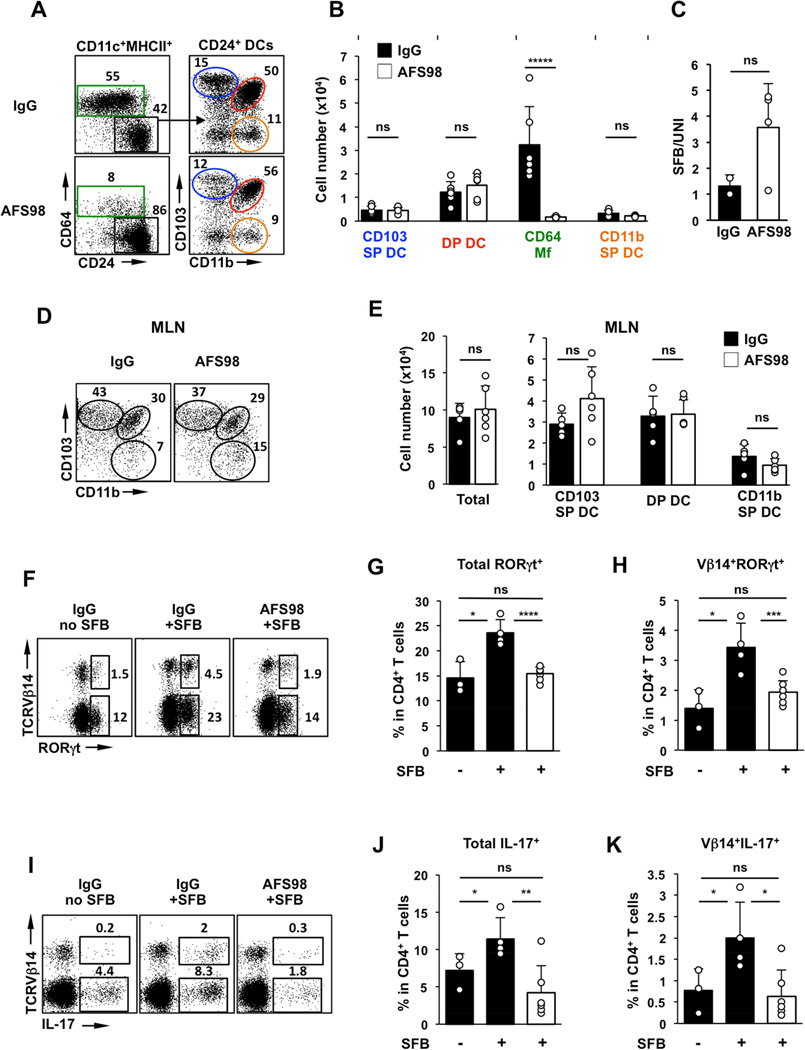
Specific depletion of CD64 Mfs leads to loss of SFB-mediated Th17 cell induction
To further confirm the role of CD64 Mfs, we sought to implement an independent depletion model. In contrast to DCs, intestinal Mf development and maintenance depends on CSF1R (also known as M-CSFR) (Bogunovic et al., 2009). Injection of a CSF1R-blocking antibody (clone AFS98) can specifically deplete intestinal Mfs in a dose-dependent manner without affecting resident DC subsets (Koscso et al., 2015; Mortha et al., 2014; Muller et al., 2014). We therefore treated WT C57BL/6 mice with a high dose of AFS98, or control IgG, prior to SFB colonization. As shown in Figure 6, AFS98 treatment led to a significant and specific depletion of intestinal Mfs. The average depletion was ~95% in the CD64 Mf fraction. In contrast, LP DC subsets, including CD103 SP DCs, DP DCs, and CD11b SP DCs were not affected by this treatment (Figure 6A,B and S6B and Table S1). Moreover, we did not detect any noticeable defects in the number and phenotype of migratory DC subsets in the MLN (Figure 6D,E). SFB colonization led to Th17 cell induction in mice treated with control IgG 8 days after introduction of the bacteria, which included induction of CD4+RORγt+ and CD4+IL-17+ cells and induction of SFB-specific Th17 cells as demonstrated by the induction of Vβ14+RORγt+ and Vβ14+IL-17+ cells (Figure 6F–K). In contrast, in mice treated with AFS98, RORγt+ and IL-17+ Th17 cells, as well as SFB-specific Th17 cells were significantly reduced and were similar to the levels in SFB-negative controls (Figure 6F–K). These results demonstrate that intestinal CD64 Mfs are essential for initiation of antigen-specific Th17 cell responses to an intestinal commensal.
Figure 6. Treatment with CSF1R–blocking antibody impedes Th17 responses to SFB.
(A,B) MNP subsets in the SI LP of C57BL/6 mice treated with high dose of anti-CSF1R monoclonal antibody (AFS98) or control IgG four days before SFB colonization. (C) SFB levels in feces of AFS98 and control IgG treated mice normalized to total bacterial DNA (UNI). (D,E) Total cell numbers and cell numbers in MNP subsets in the migratory DC fraction of mesenteric lymph nodes (MLN). (F–H) RORγt+ Th17 cells in SI LP 8 days after SFB gavage. Plots gated on TCRβ+CD4+ cells. (I–K) IL-17+ Th17 cells in SI LP on Day 8 post SFB gavage. Plots gated on TCRβ+CD4+ cells.
Discussion
The functional specialization of intestinal MNP subsets is important in regulating steady-state homeostasis and inflammatory immune responses. CD103+ DCs express CCR7 and migrate to MLN to deliver antigens for T cell priming (Bogunovic et al., 2009; Cerovic et al., 2015; Johansson-Lindbom et al., 2005; Koscso et al., 2015; Schulz et al., 2009). Previous studies have shown that purified DP DCs are efficient in skewing non-commensal transgenic CD4 T cells toward Th17 cell differentiation in vitro (Denning et al., 2011). Moreover, loss of DP DCs has been associated with a decrease in Th17 cells in the LP (Lewis et al., 2011; Persson et al., 2013; Schlitzer et al., 2013; Welty et al., 2013). However, the induction of Th17 cell responses by SFB was not examined in these studies. Our results clearly show that CD103+ DCs, including DP DCs, are not required for presentation of SFB antigens and induction of mucosal Th17 cell responses by SFB. Therefore, different APC subsets may mediate Th17 cell induction in response to different microbial antigens. For example, CD103+ DCs mediate Th17 cell responses to an intestinal pathogen (Schreiber et al., 2013). Whether Th17 cells induced by different microbes, e.g. commensal versus pathogenic bacteria, are phenotypically and functionally different will be important to examine in future studies. In the case of SFB, Langerin-DTA/BATF3-deficient (DKO) mice, which lack all CD103+ DCs and Flt3L-deficient mice, which lack pre-DC-derived DCs showed normal induction of antigen-specific Th17 cells by SFB. Because CD103+ DCs were absent in LP and MLN of DKO mice we conclude that they are not required for SFB-specific CD4 T cell priming and Th17 differentiation. In Flt3L-deficient mice, all examined DC subsets were drastically decreased in both LP and MLNs. This had a marked effect on mucosal CD4 T cell priming, for example leading to a significant decrease in Foxp3+ regulatory T cell numbers (data not shown) in agreement with published studies (Darrasse-Jeze et al., 2009). Despite this profound loss of DCs we did not observe any effects on the levels of SFB-specific Th17 cells (Figure 3), which were also induced with normal kinetics after SFB gavage (data not shown). However, small numbers of CD24+ MNPs were still present in Flt3L-deficient mice (Figure 3) and we cannot, therefore, exclude a role for these cells in Th17 cell priming.
In contrast to DC-depletion models, depletion of CD64 Mfs led to loss of SFB-specific Th17 cell responses even in the presence of conventional DCs, including migratory DCs in the MLN. In addition, exogenous monocytes were able to rescue Th17 cell defects in DT treated CCR2-DTR mice. Donor monocytes differentiated and reconstituted exclusively CD64 Mfs in the intestine and we did not detect significant contribution to any other LP or MLN MNP subsets. These experiments confirm that intestinal Mfs drive Th17 cell responses in CCR2-DTR mice and are therefore required for this response. This was further supported by our antibody depletion experiments, where specific depletion of intestinal Mfs led to similar loss of SFB-induced Th17 cell responses, even in the presence of normal numbers of all other LP and MLN DC subsets. The CD11b+CD103−CD64+ Mfs may be a heterogeneous population of monocyte-derived cells. These cells express high levels of CX3CR1 (Figure S1B), high levels of MHCII and co-stimulatory molecules, and have also been referred to as monocyte-derived DCs in early studies. However, in contrast to conventional DCs, they depend on CSF1, and express variable levels of Mf markers, such as CD64 and F4/80. Unlike other tissue-resident Mfs (Hashimoto et al., 2013; Yona et al., 2013), these cells are short-lived and are replenished by blood monocytes in vivo (Bain et al., 2014; Bogunovic et al., 2009; Varol et al., 2009), which explains their rapid depletion in CCR2-DTR mice ((Kinnebrew et al., 2012) and Figure 4) and reconstitution by transferred BM monocytes ((Varol et al., 2007) and Figure 5). Combined, our data suggest that CD64 Mfs are essential for initiation of Th17 cell responses to SFB. Because depletion of Mfs led to loss not only of Th17 cell differentiation, but also of SFB-specific responses, we conclude that Mfs are required for the initial acquisition of bacterial antigens from this commensal.
Although Mfs were essential, none of the examined models contained only Mfs in the absence of all DCs and therefore Mfs may not be the only MNP subset that participates in the process of Th17 cell induction by SFB. Mfs may collaborate with or support the function of a subset of DCs for optimal Th17 cell responses. Based on our results, such a subset must be contained in the CD11b SP DC fraction. CD11b SP DCs are a phenotypically and developmentally heterogeneous subset that is relatively understudied. They express both DC (CD24, CD26) and monocyte (CX3CR1) markers (Figure S1B). A proportion of CD11b SP DCs, express CCR2 and represent a pre-DC-derived, Flt3L-dependent subset, that was recently shown to promote IL-17 production by CD4 T cells in vitro (Scott et al., 2014). CCR2+ CD11b SP DCs were depleted in CCR2-DTR treated mice (Figure S6A) that lack Th17 cell induction, however they depend on Flt3L and are presumably absent in Flt3L-deficient mice (Scott et al., 2014), which showed normal Th17 cell induction (Figure 3), suggesting that CCR2+ CD11b SP DCs are not required for Th17 cell induction by SFB. In addition, CCR2+ CD11b SP DCs were not depleted in AFS98 treated mice, which showed loss of Th17 cells (Figure S6B), demonstrating that they are not sufficient for the process. However, it is formally possible that other CD103−CX3CR1+ MNPs, refractory to depletion in our DC models, participate in Th17 induction together with intestinal Mf.
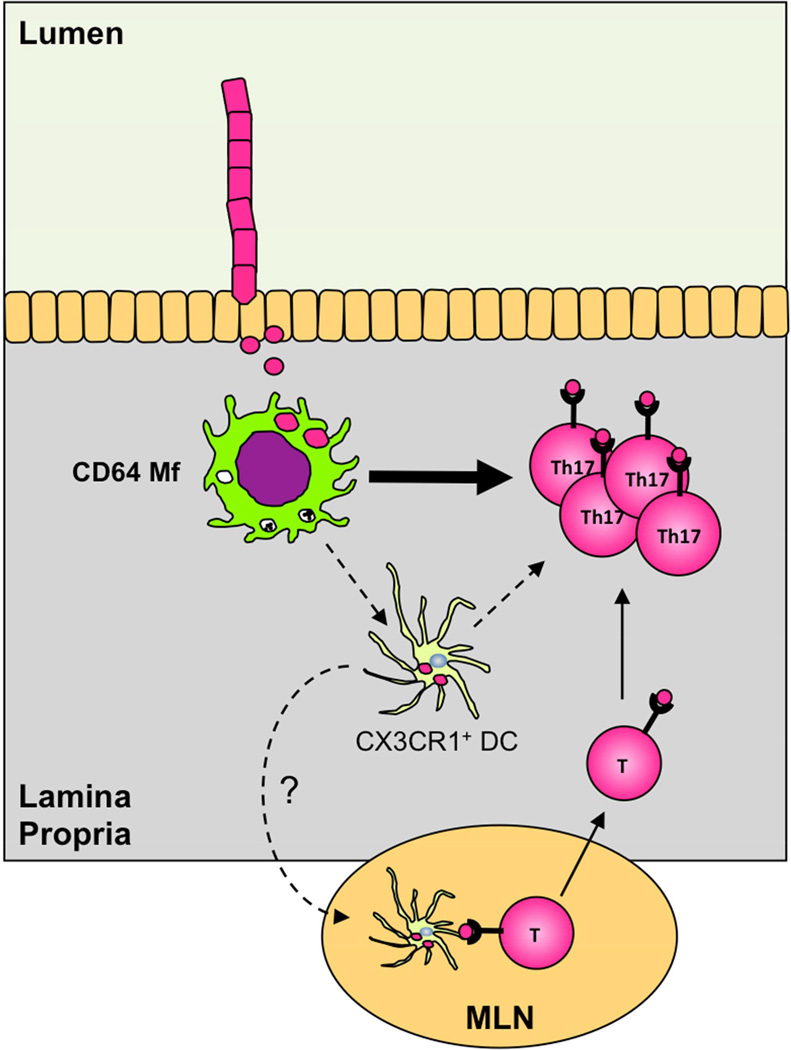
Combined, our results demonstrate that monocyte-derived CX3CR1+ MNPs (that include Mfs and possibly a subset of CD11b SP DCs) prime SFB-specific T cells and direct Th17 cell differentiation and that CD64 Mfs are required and play a central role in initiating this response (Figure 7).
Figure 7. Central role of intestinal Mfs in generation of commensal-induced Th17 cells.
Intestinal Mfs acquire antigens from epithelium-associated SFB and initiate SFB-specific Th17 cell responses. CD103+ DCs are dispensable for the induction of Th17 cells. Intestinal Mfs may participate in the Th17 cell differentiation stage locally in the LP or collaborate with CX3CR1+ DCs for antigen transfer into MLN or Th17 cell priming/maintenance in the LP.
The detailed roles of intestinal Mfs and other CX3CR1+ cells, such as CD11b SP DCs, will be important to elucidate in future studies. Our results support a model in which intestinal Mfs first acquire SFB antigens and subsequently induce CD4 T cell priming and Th17 cell differentiation possibly with a contribution from a subset of CD11b SP DCs. Because Mfs are generally resident to the LP, CD11b SP DCs may be required for transport of Mf-acquired SFB antigens to MLN for priming of T cell responses. Indeed, evidence for such mode of antigen transfer in SI LP has recently been reported (Mazzini et al., 2014). CX3CR1+ Mfs have also been shown to migrate to MLN under certain conditions (Diehl et al., 2013) and may deliver SFB antigens without prior transfer to DCs in the LP similarly to what has been reported for lung monocytes (Samstein et al., 2013). At the same time, we have recently shown that induction of Th17 cell responses by SFB occurs normally in MLN and PP-deficient mice (Goto et al., 2014), and therefore, delivery of SFB antigens to MLN may not be required for SFB-specific Th17 cell responses. Instead, intestinal Mfs may promote T cell priming and Th17 cell induction locally in the LP (Figure 7). Mfs may also provide trophic signals for certain DC subsets or modify DC migration or function. Indeed, we observed a relative increase in host-derived migratory DCs in MLN following monocyte transfer (Figure 5D).
How Mfs acquire SFB antigens and whether they also participate in later stages of Th17 cell differentiation remains to be ascertained. A distinguishing feature of SFB is their close association with the intestinal epithelium (Klaasen et al., 1992). Indeed, SFB represent the majority of mucosa-associated bacteria in laboratory mice (Farkas et al., 2015). At the same time, intestinal Mfs are located close to the epithelial layer and have been shown to extend dendrites into the gut lumen (Niess et al., 2005; Rescigno et al., 2001). Thus intestinal Mfs may be perfectly positioned to acquire SFB antigens.
Our data demonstrate a crucial in vivo function of intestinal Mfs in controlling effector T cell-homeostasis to luminal bacteria. Identification of the exact mechanisms of antigen acquisition and the location of T cell priming will be important future questions to address. Regardless of the details, this mechanism must be distinct from conventional sampling of luminal antigens by DCs at steady state or by the DC/Mf-mediated acute immune response to invasive pathogens. Because of the specific nature of the interaction of SFB with the host, we propose that this pathway may represent a more general mechanism for inducing localized effector Th17 cell responses to mucosa-associated non-invasive bacteria.
Experimental Procedures
Mice
Langerin-DTA, Batf3−/−, Notch2F/F, CX3CR1-GFP and CD11c-Cre mice were obtained from the Jackson Laboratory. Flt3l−/− mice were obtained from Taconic farms and derived SFB-free by antibiotic treatment of a founder breeding pair, followed by fecal transplantation of Jackson (SFB-negative) microbiota. CCR2-DTR and CCR2-GFP mice have been previously described (Hohl et al., 2009). CCR2-DTR mice were re-derived by embryo transfer and kept SFB-negative in our colony. All mice were bred and housed under specific pathogen-free conditions at Columbia University Medical Center under IACUC approved guidelines. To control for microbiota and cage effects, all experiments were performed with littermate control animals housed in the same cage.
SFB colonization and Th17 cell induction
All mice, regardless of origin, were screened at multiple points for the presence and levels of SFB by quantitative PCR (Farkas et al., 2015). Bacterial genomic DNA isolation from fecal pellets and quantitative PCR for the SFB 16S rRNA gene were performed as previously described (Farkas et al., 2015; Ivanov et al., 2009). SFB colonization was performed by oral gavage with SFB-containing fecal pellets. To control for SFB levels in the feces used for gavage, as well as for other constituents of the microbiota between experiments, all gavages were performed with frozen stocks from a single batch of feces obtained from 10 SFB-positive Taconic B6 mice. Control mice were gavaged with fecal pellets from SFB-negative littermates in our colony or with PBS. SFB colonization levels were confirmed by quantitative PCR and normalized to levels of total bacteria (UNI). SI LP Th17 cell induction was assessed 8–10 days after gavage unless otherwise noted.
Lamina propria cell isolation and in vitro co-culture experiments
Lamina propria (LP) lymphocytes, intracellular cytokine staining, and RORγt staining were performed as previously described (Ivanov et al., 2009). LP CD4+ T cells were purified by positive selection using anti-CD4 magnetic microbeads and MACS columns (Miltenyi Biotec). 3–5 × 104 CD4 T cells were co-cultured in 96-well U-bottom plates with 5 × 104 MACS purified splenic CD11c+ cells as APCs in the presence or absence of autoclaved bacterial lysates prepared from feces of SFB-monocolonized mice (SFB) or SFB-negative Jackson C57BL/6 mice (Jax) as previously described (Farkas et al., 2015; Goto et al., 2014). T cell proliferation was assessed 72 hours later by counting the number of live proliferated CD4 T cells.
DT treatment for ablation of intestinal Mfs
SFB-negative CCR2-DTR mice and littermate controls were treated with 20 ng/g diphtheria toxin (DT) i.p. on Day 0 and every 48 or 60 hours after that for the duration of the experiment (a total of 6 or 5 injections respectively). On Day 2 some mice were gavaged twice with SFB-containing fecal homogenates. Th17 cell induction was examined on Day 10.
Adoptive transfers
SFB-negative CD45.2 CCR2-DTR mice were treated with DT on Day 0 and every 60 hours after that (total of 5 injections). On Day 1.5 some of the mice received 5–10 × 106 Lin−GFP+ bone marrow monocytes, purified from congenic CD45.1 CCR2-GFP mice (Hohl et al., 2009) or Lin−Ly6Chi bone marrow monocytes from CD45.1 C57BL/6 mice by cell sorting. Transfer of a large number of BM monocytes was required to reconstitute the LP Mf compartment in monocyte-depleted CCR2-DTR mice to significant levels. Recipient mice were gavaged with SFB on Day 2 and DC subsets and Th17 cell induction were examined on Day 12. The Lin(eage) cocktail included B220, CD3, NK1.1, CD11c, and CD117 (c-Kit). Sorting was performed on a FACS Aria II (BD).
Macrophage depletion
For macrophage depletion, four days prior to SFB colonization, SFB-negative C57BL/6 mice were injected intra-peritoneally with 150 ug/g of body weight of CSF1R blocking antibody (clone AFS98 (Sudo et al., 1995)), purified from a hybridoma as described earlier (Hashimoto et al., 2011)
Cell numbers and statistics
To compensate for differences in yield between experiments, in some figures numbers of lamina propria and mesenteric lymph node mononuclear cell subsets are represented as percentage of total live single cells (gate R1 in Figure S1B). Significance was determined by the Student’s unpaired two-tailed t test unless otherwise noted. P values were represented on figures as follows: ns, p ≥ 0.05, * p < 0.05, ** p < 0.01, *** p < 0.005, **** p < 0.001, ***** p < 0.0005, ****** p < 0.0001. Error bars on all figures represent standard deviation of the mean.
Supplementary Material
Highlights.
Intestinal CD103 DCs are dispensable for induction of Th17 cells by a gut commensal
Intestinal CX3CR1 macrophages are required for Th17 cell induction by SFB
Intestinal CX3CR1 macrophages are required for a commensal antigen-specific response
Acknowledgments
We thank Ingrid Leiner and Eric Pamer at the Memorial Sloan-Kettering Cancer Center for providing CCR2-GFP and CCR2-DTR mice. We thank Lei Ding for expert advice in BM experiments. We thank Darya Esterhazy and Daniel Mucida at the Rockefeller University for reagents. We thank Amir Figueroa, Kristie Gordon and Siu-Hong Ho at the Columbia Microbiology, Cancer Center, and Center for Translational Immunology Flow Cytometry Cores for cell sorting. We thank Boris Reizis and Steve Reiner for invaluable advice and scientific discussions. This work was supported by the National Institutes of Health R01-DK098378 to I.I.I., R01-AI093808 to T.M.H., and by the Crohn’s and Colitis Foundation of America SRA#259540 to I.I.I. I.I.I. is a Pew Scholar in the Biomedical Sciences, supported by the Pew Charitable Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nature immunology. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal immunology. 2013;6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M, Mortha A, Muller PA, Merad M. Mononuclear phagocyte diversity in the intestine. Immunol Res. 2012 doi: 10.1007/s12026-012-8323-5. [DOI] [PubMed] [Google Scholar]
- Cerovic V, Bain CC, Mowat AM, Milling SW. Intestinal macrophages and dendritic cells: what’s the difference? Trends Immunol. 2014;35:270–277. doi: 10.1016/j.it.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Cerovic V, Houston SA, Scott CL, Aumeunier A, Yrlid U, Mowat AM, Milling SW. Intestinal CD103(-) dendritic cells migrate in lymph and prime effector T cells. Mucosal immunology. 2013;6:104–113. doi: 10.1038/mi.2012.53. [DOI] [PubMed] [Google Scholar]
- Cerovic V, Houston SA, Westlund J, Utriainen L, Davison ES, Scott CL, Bain CC, Joeris T, Agace WW, Kroczek RA, Mowat AM, Yrlid U, Milling SW. Lymph-borne CD8alpha+ dendritic cells are uniquely able to cross-prime CD8+ T cells with antigen acquired from intestinal epithelial cells. Mucosal immunology. 2015;8:38–48. doi: 10.1038/mi.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. The Journal of experimental medicine. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Norris BA, Medina-Contreras O, Manicassamy S, Geem D, Madan R, Karp CL, Pulendran B. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. Journal of immunology. 2011;187:733–747. doi: 10.4049/jimmunol.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A, Schwab SR, Littman DR. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature. 2013;494:116–120. doi: 10.1038/nature11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung SS, Murphy TL, Hildner K, Murphy KM. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. The Journal of experimental medicine. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farache J, Zigmond E, Shakhar G, Jung S. Contributions of dendritic cells and macrophages to intestinal homeostasis and immune defense. Immunology and cell biology. 2013;91:232–239. doi: 10.1038/icb.2012.79. [DOI] [PubMed] [Google Scholar]
- Farkas AM, Panea C, Goto Y, Nakato G, Galan-Diez M, Narushima S, Honda K, Ivanov II. Induction of Th17 cells by segmented filamentous bacteria in the murine intestine. J Immunol Methods. 2015 doi: 10.1016/j.jim.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, Stanley ER, Nussenzweig M, Merad M. The origin and development of nonlymphoid tissue CD103+ DCs. The Journal of experimental medicine. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, Laufer TM, Ignatowicz L, Ivanov II. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger JR, Askenase MH, Guimont-Desrochers F, da Fonseca DM, Belkaid Y. Contextual functions of antigen-presenting cells in the gastrointestinal tract. Immunological reviews. 2014;259:75–87. doi: 10.1111/imr.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D, Chow A, Greter M, Saenger Y, Kwan WH, Leboeuf M, Ginhoux F, Ochando JC, Kunisaki Y, van Rooijen N, Liu C, Teshima T, Heeger PS, Stanley ER, Frenette PS, Merad M. Pretransplant CSF-1 therapy expands recipient macrophages and ameliorates GVHD after allogeneic hematopoietic cell transplantation. The Journal of experimental m edicine. 2011;208:1069–1082. doi: 10.1084/jem.20101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell host & microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. The Journal of experimental medicine. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, Flavell RA, Littman DR, Pamer EG. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaasen HL, Koopman JP, Poelma FG, Beynen AC. Intestinal, segmented, filamentous bacteria. FEMS microbiology reviews. 1992;8:165–180. doi: 10.1111/j.1574-6968.1992.tb04986.x. [DOI] [PubMed] [Google Scholar]
- Koscso B, Gowda K, Schell TD, Bogunovic M. Purification of dendritic cell and macrophage subsets from the normal mouse small intestine. J Immunol Methods. 2015 doi: 10.1016/j.jim.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, Klinakis A, Charo IF, Jung S, Gommerman JL, Ivanov II, Liu K, Merad M, Reizis B. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35:780–791. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity. 2014;40:248–261. doi: 10.1016/j.immuni.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M, Bogunovic M. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nature immunology. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hagerbrand K, Marsal J, Gudjonsson S, Hakansson U, Reizis B, Kotarsky K, Agace WW. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38:958–969. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature immunology. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. The Journal of experimental medicine. 2012;209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samstein M, Schreiber HA, Leiner IM, Susac B, Glickman MS, Pamer EG. Essential yet limited role for CCR2(+) inflammatory monocytes during Mycobacterium tuberculosis-specific T cell priming. Elife. 2013;2:e01086. doi: 10.7554/eLife.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, Hoeffel G, Malleret B, Heiseke A, Chew S, Jardine L, Purvis HA, Hilkens CM, Tam J, Poidinger M, Stanley ER, Krug AB, Renia L, Sivasankar B, Ng LG, Collin M, Ricciardi-Castagnoli P, Honda K, Haniffa M, Ginhoux F. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38:970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber HA, Loschko J, Karssemeijer RA, Escolano A, Meredith MM, Mucida D, Guermonprez P, Nussenzweig MC. Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium. The Journal of experimental medicine. 2013;210:2025–2039. doi: 10.1084/jem.20130903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. The Journal of experimental medicine. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CL, Bain CC, Wright PB, Sichien D, Kotarsky K, Persson EK, Luda K, Guilliams M, Lambrecht BN, Agace WW, Milling SW, Mowat AM. CCR2CD103 intestinal dendritic cells develop from DC-committed precursors and induce interleukin-17 production by T cells. Mucosal immunology. 2014 doi: 10.1038/mi.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T, Nishikawa S, Ogawa M, Kataoka H, Ohno N, Izawa A, Hayashi S. Functional hierarchy of c-kit and c-fms in intramarrow production of CFU-M. Oncogene. 1995;11:2469–2476. [PubMed] [Google Scholar]
- Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, Woltman AM, Reyal Y, Bonnet D, Sichien D, Bain CC, Mowat AM, Reis e Sousa C, Poulin LF, Malissen B, Guilliams M. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. European journal of immunology. 2012;42:3150–3166. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
- Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. The Journal of experimental medicine. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Welty NE, Staley C, Ghilardi N, Sadowsky MJ, Igyarto BZ, Kaplan DH. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. The Journal of experimental medicine. 2013;210:2011–2024. doi: 10.1084/jem.20130728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, Sczesnak A, Liao JJ, Torres VJ, Jenkins MK, Lafaille JJ, Littman DR. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152–156. doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond E, Jung S. Intestinal macrophages: well educated exceptions from the rule. Trends Immunol. 2013;34:162–168. doi: 10.1016/j.it.2013.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.