Abstract
Background
Speckle-tracking echocardiographic (STE) measures of right ventricular (RV) function appear to improve after transcatheter pulmonary valve (TPV) implantation. Measures of exercise function, such as ventilatory efficiency (the VE/VCO2 slope), have been shown to be prognostic of mortality in patients that may require TPV. Our objective was to evaluate the correlation between STE measures of RV function and changes in VE/VCO2 after TPV placement.
Methods
STE and cardiopulmonary exercise testing were performed at baseline and 6 months after TPV placement in 24 patients from 4 centers. Conventional echocardiographic measures of RV function were also assessed. Echocardiograms and exercise stress tests were interpreted by single blinded observers at separate Core laboratories.
Results
All patients demonstrated relief of pulmonary regurgitation and stenosis after TPV implantation. Improvements in RV longitudinal strain (−16.9±3.5% vs. −19.7±4.3%, p<0.01) and strain rate (−0.9±0.4s−1 vs. −1.2±0.4s−1, p<0.01) were noted. The VE/VCO2 slope improved (32.4±5.7 vs. 31.5±8.8, p=0.03). No other significant echo or exercise changes were found. Upon multivariable regression, the change in VE/VCO2 was independently associated with change in RV longitudinal early diastolic strain rate (p<0.001) and tricuspid A velocity (p<0.001). Pre-intervention RV longitudinal strain was found to be a predictor of change in VE/VCO2 after TPVI (r = −0.60, p<0.001).
Conclusion
STE measures of RV function appear to hold the potential for being used as predictors of improved outcomes in patients requiring TPV implantation. Future studies should directly assess the prognostic significance of STE measures of RV function in this population.
Keywords: speckle-tracking echocardiography, transcatheter pulmonary valve implantation, congenital heart disease
Introduction
Patients with congenital heart disease requiring right ventricular (RV) to pulmonary artery (PA) conduits frequently develop free pulmonary regurgitation, severely dilated RVs, and decreased ventricular function over time.1 Measures of ventilatory efficiency derived from exercise testing, such as the VE/VCO2 slope, have been shown to be prognostic of mortality in these patients and are improved after pulmonary valve replacement secondary to improvement in effective stroke volume.2-7
The relationship between echocardiographic measures of ventricular function and these exercise derived surrogates of outcome are unknown. These patients show little improvement in ventricular function when assessed by traditional echocardiographic markers.8 Speckle-tracking echocardiography (STE) measures of ventricular function have been shown to be more sensitive than conventional measures in many disease processes, including in those patients undergoing transcatheter pulmonary valve implantation (TPVI).9-12 However, the relationship between STE measures of RV function before and after TPVI and exercise measures are unknown in this population. The objective of this study was to determine the usefulness of assessing RV STE measures of function by assessing their relationship with changes in cardiopulmonary exercise function both before and after TPVI. We hypothesized that RV STE measures of cardiac function would correlate with changes seen in exercise function after TPVI.
Methods
This was a retrospective, secondary analysis of data collected during The COngenital Multicenter trial of Pulmonic vAlve regurgitation Studying the SAPIEN interventIONal transcatheter heart valve (COMPASSION) trial. COMPASSION is a prospective, non-randomized, multi-center study to assess the safety and efficacy of the SAPIEN transcatheter heart valve for the treatment of dysfunctional RV-PA conduits. Early phase 1 results have shown good feasibility, effectiveness, and safety.13 Patients included in COMPASSION were enrolled prospectively from 4 participating centers. Inclusion criteria included: (1) Weight equal to or exceeding 35 kilograms. (2) In situ conduit size between 16 mm and 24 mm in diameter. (3) Moderate or severe pulmonary regurgitation defined as 3+ pulmonary regurgitation by echocardiogram and/or RV-PA conduit obstruction with a mean gradient of >35 mmHg. (4) Peak VO2 or VE/VCO2 less than 70% predicted. Informed consent was obtained from all potential subjects and/or their legal guardians. The Institutional Review Board at each participating institution approved the trial.
Procedure
The protocol for valve implantation has been reported previously and is summarized here for convenience.13 Procedures were performed under general anesthesia with biplane fluoroscopic guidance. The minimum diameter of the conduit was assessed by angiography. Risk for coronary compression was assessed with aortic root angiography or selective coronary angiography with simultaneous inflation of a noncompliant balloon in the conduit. Prestenting of the conduit with a bare metal stent was performed. A 23 mm or 26 mm SAPIEN transcatheter heart valve was then implanted over a stiff guidewire and expanded via balloon inflation.
Echocardiographic protocol
Analysis of echocardiograms submitted to the COMPASSION core laboratory was performed. Echocardiograms were acquired by experienced sonographers at each center following a protocol which included a complete set of standardized views to evaluate ventricular function. The image acquisition protocol was developed by the echocardiography core laboratory. On-site or web-based training to the local SAPIEN TPV implantation sites was provided. Echocardiograms utilized for this analysis were performed at baseline prior to TPV implantation, prior to discharge after TPV implantation, 30-day follow-up, and 6-month follow-up. All studies were performed under baseline physiologic conditions, not under the influence of anesthesia. Measures were recorded at end expiration with quiet respiration. Pre-TPV echos were performed ≤ 1 week prior to TPV implantation. Echocardiograms were in DICOM format. All measurements were made off line by a single reviewer and averaged over 3 beats. Pulmonary regurgitation was graded from 0 to 4 based on jet width:annulus ratio and flow reversal in the branch pulmonary arteries as follows: 0 = no regurgitation, 1 = jet width:annulus < 0.25, 2 = jet width:annulus between 0.25 and 0.5, 3 = jet width:annulus between 0.5 and 0.7, 4 = jet width:annulus > 0.7 with flow reversal in the branch pulmonary arteries.14, 15 Tricuspid valve regurgitant orifice area was calculated from the apical four chamber and parasternal short axis windows.
Two-dimensional, spectral, and tissue Doppler measures of myocardial function
From a standard apical four-chamber window, RV fractional area change (FAC) was defined as ([end-diastolic area – end-systolic area]/end-diastolic area) x 100. Tricuspid annular plane systolic excursion (TAPSE) was obtained, and indexed TAPSE was calculated as ([RV end-diastolic length – RV end-systolic length]/RV end-diastolic length). Pulsed tissue Doppler imaging (TDI) S’ velocities at the tricuspid valve annulus and interventricular septum were obtained from the apical four-chamber view.
To evaluate diastolic function, Doppler velocities of transtricuspid flow (E and A) were obtained from an apical four-chamber window. Tissue Doppler velocities of the tricuspid annulus and interventricular septum (E’ and A’) were obtained. Derived ratios (E:A, E:E’) were calculated.
Speckle-tracking echocardiography measures of myocardial function
Speckle-tracking was performed as a secondary analysis of echocardiograms submitted to COMPASSION echocardiography core laboratory. A single, blinded observer performed offline analysis of DICOM images using vendor-independent software (2D Cardiac Performance Analysis v. 3.0, TomTec Imaging Systems, Inc, Munich, Germany). Myocardial motion was tracked through the cardiac cycle, calculating myocardial deformation from echogenic speckles in the B-mode image. Endocardium and epicardium were manually traced in the RV from the lateral tricuspid annulus to the septal component of the tricuspid annulus (Figure 1). The septum was included secondary to its importance to global RV function.16 End-systole was defined as end ejection of the pulmonic valve for the RV and of the aortic valve for the LV using spectral Doppler. Speckle-tracking measures of deformation from the apical 4-chamber view included peak longitudinal strain, strain rate, and early diastolic strain rate. Left ventricular measures of deformation from the apical 4-chamber view were also assessed. Global deformation measurements were calculated as an average of 6 segments. Tracking was visually assessed, and deformation curves were not accepted if greater than one segment demonstrated inadequate tracking. It should be noted that longitudinal strain is by convention expressed using negative numbers. When describing relative differences, the absolute value of the strain amplitude (ignoring the minus sign) is referenced. For example, a strain of −23% represents better function than a strain of −13%.
Figure 1. STE of the RV.
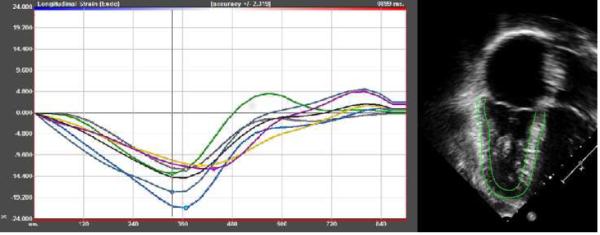
Two-dimensional speckle tracking echocardiogram of RV longitudinal strain in a patient with Tetralogy of Fallot with mixed conduit stenosis and insufficiency. Endocardial and epicardial traces are shown.
Exercise protocol
All patients underwent a symptom limited cardiopulmonary exercise test with progressive protocols using either a bicycle ergometer or treadmill depending on the available equipment in the individual centers. Patients had a rest period to capture baseline, then a warm up period without load, followed by an increase of load depending on the expected individual physical capacity as estimated by the investigator. The end of the exercise test was marked by symptom limitation and was followed by a recovery period. All exercise tests were analyzed by a blinded observer in a separate COMPASSION cardiopulmonary testing Core laboratory.
The exercise test used breath-by-breath gas exchange analysis via a metabolic cart. The primary exercise measure of interest was ventilatory efficiency as analyzed by the VE/VCO2 slope. Peak oxygen uptake (VO2) was defined as the highest mean uptake during exercise. Anaerobic threshold (AT) was determined by use of the modified V-slope method. Peak O2 pulse was defined as peak VO2 divided by peak heart rate.
Statistical Analysis
Paired t-tests were used to assess for changes in exercise test variables between baseline and 6 months. For echocardiograms, to determine the trend from time 0 to time 3, repeated measures ANOVA with a Greenhouse-Geisser correction was conducted on all individuals with measurements for each of the four time points. Post hoc comparisons using the Bonferroni correction were then performed in those variables which showed a statistically significant repeated measure ANOVA. Missing data was not imputed as numbers were sufficient to conduct appropriate analyses. Pearson's correlation and multiple variable stepwise linear regression were used to assess for a linear relationship between echocardiographic variables and exercise function. Independent variables assessed with stepwise regression techniques included baseline and % change in: RV size (end-diastolic and systolic area), conduit peak and mean gradients, and all measures of RV and LV systolic function as described above. Age and sex were also included in the analysis. Results of multivariable analysis are reported as partial correlations - its purpose is to quantify the association between two variables while eliminating the variance from other variables in the model. A receiver operating characteristic (ROC) curve was developed to assess the sensitivity and specificity of optimal cutoff values for the pre-operative echocardiographic variable that best predicts an improvement in exercise function. Intra- and inter- observer variability was assessed by absolute percent error of the mean (the difference between the two measurements was divided by the mean of those two measurements) and by intraclass correlation coefficient (ICC) using a random effects model measuring absolute agreement. An ICC of ≥ 0.75 was deemed acceptable intra- or inter-observer variability. A p-value < 0.05 was considered significant. Statistics were analyzed using SPSS v. 22 (IBM, New York, NY, USA).
Results
The first 33 patients from 4 centers who had successful SAPIEN TPV implantation in the COMPASSION trial were eligible. A total of 132 echocardiograms were performed. Of these, 17 echocardiograms were excluded for inability to perform STE due to inadequate RV free wall and/or apical segment capture in the echocardiographic window, or, for inadequate apical four-chamber windows. These 17 echocardiograms came from a total of 9 patients. Therefore, 24 patients had echocardiograms suitable for speckle-tracking analysis (≤ 2 segments were excluded from the RV analysis) at each of the four time points so that a comprehensive analysis of changes in ventricular function after TPVI could be performed. Demographic data from these patients are presented in Table 1. After TPV implantation no patient had greater than mild stenosis or regurgitation.
Table I.
Patient Demographics
| Age, yrs | 32.3 ± 17.0 |
| Weight, kg | 73.5 ± 24.1 |
| Male/female | 17/7 |
| Diagnosis | |
| Tetralogy of Fallot | 12 |
| Ross procedure | 7 |
| Other | 5 |
| Conduit dysfunction | |
| Regurgitation only | 7 |
| Mixed | 17 |
| Pulmonary stenosis grade | |
| None (<16 mmHg) | 4 |
| Mild (16-30 mmHg) | 3 |
| Moderate (31–45 mmHg) | 8 |
| Severe (>45 mmHg) | 9 |
| Pulmonary regurgitation grade | |
| None | 0 |
| Trivial | 0 |
| Mild | 1 |
| Moderate | 1 |
| Severe | 22 |
Pulmonary stenosis was graded based on net peak gradient. Pulmonary regurgitation was graded based on jet width:annulus ratio and flow reversal in the branch pulmonary arteries as follows: None = no regurgitation, trivial = jet width:annulus < 0.25, mild = jet width:annulus between 0.25 and 0.5, moderate = jet width:annulus between 0.5 and 0.7, severe = jet width:annulus > 0.7 with flow reversal in the branch pulmonary arteries.
Changes after TPV implantation
Peak and mean gradients through the conduit, pulmonary regurgitation grade, RV enddiastolic and systolic areas, and tricuspid regurgitation velocity decreased between baseline and six months (Table 2). No significant changes were detected in the conventional measures of RV systolic or diastolic function at 6 month follow-up with the exception of an increase in tricuspid inflow Doppler A velocity (Table 3). Statistically significant improvements in RV longitudinal strain and strain rate were noted while changes in RV early diastolic strain rate trended toward significance (Table 4). The VE/VCO2 slope and O2 pulse improved between baseline and 6 months; in contrast, there were no statistically significant changes in peak VO2, VO2 at AT, or respiratory exchange ratio at AT (Table 5).
Table 2.
Pulmonary and tricuspid valve function and right ventricular size after TPV implantation
| Measure | Baseline (time 0) | Discharge (time 1) | 30-day follow-up (time 2) | 6-month follow-up (time 3) | ANOVA (p-value) | Multiple Comparison (p < 0.05) |
|---|---|---|---|---|---|---|
| Conduit stenosis peak gradient (mm Hg) | 42.4 ± 5.5 | 22.1 ± 2.1 | 22.3 ± 3.1 | 20.7 ± 2.6 | <0.01 | Time 0 vs 1, 2, 3 |
| Conduit stenosis mean gradient (mm Hg) | 24.1 ± 3.2 | 13.2 ± 1.3 | 13.1 ± 1.8 | 12.3 ± 1.7 | <0.01 | Time 0 vs 1, 2, 3 |
| RV end-diastolic area (cm^2) | 41.4 ± 1.9 | 42.3 ± 2.3 | 37.6 ± 2.0 | 37.1 ± 1.6 | <0.01 | Time 0 vs 2, 3 Time 1 vs 2, 3 |
| RV end-systolic area (cm^2) | 29.3 ± 1.3 | 29.7 ± 2.0 | 26.1 ± 1.6 | 25.4 ± 1.0 | <0.01 | Time 0 vs 3 Time 1 vs 2, 3 |
| TR peak gradient (mm Hg) | 56.2 ± 5.1 | 47.1 ± 3.2 | 40.2 ± 2.6 | 40.9 ± 2.6 | <0.01 | Time 0 vs 2, 3 Time 1 vs 2 |
| Indexed TR jet area (cm^2/m^2) | 0.18 ± 0.20 | 0.11 ± 0.12 | 0.11 ± 0.17 | 0.10 ± 0.09 | <0.01 | Time 0 vs 1, 2, 3 |
| Tricuspid Valve Annulus Z-score | 1.36 ± 0.94 | 1.41 ± 0.99 | 1.49 ± 1.00 | 1.64 ± 0.80 | 0.89 | none |
Values are mean ± SD, p-values, or time-points where differences in measures were statistically significant by multiple comparison test. RV = right ventricular, TR = tricuspid regurgitation.
Table 3.
Changes in conventional measures of cardiac function after TPV implantation
| Measure | Baseline (time 0) | Discharge (time 1) | 30-day Follow-up (time 2) | 6-month Follow-up (time 3) | ANOVA (p-value) | Multiple Comparison (p < 0.05) |
|---|---|---|---|---|---|---|
| RV FAC (%) | 29.0 ± 1.9 | 30.1 ± 1.7 | 29.2 ± 2.0 | 31.4 ± 1.1 | 0.72 | n/a |
| TAPSEi (%) | 0.14 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.48 | n/a |
| TDI: Tricuspid S (cm/sec) | 7.7 ± 0.5 | 9.1 ± 0.4 | 8.0 ± 0.4 | 8.2 ± 0.4 | <0.01 | Time 0 vs 1 Time 1 vs 2 |
| RV Doppler E (cm/sec) | 72.6 ± 5.5 | 77.9 ± 5.1 | 76.6 ± 5.7 | 70.3 ± 3.7 | 0.31 | n/a |
| RV Doppler A (cm/sec) | 44.9 ± 4.0 | 59.1 ± 5.5 | 51.1 ± 4.4 | 49.2 ± 4.9 | 0.02 | n/a |
| RV Doppler E:A | 1.8 ± 0.2 | 1.5 ± 0.2 | 1.6 ± 0.2 | 1.7 ± 0.2 | 0.37 | n/a |
| TDI: Tricuspid e' (cm/sec) | 7.9 ± 2.1 | 8.9 ± 2.1 | 7.8 ± 1.7 | 7.9 ± 1.7 | <0.01 | Time 0 vs 1 Time 1 vs 2 |
| TDI: Tricuspid E:e' | 7.4 ± 0.9 | 8.4 ± 0.7 | 9.3 ± 1.2 | 8.3 ± 0.8 | 0.29 | n/a |
Results are reported in mean ± standard deviation. FAC = fractional area change, RV = right ventricle, TAPSEi = indexed tricuspid annular systolic plane excursion, TDI = tissue Doppler imaging.
Table 4.
Changes in STE Measures of Function
| Measure | Baseline (time 0) | Discharge (time 1) | 30-day Follow-up (time 2) | 6-month Follow-up (time 3) | ANOVA (p-value) | Multiple Comparison (p < 0.05) |
|---|---|---|---|---|---|---|
| Global RV LS (%) | −16.9 ± 0.7 | −17.3 ± 1.0 | −17.8 ± 0.6 | −19.6 ± 0.9 | < 0.01 | Time 0 vs 3 |
| Global RV LSR | −0.87 ± 0.09 | −1.03 ± 0.07 | −1.03± 0.05 | −1.16 ± 0.08 | 0.01 | Time 0 vs 3 |
| Global RV LEDSR (s−1) | 1.11 ± 0.10 | 1.16 ± 0.11 | 1.12 ± 0.09 | 1.31 ± 0.10 | 0.15 | n/a |
| RV free wall LS (%) | −17.0 ± 3.7 | −17.2 ± 6.1 | −19.1 ± 4.8 | −21.9 ± 6.2 | < 0.01 | Time 0 vs 3 Time 1 vs 3 |
| RV free wall LSR (s−1) | −0.98 ± 0.33 | −1.04 ± 0.39 | −1.11 ± 0.30 | −1.31 ± 0.68 | 0.04 | Time 0 vs 3 |
| RV free wall LEDSR (s−1) | 1.10 ± 0.48 | 1.16 ± 0.56 | 1.27 ± 0.61 | 1.43 ± 0.64 | 0.11 | n/a |
| Septal LS (%) | −16.0 ± 3.9 | −16.5 ± 4.5 | −15.9 ± 2.9 | −17.8 ± 5.4 | 0.30 | n/a |
| Septal LSR (s−1) | −0.91 ± 0.24 | −1.01 ± 0.35 | −0.93 ± 0.27 | −1.05 ± 0.42 | 0.22 | n/a |
| Septal LEDSR (s−1) | 1.10 ± 0.46 | 1.08 ± 0.49 | 0.97 ± 0.34 | 1.17 ± 0.46 | 0.29 | n/a |
| Global LV LS (%) | −16.2 ± 0.8 | −18.5 ± 0.8 | −18.0 ± 1.1 | −18.2 ± 0.9 | 0.01 | Time 0 vs 3 |
| Global LV LSR (s−1) | −1.13 ± 0.09 | −1.18 ± 0.07 | −1.11± 0.08 | −1.06 ± 0.15 | 0.69 | n/a |
| Global LV LEDSR (s−1) | 1.28 ± 0.08 | 1.34 ± 0.11 | 1.30 ± 0.10 | 1.32 ± 0.09 | 0.90 | n/a |
Results are reported in mean ± standard deviation. LEDSR = longitudinal early diastolic strain rate, LS = longitudinal strain, LSR = longitudinal strain rate, LV = left ventricular, RV = right ventricular.
Table 5.
Changes in Exercise Parameters
| Measure | Baseline | 6-month | p-value |
|---|---|---|---|
| Peak VO2 (mL/kg/mm) | 24.1 ± 10.3 | 25.5 ± 8.2 | 0.18 |
| VO2 at AT (mL/kg/min) | 14.1 ± 5.2 | 15.0 ± 5.1 | 0.53 |
| RER at AT | 0.89 ± 0.09 | 0.91 ± 0.10 | 0.94 |
| VE/VCO2 at AT | 32.4 ± 5.7 | 29.5 ± 8.8 | 0.02 |
| O2 Pulse (mL/beat) | 10.9 ± 3.4 | 12.1 ± 3.8 | 0.01 |
Results are reported in mean ± standard deviation. AT = anaerobic threshold, RER = respiratory exchange ratio.
Changes in echocardiographic measures vs. changes in exercise measures
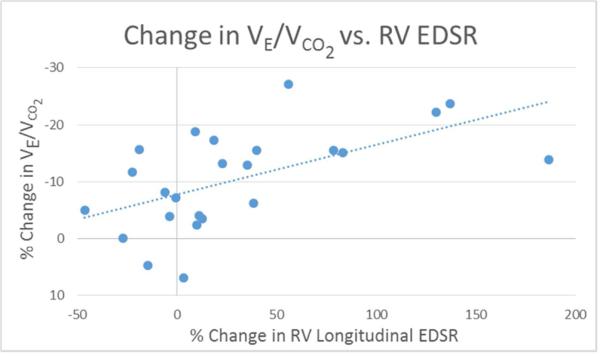
Changes in conventional echocardiographic measures of RV function did not correlate with changes in measures of exercise function. Further, changes in echocardiographic measures of RV size, conduit stenosis, conduit insufficiency, and tricuspid regurgitant jet gradient did not correlate with changes in measures of exercise function. The change in VE/VCO2 correlated with the change in RV longitudinal strain (r = 0.54, p = 0.02) (Figure 2) and early diastolic strain rate (r = −0.59, p = 0.01) (Figure 3). No other correlations were found between changes in STE measures of function and exercise measures of function. No correlations were found between changes in LV measures of deformation and changes in exercise function variables. Upon multiple variable regression, only % change in RV early diastolic strain rate and tricuspid valve inflow Doppler A velocity demonstrated a statistically significant relationship with % change in VE/VCO2 (Table 6). No variables demonstrated collinearity (variance inflation factors for all variables < 5).
Figure 2. Percent change in RV LS vs. VE/VCO2.
Scatter plot with linear regression line showing the relationship between % change in VE/VCO2 and RV longitudinal strain.
Figure 3. Percent change in RV EDSR vs. VE/VCO2.
Scatter plot with linear regression line showing the relationship between % change in VE/VCO2 and RV longitudinal strain.
Table 6.
Results of Stepwise Multivariable Linear Regression Analysis Assessing Changes in Echocardiographic Measures of Pulmonary Valve Function, RV Size, and RV Function to % Change in VE/VCO2
| Model | Variable | B | SE | β | t | Partial R | p | F | R | R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.03 | 6.35 | −0.57 | 0.33 | ||||||
| Constant | 7.33 | 2.44 | 3.00 | 0.01 | ||||||
| % change RV EDSR | −0.13 | 0.05 | −0.57 | −2.52 | 0.57 | 0.03 | ||||
| 2 | <0.01 | 10.63 | −0.80 | 0.64 | ||||||
| Constant | 8.70 | 1.91 | 4.55 | <0.01 | ||||||
| % change RV EDSR | −0.14 | 0.04 | −0.61 | −3.53 | 0.71 | <0.01 | ||||
| % change TV A velocity | 0.12 | 0.04 | 0.56 | 3.22 | 0.68 | <0.01 | ||||
Results of stepwise multiple variable linear regression analysis above. No other measures of changes in pulmonary valve function, RV size, and RV function met criteria for entrance into the model. A p value of < 0.05 was considered significant. EDSR = early diastolic strain rate, RV = right ventricular, TV = tricuspid valve.
Pre-intervention echocardiographic measures vs. changes in exercise measures
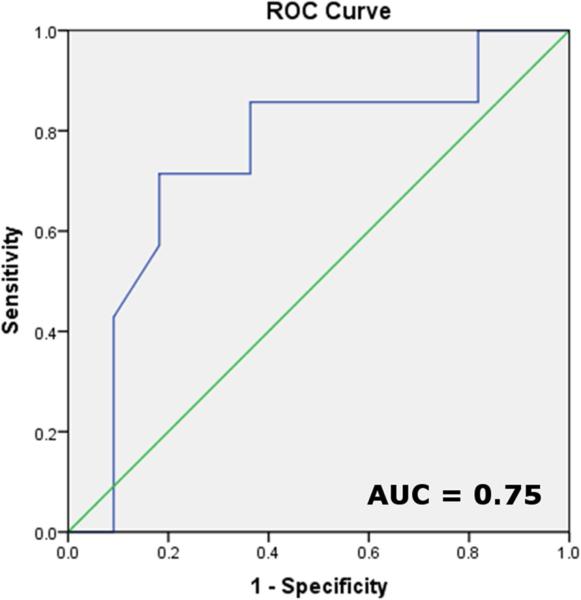
Pre-TPVI echocardiographic measures of RV size, conduit insufficiency, and tricuspid regurgitant jet gradient did not correlate with changes in measures of exercise function. The change in VE/VCO2 correlated with the pre-TPVI RV longitudinal strain (r = −0.60, p < 0.01) and conduit mean gradient (r = −0.48, p = 0.05). No other correlations were found between changes in STE measures of function and exercise measures of function. Pre-TPVI mitral Doppler E:A (r = −0.71, p < 0.01), tricuspid E:E’ (r = −0.56, p = 0.01), and tricuspid septal S velocity (r = 0.57, p < 0.01) correlated with change in O2 pulse. No correlation between baseline LV measures of deformation and changes in exercise function variables were found. Upon multiple variable regression, only pre-TPVI RV longitudinal strain demonstrated a statistically significant relationship with % change in VE/VCO2 (Table 7). No variables demonstrated collinearity (variance inflation factors for all variables < 5). ROC curve for pre-TPVI RV longitudinal strain to predict an improvement in VE/VCO2 greater than 5% of baseline showed a c-statistic (area under the curve) of 0.75 (Figure 4).
Table 7.
Results of Stepwise Multivariable Linear Regression Analysis Assessing Pre-intervention Echocardiographic Measures of Pulmonary Valve Function, RV Size, and RV Function to % Change in VE/VCO2 Post-intervention
| Model | Variable | B | SE | β | t | P | F | R | R2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.01 | 8.05 | 0.59 | 0.35 | |||||
| Constant | 35.84 | 9.33 | 3.84 | <0.01 | |||||
| RV LS | 1.61 | 0.57 | 0.59 | 2.84 | 0.01 |
Results of stepwise multiple variable linear regression analysis above. All other measures of pre-intervention pulmonary valve function, RV size, and RV function did not meet criteria for entrance into the model. A p value of < 0.05 was considered significant. EDSR = early diastolic strain rate, RV = right ventricular, TV = tricuspid valve.
Figure 4. ROC curve.
Receiver operating characteristic analysis to depict pre-intervention RV longitudinal strain's ability to predict a > 5% increase in post-intervention VE/VCO2, area under the curve = 0.75.
STE observer variability
Intra- and inter-observer variability of STE measures of deformation were performed in 25% (n = 24) of the studies. Results are presented in Table 8. All observer measurement variability was deemed acceptable.
Table 8.
Intraobserver and interobserver variability of speckle-tracking measures of ventricular function
| Intraobserver % Error of the Mean (%) | Intraobserver ICC (r value) | Interobserver % Error of the Mean (%) | Interobserver ICC (r value) | |
|---|---|---|---|---|
| RV LS | 0.1 (−5.2 – 2.9) | 0.93 | 3.1 (−10.5 – 3.6) | 0.75 |
| RV LSR | 0.4 (−9.1 – 10.3) | 0.88 | −3.3 (−13.4 – −0.2) | 0.77 |
| RV LEDSR | −3.7 (−10.6 – 5.2) | 0.94 | 5.1 (−6.2 – 15.5) | 0.87 |
| LV LS | 0.9 (−4.2 – 4.6) | 0.9 | 2.9 (−2.5 – 12.3) | 0.86 |
| LV LSR | −1.3 (−14.6 – 16) | 0.84 | 2.4 (−9.1 – 16) | 0.81 |
| LV LEDSR | 3.0 (−8.2 – 15) | 0.82 | 7.7 (−1.6 – 16) | 0.84 |
Values reported as median (interquartile range) or correlation r-values. All p-values were < 0.01. ICC = intraclass correlation coefficient, LEDSR = longitudinal early diastolic strain rate, LS = longitudinal strain, LSR = longitudinal strain rate, LV = left ventricular, RV = right ventricular.
Discussion
Speckle-tracking measures of deformation have been shown to be more sensitive than conventional measures in detecting changes in myocardial function in multiple disease processes.9-11 We have reported similar findings in the current cohort.12 However, the clinical utility of detecting these changes by STE is unknown in this population. A number of groups have shown that measures of exercise function, specifically peak VO2 and VE/VCO2, predict risk of morbidity and mortality in patients with repaired Tetralogy of Fallot and other forms of congenital heart disease.4-7, 17 Therefore, to assess STE measures’ potential to be used as surrogates for outcome, we assessed the correlation between STE measures of deformation and changes in measures of exercise function after TPVI. We found that changes in STE early diastolic strain rate correlated with changes in VE/VCO2. In addition, pre-TPVI RV longitudinal stain correlated with VE/VCO2 response to TPVI. These findings support the potential clinical usefulness of assessing RV function using STE prior to and after TPVI.
Patients with dysfunctional RV to PA conduits exhibit decreased VO2, VE/VCO2, and O2 pulse upon exercise testing and are often symptomatic.18 Despite the fact that patient symptoms improved significantly in this cohort as previously reported (85% of patients were NYHA Class II-IV pre-TPVI vs. 15% post-TPVI),13 patients in the current study showed no change in peak VO2, VO2 at AT, or RER after TPVI. This is in line with previous studies that showed similar findings.2, 3, 6, 19-21 Many studies use VO2 as the primary outcome of interest after pulmonary valve replacement. However, using VO2 as the primary measure of cardiovascular health in this population may be misleading. This is because VO2 is influenced by both respiratory and cardiovascular health, with no way to differentiate between the two. In fact, the majority of patients with chronic pulmonary regurgitation have a primary respiratory limitation to peak VO2, rather than a cardiovascular limitation.22 Pulmonary valve replacement does not improve this respiratory limitation, therefore VO2 is unchanged.
In contrast, VE/VCO2 accounts for both respiratory (VE) and cardiovascular (VCO2) influences to exercise function. It will detect changes in the cardiovascular contribution to exercise function even if the respiratory component is unchanged. Improvement in VE/VCO2 has been reported after both surgical and transcatheter pulmonary valve replacement in patients with predominantly pulmonary regurgitation or mixed disease, similar to the findings in the current study.2, 3 VE/VCO2 is a measure of ventilatory efficiency and thought to improve after TPVI secondary to improved effective RV stroke volume and/or cardiac output. This is supported by the fact that a surrogate of LV stroke volume, the peak O2 pulse, improved in our study, and others,19, 23 after TPVI suggesting improved LV preload.
Percent change in RV longitudinal strain and early diastolic strain rate correlated with percent change in VE/VCO2. Multivariable analysis revealed that only diastolic measures demonstrated a relationship with change in VE/VCO2. Diastolic function has been suggested to be associated with exercise capacity in this patient group by others.21 These findings suggest that change in RV diastolic function may be an important determinant of the change in cardiac output that drives resultant VE/VCO2 after TPVI. Therefore, these measurements of RV diastolic function may be clinically useful to follow before and after TPVI.
There were no significant correlations between traditional measures of RV function and exercise function in the current study. Menon et al. reported similar findings in a group with pulmonary regurgitation.24 Hasan et al. showed that there are correlations between RV STE longitudinal strain and peak VO2 both before and after TPVI in a group with obstructed conduits.23 These studies support the additive value of STE over conventional echocardiography in this population.
While STE measures of deformation are more load-resistant that conventional measures of function, they are not load independent. The association we found between STE measures and VE/VCO2 may be confounded by the loading changes after TPVI. Unfortunately, as reported previously by Kenny et al,13 change in RV volumes and pulmonary regurgitant fraction were available in less than half of patients (n = 13) in the initial cohort (n = 33) due to artifact from the stainless steel stent. In patients with MRI data, right ventricular volumes (130 ± 63 ml/m2 to 87 ± 20 ml/m2, p = 0.02) and pulmonary regurgitant fraction (29 ± 18% to 3.5±5.4%, p < 0.01) improved following TPVI. To attempt to determine whether changes in RV STE measures of deformation were indeed representative of changes in RV performance, we attempted to control for changes in RV size by including echocardiographic end-diastolic area and end-systolic area in the multivariable model; they were not predictive of changes in exercise parameters. In addition, RV STE measures of deformation did not change with acute changes in loading conditions, i.e. no change was seen between baseline and discharge after TPVI. There was also no change at one month follow-up when RV size reached its nadir. RV STE measures of deformation did not improve until six months, providing evidence that RV remodeling, and not changes in loading conditions, were responsible for improved strain and strain rate. However, as STE measures of deformation are measures of pump function, and not contractility, they are inherently influenced by factors that could not be absolutely controlled for, such as changes in loading conditions, our heterogenous population, and the degree of RV hypertrophy. It is feasible, therefore, that the improvement in RV strain and strain rate may be confounded by these other factors and may not actually represent improvement in RV contractility.
Pre-intervention RV longitudinal strain was found to be a predictor of change in VE/VCO2 after TPVI in both univariate and multivariable analysis. Those with a lower RV strain at baseline showed the greatest improvement in exercise function post-TPVI. In a cohort identical to the one in this study, a baseline RV longitudinal strain greater than −18% would have an 82% sensitivity and 71% specificity in detecting a positive response to TPVI as defined by a > 5% improvement in VE/VCO2. However, this is an ideal scenario, the diagnostic performance of RV strain in predicting improvement in VE/VCO2 needs to be independently assessed in a separate cohort. Sabate Rotes et al. found RV longitudinal strain as the only independent predictor for an improvement in NYHA heart failure class after surgical pulmonary valve replacement.25 Traditional echocardiographic surrogates for MRI EF, such as RV FAC, did not show a similar relationship. It appears RV longitudinal strain holds potential as a pre-intervention predictor of outcomes and deserves further study. It may be especially useful in patients with borderline RV volumes and normal EF who have pulmonary regurgitation or mixed disease.
Limitations
There were limitations to this study. The sample was small and heterogeneous in age, diagnosis, and type of conduit dysfunction. The primary diagnosis in five patients were not available as they did not have Tetralogy of Fallot or the Ross procedure. This may have implications as regional abnormalities in RV function can be expected if reconstruction of the RV outflow tract was performed, leaving regional of measures of function, such as TAPSE, inaccurate as a measure of global RV function. This was a secondary analysis of prospectively collected data. Thus, the echocardiographic protocol was not designed with STE in mind. This resulted in nine patients being excluded from this analysis. Most patients were excluded for RV free wall and apical segments being inadequately captured in the echocardiographic window. Future protocols using STE post TPV implantation should stress the importance of optimal image acquisition including all RV segments. The percentage of success in obtaining adequate images for speckle-tracking analysis of the RV should then be assessed. In addition, echocardiograms were compressed and stored at 30 frames per second when read by the core lab. While strain measures are accurate at this frame rate, strain rate can be underestimated, which may confound our results.26, 27 We were only able to measure longitudinal strain. Circumferential strain has been shown to be important in pressure-loaded RVs,28 but could not be measured due to limitations in the acquisition protocol.
It is known that patients with conduit stenosis display more improved exercise function after valve replacement than patients with pulmonary regurgitation.19 However, many of the patients in this cohort had mixed disease, which makes it difficult to compare our exercise results with previous studies as those with mixed disease may display traits of both those with stenosis only and regurgitation only.3 We measured RV size using RV areas from the apical four chamber windows. While echocardiography is certainly not the gold standard method to measure RV size, it has shown good correlation to MRI values.29, 30 This may become more important as the use of TPVI increases, as stainless steel stent implantation can decrease the success rate of obtaining RV volumes by MRI.13 Many known factors that influence outcomes in these patients were not accounted for secondary to unavailability of data or due to the small, heterogeneous nature of the sample, including age at first operation, age since last operative repair, type of conduit dysfunction, type of congenital heart disease, RV volumes derived from MRI, degree of impairment in ventriculo-ventricular interactions, evidence of post-systolic shortening, forward flow in the pulmonary artery during atrial systole, other echocardiographic surrogates of right atrial pressure, QRS duration, arrhythmias, and evidence of mechanical dyssynchrony.2, 22, 31-34 Larger studies will be needed to determine the usefulness of RV STE measures of deformation in the peri-TPVI period when these other factors are accounted for. The lack of an age-matched control group does not allow us to have a frame of reference when assessing the effect TPVI on RV function in relation to healthy individuals.
Conclusion
Improvements are seen in exercise function, as measured by the VE/VCO2 ratio, six months post-TPVI in patients with conduits that are purely regurgitant or display mixed disease. Improvements in RV diastolic function may contribute to the changes in exercise performance seen after TPVI. Abnormal pre-intervention RV longitudinal strain may be a predictor for improved exercise function after TPVI. STE measures of RV function appear to hold the potential for being used as predictors of improved clinical outcomes in patients requiring TPV implantation. Future studies should directly assess the prognostic significance of STE measures of RV function in this population.
Highlights.
Improvements are seen in RV STE measures of deformation and VE/VCO2 six months post-TPVI in patients with conduits that are purely regurgitant or display mixed disease.
Changes in STE measures of RV diastolic function correlate with changes seen in VE/VCO2 after TPVI.
Patients with decreased pre-intervention RV longitudinal strain have a higher likelihood to display improved VE/VCO2 after TPVI than those without.
STE measures of RV function appear to hold the potential for being used as predictors of improved outcomes in patients requiring TPV implantation.
Acknowledgements
The authors would like to acknowledge Jeremy Gorelick for his statistical expertise and Lazar Mandinov, MD, PhD. This study was funded by Edwards Lifesciences, LLC. Dr. Chowdhury received funding from NIH/NHLBI grant 5 T32 HL07710-19.
Abbreviations
- AT
anaerobic threshold
- FAC
fractional area change
- PA
pulmonary artery
- STE
speckle-tracking echocardiography
- TAPSE
tricuspid annular plane systolic excursion
- TDI
tissue Doppler imaging
- TPVI
transcatheter pulmonary valve implantation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caldarone CA, McCrindle BW, Van Arsdell GS, Coles JG, Webb G, Freedom RM, et al. Independent factors associated with longevity of prosthetic pulmonary valves and valved conduits. J Thorac Cardiovasc Surg. 2000;120:1022–30. doi: 10.1067/mtc.2000.110684. discussion 1031. [DOI] [PubMed] [Google Scholar]
- 2.Frigiola A, Tsang V, Bull C, Coats L, Khambadkone S, Derrick G, et al. Biventricular response after pulmonary valve replacement for right ventricular outflow tract dysfunction: is age a predictor of outcome? Circulation. 2008;118:S182–90. doi: 10.1161/CIRCULATIONAHA.107.756825. [DOI] [PubMed] [Google Scholar]
- 3.Coats L, Khambadkone S, Derrick G, Hughes M, Jones R, Mist B, et al. Physiological consequences of percutaneous pulmonary valve implantation: the different behaviour of volume and pressure-overloaded ventricles. Eur Heart J. 2007;28:1886–93. doi: 10.1093/eurheartj/ehm181. [DOI] [PubMed] [Google Scholar]
- 4.Sutton NJ, Peng L, Lock JE, Lang P, Marx GR, Curran TJ, et al. Effect of pulmonary artery angioplasty on exercise function after repair of tetralogy of Fallot. Am Heart J. 2008;155:182–6. doi: 10.1016/j.ahj.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Giardini A, Specchia S, Tacy TA, Coutsoumbas G, Gargiulo G, Donti A, et al. Usefulness of cardiopulmonary exercise to predict long-term prognosis in adults with repaired tetralogy of Fallot. Am J Cardiol. 2007;99:1462–7. doi: 10.1016/j.amjcard.2006.12.076. [DOI] [PubMed] [Google Scholar]
- 6.Babu-Narayan SV, Diller GP, Gheta RR, Bastin AJ, Karonis T, Li W, et al. Clinical outcomes of surgical pulmonary valve replacement after repair of tetralogy of Fallot and potential prognostic value of preoperative cardiopulmonary exercise testing. Circulation. 2014;129:18–27. doi: 10.1161/CIRCULATIONAHA.113.001485. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulos K, Okonko DO, Diller GP, Broberg CS, Salukhe TV, Babu-Narayan SV, et al. Abnormal ventilatory response to exercise in adults with congenital heart disease relates to cyanosis and predicts survival. Circulation. 2006;113:2796–802. doi: 10.1161/CIRCULATIONAHA.105.594218. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury SM, Hijazi ZM, Rhodes J, Kar S, Makkar R, Mullen M, et al. Early echocardiographic changes after percutaneous implantation of the Edwards SAPIEN transcatheter heart valve in the pulmonary position. Echocardiography. 2013;30:786–93. doi: 10.1111/echo.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellavia D, Abraham TP, Pellikka PA, Al-Zahrani GB, Dispenzieri A, Oh JK, et al. Detection of left ventricular systolic dysfunction in cardiac amyloidosis with strain rate echocardiography. J Am Soc Echocardiogr. 2007;20:1194–202. doi: 10.1016/j.echo.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Mori K, Hayabuchi Y, Inoue M, Suzuki M, Sakata M, Nakagawa R, et al. Myocardial strain imaging for early detection of cardiac involvement in patients with Duchenne's progressive muscular dystrophy. Echocardiography. 2007;24:598–608. doi: 10.1111/j.1540-8175.2007.00437.x. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Xie M, Wang X, Lv Q, Lu X, Yang Y, et al. Evaluation of right ventricular global longitudinal function in patients with tetralogy of fallot by two-dimensional ultrasound speckle tracking imaging. J Huazhong Univ Sci Technolog Med Sci. 2010;30:126–31. doi: 10.1007/s11596-010-0123-3. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury SM, Hijazi ZM, Rhodes JF, Kar S, Makkar R, Mullen M, et al. Changes in Speckle Tracking Echocardiography Measures of Ventricular Function after Percutaneous Implantation of the Edwards SAPIEN Transcatheter Heart Valve in the Pulmonary Position. Echocardiography. 2014 doi: 10.1111/echo.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenny D, Hijazi ZM, Kar S, Rhodes J, Mullen M, Makkar R, et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. J Am Coll Cardiol. 2011;58:2248–56. doi: 10.1016/j.jacc.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 14.Puchalski MD, Askovich B, Sower CT, Williams RV, Minich LL, Tani LY. Pulmonary regurgitation: determining severity by echocardiography and magnetic resonance imaging. Congenit Heart Dis. 2008;3:168–75. doi: 10.1111/j.1747-0803.2008.00184.x. [DOI] [PubMed] [Google Scholar]
- 15.Mercer-Rosa L, Yang W, Kutty S, Rychik J, Fogel M, Goldmuntz E. Quantifying pulmonary regurgitation and right ventricular function in surgically repaired tetralogy of Fallot: a comparative analysis of echocardiography and magnetic resonance imaging. Circ Cardiovasc Imaging. 2012;5:637–43. doi: 10.1161/CIRCIMAGING.112.972588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klima U, Guerrero JL, Vlahakes GJ. Contribution of the interventricular septum to maximal right ventricular function. Eur J Cardiothorac Surg. 1998;14:250–5. doi: 10.1016/s1010-7940(98)00179-1. [DOI] [PubMed] [Google Scholar]
- 17.Robbins M, Francis G, Pashkow FJ, Snader CE, Hoercher K, Young JB, et al. Ventilatory and heart rate responses to exercise : better predictors of heart failure mortality than peak oxygen consumption. Circulation. 1999;100:2411–7. doi: 10.1161/01.cir.100.24.2411. [DOI] [PubMed] [Google Scholar]
- 18.Giardini A, Specchia S, Coutsoumbas G, Donti A, Formigari R, Fattori R, et al. Impact of pulmonary regurgitation and right ventricular dysfunction on oxygen uptake recovery kinetics in repaired tetralogy of Fallot. Eur J Heart Fail. 2006;8:736–43. doi: 10.1016/j.ejheart.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Lurz P, Giardini A, Taylor AM, Nordmeyer J, Muthurangu V, Odendaal D, et al. Effect of altering pathologic right ventricular loading conditions by percutaneous pulmonary valve implantation on exercise capacity. Am J Cardiol. 2010;105:721–6. doi: 10.1016/j.amjcard.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 20.Sabate Rotes A, Johnson JN, Burkhart HM, Eidem BW, Allison TG, Driscoll DJ. Cardiorespiratory Response to Exercise before and after Pulmonary Valve Replacement in Patients with Repaired Tetralogy of Fallot: A Retrospective Study and Systematic Review of the Literature. Congenit Heart Dis. 2014 doi: 10.1111/chd.12207. [DOI] [PubMed] [Google Scholar]
- 21.Frigiola A, Giardini A, Taylor A, Tsang V, Derrick G, Khambadkone S, et al. Echocardiographic assessment of diastolic biventricular properties in patients operated for severe pulmonary regurgitation and association with exercise capacity. Eur Heart J Cardiovasc Imaging. 2012;13:697–702. doi: 10.1093/ehjci/jes002. [DOI] [PubMed] [Google Scholar]
- 22.Sterrett LE, Ebenroth ES, Query C, Ho J, Montgomery GS, Hurwitz RA, et al. Why Exercise Capacity Does Not Improve After Pulmonary Valve Replacement. Pediatr Cardiol. 2014 doi: 10.1007/s00246-014-0942-2. [DOI] [PubMed] [Google Scholar]
- 23.Hasan BS, Lunze FI, Chen MH, Brown DW, Boudreau MJ, Rhodes J, et al. Effects of transcatheter pulmonary valve replacement on the hemodynamic and ventricular response to exercise in patients with obstructed right ventricle-to-pulmonary artery conduits. JACC Cardiovasc Interv. 2014;7:530–42. doi: 10.1016/j.jcin.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Menon SC, Kaza AK, Puchalski MD. Effect of ventricular size and function on exercise performance and the electrocardiogram in repaired tetralogy of Fallot with pure pulmonary regurgitation. Ann Pediatr Cardiol. 2012;5:151–5. doi: 10.4103/0974-2069.99617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rotes AS. Long-Term Follow-Up in Repaired Tetralogy of Fallot: Can Deformation Imaging Help Identify Optimal Timing of Pulmonary Valve Replacement? Journal of the American Society of Echocardiography. 2014 doi: 10.1016/j.echo.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Risum N, Ali S, Olsen NT, Jons C, Khouri MG, Lauridsen TK, et al. Variability of global left ventricular deformation analysis using vendor dependent and independent two-dimensional speckle-tracking software in adults. J Am Soc Echocardiogr. 2012;25:1195–203. doi: 10.1016/j.echo.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Koopman LP, Slorach C, Manlhiot C, McCrindle BW, Jaeggi ET, Mertens L, et al. Assessment of myocardial deformation in children using Digital Imaging and Communications in Medicine (DICOM) data and vendor independent speckle tracking software. J Am Soc Echocardiogr. 2011;24:37–44. doi: 10.1016/j.echo.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Tham EB, Smallhorn JF, Kaneko S, Valiani S, Myers KA, Colen TM, et al. Insights into the evolution of myocardial dysfunction in the functionally single right ventricle between staged palliations using speckle-tracking echocardiography. J Am Soc Echocardiogr. 2014;27:314–22. doi: 10.1016/j.echo.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Chaowalit N, Durongpisitkul K, Krittayaphong R, Komoltri C, Jakrapanichakul D, Phrudprisan S. Echocardiography as a Simple Initial Tool to Assess Right Ventricular Dimensions in Patients with Repaired Tetralogy of Fallot before Undergoing Pulmonary Valve Replacement: Comparison with Cardiovascular Magnetic Resonance Imaging. Echocardiography. 2012;29:1239–46. doi: 10.1111/j.1540-8175.2012.01766.x. [DOI] [PubMed] [Google Scholar]
- 30.Neukamm C, Try K, Norgard G, Brun H. Right ventricular volumes assessed by echocardiographic three-dimensional knowledge-based reconstruction compared with magnetic resonance imaging in a clinical setting. Congenit Heart Dis. 2014;9:333–42. doi: 10.1111/chd.12146. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz MC, Rome JJ, Gillespie MJ, Whitehead K, Harris MA, Fogel MA, et al. Relation of left ventricular end diastolic pressure to right ventricular end diastolic volume after operative treatment of tetralogy of fallot. Am J Cardiol. 2012;109:417–22. doi: 10.1016/j.amjcard.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Li SN, Yu W, Lai CT, Wong SJ, Cheung YF. Left ventricular mechanics in repaired tetralogy of Fallot with and without pulmonary valve replacement: analysis by three-dimensional speckle tracking echocardiography. PLoS One. 2013;8:e78826. doi: 10.1371/journal.pone.0078826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiewak M, Malek LA, Petryka J, Mazurkiewicz L, Marczak M, Biernacka EK, et al. Determinants of left- and rightventricular ejection fractions in patients with repaired tetralogy of Fallot: a cardiac magnetic resonance imaging study. Pol Arch Med Wewn. 2013;123:539–46. doi: 10.20452/pamw.1929. [DOI] [PubMed] [Google Scholar]
- 34.Zdradzinski MJ, Qureshi AM, Stewart R, Pettersson G, Krasuski RA. Comparison of long-term postoperative sequelae in patients with tetralogy of Fallot versus isolated pulmonic stenosis. Am J Cardiol. 2014;114:300–4. doi: 10.1016/j.amjcard.2014.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]