Abstract
Cancer patients suffer high levels of affective and cognitive disturbances, which have been attributed to diagnosis-related distress, impairment of quality of life, and side effects of primary treatment. An inflammatory microenvironment is also a feature of the vast majority of solid tumors. However, the ability of tumor-associated biological processes to affect the central nervous system (CNS) has only recently been explored in the context of symptoms of depression and cognitive disturbances. In this review, we summarize the burgeoning evidence from rodent cancer models that solid tumors alter neurobiological pathways and subsequent behavioral processes with relevance to affective and cognitive disturbances reported in human cancer populations. We consider, in parallel, the evidence from human clinical cancer research demonstrating that affective and cognitive disturbances are common in some malignancies prior to diagnosis and treatment. We further consider the underlying neurobiological pathways, including altered neuroinflammation, tryptophan metabolism, prostaglandin synthesis and associated neuroanatomical changes, that are most strongly implicated in the rodent literature and supported by analogous evidence from human cancer populations. We focus on the implications of these findings for behavioral researchers and clinicians, with particular emphasis on methodological issues and areas of future research.
Keywords: cancer, depression, cognition, cytokines
1. Introduction
Cancer patients suffer from a high prevalence of depression, anxiety, and cognitive disturbances (Evans et al., 2005). In fact, these disturbances are common among numerous other populations with chronic, inflammatory disease (e.g, cardiovascular disease, metabolic disease, HIV/AIDS), a fact that has stimulated discussion of potential shared biological mechanisms (Irwin & Miller, 2007; Lee et al., 2004). In the field of cancer research, it is widely acknowledged that various interrelated factors may underlie these behavioral comorbidities: tumor biology, distress associated with the cancer diagnosis, and/or cancer treatments (e.g., “chemobrain”). However, in spite of a recent burst of research activity in this area, these factors are rarely differentiated and their likely mechanistic interactions remain either entangled or ignored (Dantzer et al., 2012). For example, many clinical cancer studies do not encompass pre-diagnosis or pre-treatment psychological or physiological measures and most rodent studies of the impact of cancer treatment on the central nervous system (CNS) use tumor-free models. This results in a knowledge gap concerning the independent impact of tumor-associated biological processes on affective and cognitive symptoms despite the acknowledgment that tumors are capable of exerting an influence on the CNS (Cleeland et al., 2003; Lee et al., 2004), and that inflammation is inherent to all phases of tumor growth and metastases (Colotta et al., 2009). Addressing this deficit will help to identify specific underlying biological causes and underlying mechanisms of cancer-associated affective and cognitive disturbances, with the potential to improve treatment.
This review addresses this knowledge gap by presenting the current literature on the potential for tumor-associated biological processes to affect brain function (e.g., affect and cognition) independent of cancer-associated stress and treatments. We focus on behavioral changes that are most strongly associated with the presence of a tumor outside of the CNS and on identifying underlying mechanisms using data from human and rodent research. Direct effects of tumor associated biological processes on the brain would indicate that patients suffer affective and cognitive disturbances prior to the receipt of treatment. The clinical impact of psychological comorbidities should not be underestimated in the context of cancer. Both quality of life and adherence to treatment plans significantly deteriorate when depression and other mood disturbances are present (DiMatteo, 2003). Poor treatment adherence is a significant risk factor for decreased quality of life and increased mortality (Gripp et al., 2007). Isolating the specific influence of tumor biology on CNS processes and empirically testing how tumor biology interacts with the biology of stress and cancer treatments may allow for tailored treatment of cancer-associated affective and cognitive comorbidities. This review first provides a brief summary of inflammation-induced changes in behavior, and then summarizes the quickly growing body of behavioral research in rodent cancer models and clinical investigations of cancer patients prior to treatment. Evidence for affective and somatic symptoms of depression, cognitive impairment, and their mechanistic correlates are reviewed.
1.1 Inflammation-Induced Behavioral Changes
There is now considerable evidence that inflammatory pathways modulate affective and cognitive processes (Dantzer et al., 2008; Maes et al., 2012; Miller et al., 2009). Various aspects of this concept have been termed the “cytokine” or “macrophage theory of depression” and “sickness behavior.” These concepts are primarily supported by literatures demonstrating that: 1) pathways exist between the periphery and CNS that allow for modulation of neural structure and function (e.g., enhanced permeability of the blood-brain-barrier and signaling via the vagus nerve; Dantzer et al., 1999), 2) acute cytokine or bacterial mimetic injection in rodents (i.p. lipopolysaccharide [LPS]; Dantzer et al., 2008) induces behavioral changes that are reversible by anti-inflammatory pharmacologic treatment, 3) inflammatory/immune responses and markers are elevated in patients with psychological disorders such as major depressive disorder (Maes et al., 2012; Miller et al., 2009) and, 4) anti-inflammatory drugs can be efficacious in the treatment of some psychiatric disorders (Köhler et al., 2014). For example, patients with clinical depression display elevations in proinflammatory cytokine concentrations in the blood and cerebral spinal fluid, increases in other circulating inflammatory effector and target molecules (e.g., prostaglandins, acute phase proteins, chemokines, cell adhesion molecules; Maes et al., 2012) and altered adaptive immune cell function (Howren, Lamkin, & Suls, 2009; Miller, 2010). Cytokine-based treatment of cancers (i.e., interferon-alpha) also result in considerable increases in depression (Capuron et al., 2002; Udina et al., 2012), which resolve when the treatment is terminated.
Studies using animal models have delineated inflammatory mechanisms underlying changes in behavior, including sickness behavior, affective symptoms, and cognitive function. However, the majority of the research in this area has focused on models of acute illness. The canonical acute bacterial infection model, a single, sub-toxic i.p. injection of LPS (a component of the cell wall of E. coli bacteria), causes proinflammatory cytokine production primarily by first-responder monocytes in the peritoneal cavity. Then, through both neural and humoral signaling pathways (Dantzer et al., 2008; Quan & Banks, 2007; Quan, 2014), cytokine production rapidly ensues in the brain (hippocampus, hypothalamus, forebrain) and stimulates the production or activation of other inflammatory effectors (IDO, iNOS, Nf-kB, COX; Quan et al., 1998). The roles of these inflammatory markers in cytokine-induced behavioral changes, and consistent with clinical research in depressed patients, are summarized in Box 1. Acute sickness behaviors (e.g., lethargy, social withdrawal, fever, anorexia) akin to “somatic” or “vegetative” symptoms of depression, subsequent affective-like behaviors, and impaired learning and memory (Pugh et al., 1998) follow for several days after LPS treatment. The experimental manipulation of cytokines in these models (e.g., pharmacologic blockade, cytokine gene knockout) has established that brain-production of cytokines are necessary and sufficient for the subsequent behavioral changes (Dantzer et al., 1998, 1999; Kent et al., 1992). As with all preclinical studies, there is the assumption that affective-like behaviors assessed by rigorously validated rodent behavioral tests (Dantzer et al., 2012) are analogous to some affective symptoms of depression in human beings.
A longer-enduring infectious disease model (i.p., injection of Bacillus Calmette-Guerin [BCG]) has been used to probe for differences in chronic versus acute bacterial illness. Like the LPS model, BCG induces depressive-like behaviors after overt sickness behaviors have subsided. Coincident with the behavioral changes, the BCG model triggers brain cytokine and IDO production (Lestage et al., 2002; Moreau et al., 2005). While longer-lasting than the LPS model, the behavioral and inflammatory consequences of BCG infection still last only 3–4 weeks (Moreau et al., 2008).
In summary, the majority of the mechanistic postulating about mental comorbidities within the cancer context (and other chronic inflammatory diseases) logically focuses on neuroinflammatory pathways (Cleeland et al., 2003; Dantzer et al., 2012; Illman et al.; Lee et al., 2004). Peripheral tumors and their microenvironment are sources of various cytokines and potentially use neural and/or humoral signaling pathways similar to peripheral infection to gain access to the brain. A review of the current literature that best encapsulates the purely biological influence of peripheral tumors on brain physiology and depression and cognition follows. Here we organized the corresponding behavioral data from rodent and human research and discuss the putative inflammatory mechanisms associated with these behaviors. Finally, we discuss how this compilation of data may be used to modify current treatment strategies and future research design in an effort to move towards more individualized, mechanism-based treatment approaches.
2. Rodent Models of Cancer
Recent basic research in rodents provides compelling evidence that primary tumors and tumor-associated inflammation alter brain physiology and behavior. Essentially, these models are comparable to patients with cancer prior to treatment, though both human and rodent research reflects considerable heterogeneity in the type and timing of neoplasms investigated. Rodent modeling allows for both the differentiation of potentially additive/synergistic biopsychological factors and for the empirical study of underlying mechanisms. For the purpose of this review, we focused on PubMed primary reports in the English language of rodent models with malignant primary tumors outside of the CNS, using the search terms [“rodent;” “cancer” or “tumor;” “inflammation” or “cytokine” or “neuroinflammation” or “serotonin;” “behavior” or “cognition” or “learning” or “affect” or “depression” or “anxiety, indexed from “no determined start date” to January, 2015]. Additional criteria were that the reports included tumor-free control groups and groups in which both cancer therapies and psychosocial stressors were absent.
2.1 Evidence for Inflammation in Tumor-Bearing Rodent Models
In rodent models of solid neoplasms, tumor cells, surrounding stromal cells, and leukocytes recruited to the tumor site produce many inflammatory mediators (cytokines, chemokines) in the tumor microenvironment (reviewed in Allavena et al., 2008; Candido & Hagemann, 2013; Coussens & Werb, 2002). Commonly identified inflammatory mediators include various cytokines, chemokines, and their receptors (e.g., IL-1, TNFα, IL-6, IL-10, IL-12, TGFβ, MIP1α, CXCR4; Candido & Hagemann, 2013; Turrin et al., 2004), prostaglandin E2, NF-κB and STAT3 signaling cascades, and enzymes such as IDO, COX, and iNOS (reviewed in Cesario et al., 2011; Karin et al., 2002; Landskron et al., 2014; Munn & Mellor, 2013; Uyttenhove et al., 2003). Cytokine production in the tumor microenvironment is sometimes significant enough to be detectable in the general circulation of experimental models (Fang et al., 2012; Lamkin et al., 2011; Norden et al., 2014; Uomoto et al., 1998; Yang et al., 2014). However, this detection appears to be dependent upon the type of tumor and the timing of the blood sampling relative to tumor growth, as elevations in circulating cytokines do not necessarily accompany elevations in tumor cytokines, brain cytokines, or behavioral changes in these models (Catalano et al., 2003; Pyter et al., 2009). Given the known interactions between the HPA axis and inflammatory responses, it is important to note that solid tumors either increase or have no effect on basal circulating corticosterone concentrations (Azpiroz et al., 2008; Bojková et al., 2011; Chuluyan et al., 2000; Pyter et al., 2009; Yang et al., 2014), decrease stressor-triggered corticosterone responses (Pyter et al., 2009), and increase glucocorticoid receptor expression in brain regions involved in the HPA axis negative feedback loop (Pyter et al., 2009) in some rodent models.
2.2 Sickness, Affective-Like and Cognitive Impairment Behaviors in Tumor-Bearing Rodent Models
In rodents, many somatic-like symptoms of depression have considerable overlap with “sickness behaviors” (e.g., food intake, physical activity, motivation for social interaction). Several tumor models have been developed specifically for their extreme negative effects on food intake, weight loss, and muscle wasting (Emery, 1999; Tisdale, 2002). More broadly, however, in studies of tumor models that focus on other behaviors (e.g., affective-like), weight loss ranges widely from significant (Lamkin et al., 2011; Norden et al., 2014; Wang et al., 2001; Yang et al., 2014) to absent (Coma et al., 2003; Pyter et al., 2009), depending on the tumor type, the rate of tumor growth and the time of assessment. In one study, total weight loss includes a specific reduction in brain mass (Coma et al., 2003).
The weight loss observed in some models is coincident with electrophysiological muscle fatigue (Aulino et al., 2010; Baltgalvis et al., 2010; Murphy et al., 2012; Norden et al., 2014), reduced locomotor activity (Lamkin et al., 2011; Murphy et al., 2012; Xiu et al., 2010), and reduced voluntary wheel running (Baltgalvis et al., 2010; Wood et al., 2006). Reduced locomotor activity may reflect fatigue or physical limitations due to the tumor location and/or changes in areas of the CNS that regulate movement (Dantzer et al., 2012). While the tumor may infringe on physical movement or muscle endurance in some models, thereby confounding results of affective-like or cognitive behavioral testing, depressive-like behaviors persist in other tests that are virtually independent of locomotion (e.g., sucrose anhedonia, see below). In contrast, the effects of peripheral tumors on sleep, a component of fatigue, remain understudied. Finally, limited investigation of social withdrawal, a common component of sickness behavior, indicates that tumor-bearing rodents are no less interested in social interactions (Pyter et al., 2009) or E2-primed lordosis with a mate (Walf & Frye, 2010) than their tumor-free counterparts. In contrast to tumor-associated sickness behaviors, the vast majority of studies to examine affective-like or cognitive behaviors in cancer models were published within the last five years, excepting some research in the 1970s–80s on learned taste aversion as it relates to cancer anorexia. Based on the previously described criteria, all but one of the 13 reports that assessed affective-like behavior found significant increases in depressive-like behavior in a broad range of solid tumor models and one leukemia model relative to tumor-free controls (Table 1). Therefore, it appears that varying tumor types and induction methods produce similar affective-like consequences s, though more studies would be necessary to determine if model type influences behavioral outcomes. One of the standardized tests validated to assess depressive-like behavior (Krishnan & Nestler, 2008) is a tail suspension test in which mice are suspended by their tail and the latency and duration of immobility (versus active struggling) is quantified over a 5-min period. Immobility behavior in this context is hypothesized to be analogous to clinical symptoms of despair. As evidence of this test’s predictive validity, pharmacological treatment with antidepressants for several weeks prior to testing ameliorates the immobility behaviors. For example, mice that are subcutaneously inoculated with murine hepatoma cells display significant increases in immobility duration using the tail suspension test 12 days after inoculation, a behavioral change that is not observed earlier (6 or 8 days after inoculation), when tumor size is relatively small (Qi et al., 2009). Other reported increases in depressive-like behaviors in tumor-bearing rodents include what is termed “behavioral despair” using the Porsolt forced swim test and reduced preference for sucrose-infused drinking water (i.e., sucrose anhedonia). In rodents, the absence of preferential consumption of sucrose solutions over tap water is hypothesized to be homologous to symptoms of anhedonia in depressed patients. Tumor-bearing animals consistently display reductions in sucrose preference (DeWys, 1974; Godbout et al., 2005; Lamkin et al., 2011; Pyter et al., 2009; Smith et al., 1994; Xiu et al., 2010), which do not appear to be driven by changes in nutritional motivation nor changes in taste thresholds, as preference for a saccharine solution (non-nutritive) is also reduced and aversion to noxious solutions (e.g., quinine, hydrochloric acid) remains unchanged (Smith et al., 1994).
Table 1.
Affective-like and cognitive behavioral and inflammatory consequences of peripheral tumor growth in rodents.
| Publication | Tumor model | Behavioral Tests | Putative Behavioral Correlate | Peripheral Inflammatory Measures | Central Inflammatory Measures | ||
|---|---|---|---|---|---|---|---|
| Yang 2012 | mouse breast (SI; sc) | Tail suspension | ⬆ depressive-like behavior (learned helplessness/behavioral despair) | ⬆ | hippocampal COX-2 and iNOS expression | ||
|
| |||||||
| Yang 2014 | mouse colorectal (SI; sc) | Tail suspension | ⬆ depressive-like behavior (learned helplessness/behavioral despair) | ⬆ blood IL-6 | ⬆ | hippocampal IL-6 and TNFa mRNA | |
| ⬇ | cell proliferation and neurogenesis markers and qualitatively shorter dendrites in dentate gyrus | ||||||
| ⬇ | hippocampal BDNF and COX-2 mRNA | ||||||
|
| |||||||
| Fang 2012 | mouse colorectal (SI; sc) | Tail suspension | ⬆ depressive-like behavior (learned helplessness/behavioral despair) | ⬆ blood IL-12 (initially) | ⬇ | binding ratio of 123I-ADAM in various SERT-rich in relevant brain regions | |
|
| |||||||
| Qi 2009 | mouse hepatoma (SI; ip) | Tail suspension | ⬆ depressive-like behavior (learned helplessness/behavioral despair) | ⬇ | whole brain SOD activity (reversible by fluoxetine) | ||
| ⬇ | hippocampal GMFβ and BDNF mRNA (reversible by fluoxetine) | ||||||
|
| |||||||
| Norden 2014 | mouse colorectal (SI; sc) | Tail suspension | ⬆ depressive-like behavior (learned helplessness/behavioral despair) | ⬆ blood IL-6 | ⬆ | cortical microglial activation (reversible by minocycline) | |
| Sucrose anhedonia | ⬆ depressive-like behavior (anhedonia) | ⬆ | hippocampal and cortical IL-1β and IL-6 (reversible by minocycline) | ||||
|
| |||||||
| DeWys 1974 | rat breast (SI; sc?) | Sucrose anhedonia | ⬆ depressive-like behavior (anhedonia) | ||||
|
| |||||||
| Lamkin 2010 | mouse ovarian (SI; ip) | Sucrose anhedonia | ⬆ depressive-like behavior (anhedonia) | ⬆ blood IL-6, TNFα, IL-10 | |||
|
| |||||||
| Xiu 2010 | rat breast (SI; sc) | Sucrose anhedonia | ⬆ depressive-like behavior (anhedonia) | ||||
|
| |||||||
| Smith 1994 | rat sarcoma (implant; sc) | Sucrose/saccharine anhedonia | ⬆ depressive-like behavior (anhedonia) | ||||
|
| |||||||
| Pyter 2009 | rat breast (carcinogen) | Porsolt forced swim | ⬆ depressive-like behavior (learned helplessness/behavioral despair) | ⬆ tumor IL-1β and GM-CSF mRNA | ⬆ | hippocampal IL-1β, IL-6, TNFα, and IL-10 | |
| Sucrose anhedonia | ⬆ depressive-like behavior (anhedonia) | ||||||
|
| |||||||
| Papiez 2009 | rat myeloid leukemia (SI; ip) | Porsolt forced swim | ⬆ depressive-like behavior (learned helplessness/behavioral despair) | ⬇ | cortical and hippocampal antioxident potential | ||
| ⬆ | cortical and hippocampal lipid peroxidation, mRNA response to oxidative stress challenge | ||||||
| ⬆ | cortical and hippocampal SOD activity (but initial decrease in hippocampus) | ||||||
| ⬆ | cortical 5-HT2a denstity and binding affinity of β-adrenergic receptors | ||||||
|
| |||||||
| Lebena 2014 | mouse melanoma (SI; iv) | tail suspension | ⬆ depressive-like behavior (learned helplessness/behavioral despair) | ⬆ blood IL-6 for large tumors | ⬆ | hippocampal IL-6 and TNFα mRNA | |
| ⬇ | striatal DOPAC, DA, 5-HT; prefrontal 5-HIAA, 5-HIAA/5- HT | ||||||
|
| |||||||
| Vegas 2004 | mouse melanoma (SI; iv) | Social defeat | ⬆ potentially depressive-like behavior (social avoidance) | ⬇ | hypothalamic 5-HT | ||
| ⬆ | hypothalamic 5-HIAA/5-HT ratio | ||||||
|
| |||||||
| Vegas 2004 | mouse melanoma (SI; iv) | Social defeat | ⬆ anxiety-like behavior (exploration) | ⬇ ⬆ |
hypothalamic 5-HT | ||
| ⬆ | hypothalamic 5-HIAA/5-HT ratio | ||||||
|
| |||||||
| Pyter 2009 | rat breast (carcinogen) | Marble burying | ⬆ anxiety-like behavior (obsessive- complusive) | ⬆ tumor IL-1β and GM-CSF mRNA | hippocampal IL-1β, IL-6, TNFα, and IL-10 | ||
|
| |||||||
| Qi 2009 | mouse hepatoma (SI; ip) | Total locomotor activity | ⬆ sickness behavior (lethargy) | ⬇ anxiety-like behavior (hyperactivity) | ⬇ | whole brain SOD activity (reversible by fluoxetine) | |
| ⬇ | hippocampal GMFβ and BDNF mRNA (reversible by fluoxetine) | ||||||
|
| |||||||
| Fang 2012 | mouse colorectal (SI; sc) | Total locomotor activity Total | ⬆ sickness behavior (lethargy) | ⬇ anxiety-like behavior (hyperactivity) | ⬆ blood IL-12 (initially) | ⬇ | binding ratio of 123I-ADAM in various SERT-rich in relevant brain regions |
|
| |||||||
| Xiu 2010 | rat breast (SI; sc) | locomotor activity | ⬆ sickness behavior (lethargy) | ⬇ anxiety-like behavior (hyperactivity) | |||
|
| |||||||
| Lamkin 2010 | mouse ovarian (SI; ip) | Total locomotor activity | ⬆ sickness behavior (lethargy) | ⬇ anxiety-like behavior (hyperactivity) | ⬆ blood IL-6, TNFα, IL-10 | ||
|
| |||||||
| Papiez 2009 | rat myeloid leukemia (SI; ip) | Total locomotor activity | ⬆ sickness behavior (lethargy) | ⬇ anxiety-like behavior (hyperactivity) | ⬇ | cortical and hippocampal antioxident potential | |
| ⬆ | cortical and hippocampal lipid peroxidation, mRNA response to oxidative stress challenge | ||||||
| ⬆ | cortical and hippocampal SOD activity (but initial decrease in hippocampus) | ||||||
| ⬆ | cortical 5-HT2a denstity and binding affinity of β-adrenergic receptors | ||||||
|
| |||||||
| Yang 2014 | mouse colorectal (SI; sc) | Novel object recognition | ⬇ cognition (temporal lobe-dependent learning and memory) | ⬆ | hippocampal IL-6 and TNFa mRNA | ||
| ⬇ | cell proliferation and neurogenesis markers and qualitatively shorter dendrites in dentate gyrus | ||||||
| ⬇ | hippocampal BDNF and COX-2 mRNA | ||||||
|
| |||||||
| Pyter 2010 | rat breast (carcinogen) | Radial arm maze | ⬇ cognition (hippocampal-dependent learning and memory - aversive) | ⬆ | hippocampal IL-1β mRNA | ||
| Novel object recognition | ⬇ cognition (temporal lobe-dependent learning and memory) | ||||||
|
| |||||||
| Bernstein 1985 | rat leydigcell and breast (both implant; sc) | Taste aversion (in advanced disease) | ⬇ cognition (classical conditioning with tumor as unconditioned stimulus) | ||||
|
| |||||||
| Treneer 1987 | rat leydig cell (implant; sc) and tumor resection | Taste aversion (reversal learning) | ⬇ cognition (classical conditioning with tumor as unconditioned stimulus) | ||||
|
| |||||||
| Bernstein 1980 | rat sarcoma (implant; sc) | Taste aversion | ⬆ cognition (classical conditioning with tumor as unconditioned stimulus) | ||||
|
| |||||||
| Bernstein 1983 | rat leydig cell (implant; sc) | Taste aversion | ⬆ cognition (classical conditioning with tumor as unconditioned stimulus) | ||||
N/C = No change
SI = syngeneic inoculation
sc = subcutaneous
iv = intravenous
ip = intraperitoneal
OVX = ovariectomized
In addition to being a measure of fatigue, total locomotor activity in an open field also assesses whether hyperactivity, a hypothesized indicator of anxiety-like behavior, is manifest in tumor-bearing models. In contrast, hypoactivity (i.e., lethargy) is noted in several tumor models (Table 1). An arguably more anxiety-specific measure gleaned from the same open field paradigm is central tendency, the conflict between rodents’ exploration of the bright, exposed center of the open field versus remaining in close proximity to the relatively protected, dark edges of the field (Crawley, 2006). Central tendency is not affected by the presence of mammary tumors in rats (Pyter et al., 2009). A similar light-dark conflict is presented using the elevated plus maze test for anxiety-like behavior, and the results in tumor-bearing rodents indicate that there is no apparent deficit in this behavior either (Pyter et al., 2009; Walf & Frye, 2010). In contrast, obsessive-compulsive anxiety-like behavior is significantly elevated in tumor-bearing rats (Pyter et al., 2009). Finally, one study indicates that behavioral responses of tumor-bearing mice to a social defeat paradigm, in which a highly aggressive conspecific interacts with the tumor-bearing subject, are characterized by increased avoidance of social interactions and decreased non-social exploration (Vegas et al., 2004). The authors argue that these behaviors may be consistent with avoidance behaviors, which are typical symptoms of depressed individuals.
Cognition is primarily assessed through tests of learning and memory in non-human animals. Four out of seven of the reports included in the present search focus on learned taste aversion in the context of cancer-associated anorexia and cachexia. By manipulating the type of chow that was administered throughout the tumor induction and growth paradigm, these studies inadvertently examined the effects of tumors on classical conditioning. Rats implanted with sarcoma, leydig cell, or mammary tumors successfully develop a learned taste aversion to novel diets (conditioned stimulus) eaten during tumor growth (unconditioned stimulus) (Bernstein & Sigmundi, 1980; Bernstein, 1985; Treneer & Bernstein, 1987); but see one experiment using mammary tumors (Bernstein & Fenner, 1983). Extinction of this taste aversion (i.e., the learned acquisition of a new relationship between the same two stimuli), at least 25 days after the tumor is removed, however, is impaired relative to both tumor-free controls on the same diet scheme and tumor-bearing controls that receive a novel (and therefore, preferred) diet post-tumor excision (Bernstein, 1985; Treneer & Bernstein, 1987). This suggests that: 1) although the pairing of the conditioned and unconditioned stimuli is longer in duration and less intense than other classical conditioning paradigms, the aversions developed are quite robust, and 2) the resistance to extinction in rats that previously had tumors may reflect an impairment in reversal learning. Finally, in advanced stages of both mammary and leydig cell tumor progression, the learned taste aversion spontaneously dissipates, indicating that the taste aversion memory may become impaired or disrupted over prolonged or advanced stages of tumor development (Bernstein, 1985).
The remaining studies on cognitive function in tumor-bearing models examined other modalities of learning and memory using a variety of standardized behavioral tests. For example, the ability to distinguish between novel and familiar objects is remarkably impaired in tumor-bearing rats and mice (Pyter et al., 2010; Yang et al., 2014). Using a fear-based classical conditioning paradigm, tumor-bearing rats and mice tend to display modest impairments in passive auditory conditioning (Pyter et al., 2010), but not in contextual conditioning (Pyter et al., 2010; Yang et al., 2012). In terms of hippocampal-based learning, rats with tumors make more long-term, reference memory errors in an appetitive radial arm maze task, while working memory remains similar to tumor-free controls. Long-term memory in an aversive water maze test is unchanged by the presence of a tumor (Pyter et al., 2010).
2.3 Neuroinflammatory Correlates of Sickness, Affective-Like, and Cognitive Impairment Behaviors in Tumor-Bearing Rodent Models
The evidence for the direct involvement of inflammatory cytokines (IL-1, IL-6, TNFα, INF-γ) in the induction of the somatic behaviors of anorexia/cachexia is quite prevalent (for reviews see Dianliang, 2009; Matthys & Billiau, 1997; Plata-Salamán, 2000; Tisdale, 2002). Given the high likelihood of mechanistic overlap, much may be gained by assimilating the previously discreet older literature on metabolism and the more recent affective/cognitive research in cancer models.
From the metabolic work, it is evident that many cancer cachexia models display elevated proinflammatory cytokine and cytokine receptor proteins and transcripts in the whole brain (Catalano et al., 2003), within brain regions known to regulate affective-like and cognitive behaviors (e.g., hippocampus, hypothalamus, cortex, brainstem, cerebellum; Chance et al., 2003; Plata-Salamán, 2000; Turrin et al., 2004; Wang et al., 2001), and even in CSF (Opara et al., 2005) relative to pair-fed tumor-free controls or simply tumor-free controls. The null-finding exceptions are rare (Carson et al., 1998; Wang et al., 2001). Mounting evidence indicates that this central cytokine production is tumor-initiated rather than host-derived (Baltgalvis et al., 2010; Cahlin et al., 2000; Kawamura et al., 1999; Rebeca et al., 2008). Notably, evidence of elevations in circulating cytokines need not be present for tumor-induced inflammation to be transduced into brain cytokine production (Quan, 2014). In cancer models of depressive-like behavior and select cognitive impairments, elevations in brain proinflammatory cytokine production are observed primarily in the cortex and hippocampus (Norden et al., 2014; Pyter et al., 2010; Pyter et al., 2014; Pyter et al., 2009; Yang et al., 2014).
Local brain cytokine production, in turn, can influence a number of neural pathways by which tumors may induce behavioral changes (Plata-Salamán, 2000; Tisdale, 2002). Those known to overlap with the hypothesized mechanisms underlying cytokine-induced affective, somatic and cognitive behavior involve: iNOS and COX enzymatic activities, neuronal death, and tryptophan metabolism. Briefly, the negative behavioral consequences of tumors are associated with elevations in iNOS expression among various hypothalamic regions (Wang et al., 2001; Yang et al., 2012) and elevations in cerebral blood vessel expression of COX-1 (Ruud et al., 2013; not COX-2 (Wang et al., 2001). The results from measuring constitutive hippocampal COX-2 expression in tumor-bearing models with affective-like behavior or cognitive impairment are mixed (Yang et al., 2012, 2014).. Evidence of microglial activation has also been detected in the hippocampus and cortex of tumor-bearing rodents (Norden et al., 2014; Pyter et al., 2014; but see Yang et al., 2012). Many of these elevations in neuroinflammatory markers are attenuated by the systemic administration of the anti-inflammatory agent, minocycline (Norden et al., 2014). Furthermore, adding a peripheral inflammatory challenge (i.p. LPS injection) to a rat model of mammary cancer exacerbates the neuroinflammatory signaling responses of IL-1β, IDO, and markers of NF-κB and microglial cell activation among various brain regions that regulate affective-like and cognitive behavior (Pyter et al., 2014). These results suggest that both baseline neuroinflammation and neuroinflammatory responses are altered by the presence of a peripheral tumor.
Peripheral tumors associated with poor novel object recognition also reduce cell proliferation, neurogenesis, and dendritic length (qualitatively) in the dentate gyrus of the hippocampus (Yang et al., 2014, but see Yang et al., 2012). Growth factors associated with neurogenesis, GMFβ and BDNF, are reduced or remain unaltered in the hippocampus in tumor-bearing rodents that display cognitive impairments or depressive-like behavior (Qi et al., 2009; Pyter et al 2010; Yang et al 2014) Neural redox homeostasis may underlie these changes in brain plasticity as reduced oxidase potential is detected in brains of tumor-bearing mice with depressive-like behavior (Chen et al., 2006; Papiez et al., 2009; Qi et al., 2009), which is reversible with antidepressant treatment (Qi et al., 2009).
Weight loss in a tumor-bearing model is also associated with cerebellar atrophy and reduced numbers and size of Purkinje neurons (Michalak et al., 2006), suggesting that neuronal apoptosis in the cerebellum may be a consequence of peripheral tumor growth. Other pathways downstream of brain cytokine production that are observed to be modulated in these models of cancer cachexia, but may be less relevant to affect/cognition, include changes in neuronal K+ channel expression (Coma et al., 2003; Vicente et al., 2004) and in metabolic neurohormone synthesis (Chance et al., 2003).
Lastly, altered amino acid production and turnover in the brain, the particularly relevant tryptophan catabolism (reviewed in Laviano et al., 1996), is reported in rodent cancer models. Brain tryptophan concentrations are consistently higher in cachexic tumor-bearing rodents relative to pair-fed, tumor-free controls (Chance et al., 1988; Chance et al., 1983; Krause et al., 1979), even days prior to gross tumor palpability (Muscaritoli et al., 1996). The tryptophan metabolite and target of many antidepressant treatments, serotonin (5-HT), is also increased in brain tissue of tumor-bearing rodents (Chance et al., 1988, 1983; Muscaritoli et al., 1996), but see (Krause et al., 1979). Slightly different from the cachexia literature, however, whole brain 5-HT concentrations and SERT binding are decreased, whereas indicators of 5-HT turnover (5-HIAA, 5-HT/5-HIAA ratio;(Vegas et al., 2004) remain unchanged in tumor-bearing mice with depressive-like behavior. This may be counterintuitive given that depressive-like behavior is associated with reduced brain 5-HT concentrations (Muller & Schwarz 2007). However, 1) 5-HT concentrations do not reflect 5-HT release and, 2) turnover (or catabolism) of 5-HT may be simultaneously elevated as demonstrated by the concurrent elevations in brain 5-HT metabolites (e.g., 5-HIAA) and 5-HIAA:5-HT ratios (Chance et al., 1988, 1983; Chuluyan et al., 2000; Krause et al., 1979; Muscaritoli et al., 1996; Uomoto et al., 1998). Tumor resection resolves many of these alterations in brain amine concentrations (Chance et al., 1988). Future determination of the activity of the kynurenine pathway downstream from tryptophan in tumor-bearing models is necessary to elucidate the details of these potential neurotransmitter mechanisms.
Arguably most consistent with data attainable in humans, a couple of studies have linked elevated circulating cytokines and sickness behavior in these rodent models. For example, significant negative correlations were found between blood IL-6 concentrations and voluntary wheel running, cachexia (Wood et al., 2006), and locomotion (Lamkin et al., 2011). Circulating tryptophan or relevant tryptophan ratios (to 5-HT metabolites) are also altered in cancer anorexia models (Chance et al., 1988, 1983; Krause et al., 1979; Meguid et al. 1998; Muscaritoli et al., 1996) and are significantly correlated with the onset of a palpable tumor (Källberg et al., 2010) and the reduction in food intake (Muscaritoli et al., 1996).
3. Cancer Patients Prior To Treatment
A separate line of inquiry concerns cancer patients prior to treatment (i.e., prior to surgical resection of tumor, chemotherapy, radiation). Pre-treatment data provides important insights into both biological consequences of tumor development and attendant psychological and behavioral symptoms. For this review, we focus primarily on English language original reports indexed in Pubmed that considered inflammatory factors and/or psychological and behavioral symptoms in cancer patients prior to treatment. Search terms included: “depression,” or “anxiety,” or “cognition,” or “neuropsychological test,” or “cancer,” or “tumor,” indexed from “no determined start date” to January, 2015. Studies that only examined these factors in patients during or following treatment were not included. For psychological and behavioral factors, we focused primarily on research that consider symptoms prior to diagnosis, as the diagnosis itself represents a major psychosocial stressor capable of inducing both depressive and immune consequences. For studies of cognitive impairment in cancer patients prior to treatment we focused on studies that assessed multiple cognitive domains and, due to the small number of available studies, included studies in which patients were post-surgery but pre-chemotherapy/radiation in addition to studies of patients prior to all treatment.
3.1 Inflammation in Cancer Patients Prior to Treatment
Similar to in rodent cancer models, tumor-derived inflammation in humans originates from tumor cells, the surrounding stromal cells (Gogusev et al., 1993; Kinoshita et al., 1999; Offner et al., 1995; Pusztai et al., 1994; Watson et al., 1990) or tumor-associated macrophages (TAMS), and infiltrating T-cells (Allavena et al., 2008). Circulating pro-inflammatory cytokines are elevated in various cancer populations prior to any treatment including ovarian cancer (Lutgendorf et al., 2008), colorectal cancer (Belluco et al., 2000), lung cancer (Wojciechowska-Lacka, et al., 1996), breast cancer (Benoy et al., 2002), pancreatic cancer (Okada et al., 1998), gastric cancer (Kim et al., 2003), prostate cancer (Michalaki et al., 2004), head and neck cancer (Riedel et al., 2005), renal cell carcinoma (Yoshida et al., 2002), and bladder cancer (Mahmoud El-Salahy, 2002). High concentrations of circulating inflammatory cytokines such as IL-6 and TNF-α are associated with later stage disease (Belluco et al., 2000; Kim et al., 2003; Kozlowski et al., 2003; Michalaki et al., 2004; Okada et al., 1998; Yoshida et al., 2002), poorly differentiated tumors (Goswami et al., 2013; Kaminska et al., 2005; Ljungberg et al., 1997; Mahmoud El-Salahy, 2002; Yoshida et al., 2002) and tumor size/volume (Chung & Chang, 2003; Kaminska et al., 2005; Plante et al., 1994) in a variety of malignancies. Elevations of circulating pro-inflammatory cytokines have also been associated with less common indices of disease severity including extent of tumor necrosis in colorectal cancer (Guthrie et al., 2013; Richards et al., 2012). Spontaneous release of pro-inflammatory cytokines in cultures of primary tumor further suggests that circulating levels of inflammatory molecules are at least partially tumor-derived in some malignancies (Burger et al., 1995; Toutirais et al., 2003; Wigmore et al., 2002). Inflammatory cytokines are also elevated in ascites fluid proximal to the tumor in ovarian cancer patients (Moradi et al., 1993) and malignant pleural effusions in lung cancer (Yamaguchi, 2000). In ovarian cancer, levels of IL-6 from ascites are strongly associated with IL-6 in plasma;(Costanzo et al., 2005), similar to that of malignant pleural effusions from lung cancer patients (Yamaguchi, 2000). Other systemic effects of tumor-associated inflammation have been noted, especially alteration of the hypothalamic-pituitary-adrenal (HPA) axis. Altered cortisol patterns have been identified in ovarian cancer patients (Weinrib et al., 2010), endometrial cancer patients (Sannes et al., 2013), and oral cancer patients (Bernabé et al., 2012) prior to treatment. In ovarian cancer patients, these patterns are significantly associated with IL-6 concentrations in the ascites fluid (Schrepf et al., 2015) and in plasma (Lutgendorf et al., 2008).
In addition to pro-inflammatory cytokines, cancer patients prior to treatment show altered patterns of tryptophan metabolism. IDO activity is associated with tumor induction and is often highly expressed in solid tumors (Munn & Mellor, 2013). Higher kynurenine/tryptophan ratios and lower tryptophan concentrations in blood compared to healthy controls have been reported in colorectal cancer, malignant melanoma, non-small cell and small cell lung cancer, ovarian cancer, endometrial cancer, vulvar cancer, and breast cancer patients (de Jong et al., 2011; Engin et al., 2010; Huang et al., 2002; Lyon et al., 2011; Sperner-Unterweger et al., 2011; Suzuki et al., 2010; Weinlich et al., 2007), although the opposite pattern was noted in a study of primary cervical cancer patients (Fotopoulou et al., 2011). Thus, it appears that increased IDO activity in peripheral blood is associated with many malignancies prior to treatment.
3.2 Affective/Somatic Symptoms of Depression and Cognitive Impairment in Cancer Patients Prior to Treatment
Whether a clinical diagnosis of depression is associated with subsequent increased incidence of cancer in the following years remains a controversial subject (Oerlemans et al., 2007). However, for some malignancies, changes in affect and somatic symptoms are noted in the months prior to and at the time of a diagnosis of cancer, consistent with the time frame of tumor development. This review will focus on these symptoms.
In pancreatic cancer, there is strong evidence that affective symptoms of depression may manifest prior to diagnosis (Green & Austin, 1993; Joffe et al., 1986; Sebti et al., 2014). One study reported that clinically relevant depression occurs in 50% of patients in the year prior to diagnosis (Joffe et al., 1986). Among commonly reported symptoms in pancreatic cancer patients prior to diagnosis, affective symptoms like crying spells and feelings of hopelessness were common in addition to somatic symptoms like loss of appetite, fatigue, anorexia, and insomnia (Green & Austin, 1993).
Symptoms that precede a diagnosis of ovarian cancer overlap considerably with somatic symptoms of depression. Case control studies using retrospective recall of symptoms report that fatigue (O.R. 3.9, 95% CI 2.5–6.1), weight loss or weight gain (OR 14.2, 95% CI 8.2–24.5), and loss of appetite (OR 8.8, 95% CI 4.3–18.2) are much more commonly reported by ovarian cancer patients prior to diagnosis than controls visiting the same clinics (Olson, 2001; Vine et al., 2003). Fatigue and weight loss/gain are also more common amongst those with advanced (stages III–IV) versus early (stages I–II) of ovarian cancer. The onset of these symptoms was most often reported as 2–7 months prior to diagnosis, suggesting that they are not part of pre-existing condition, but rather are consistent with the timeframe of tumor development. Another study examining medical records (and hence free of recall bias) found that loss of appetite, fatigue and anxiety are all significantly more common in ovarian cancer patients compared to controls in the 6 months preceding diagnosis (Friedman et al., 2005).
In a retrospective study of symptoms prior to lung cancer diagnosis, most symptoms were reported as occurring in the 12 months prior to diagnosis, and included 68% of patients reporting fatigue, 64% change in appetite, 59% changes in sleep, and 50% weight loss (Corner et al., 2005). These results were confirmed by a case-control analysis of symptoms recorded in primary care settings in the two years prior to a lung cancer diagnosis that found fatigue, loss of appetite, and loss of weight are more commonly reported by patients than controls (fatigue OR 2.3, 95% CI 1.9–2.9; loss of appetite OR 4.8, 95% CI 3.3–7.0; loss of weight OR 6.2, 95% CI 4.5–8.6; Hamilton et al., 2005). Importantly, when the final 180 days prior to diagnosis were excluded from the analyses fatigue, loss of appetite, and loss of weight no longer distinguish cases from controls. This suggests that these symptoms manifest relatively close to the time of diagnosis.
In women awaiting surgery for suspected ovarian cancer, symptoms of somatic and affective depression are more prevalent in those subsequently diagnosed with ovarian cancer than in those with tumors of low malignant potential, despite facing presumably similar levels of distress and no differences in levels of interpersonal difficulties (Lutgendorf, 2008). Nonetheless, it is possible that symptoms of invasive disease (e.g. bowel distension) may have contributed to suspicion of ovarian cancer and consequent symptoms of depression, though this possibility warrant further investigation. One study of women presenting at an outpatient clinic subsequently diagnosed with breast cancer found that 28% met criteria for state anxiety and depression prior to diagnosis though the prevalence of these affective disturbances were not reported for women who were subsequently found to be free of cancer (Van Esch et al., 2012). In contrast, another report using assessment of affective symptoms in women prior to diagnosis either with breast cancer or benign disease found that neither anxiety nor depression were higher in cancer patients than women with benign disease in the two years prior (Eskelinen & Ollonen, 2011). Similarly, another study that examined physical and mental well-being in the year before a prostate cancer diagnosis found no differences between men that were subsequently diagnosed with prostate cancer and matched healthy controls (Reeve et al., 2012). Therefore, increased symptoms of depression, and specifically affective symptoms, are not universal prodromal features of cancer.
That somatic symptoms may be more pronounced in cancer patients prior to treatment is unsurprising. In a large-scale mixed cancer population (n=213) post-diagnosis but pre-treatment somatic symptoms are responsible for most of the variance in Beck’s Depression Inventory scores when compared to healthy controls (Wedding et al., 2007). More specifically, the effect sizes (Cohen’s D) for the difference in affective symptoms averaged 0.12 between cancer patients and controls versus 0.34 for somatic items. Interestingly, some affective items are considerably less pronounced in cancer patients (i.e. sense of failure, d=−2.35, guilt, d=−1.00), while every somatic item except loss of libido is more prevalent in cancer patients. Comparing patients with solid tumors to those with haematological malignancies reveals no differences in affective items or total depression scores, but significantly higher somatic scores in patients with solid tumors (Wedding et al., 2007).
A relatively small number of published studies have examined neuropsychological test performance in cancer patients prior to treatment. Eleven total studies were identified: 5 in breast cancer samples (Ahles et al., 2008; Hermelink et al., 2007; Hurria et al., 2006; Mandelblatt et al, 2014; Wefel et al., 2004), and 1 each in samples of prostate cancer (Green et al., 2004), ovarian cancer (Mayerhofer et al., 2000), testicular cancer (Wefel et al., 2011), head and neck cancer (Bond et al., 2012), lung cancer (Meyers et al., 1995), and leukemia (Meyers et al., 2005). Of these, five were in samples prior to the receipt of any treatment and five were samples in which some patients had completed surgery but not chemotherapy or radiation. Sample sizes ranged between 18 and 110 participants.
Reviewing the areas of cognitive impairment tested across studies resulted in enough crossover in several domains by percent of sample impaired to warrant comparison across studies: global impairment (6 studies), verbal learning -- both total recall (5 studies) and delayed recall (4 studies), manual dexterity (4 studies), executive function (5 studies), visual scanning (5 studies), verbal fluency (5 studies), processing speed (4 studies) and auditory attention (3 studies) (Table 2). Some studies that did not report percentages of impaired patients are discussed but could not contribute to mean and median calculations. Mean and median percentages of cognitive impairment in cancer patients across studies were as follows: global impairment, 32% (median: 33%); total verbal learning 31.7% (median 37.7%); delayed recall 39.3% (median 33%); executive functioning 23.3% (median 22%); manual dexterity 24.5% (median 24.5%); visual scanning 18.9% (median 16%); verbal fluency 10.6 % (median 10%); processing speed 7.8% (median 6.2%); auditory attention 5.4% (median 5%). Several studies also compared cancer patients prior to treatment to controls or normative means using group means rather than percentage of sample impaired, and these generally revealed fewer differences. These results reveal a broadly consistent pattern of impairment. Approximately one-third of patients demonstrated impairment in total recall and delayed recall on verbal learning tasks, and one-quarter demonstrated impairment on manual dexterity and executive functioning tasks. Approximately 20% demonstrated impairment on visual scanning tasks, while approximately 10% or fewer were impaired on verbal fluency, processing speed, and auditory attention tasks. The percentages of impaired patient in the verbal learning, executive function, manual dexterity, and visual scanning domains were considerably higher than what would be expected were the samples drawn from a healthy population, while verbal fluency, processing speed and auditory attention did not appear to differ substantially from normative expectations. Verbal learning (either total recall or delayed recall) was the most impaired domain in every study in which it was assessed, while processing speed and auditory attention were the least impaired domains in all but one study.
Table 2.
Study characteristics and results of neuropsychological tests in cancer patients prior to chemotherapy/radiation.
| First Author, Year | Ahles 2008 | Bond 2012 | Green 2004 | Hermelink 2007 | Hurria 2006 |
|---|---|---|---|---|---|
| Sample | Breast cancer | Head and Neck cancer | Prostate cancer | Breast cancer | Breast cancer |
| Time | Post-surgery/Pre-chemotherapy/radiation | Pre-Treatment | Pre-Treatment | Pre-treatment | Post-surgery/Pre- chemotherapy/radiation |
| Inclusion criteria | Stage 1–3A, 18–70 years | 21 or older | Non-localized prostate cancer | Non-metastatic breast cancer | Newly diagnosed Stage 1–3 breast cancer, age 65 or older |
| Exclusion criteria | CNS disease, previous cancer/cancer treatment, neurologic disorder, substance abuse, brain injury, psychiatric disorder | Brain metastases, intracranial radiation, previous cancer | Previous hormonal therapy, severe LUTS, abnormal serum testosterone | None listed | Metastatic disease, psychiatric disorder, previous cancer treatment |
| n | 110 | 70 | 77 | 109 | 31 |
| Definition of Global Impairment | 2 domains below 1.5 S.D. of healthy controls, or 1 domain 2.0 S.D. below* | Average Global Deficit Score ≥ 0.5 | N/A | ≤ 5% normative mean on 2 or more domains | 2 domains below 2.0 S.D. below normative means |
| Global Impairment | 22% | 47 % | N/A | 31% | 11% |
| Definition of Impairment on individual tests | Below 1.5 S.D. of healthy controls* | T-score <40 By normative means | Comparison to healthy control | Mean comparison to HC | N/A |
| Verbal Learning | n/a | 25% | < Healthy controls | Not different from norms | n/a |
| Test | n/a | RAVLT total | N/A | n/a | |
| Delayed Recall | n/a | 21% | N/A | N/A | n/a |
| Test | n/a | RAVLR DR | N/A | N/A | n/a |
| Auditory Attention | n/a | N/A | N/A | Not different from norms | n/a |
| Test | n/a | N/A | N/A | Digit Span | n/a |
| Processing Speed | n/a | 19% | <Healthy controls | >Norms | n/a |
| Test | n/a | SDMT | N/A | N/A | n/a |
| Executive | n/a | 22% | Marginal < Healthy Controls | <Norms | n/a |
| Test | n/a | TMT-B | Stoop Inhibitory | TMT-B | n/a |
| Verbal fluency | n/a | 17% | N/A | <Norms | n/a |
| Test | n/a | AVF | N/A | RWT | n/a |
| Visual Scanning | n/a | 16 % | N/A | Not Different from Norms | n/a |
| Test | n/a | TMT-a | N/A | TMT-A | n/a |
| Dexterity | n/a | N/A | N/A | N/A | n/a |
| Test | n/a | N/A | N/A | N/A | n/a |
| First Author, Year | Mayerhofer 2000 | Meyers 2005 | Meyers 1995 | Wefel 2011 | Wefel 2004 |
|---|---|---|---|---|---|
| Sample | Epithelial ovarian cancer | Myelogenous Leukemia/Myelodysplastic Syndrome (AML/MDS) | Small cell lung cancer | nonseminomatous germ cell tumors (NSGCT)of the testis | Breast cancer |
| Time | Pre-treatment | Pre-treatment | Pre-treatment | Post-surgery/pre-chemotherapy | 50% pre-surgery, all pre- chemotherapy/radiation |
| Inclusion criteria | Newly diagnosed EOC, normal blood panel | Newly diagnosed AML/MDS | Newly diagnosed limited sclc, ≥ 8th grade education | Newly diagnosed NSGCT, age 18–50 | Breast carcinoma, 18 + years, ≥ 8 years of education |
| Exclusion criteria | Neurologic disorder, psychiatric disorder, brain metastases, substance abuse, cognition altering medication | N/A | psychiatric disorder, substance abuse | Neurologic illness, brain injury, psychiatric disorder, brain metastasis | evidence of metastases, psychiatric disorder, previous cancer, cognition altering medication |
| n | 28 | 54 | 21 | 69 | 18–84** |
| Definition of Global Impairment | n/a | n/a | n/a | 2 domains below 1.5 S.D. of normative means, or 1 domain 2.0 S.D. below | 2 domains below 1.5 S.D. of normative means, or 1 domain 2.0 S.D. below |
| Global Impairment | n/a | n/a | n/a | 46 % | 35% |
| Definition of Impairment on individual tests | Median values compared to normative means | 2 S.D. below normative mean | 1.5 S.D. below normative mean | 1.5 S.D. below normative mean | 1.5 S.D. below normative means |
| Verbal Learning | n/a | 44% | 38% | 37.7% | 14% |
| Test | n/a | HVLT | VSRT | HVLT | HVLT, VSRT |
| Delayed Recall | n/a | 41% | 70% | N/A | 25 |
| Test | n/a | HVLT-DR | VSRT-DR | N/A | VSRT-DR |
| Auditory Attention | n/a | 7% | 5% | 4.3% | N/A |
| Test | n/a | Digit Span | Digit Span | Digit Span | N/A |
| Processing Speed | Not different than norms | 8% | 0% | 4.3% | N/A |
| Test | Alphabet cross-out test | Digit Symbol | Digit Symbol | Digit Symbol | N/A |
| Executive | n/a | 29 % | 38 % | 21.7% | 6% |
| Test | n/a | TMT-B | TMT-B | TMT-B | TMT-B |
| Verbal fluency | n/a | 17 % | 10 % | 2.9% | 6% |
| Test | n/a | COWA | COWA | COWA | COWA |
| Visual Scanning | n/a | 28 % | 33 % | 4.3% | 13% |
| Test | n/a | TMT-A | TMT-A | TMT-A | TMT-A |
| Dexterity | < than norms | 37 % | 31.5 % | 17.4% | 12% |
| Test | Fine motoric test | Pegboard | Pegboard | Pegboard | Pegboard |
Estimated by Monte Carlo simulation
employed a mixed sample ranging in size from 18–84 participants
Methodological differences appeared to play a large role in the differential findings. For instance, studies which examined sample means in comparison to healthy controls or normative means appeared to find fewer differences than studies which determined if individual participants met criteria for impairment. For instance, one 2007 study of breast cancer patients found no significant difference in verbal learning using group means compared to normative means (Hermelink et al., 2007) while a 2004 study of breast cancer patients using individual scores compared to normative means found that the number of patients impaired on verbal learning tasks was twice what would be expected in a normal, healthy population (Wefel et al., 2004). As it would appear that many of the sample means studied would fall within a normal performance range according to normative means, group means likely mask individuals with impaired performance. This further suggests that cognitive impairment affects a particular, vulnerable subsample of cancer patients prior to treatment.
Studies that consider moderating factors reveal inconsistent results. For instance, 3 of 4 studies that considered fatigue as a moderating factor did not find it to be associated with any test results, while the fourth found it to be associated only with processing speed and global impairment. Similarly, depression was not related to neuropsychological test results in three of five studies, but was associated with processing speed in two studies and both verbal learning and global impairment in another. In one study of breast cancer, medical comorbidities were the only factor associated with worse neurocognitive performance, such that having 2 or more comorbidities was associated with a nearly 9 fold increase in the risk for cognitive impairment (Mandelblatt et al., 2014).
3.3 Inflammatory Correlates of Somatic/ Affective Symptoms of Depression and Cognitive Impairment
Elevations in peripheral markers of inflammation are linked to the elevations in somatic and affective symptoms in cancer patients prior to treatment in a variety of malignancies. In ovarian cancer patients with invasive disease, elevated IL-6 in plasma and ascites is associated with greater somatic, but not affective, symptoms of depression prior to diagnosis (Lutgendorf et al., 2008). In breast cancer patients prior to the receipt of treatment, a diagnosis of depression by structured interview (Structured Clinical Interview for DSM-IV, SCID) is associated with both elevated plasma IL-6 and abnormal dexamethasone suppression tests compared to non-depressed patients (Soygur et al., 2007). Similarly, levels of soluble IL-6 receptor (sIL-6r) and CRP are positively associated with fatigue and affective symptoms of depression in breast cancer patients prior to treatment (Courtier et al., 2013; Pertl et al., 2013). In patients with peritoneal carcinomas, serum levels of IL-6 and CRP are associated with greater fatigue and somatic symptoms of depression, but not affective symptoms (Low et al., 2014). In prostate cancer patients with non-metastatic disease elevated TNF-α levels in serum are associated with fatigue, depression and anxiety (HADS) (Dirksen et al., 2013). In malignant melanoma patients, sIL6-r levels in serum are negatively associated with self-reported combined affective and somatic symptoms (Self-Reported Depression Scale; SDS) and sTNF-r levels positively associated (Friebe et al., 2007) In metastatic colorectal cancer patients serum IL-6 levels are positively associated with loss of appetite and nausea/vomiting, TGF-β levels are positively associated with loss of appetite and fatigue (Rich et al., 2005). In advanced lung cancer patients higher Glasgow Prognostic Scores (a composite measure of abnormal albumin and CRP levels in blood) are correlated with both depression and anxiety levels (HADS) (Giannousi et al., 2012) and serum CRP levels with fatigue (Brown et al., 2005). However, in a study of colorectal cancer patients, levels of TNF-α and CRP in plasma are not associated with baseline depressive symptoms (Archer et al., 2012). Similarly, in a study of depressed versus non-depressed hepatobilary carcinoma patients, levels of TNF-α and IFN-γ in plasma did not differ between groups (Steel et al., 2007).
IDO activity has also been linked to somatic and depressive symptoms in cancer patients. Reduced serum tryptophan is associated with more severe somatic symptoms (RSC) but not anxiety or depression scales in a sample of colorectal cancer patients (Huang et al., 2002). In pancreatic cancer patients, a lower kynurenic acid:tryptophan ratio in serum is associated with worse symptoms of anxiety and depression (Botwinick et al., 2014). Regarding cognitive function, only one study has examined inflammatory correlates of impairment in cancer patients prior to treatment and found that levels of IL-6 were found to be associated with poorer executive functioning and levels of IL-8 were inversely associated with measures of memory (Meyers, et al., 2005).
4. Conclusions and Limitations
Viewed in tandem, the rodent and human literatures suggest that similar inflammatory mechanisms and behaviors, which were initially established with respect to behavioral changes following acute immune challenges (i.e. sickness behavior, cognitive impairment), are also features of chronic disease (i.e., cancer). Differences between cancer-associated behavioral changes and acute sickness behavior are likely due to differing time scales, the magnitude of inflammation, and subtle differences in underlying mechanisms. One possibility is that tumor growth, in early stages, represents a slow-priming effect on the immune system rather than full activation of conserved sickness responses. In fact, mounting a full sickness response over the span of tumor growth would not be viable given the high energetic cost of such behaviors (fever, anorexia). The sensitized neuroinflammatory responses to LPS administration in tumor-bearing rats (Pyter et al., 2014) and microglial activation (Norden et al., 2014; Pyter et al., 2014) support this priming hypothesis.
Significant gaps in knowledge remain. Sleep dysregulation is clearly prevalent in human cancer patients prior to treatment, but little information is known about sleep and associated mechanisms in tumor-bearing rodents. While models of tumor-bearing rodents suggest that some anxiety-like behaviors may be enhanced, little is known about symptoms of anxiety and inflammation in human cancer patients prior to diagnosis. Methodological disparities have thus far limited inference from behavioral results in tumor-bearing rodents. For instance, models which clearly result in reduced locomotion are not ideal for behavioral tests that required considerable movement such as the tail-suspension and Porsolt forced swim test. Similarly, models which result in wasting and anorexia are not ideal for behavioral tests predicated on food/consumption rewards. Additionally, inflammatory correlates of cognitive impairment have not been established in cancer patients prior to treatment. In other clinical populations, elevated peripheral biomarkers of inflammation (CRP, IL-6) have been associated with neurocognitive decline such as delayed verbal learning tasks (Bettcher et al., 2012; Grassi-Oliveira et al., 2011; Hudetz et al., 2011) and impaired executive functioning (Hoshi et al., 2010; Mooijaart et al., 2013). Increased markers of indoleamine-2,3-dioxygenase activity in peripheral blood have been linked to poorer global cognitive performance in stroke survivors and cardiac patients following surgery (Forrest et al., 2011; Gold et al., 2011). Some of these effects may be hippocampus-dependent, as damage limited to the hippocampus has previously been associated with impaired recognition memory and verbal learning in patients suffering hypoxia-induced brain damage (Hopkins et al., 1995) and hippocampal impairments generally produce worse performance on NOR tests in rodent models, though this relationship continues to be explored (Antunes & Biala, 2012).
This work has several implications for future research. The appearance of somatic symptoms in the months prior to a cancer diagnosis again raises the possibility of early detection for some malignancies. Against this proposition is the fact that most of these symptoms appear to be non-specific and experienced by a wide swath of the population. However, this conclusion is based on the limited survey of symptoms that are discovered in clinic visits. It is unlikely symptoms of anxiety and depression are widely monitored. It is possible, therefore, that somatic, depressive, or cognitive symptoms may have utility in combination with physiologic measures to contribute algorithmically to characterizing high-risk patients.
Measures of depression in cancer patients, even those that attempt to exclude somatic symptoms, likely capture considerable influence of tumor-derived processes in some malignancies. This is an issue that has posed a considerable challenge to researchers interested in affective disturbances in cancer patients. While depressive symptoms are sometimes modeled as a unitary construct, in cancer populations it appears that there are differential associations between somatic and affective symptoms of depression and measures of inflammation (Lutgendorf et al., 2008). Many theories of depression and sickness behavior emphasize putative shared inflammatory mechanisms, however, there is also evidence that affective and somatic symptoms do not always occur in tandem in acute and chronic illness, and that specific presentations (e.g., melancholic vs. vegetative depression) may be undergirded by independent, albeit associated, mechanisms (Maes et al., 2012). We would advocate, therefore, the use of measures or structured interviews that allow both somatic and depressive symptoms to be measured, rather than measures which aggregate both or exclude somatic symptoms. In cancer populations both affective and somatic symptoms have important and sometimes divergent associations with quality of life outcomes throughout the trajectory of treatment (Reuter et al., 2004; Teng et al., 2014).
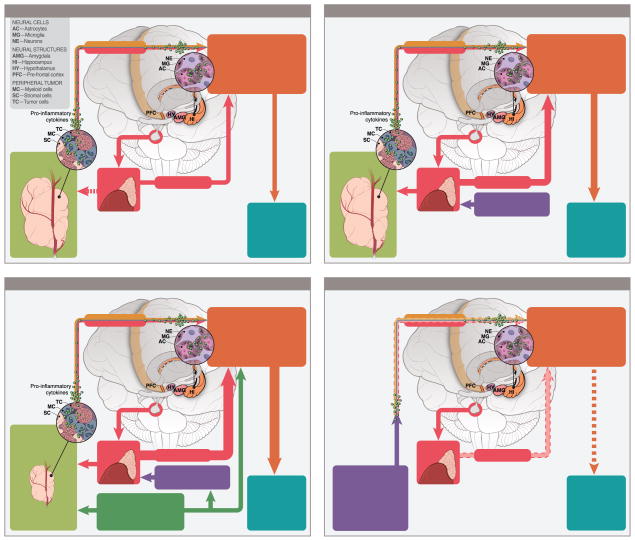
The understanding that cancer may fundamentally alter behavioral processes prior to any treatment should challenge existing paradigms and lead to changes in rodent models of cancer treatment and protocols for observational studies of cancer patients. The physiology and behavior of pre-diagnosis cancer patients may already be compromised before the advent of other challenges associated with cancer and cancer treatment. Figure 1 represents a timeline of various factors involved in the cancer experience that may influence the associated behavioral outcomes. Notably, tumors, cancer-associated stressors, and cancer treatments can independently influence relevant neuroinflammatory pathways over chronological stages of cancer, as well, as their likely synergistic/additive biological interactions. Finally, persistent negative behavioral symptoms in cancer survivors suggest that these neuroimmunological pathways are likely altered over the long term (Figure 4D). Many rodent models of cancer treatment-related symptoms (CTRS) do not employ tumor-bearing animals. Given that there is now a substantial body of work suggesting that many of the behaviors of interest and associated physiological and CNS processes of interest to CTRS researchers are altered by the presence of tumors, this modeling approach is no longer adequate. Further, the immunologic changes secondary to cancer and the immunologic changes secondary to treatment are not necessarily additive, but may be interactive. In fact, these interactions have begun to be explored in the context of fatigue and cognitive complaints in breast cancer patients (Bower, 2014; Bower et al, 2013: Bower, 2007; Ganz, 2013). Cancer represents a state of immunologic compromise; this is demonstrated potently by the differential response to subsequent acute immune challenges (i.e., LPS) seen in tumor-bearing rodents, and by the fact that many alterations of the immune system (e.g., enhanced production of pro-inflammatory cytokines) associated with cancer-treatment symptoms are the same as those that are clearly altered by tumor-related processes. That tumor bearing rats have an enhanced neuroinflammatory signaling cascade response to LPS challenge may be an important finding for cancer treatment symptom research, as Toll-Like receptor 4, which is responsive to LPS, also responds to damage-associated molecular patterns (DAMPs) that are thought to be more prevalent following some cancer therapies (Krysko et al., 2013). It is worth considering that predisposing genetic factors may increase the risk of both cancer and worse symptoms associated with the disease and treatment. Studies of genetic polymorphisms and symptom profiles in cancer patients pre-treatment may help to identify vulnerable patients. In fact, a recent investigation of breast cancer patients prior to treatment revealed that variations in cytokine genes are associated with subsyndromal depressive symptoms through the treatment trajectory (Saad et al., 2014).
Figure 1.
Proposed causes underlying cancer-associated behavioral alterations over the cancer process: (A) pre-cancer diagnosis, (B) post-cancer diagnosis, pre-treatment, (C) during cancer treatment, and (D) post-treatment, cancer remission.
Moving forward, baseline measurements and monitoring of immune processes prior to treatment are an important step in observational studies of cancer patients at risk for cancer treatment symptoms. At least one meta-analysis of cancer treatment related cognitive impairment found no evidence for treatment associated impairment in studies which included a baseline measurement of cognitive function compared to those that used non-treatment controls (Anderson-Hanley et al., 2003). The lack of studies considering inflammatory processes and cognitive function in cancer patients is an urgent concern. Future research should incorporate in vitro challenges of isolated circulating immune cells from cancer patients prior to treatment in order to provide information about facets of immune compromise not inferable from systemic markers of inflammation. Additionally, there is evidence that depressive episodes may impact inflammatory pathways, as inflammatory markers are seen to increase with greater numbers of depressive episodes (Maes et al., 2012). It is possible, therefore, that inflammatory mechanisms associated with cancer-treatment symptoms may be altered by tumor-associated inflammation and depressive episodes triggered by diagnosis prior to the impact of treatment. Non-cancer controls, particularly those facing the same pre-surgical stress but subsequently found not to have malignant disease, can provide critical information about the relationship between tumor-associated biological mechanisms and psychological comorbidities. Well-designed imaging studies may play an important role in future research, as diffusion tensor imaging techniques have been able to identify structural alterations that predict future remission of depression following antidepressant treatment (Korgaonkar et al., 2014) and transition to chronic pain (Mansour et al., 2013) suggesting that neural signatures may be an important and underused marker of vulnerable phenotypes in cancer. Structural (e.g. reduced fractional anisotropy in the hippocampal formation) or functional (e.g. reduced functional connectivity in the prefrontal cortex) changes in networks associated with executive function and verbal learning are strong candidates. In fact, a recent fMRI study found that pre-treatment dysfunction in executive networks was more strongly associated with post-treatment fatigue and cognitive dysfunction than receipt of chemotherapy in a longitudinal study of breast cancer patients (Askren et al., 2014). As it would appear that a substantial proportion of cancer patients pre-treatment suffer from deficits in memory tasks, clinicians should consider what potential impact this may have on information provided to patients at this critical juncture. Patient retention of treatment-related information should be assessed in cancer patients prior to treatment to determine if alternative strategies for communication and retention need to be developed.
Highlights.
We review evidence of peripheral tumor-associated biological process on the CNS
Tumors exert considerable influence on behavior via inflammatory pathways
Cancer patients may experience affective and cognitive symptoms due to tumor
Acknowledgments
We thank Anthony Baker for creating Figure 1. The authors are supported by grants CA140933 (S.K.) and CA177325 (A.S.) from the National Cancer Institute, and Ohio State Medical Center (L.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS, Kaufman PA. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Research and Treatment. 2008;110(1):143–52. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunological Reviews. 2008;222:155–61. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Critical Reviews in Oncology/hematology. 2008;66(1):1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. Journal of the International Neuropsychological Society : JINS. 2003;9(7):967–82. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cognitive Processing. 2012;13(2):93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer JA, Hutchison IL, Dorudi S, Stansfeld SA, Korszun A. Interrelationship of depression, stress and inflammation in cancer patients: a preliminary study. Journal of Affective Disorders. 2012;143(1–3):39–46. doi: 10.1016/j.jad.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Askren MK, Jung M, Berman MG, Zhang M, Therrien B, Peltier S, Cimprich B. Neuromarkers of fatigue and cognitive complaints following chemotherapy for breast cancer: a prospective fMRI investigation. Breast Cancer Research and Treatment. 2014;147(2):445–55. doi: 10.1007/s10549-014-3092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulino P, Berardi E, Cardillo VM, Rizzuto E, Perniconi B, Ramina C, Coletti D. Molecular, cellular and physiological characterization of the cancer cachexia-inducing C26 colon carcinoma in mouse. BMC Cancer. 2010;10:363. doi: 10.1186/1471-2407-10-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiroz A, De Miguel Z, Fano E, Vegas O. Relations between different coping strategies for social stress, tumor development and neuroendocrine and immune activity in male mice. Brain, Behavior, and Immunity. 2008;22(5):690–8. doi: 10.1016/j.bbi.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Baltgalvis KA, Berger FG, Peña MMO, Mark Davis J, White JP, Carson JA. Activity level, apoptosis, and development of cachexia in Apc(Min/+) mice. Journal of Applied Physiology (Bethesda, Md : 1985) 2010;109(4):1155–61. doi: 10.1152/japplphysiol.00442.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clinical Science (London, England : 1979) 1998;94(6):557–72. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Belluco C, Nitti D, Frantz M, Toppan P, Basso D, Plebani M, Jessup JM. Interleukin-6 blood level is associated with circulating carcinoembryonic antigen and prognosis in patients with colorectal cancer. Annals of Surgical Oncology. 2000;7(2):133–8. doi: 10.1007/s10434-000-0133-7. [DOI] [PubMed] [Google Scholar]
- Benoy I, Salgado R, Colpaert C, Weytjens R, Vermeulen PB, Dirix LY. Serum Interleukin 6, Plasma VEGF, Serum VEGF, and VEGF Platelet Load in Breast Cancer Patients. Clinical Breast Cancer. 2002;2(4):311–315. doi: 10.3816/CBC.2002.n.008. [DOI] [PubMed] [Google Scholar]
- Bernabé DG, Tamae AC, Miyahara GI, Sundefeld MLM, Oliveira SP, Biasoli ÉR. Increased plasma and salivary cortisol levels in patients with oral cancer and their association with clinical stage. Journal of Clinical Pathology. 2012;65(10):934–9. doi: 10.1136/jclinpath-2012-200695. [DOI] [PubMed] [Google Scholar]
- Bernstein IL. Learned food aversions in the progression of cancer and its treatment. Annals of the New York Academy of Sciences. 1985;443:365–80. doi: 10.1111/j.1749-6632.1985.tb27086.x. [DOI] [PubMed] [Google Scholar]
- Bernstein IL, Fenner DP. Learned food aversions: heterogeneity of animal models of tumor-induced anorexia. Appetite. 1983;4(2):79–86. doi: 10.1016/s0195-6663(83)80004-x. [DOI] [PubMed] [Google Scholar]
- Bernstein IL, Sigmundi RA. Tumor anorexia: a learned food aversion? Science (New York, NY) 1980;209(4454):416–8. doi: 10.1126/science.6930106. [DOI] [PubMed] [Google Scholar]
- Bettcher BM, Wilheim R, Rigby T, Green R, Miller JW, Racine CA, Kramer JH. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain, Behavior, and Immunity. 2012;26(1):103–8. doi: 10.1016/j.bbi.2011.07.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthé RM, Crestani F, Kelley KW, Dantzer R. Mechanisms of the behavioral effects of interleukin 1. Role of prostaglandins and CRF. Annals of the New York Academy of Sciences. 1992;650:268–75. doi: 10.1111/j.1749-6632.1992.tb49135.x. [DOI] [PubMed] [Google Scholar]
- Bojková B, Garajová M, Péč M, Kubatka P, Kajo K, Mokáň M, Ahlers I. Metabolic effects of pioglitazone in chemically-induced mammary carcinogenesis in rats. Pathology Oncology Research: POR. 2011;17(4):887–92. doi: 10.1007/s12253-011-9399-2. [DOI] [PubMed] [Google Scholar]
- Botwinick IC, Pursell L, Yu G, Cooper T, Mann JJ, Chabot JA. A biological basis for depression in pancreatic cancer. HPB: The Official Journal of the International Hepato Pancreato Biliary Association. 2014;16(8):740–3. doi: 10.1111/hpb.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behavior and Immunity. 2007;21(7):863–71. doi: 10.1016/j.bbi.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Castellon S, Arevalo J, Cole SW. Cytokine genetic variations and fatigue among patients with breast cancer. Journal of Clinical Oncology. 2013;31(13):1656–61. doi: 10.1200/JCO.2012.46.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nature Reviews Clinical Oncology. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DJF, McMillan DC, Milroy R. The correlation between fatigue, physical function, the systemic inflammatory response, and psychological distress in patients with advanced lung cancer. Cancer. 2005;103(2):377–82. doi: 10.1002/cncr.20777. [DOI] [PubMed] [Google Scholar]
- Burger RA, Grosen EA, Ioli GR, Van Eden ME, Park M, Berman ML, Gatanaga T. Spontaneous release of interleukin-6 by primary cultures of lymphoid and tumor cell populations purified from human ovarian carcinoma. Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research. 1995;15(3):255–60. doi: 10.1089/jir.1995.15.255. [DOI] [PubMed] [Google Scholar]
- Cahlin C, Körner A, Axelsson H, Wang W, Lundholm K, Svanberg E. Experimental cancer cachexia: the role of host-derived cytokines interleukin (IL)-6, IL-12, interferon-gamma, and tumor necrosis factor alpha evaluated in gene knockout, tumor-bearing mice on C57 Bl background and eicosanoid-dependent cachexia. Cancer Research. 2000;60(19):5488–93. [PubMed] [Google Scholar]
- Candido J, Hagemann T. Cancer-related inflammation. Journal of Clinical Immunology. 2013;33(Suppl 1):S79–84. doi: 10.1007/s10875-012-9847-0. [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2002;26(5):643–52. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Carson LF, Roy S, Cain K, Charboneau R, DeTurris S, Ramakrishin S, Barke RA. The central response to ovarian carcinoma simulates the response to sepsis. The Journal of Surgical Research. 1998;75(2):97–102. doi: 10.1006/jsre.1997.5183. [DOI] [PubMed] [Google Scholar]
- Cassano P, Hidalgo A, Burgos V, Adris S, Argibay P. Hippocampal upregulation of the cyclooxygenase-2 gene following neonatal clomipramine treatment (a model of depression) The Pharmacogenomics Journal. 6(6):381–7. doi: 10.1038/sj.tpj.6500385. [DOI] [PubMed] [Google Scholar]
- Catalano MG, Fortunati N, Arena K, Costelli P, Aragno M, Danni O, Boccuzzi G. Selective up-regulation of tumor necrosis factor receptor I in tumor-bearing rats with cancer-related cachexia. International Journal of Oncology. 2003;23(2):429–36. [PubMed] [Google Scholar]
- Cesario A, Rocca B, Rutella S. The interplay between indoleamine 2,3-dioxygenase 1 (IDO1) and cyclooxygenase (COX)-2 in chronic inflammation and cancer. Current Medicinal Chemistry. 2011;18(15):2263–71. doi: 10.2174/092986711795656063. [DOI] [PubMed] [Google Scholar]
- Chakraborti AK, Garg SK, Kumar R, Motiwala HF, Jadhavar PS. Progress in COX-2 inhibitors: a journey so far. Current Medicinal Chemistry. 2010;17(15):1563–93. doi: 10.2174/092986710790979980. [DOI] [PubMed] [Google Scholar]
- Chance WT, Cao L, Nelson JL, Foley-Nelson T, Fischer JE. Reversal of neurochemical aberrations after tumor resection in rats. American Journal of Surgery. 1988;155(1):124–30. doi: 10.1016/s0002-9610(88)80269-1. [DOI] [PubMed] [Google Scholar]
- Chance WT, Sheriff S, Dayal R, Balasubramaniam A. Refractory hypothalamic alpha-mSH satiety and AGRP feeding systems in rats bearing MCA sarcomas. Peptides. 2003;24(12):1909–19. doi: 10.1016/j.peptides.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Chance WT, von Meyenfeldt M, Fischer JE. Serotonin depletion by 5,7-dihydroxytryptamine or para-chloroamphetamine does not affect cancer anorexia. Pharmacology, Biochemistry, and Behavior. 1983;18(1):115–21. doi: 10.1016/0091-3057(83)90260-5. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang W, Lu T, Li J, Zheng Y, Kong L. Morphological and genetic characterization of a cultivated Cordyceps sinensis fungus and its polysaccharide component possessing antioxidant property in H22 tumor-bearing mice. Life Sciences. 2006;78(23):2742–8. doi: 10.1016/j.lfs.2005.10.047. [DOI] [PubMed] [Google Scholar]
- Chuluyan HE, Wolcott RM, Chervenak R, Dunn AJ. Catecholamine, indoleamine and corticosteroid responses in mice bearing tumors. Neuroimmunomodulation. 2000;8(3):107–13. doi: 10.1159/000054269. 54269. [DOI] [PubMed] [Google Scholar]
- Chung YC, Chang YF. Serum interleukin-6 levels reflect the disease status of colorectal cancer. Journal of Surgical Oncology. 2003;83(4):222–6. doi: 10.1002/jso.10269. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, Lee BN. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97(11):2919–25. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- Coma M, Vicente R, Busquets S, Carbó N, Tamkun MM, López-Soriano FJ, Felipe A. Impaired voltage-gated K+ channel expression in brain during experimental cancer cachexia. FEBS Letters. 2003;536(1–3):45–50. doi: 10.1016/s0014-5793(03)00009-7. [DOI] [PubMed] [Google Scholar]
- Corner J, Hopkinson J, Fitzsimmons D, Barclay S, Muers M. Is late diagnosis of lung cancer inevitable? Interview study of patients’ recollections of symptoms before diagnosis. Thorax. 2005;60(4):314–9. doi: 10.1136/thx.2004.029264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, Lubaroff DM. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104(2):305–13. doi: 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- Courtier N, Gambling T, Enright S, Barrett-Lee P, Abraham J, Mason MD. Psychological and immunological characteristics of fatigued women undergoing radiotherapy for early-stage breast cancer. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer. 2013;21(1):173–81. doi: 10.1007/s00520-012-1508-6. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. What’s Wrong With My Mouse?: Behavioral Phenotyping of Transgenic and Knockout Mice: Second Edition. John Wiley & Sons, Inc; 2006. What’s Wrong With My Mouse?: Behavioral Phenotyping of Transgenic and Knockout Mice: Second Edition; pp. 1–523. [Google Scholar]
- Dantzer R, Aubert A, Bluthé RM, Gheusi G, Cremona S, Layé S, Kelley KW. Mechanisms of the behavioural effects of cytokines. Advances in Experimental Medicine and Biology. 1999;461:83–105. doi: 10.1007/978-0-585-37970-8_6. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthé RM, Gheusi G, Cremona S, Layé S, Parnet P, Kelley KW. Molecular basis of sickness behavior. Annals of the New York Academy of Sciences. 1998;856:132–8. doi: 10.1111/j.1749-6632.1998.tb08321.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nature Reviews Clinical Oncology. 2012;9(7):414–26. doi: 10.1038/nrclinonc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong RA, Nijman HW, Boezen HM, Volmer M, Ten Hoor KA, Krijnen J, Kema IP. Serum tryptophan and kynurenine concentrations as parameters for indoleamine 2,3-dioxygenase activity in patients with endometrial, ovarian, and vulvar cancer. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society. 2011;21(7):1320–7. doi: 10.1097/IGC.0b013e31822017fb. [DOI] [PubMed] [Google Scholar]
- DellaGioia N, Hannestad J. A critical review of human endotoxin administration as an experimental paradigm of depression. Neuroscience and Biobehavioral Reviews. 2010;34(1):130–43. doi: 10.1016/j.neubiorev.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWys WD. A spectrum of organ systems that respond to the presence of cancer. Abnormalities of taste as a remote effect of a neoplasm. Annals of the New York Academy of Sciences. 1974;230:427–34. doi: 10.1111/j.1749-6632.1974.tb14476.x. [DOI] [PubMed] [Google Scholar]
- Dianliang Z. Probing cancer cachexia-anorexia: recent results with knockout, transgene and polymorphisms. Current Opinion in Clinical Nutrition and Metabolic Care. 2009;12(3):227–31. doi: 10.1097/MCO.0b013e328329d14b. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR. Future directions in research on consumer-provider communication and adherence to cancer prevention and treatment. Patient Education and Counseling. 2003;50(1):23–6. doi: 10.1016/s0738-3991(03)00075-2. [DOI] [PubMed] [Google Scholar]
- Dirksen SR, Kirschner KF, Belyea MJ. Association of Symptoms and Cytokines in Prostate Cancer Patients Receiving Radiation Treatment. Biological Research for Nursing. 2013;16(3):250–257. doi: 10.1177/1099800413490228. [DOI] [PubMed] [Google Scholar]
- Donzis EJ, Tronson NC. Modulation of learning and memory by cytokines: Signaling mechanisms and long term consequences. Neurobiology of Learning and Memory. 2014;115C:68–77. doi: 10.1016/j.nlm.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery PW. Cachexia in experimental models. Nutrition (Burbank, Los Angeles County, Calif) 15(7–8):600–3. doi: 10.1016/s0899-9007(99)00095-7. [DOI] [PubMed] [Google Scholar]
- Engin AB, Ozkan Y, Fuchs D, Yardim-Akaydin S. Increased tryptophan degradation in patients with bronchus carcinoma. European Journal of Cancer Care. 2010;19(6):803–8. doi: 10.1111/j.1365-2354.2009.01122.x. [DOI] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KRR, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biological Psychiatry. 2005;58(3):175–89. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Fang CK, Chen HW, Chiang IT, Chen CC, Liao JF, Su TP, Hwang JJ. Mirtazapine inhibits tumor growth via immune response and serotonergic system. PloS One. 2012;7(7):e38886. doi: 10.1371/journal.pone.0038886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest CM, Mackay GM, Oxford L, Millar K, Darlington LG, Higgins MJ, Stone TW. Kynurenine metabolism predicts cognitive function in patients following cardiac bypass and thoracic surgery. Journal of Neurochemistry. 2011;119(1):136–52. doi: 10.1111/j.1471-4159.2011.07414.x. [DOI] [PubMed] [Google Scholar]
- Fotopoulou C, Sehouli J, Pschowski R, VON Haehling S, Domanska G, Braicu EI, Schefold JC. Systemic changes of tryptophan catabolites via the indoleamine-2,3-dioxygenase pathway in primary cervical cancer. Anticancer Research. 2011;31(8):2629–35. [PubMed] [Google Scholar]
- Friebe A, Schwarz MJ, Schmid-Wendtner M, Volkenandt M, Schmidt F, Horn M, Schaefer M. Pretreatment levels of sTNF-R1 and sIL-6R are associated with a higher vulnerability for IFN-alpha-induced depressive symptoms in patients with malignant melanoma. Journal of Immunotherapy (Hagerstown, Md : 1997) 2007;30(3):333–7. doi: 10.1097/01.cji.0000211346.19330.c9. [DOI] [PubMed] [Google Scholar]
- Friedman GD, Skilling JS, Udaltsova NV, Smith LH. Early symptoms of ovarian cancer: a case-control study without recall bias. Family Practice. 2005;22(5):548–53. doi: 10.1093/fampra/cmi044. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Kwan L, Castellon SA, Oppenheim A, Bower JE, Silverman DH, Cole SW, Irwin MR, Ancoli-Israel S, Belin TR. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. Journal of the National Cancer Institute. 2013;105(11):791–801. doi: 10.1093/jnci/djt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannousi Z, Gioulbasanis I, Pallis AG, Xyrafas A, Dalliani D, Kalbakis K, Papandreou CN. Nutritional status, acute phase response and depression in metastatic lung cancer patients: correlations and association prognosis. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer. 2012;20(8):1823–9. doi: 10.1007/s00520-011-1282-x. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Berg BM, Krzyszton C, Johnson RW. Alpha-tocopherol attenuates NFkappaB activation and pro-inflammatory cytokine production in brain and improves recovery from lipopolysaccharide-induced sickness behavior. Journal of Neuroimmunology. 2005;169(1–2):97–105. doi: 10.1016/j.jneuroim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Gogusev J, Augusti M, Chrétien Y, Droz D. Interleukin-6 and TNFα production in human renal cell carcinoma. Kidney International. 1993;44(3):585–592. doi: 10.1038/ki.1993.285. [DOI] [PubMed] [Google Scholar]
- Gold AB, Herrmann N, Swardfager W, Black SE, Aviv RI, Tennen G, Lanctôt KL. The relationship between indoleamine 2, 3-dioxygenase activity and post-stroke cognitive impairment. Journal of Neuroinflammation. 2011;8:17. doi: 10.1186/1742-2094-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami B, Mittal P, Gupta N. Correlation of levels of IL-6 with tumor burden and receptor status in patients of locally advanced carcinoma breast. Indian Journal of Clinical Biochemistry: IJCB. 2013;28(1):90–4. doi: 10.1007/s12291-012-0234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goujon E, Layé S, Parnet P, Dantzer R. Regulation of cytokine gene expression in the central nervous system by glucocorticoids: mechanisms and functional consequences. Psychoneuroendocrinology. 1997;22(Suppl 1):S75–80. doi: 10.1016/s0306-4530(97)00009-7. [DOI] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Bauer ME, Pezzi JC, Teixeira AL, Brietzke E. Interleukin-6 and verbal memory in recurrent major depressive disorder. Neuro Endocrinology Letters. 2011;32(4):540–4. [PubMed] [Google Scholar]
- Gripp S, Moeller S, Bölke E, Schmitt G, Matuschek C, Asgari S, Willers R. Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2007;25(22):3313–20. doi: 10.1200/JCO.2006.10.5411. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress (Amsterdam, Netherlands) 2009;12(1):1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulaj E, Pawlak K, Bien B, Pawlak D. Kynurenine and its metabolites in Alzheimer’s disease patients. Advances in Medical Sciences. 2010;55(2):204–11. doi: 10.2478/v10039-010-0023-6. [DOI] [PubMed] [Google Scholar]
- Guo JY, Li CY, Ruan YP, Sun M, Qi XL, Zhao BS, Luo F. Chronic treatment with celecoxib reverses chronic unpredictable stress-induced depressive-like behavior via reducing cyclooxygenase-2 expression in rat brain. European Journal of Pharmacology. 2009;612(1–3):54–60. doi: 10.1016/j.ejphar.2009.03.076. [DOI] [PubMed] [Google Scholar]
- Guthrie GJK, Roxburgh CSD, Richards CH, Horgan PG, McMillan DC. Circulating IL-6 concentrations link tumour necrosis and systemic and local inflammatory responses in patients undergoing resection for colorectal cancer. British Journal of Cancer. 2013;109(1):131–7. doi: 10.1038/bjc.2013.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W, Peters TJ, Round A, Sharp D. What are the clinical features of lung cancer before the diagnosis is made? A population based case-control study. Thorax. 2005;60(12):1059–65. doi: 10.1136/thx.2005.045880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermelink K, Untch M, Lux MP, Kreienberg R, Beck T, Bauerfeind I, Münzel K. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer. 2007;109(9):1905–13. doi: 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Kesner RP, Goldstein M. Item and order recognition memory in subjects with hypoxic brain injury. Brain and Cognition. 1995;27(2):180–201. doi: 10.1006/brcg.1995.1016. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Yamagami H, Furukado S, Miwa K, Tanaka M, Sakaguchi M, Kitagawa K. Serum inflammatory proteins and frontal lobe dysfunction in patients with cardiovascular risk factors. European Journal of Neurology: The Official Journal of the European Federation of Neurological Societies. 2010;17(9):1134–40. doi: 10.1111/j.1468-1331.2010.02990.x. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic Medicine. 2009;71(2):171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Huang A, Fuchs D, Widner B, Glover C, Henderson DC, Allen-Mersh TG. Serum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancer. British Journal of Cancer. 2002;86(11):1691–6. doi: 10.1038/sj.bjc.6600336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz JA, Gandhi SD, Iqbal Z, Patterson KM, Pagel PS. Elevated postoperative inflammatory biomarkers are associated with short- and medium-term cognitive dysfunction after coronary artery surgery. Journal of Anesthesia. 2011;25(1):1–9. doi: 10.1007/s00540-010-1042-y. [DOI] [PubMed] [Google Scholar]
- Illman J, Corringham R, Robinson D, Davis HM, Rossi J-F, Cella D, Trikha M. Are inflammatory cytokines the common link between cancer-associated cachexia and depression? The Journal of Supportive Oncology. 3(1):37–50. [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain, Behavior, and Immunity. 2007;21(4):374–83. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Källberg E, Wikström P, Bergh A, Ivars F, Leanderson T. Indoleamine 2,3-dioxygenase (IDO) activity influence tumor growth in the TRAMP prostate cancer model. The Prostate. 2010;70(13):1461–70. doi: 10.1002/pros.21181. [DOI] [PubMed] [Google Scholar]
- Kaminska J, Nowacki MP, Kowalska M, Rysinska A, Chwalinski M, Fuksiewicz M, Chechlinska M. Clinical significance of serum cytokine measurements in untreated colorectal cancer patients: soluble tumor necrosis factor receptor type I--an independent prognostic factor. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine. 2005;26(4):186–94. doi: 10.1159/000086951. [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nature Reviews Cancer. 2002;2(4):301–10. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Kawamura I, Morishita R, Tomita N, Lacey E, Aketa M, Tsujimoto S, Ogihara T. Intratumoral injection of oligonucleotides to the NF kappa B binding site inhibits cachexia in a mouse tumor model. Gene Therapy. 1999;6(1):91–7. doi: 10.1038/sj.gt.3300819. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(19):9117–20. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Song K, Park Y, Kang Y, Lee Y, Lee K, Kim S. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. European Journal of Cancer. 2003;39(2):184–191. doi: 10.1016/S0959-8049(02)00596-8. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Ito H, Miki C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer. 1999;85(12):2526–31. doi: 10.1002/(sici)1097-0142(19990615)85:12<2526::aid-cncr6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, Krogh J. Effect of Anti-inflammatory Treatment on Depression, Depressive Symptoms, and Adverse Effects. JAMA Psychiatry. 2014;71(12):1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- Korgaonkar MS, Williams LM, Song YJ, Usherwood T, Grieve SM. Diffusion tensor imaging predictors of treatment outcomes in major depressive disorder. The British Journal of Psychiatry: The Journal of Mental Science. 2014;205(4):321–8. doi: 10.1192/bjp.bp.113.140376. [DOI] [PubMed] [Google Scholar]
- Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Roczniki Akademii Medycznej W Bialymstoku (1995) 2003;48:82–4. [PubMed] [Google Scholar]
- Krause R, James JH, Ziparo V, Fischer JE. Brain tryptophan and the neoplastic anorexia-cachexia syndrome. Cancer. 1979;44(3):1003–8. doi: 10.1002/1097-0142(197909)44:3<1003::aid-cncr2820440330>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh J, Benros ME, Jørgensen MB, Vesterager L, Elfving B, Nordentoft M. The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain, Behavior, and Immunity. 2014;35:70–6. doi: 10.1016/j.bbi.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Krysko O, Løve Aaes T, Bachert C, Vandenabeele P, Krysko DV. Many faces of DAMPs in cancer therapy. Cell Death & Disease. 2013;4:e631. doi: 10.1038/cddis.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird BJA, Scott AC, Colvin LA, McKeon AL, Murray GD, Fearon KCH, Fallon MT. Cancer pain and its relationship to systemic inflammation: an exploratory study. Pain. 2011;152(2):460–3. doi: 10.1016/j.pain.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Lamkin DM, Lutgendorf SK, Lubaroff D, Sood AK, Beltz TG, Johnson AK. Cancer induces inflammation and depressive-like behavior in the mouse: modulation by social housing. Brain, Behavior, and Immunity. 2011;25(3):555–64. doi: 10.1016/j.bbi.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. Journal of Immunology Research. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasselin J, Capuron L. Chronic low-grade inflammation in metabolic disorders: relevance for behavioral symptoms. Neuroimmunomodulation. 2014;21(2–3):95–101. doi: 10.1159/000356535. [DOI] [PubMed] [Google Scholar]
- Laviano A, Meguid MM, Yang ZJ, Gleason JR, Cangiano C, Rossi Fanelli F. Cracking the riddle of cancer anorexia. Nutrition (Burbank, Los Angeles County, Calif ) 1996;12(10):706–10. doi: 10.1016/s0899-9007(96)00164-5. [DOI] [PubMed] [Google Scholar]
- Lee BN, Dantzer R, Langley KE, Bennett GJ, Dougherty PM, Dunn AJ, Cleeland CS. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11(5):279–92. doi: 10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- Lestage J, Verrier D, Palin K, Dantzer R. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain, Behavior, and Immunity. 2002;16(5):596–601. doi: 10.1016/s0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- Lieb J, Karmali R, Horrobin D. Elevated levels of prostaglandin E2 and thromboxane B2 in depression. Prostaglandins, Leukotrienes, and Medicine. 1983;10(4):361–7. doi: 10.1016/0262-1746(83)90048-3. [DOI] [PubMed] [Google Scholar]
- Ljungberg B, Grankvist K, Rasmuson T. Serum interleukin-6 in relation to acute-phase reactants and survival in patients with renal cell carcinoma. European Journal of Cancer. 1997;33(11):1794–1798. doi: 10.1016/S0959-8049(97)00179-2. [DOI] [PubMed] [Google Scholar]
- Low CA, Bovbjerg DH, Jenkins FJ, Ahrendt SA, Choudry HA, Holtzman MP, Bartlett DL. Preoperative inflammatory biomarkers and neurovegetative symptoms in peritoneal carcinomatosis patients. Brain, Behavior, and Immunity. 2014;42:65–8. doi: 10.1016/j.bbi.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Weinrib AZ, Penedo F, Russell D, DeGeest K, Costanzo ES, Lubaroff DM. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2008;26(29):4820–7. doi: 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon DE, Walter JM, Starkweather AR, Schubert CM, McCain NL. Tryptophan degradation in women with breast cancer: a pilot study. BMC Research Notes. 2011;4:156. doi: 10.1186/1756-0500-4-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Berk M, Goehler L, Song C, Anderson G, Galecki P, Leonard B. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Medicine. 2012;10:66. doi: 10.1186/1741-7015-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud El-Salahy E. Evaluation of cytokeratin-19 & cytokeratin-20 and interleukin-6 in Egyptian bladder cancer patients. Clinical Biochemistry. 2002;35(8):607–613. doi: 10.1016/S0009-9120(02)00382-X. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Immune-to-central nervous system communication and its role in modulating pain and cognition: Implications for cancer and cancer treatment. Brain, Behavior, and Immunity. 2003;17(Suppl 1):S125–31. doi: 10.1016/s0889-1591(02)00079-x. [DOI] [PubMed] [Google Scholar]
- Mandelblatt JS, Stern RA, Luta G, McGuckin M, Clapp JD, Hurria A, Ahles T. Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2014;32(18):1909–18. doi: 10.1200/JCO.2013.54.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour AR, Baliki MN, Huang L, Torbey S, Herrmann KM, Schnitzer TJ, Apkarian AV. Brain white matter structural properties predict transition to chronic pain. Pain. 2013;154(10):2160–8. doi: 10.1016/j.pain.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masferrer JL, Seibert K, Zweifel B, Needleman P. Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(9):3917–21. doi: 10.1073/pnas.89.9.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys P, Billiau A. Cytokines and cachexia. Nutrition (Burbank, Los Angeles County, Calif ) 1997;13(9):763–70. doi: 10.1016/s0899-9007(97)00185-8. [DOI] [PubMed] [Google Scholar]
- Meguid MM, Muscaritoli M, Beverly JL, Yang ZJ, Cangiano C, Rossi-Fanelli F. The early cancer anorexia paradigm: changes in plasma free tryptophan and feeding indexes. JPEN. Journal of Parenteral and Enteral Nutrition. 16(6 Suppl):56S–59S. doi: 10.1177/014860719201600604. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104(4):788–93. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- Michalak S, Wender M, Michalowska-Wender G. Cachexia--induced cerebellar degeneration: involvement of serum TNF and MCP-1 in the course of experimental neoplastic disease. Acta Neurobiologiae Experimentalis. 2006;66(2):113–22. doi: 10.55782/ane-2006-1597. [DOI] [PubMed] [Google Scholar]
- Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. British Journal of Cancer. 2004;90(12):2312–2316. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH. Depression and immunity: a role for T cells? Brain, Behavior, and Immunity. 2010;24(1):1–8. doi: 10.1016/j.bbi.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65(9):732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooijaart SP, Sattar N, Trompet S, Lucke J, Stott DJ, Ford I, de Craen AJM. Circulating interleukin-6 concentration and cognitive decline in old age: the PROSPER study. Journal of Internal Medicine. 2013;274(1):77–85. doi: 10.1111/joim.12052. [DOI] [PubMed] [Google Scholar]
- Moradi MM, Carson LF, Twiggs LB, Weinberg JB, Haney AF, Ramakrishnan S. Serum and ascitic fluid levels of interleukin-1, interleukin-6, and tumor necrosis factor-alpha in patients with ovarian epithelial cancer. Cancer. 1993;72(8):2433–2440. doi: 10.1002/1097-0142(19931015)72:8<2433::AID-CNCR2820720822>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Moreau M, André C, O’Connor JC, Dumich SA, Woods JA, Kelley KW, Castanon N. Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain, Behavior, and Immunity. 2008;22(7):1087–95. doi: 10.1016/j.bbi.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M, Lestage J, Verrier D, Mormede C, Kelley KW, Dantzer R, Castanon N. Bacille Calmette-Guérin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. The Journal of Infectious Diseases. 2005;192(3):537–44. doi: 10.1086/431603. [DOI] [PubMed] [Google Scholar]
- Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends in Immunology. 2013;34(3):137–43. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KT, Chee A, Trieu J, Naim T, Lynch GS. Importance of functional and metabolic impairments in the characterization of the C-26 murine model of cancer cachexia. Disease Models & Mechanisms. 2012;5(4):533–45. doi: 10.1242/dmm.008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscaritoli M, Meguid MM, Beverly JL, Yang ZJ, Cangiano C, Rossi-Fanelli F. Mechanism of early tumor anorexia. The Journal of Surgical Research. 1996;60(2):389–97. doi: 10.1006/jsre.1996.0064. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Bluthé RM, May MJ, Dantzer R, Parnet P. Inactivation of the cerebral NFkappaB pathway inhibits interleukin-1beta-induced sickness behavior and c-Fos expression in various brain nuclei. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2005;30(8):1492–9. doi: 10.1038/sj.npp.1300755. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Combe C, Layé S, Tridon V, Dantzer R, Amédée T, Parnet P. Nuclear factor kappaB nuclear translocation as a crucial marker of brain response to interleukin-1. A study in rat and interleukin-1 type I deficient mouse. Journal of Neurochemistry. 2003;87(4):1024–36. doi: 10.1046/j.1471-4159.2003.02097.x. [DOI] [PubMed] [Google Scholar]
- Neveu PJ, Liège S. Mechanisms of behavioral and neuroendocrine effects of interleukin-1 in mice. Annals of the New York Academy of Sciences. 2000;917:175–85. doi: 10.1111/j.1749-6632.2000.tb05382.x. [DOI] [PubMed] [Google Scholar]
- Norden DM, Bicer S, Clark Y, Jing R, Henry CJ, Wold LE, McCarthy DO. Tumor growth increases neuroinflammation, fatigue and depressive-like behavior prior to alterations in muscle function. Brain, Behavior, and Immunity. 2014 doi: 10.1016/j.bbi.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, André C, Briley EM, Szegedi SS, Lestage J, Kelley KW. Induction of IDO by bacille Calmette-Guérin is responsible for development of murine depressive-like behavior. Journal of Immunology (Baltimore, Md : 1950) 2009;182(5):3202–12. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Molecular Psychiatry. 2009;14(5):511–22. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerlemans ME, van den Akker M, Schuurman AG, Kellen E, Buntinx F. A meta-analysis on depression and subsequent cancer risk. Clinical Practice and Epidemiology in Mental Health: CP & EMH. 2007;3(1):29. doi: 10.1186/1745-0179-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner FA, Obrist P, Stadlmann S, Feichtinger H, Klingler P, Herold M, Abendstein B. IL-6 secretion by human peritoneal mesothelial and ovarian cancer cells. Cytokine. 1995;7(6):542–7. doi: 10.1006/cyto.1995.0073. [DOI] [PubMed] [Google Scholar]
- Okada S, Okusaka T, Ishii H, Kyogoku A, Yoshimori M, Kajimura N, Kakizoe T. Elevated Serum Interleukin-6 Levels in Patients with Pancreatic Cancer. Japanese Journal of Clinical Oncology. 1998;28(1):12–15. doi: 10.1093/jjco/28.1.12. [DOI] [PubMed] [Google Scholar]
- Olson S. Symptoms of ovarian cancer. Obstetrics & Gynecology. 2001;98(2):212–217. doi: 10.1016/S0029-7844(01)01457-0. [DOI] [PubMed] [Google Scholar]
- Opara EI, Laviano A, Meguid MM. Correlation between food intake and cerebrospinal fluid interleukin 1 alpha in anorectic tumor-bearing rats. Nutrition (Burbank, Los Angeles County, Calif ) 2005;11(5 Suppl):678–9. [PubMed] [Google Scholar]
- Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. The American Journal of Psychiatry. 2006;163(9):1630–3. doi: 10.1176/appi.ajp.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Papiez MA, Dybala M, Sowa-Kucma M, Krzysciak W, Taha H, Jozkowicz A, Nowak G. Evaluation of oxidative status and depression-like responses in Brown Norway rats with acute myeloid leukemia. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009;33(4):596–604. doi: 10.1016/j.pnpbp.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Pertl MM, Hevey D, Boyle NT, Hughes MM, Collier S, O’Dwyer AM, Connor TJ. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain, Behavior, and Immunity. 2013;34:108–19. doi: 10.1016/j.bbi.2013.07.177. [DOI] [PubMed] [Google Scholar]
- Plante M, Rubin SC, Wong GY, Federici MG, Finstad CL, Gastl GA. Interleukin-6 level in serum and ascites as a prognostic factor in patients with epithelial ovarian cancer. Cancer. 1994;73(7):1882–1888. doi: 10.1002/1097-0142(19940401)73:7<1882::AID-CNCR2820730718>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Plata-Salamán CR. Central nervous system mechanisms contributing to the cachexia-anorexia syndrome. Nutrition (Burbank, Los Angeles County, Calif ) 2000;16(10):1009–12. doi: 10.1016/s0899-9007(00)00413-5. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain, Behavior, and Immunity. 1998;12(3):212–29. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Pusztai L, Clover L, Cooper K, Starkey P, Lewis C, McGee J. Expression of tumour necrosis factor α and its receptors in carcinoma of the breast. British Journal of Cancer. 1994;70(2):289–292. doi: 10.1038/bjc.1994.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, Cochrane SF, Ouwenga RL, Patel PN, Pineros V, Prendergast BJ. Mammary tumors induce select cognitive impairments. Brain, Behavior, and Immunity. 2010;24(6):903–7. doi: 10.1016/j.bbi.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, El Mouatassim Bih S, Sattar H, Prendergast BJ. Peripheral tumors alter neuroinflammatory responses to lipopolysaccharide in female rats. Brain Research. 2014;1552:55–63. doi: 10.1016/j.brainres.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, Pineros V, Galang JA, McClintock MK, Prendergast BJ. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(22):9069–74. doi: 10.1073/pnas.0811949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Ma J, Liu YM, Yang L, Peng L, Wang H, Chen HZ. Allostatic tumor-burden induces depression-associated changes in hepatoma-bearing mice. Journal of Neuro-Oncology. 2009;94(3):367–72. doi: 10.1007/s11060-009-9887-3. [DOI] [PubMed] [Google Scholar]
- Quan N. In-depth conversation: spectrum and kinetics of neuroimmune afferent pathways. Brain, Behavior, and Immunity. 2014;40:1–8. doi: 10.1016/j.bbi.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Banks WA. Brain-immune communication pathways. Brain, Behavior, and Immunity. 2007;21(6):727–35. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Quan N, Whiteside M, Herkenham M. Time course and localization patterns of interleukin-1beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience. 1998;83(1):281–93. doi: 10.1016/s0306-4522(97)00350-3. [DOI] [PubMed] [Google Scholar]
- Quan N, Whiteside M, Kim L, Herkenham M. Induction of inhibitory factor kappaBalpha mRNA in the central nervous system after peripheral lipopolysaccharide administration: an in situ hybridization histochemistry study in the rat. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(20):10985–90. doi: 10.1073/pnas.94.20.10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeca R, Bracht L, Noleto GR, Martinez GR, Cadena SMSC, Carnieri EGS, de Oliveira MBM. Production of cachexia mediators by Walker 256 cells from ascitic tumors. Cell Biochemistry and Function. 2008;26(6):731–8. doi: 10.1002/cbf.1497. [DOI] [PubMed] [Google Scholar]
- Reeve BB, Stover AM, Jensen RE, Chen RC, Taylor KL, Clauser SB, Potosky AL. Impact of diagnosis and treatment of clinically localized prostate cancer on health-related quality of life for older Americans: a population-based study. Cancer. 2012;118(22):5679–87. doi: 10.1002/cncr.27578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter K, Raugust S, Bengel J, Härter M. Depressive symptom patterns and their consequences for diagnosis of affective disorders in cancer patients. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer. 2004;12(12):864–70. doi: 10.1007/s00520-004-0694-2. [DOI] [PubMed] [Google Scholar]
- Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, Lévi F. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2005;11(5):1757–64. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- Richards CH, Roxburgh CSD, Anderson JH, McKee RF, Foulis AK, Horgan PG, McMillan DC. Prognostic value of tumour necrosis and host inflammatory responses in colorectal cancer. The British Journal of Surgery. 2012;99(2):287–94. doi: 10.1002/bjs.7755. [DOI] [PubMed] [Google Scholar]
- Riedel F, Zaiss I, Herzog D, Götte K, Naim R, Hörmann K. Serum levels of interleukin-6 in patients with primary head and neck squamous cell carcinoma. Anticancer Research. 2005;25(4):2761–5. [PubMed] [Google Scholar]
- Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Molecular Psychiatry. 2007;12(4):331–59. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- Ruud J, Nilsson A, Engström Ruud L, Wang W, Nilsberth C, Iresjö BM, Blomqvist A. Cancer-induced anorexia in tumor-bearing mice is dependent on cyclooxygenase-1. Brain, Behavior, and Immunity. 2013;29:124–35. doi: 10.1016/j.bbi.2012.12.020. [DOI] [PubMed] [Google Scholar]
- Saad S, Dunn LB, Koetters T, Dhruva A, Langford DJ, Merriman JD, Miaskowski C. Cytokine gene variations associated with subsyndromal depressive symptoms in patients with breast cancer. European Journal of Oncology Nursing: The Official Journal of European Oncology Nursing Society. 2014;18(4):397–404. doi: 10.1016/j.ejon.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar A, Gonzalez-Rivera BL, Redus L, Parrott JM, O’Connor JC. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Hormones and Behavior. 2012;62(3):202–9. doi: 10.1016/j.yhbeh.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannes TS, Jensen SE, Dodd SM, Kneipp SM, Garey Smith S, Patidar SM, Pereira DB. Depressive symptoms and cortisol variability prior to surgery for suspected endometrial cancer. Psychoneuroendocrinology. 2013;38(2):241–9. doi: 10.1016/j.psyneuen.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savonenko A, Munoz P, Melnikova T, Wang Q, Liang X, Breyer RM, Andreasson K. Impaired cognition, sensorimotor gating, and hippocampal long-term depression in mice lacking the prostaglandin E2 EP2 receptor. Experimental Neurology. 2009;217(1):63–73. doi: 10.1016/j.expneurol.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff JD, Calvano SE, Lowry SF, Androulakis IP. Transcriptional implications of ultradian glucocorticoid secretion in homeostasis and in the acute stress response. Physiological Genomics. 2012;44(2):121–9. doi: 10.1152/physiolgenomics.00128.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Annals of the New York Academy of Sciences. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BK, Barker K, Schork MA, Kluger MJ. Development of altered taste preferences in tumor-bearing rats. Appetite. 1994;23(3):219–30. doi: 10.1006/appe.1994.1055. [DOI] [PubMed] [Google Scholar]
- Soygur H, Palaoglu O, Akarsu ES, Cankurtaran ES, Ozalp E, Turhan L, Ayhan IH. Interleukin-6 levels and HPA axis activation in breast cancer patients with major depressive disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2007;31(6):1242–7. doi: 10.1016/j.pnpbp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Sperner-Unterweger B, Neurauter G, Klieber M, Kurz K, Meraner V, Zeimet A, Fuchs D. Enhanced tryptophan degradation in patients with ovarian carcinoma correlates with several serum soluble immune activation markers. Immunobiology. 2011;216(3):296–301. doi: 10.1016/j.imbio.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Starkweather AR, Lyon DE, Schubert CM. Pain and inflammation in women with early-stage breast cancer prior to induction of chemotherapy. Biological Research for Nursing. 2013;15(2):234–41. doi: 10.1177/1099800411425857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel JL, Geller DA, Gamblin TC, Olek MC, Carr BI. Depression, immunity, and survival in patients with hepatobiliary carcinoma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2007;25(17):2397–405. doi: 10.1200/JCO.2006.06.4592. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Suda T, Furuhashi K, Suzuki M, Fujie M, Hahimoto D, Chida K. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer (Amsterdam, Netherlands) 2010;67(3):361–5. doi: 10.1016/j.lungcan.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Takes RP, Rinaldo A, Silver CE, Haigentz M, Woolgar JA, Triantafyllou A, Ferlito A. Distant metastases from head and neck squamous cell carcinoma. Part I. Basic aspects. Oral Oncology. 2012;48(9):775–9. doi: 10.1016/j.oraloncology.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Teng FF, Kalloger SE, Brotto L, McAlpine JN. Determinants of quality of life in ovarian cancer survivors: a pilot study. Journal of Obstetrics and Gynaecology Canada: JOGC = Journal D’obstétrique et Gynécologie Du Canada: JOGC. 2014;36(8):708–15. doi: 10.1016/S1701-2163(15)30513-2. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ. Cachexia in cancer patients. Nature Reviews Cancer. 2002;2(11):862–71. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- Toutirais O, Chartier P, Dubois D, Bouet F, Lévêque J, Catros Quemener V, Genetet N. Constitutive expression of TGF-bêta1, interleukin-6 and interleukin-8 by tumor cells as a major component of immune escape in human ovarian carcinoma. European Cytokine Network. 2003;14(4):246–255. [PubMed] [Google Scholar]
- Treneer CM, Bernstein IL. Tumor-induced diet aversions persist after successful excision of an anorexigenic tumor. Physiology & Behavior. 1987;40(3):297–300. doi: 10.1016/0031-9384(87)90050-3. [DOI] [PubMed] [Google Scholar]
- Turrin NP, Ilyin SE, Gayle DA, Plata-Salamán CR, Ramos EJB, Laviano A, Meguid MM. Interleukin-1beta system in anorectic catabolic tumor-bearing rats. Current Opinion in Clinical Nutrition and Metabolic Care. 2004;7(4):419–26. doi: 10.1097/01.mco.0000134373.16557.92. [DOI] [PubMed] [Google Scholar]
- Udina M, Castellví P, Moreno-España J, Navinés R, Valdés M, Forns X, Martín-Santos R. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. The Journal of Clinical Psychiatry. 2012;73(8):1128–38. doi: 10.4088/JCP.12r07694. [DOI] [PubMed] [Google Scholar]
- Uomoto M, Nishibori M, Nakaya N, Takeuchi Y, Iwagaki H, Tanaka N, Saeki K. Changes in monoamine turnover in the brain of cachectic mice bearing colon-26 tumor cells. Journal of Neurochemistry. 1998;70(1):260–7. doi: 10.1046/j.1471-4159.1998.70010260.x. [DOI] [PubMed] [Google Scholar]
- Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature Medicine. 2003;9(10):1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- Van Esch L, Roukema JA, Ernst MF, Nieuwenhuijzen GAP, De Vries J. Combined anxiety and depressive symptoms before diagnosis of breast cancer. Journal of Affective Disorders. 2012;136(3):895–901. doi: 10.1016/j.jad.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Vegas O, Beitia G, Sánchez-Martin JR, Arregi A, Azpiroz A. Behavioral and neurochemical responses in mice bearing tumors submitted to social stress. Behavioural Brain Research. 2004;155(1):125–34. doi: 10.1016/j.bbr.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Vicente R, Coma M, Busquets S, Moore-Carrasco R, López-Soriano FJ, Argilés JM, Felipe A. The systemic inflammatory response is involved in the regulation of K(+) channel expression in brain via TNF-alpha-dependent and -independent pathways. FEBS Letters. 2004;572(1–3):189–94. doi: 10.1016/j.febslet.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Vine MF, Calingaert B, Berchuck A, Schildkraut JM. Characterization of prediagnostic symptoms among primary epithelial ovarian cancer cases and controls. Gynecologic Oncology. 2003;90(1):75–82. doi: 10.1016/S0090-8258(03)00175-6. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Raloxifene and/or estradiol decrease anxiety-like and depressive-like behavior, whereas only estradiol increases carcinogen-induced tumorigenesis and uterine proliferation among ovariectomized rats. Behavioural Pharmacology. 2010;21(3):231–40. doi: 10.1097/fbp.0b013e32833a5cb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Lönnroth C, Svanberg E, Lundholm K. Cytokine and cyclooxygenase-2 protein in brain areas of tumor-bearing mice with prostanoid-related anorexia. Cancer Research. 2001;61(12):4707–15. [PubMed] [Google Scholar]
- Watson JM, Sensintaffar JL, Berek JS, Martínez-Maza O. Constitutive production of interleukin 6 by ovarian cancer cell lines and by primary ovarian tumor cultures. Cancer Research. 1990;50(21):6959–65. [PubMed] [Google Scholar]
- Wedding U, Koch A, Röhrig B, Pientka L, Sauer H, Höffken K, Maurer I. Requestioning depression in patients with cancer: contribution of somatic and affective symptoms to Beck’s Depression Inventory. Annals of Oncology: Official Journal of the European Society for Medical Oncology/ESMO. 2007;18(11):1875–81. doi: 10.1093/annonc/mdm353. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA. “Chemobrain” in breast carcinoma?: a prologue. Cancer. 2004;101(3):466–75. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology (Basel, Switzerland) 2007;214(1):8–14. doi: 10.1159/000096906. [DOI] [PubMed] [Google Scholar]
- Weinrib AZ, Sephton SE, Degeest K, Penedo F, Bender D, Zimmerman B, Lutgendorf SK. Diurnal cortisol dysregulation, functional disability, and depression in women with ovarian cancer. Cancer. 2010;116(18):4410–9. doi: 10.1002/cncr.25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpé S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Molecular Psychiatry. 2005;10(6):538–44. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- Wigmore SJ, Fearon KCH, Sangster K, Maingay JP, Garden OJ, Ross JA. Cytokine regulation of constitutive production of interleukin-8 and -6 by human pancreatic cancer cell lines and serum cytokine concentrations in patients with pancreatic cancer. International Journal of Oncology. 2002;21(4):881–6. doi: 10.3892/ijo.21.4.881. [DOI] [PubMed] [Google Scholar]
- Wirleitner B, Neurauter G, Schröcksnadel K, Frick B, Fuchs D. Interferon-gamma-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Current Medicinal Chemistry. 2003;10(16):1581–91. doi: 10.2174/0929867033457179. [DOI] [PubMed] [Google Scholar]
- Wojciechowska-Lacka A, Matecka-Nowak M, Adamiak E, Lacki JK, Cerkaska-Gluszak B. Serum levels of interleukin-10 and interleukin-6 in patients with lung cancer. Neoplasma. 1996;43(3):155–8. [PubMed] [Google Scholar]
- Wood LJ, Nail LM, Perrin NA, Elsea CR, Fischer A, Druker BJ. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biological Research for Nursing. 2006;8(2):157–69. doi: 10.1177/1099800406290932. [DOI] [PubMed] [Google Scholar]
- Xiu LJ, Lin HM, Wei PK. The effect of chronic mild stress on tumor-bearing rats’ behavior and its mechanism. Neuroscience Letters. 2010;473(1):1–4. doi: 10.1016/j.neulet.2009.06.031. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T. Effect of IL-6 Elevation in Malignant Pleural Effusion on Hyperfibrinogenemia in Lung Cancer Patients. Japanese Journal of Clinical Oncology. 2000;30(2):53–58. doi: 10.1093/jjco/hyd014. [DOI] [PubMed] [Google Scholar]
- Yang M, Kim J, Kim JS, Kim SH, Kim JC, Kang MJ, Moon C. Hippocampal dysfunctions in tumor-bearing mice. Brain, Behavior, and Immunity. 2014;36:147–55. doi: 10.1016/j.bbi.2013.10.022. [DOI] [PubMed] [Google Scholar]
- Yang M, Kim JS, Kim J, Jang S, Kim SH, Kim JC, Moon C. Acute treatment with methotrexate induces hippocampal dysfunction in a mouse model of breast cancer. Brain Research Bulletin. 2012;89(1–2):50–6. doi: 10.1016/j.brainresbull.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, Behavior, and Immunity. 2011;25(2):181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- York JM, Blevins NA, Baynard T, Freund GG. Mouse testing methods in psychoneuroimmunology: an overview of how to measure sickness, depressive/anxietal, cognitive, and physical activity behaviors. Methods in Molecular Biology (Clifton, NJ) 2012;934:243–76. doi: 10.1007/978-1-62703-071-7_13. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Ikemoto S, Narita K, Sugimura K, Wada S, Yasumoto R, Nakatani T. Interleukin-6, tumour necrosis factor alpha and interleukin-1beta in patients with renal cell carcinoma. British Journal of Cancer. 2002;86(9):1396–400. doi: 10.1038/sj.bjc.6600257. [DOI] [PMC free article] [PubMed] [Google Scholar]