Abstract
Objective
Due to the lack of reliable biomarkers in diagnosing and monitoring neuropsychiatric systemic lupus erythematosus (NPSLE), the aim of this study was to examine the utility of measurements obtained through spectral domain optical coherence tomography (SD-OCT) as a biomarker for NP involvement in SLE.
Methods
Retinal nerve fiber layer (RNFL) and macula scans were performed using SD-OCT on 15 NPSLE patients, 16 SLE patients without NP symptoms (non-NP SLE), and 16 healthy controls. Macular volume and thickness of the central macula and peripapillary RNFL were compared between the groups and to scores on two validated cognitive tests.
Results
NPSLE patients did not differ significantly from non-NP SLE patients in retinal thickness or macular volume. However, SLE patients as a whole showed significant RNFL and macular thinning compared to controls. Scores on the Trail Making Test B, a test of complex attention, showed significant correlation to temporal superior and temporal inferior RNFL thickness.
Conclusion
Our results demonstrate RNFL thinning in SLE, and confirm the previous finding of high incidence of abnormal brain scans in SLE. These findings suggest that OCT measurements may be indicative of neurodegeneration in SLE and may be a useful biomarker for early cognitive impairment in SLE.
Introduction
Neuropsychiatric (NP) involvement due to systemic lupus erythematosus (SLE) is estimated to occur in 30–40% of SLE patients and is associated with significant reduction in quality of life, along with a significant increase in morbidity and mortality(1–5). Unfortunately, NPSLE remains a diagnostic challenge as there are currently no widely accepted biomarkers to identify SLE patients who have subclinical neurological involvement, or to identify whether NP symptoms are attributable to SLE. Sabbadini et al. showed that while patients with NPSLE have a higher likelihood of having abnormal magnetic resonance (MR) and computed tomography (CT) brain scans than non-NPSLE patients, those with non-NPSLE also had high rates of abnormal brain scan, indicating that the use of MR and CT scans are not sufficient to diagnose CNS involvement in lupus without the presence of other clinical symptoms (6). Ramage et al. used 18fluorodeoxyglucose PET imaging to measure blood flow and glucose uptake in the brains of 85 newly diagnosed SLE patients with and without NP symptoms and found that diffuse areas of increased 18FDG uptake (hypermetabolism) in brain white matter was associated with higher scores on a SLE disease activity index, even in patients without NP symptoms, indicating that overall lupus inflammatory activity is associated with inflammation in the white matter of patients with SLE, irrespective of clinical NP symptoms (7). Given the inconsistency of brain scan findings, NPSLE diagnosis continues to be challenging, and therefore also complicates the management of NP manifestations in lupus.
Optical coherence tomography (OCT) is a non-invasive, safe, and relatively inexpensive diagnostic method that uses the property of light reflection and interference to produce high resolution, micrometer-scale cross-sectional images of the retina (8). The retina shares the same embryonic origin as areas of the brain responsible for cognition, has a similar blood-tissue barrier as the brain, and contains unmyelinated axons, making it an ideal structure to observe changes in the brain and neurodegeneration (8,9). In addition to its use in diagnosing and monitoring ophthalmic disorders such as glaucoma and macular degeneration, OCT is well-established as a useful means of tracking neurodegenerative changes in multiple sclerosis (MS), another autoimmune disorder which targets white and gray matter and leads to demyelination, axonal dysfunction and neuronal loss (8). Additionally, studies have shown that the thickness of the RNFL and macula are inversely related to neurological disease severity in MS and spinocerebellar ataxia, and that foveal thickness is inversely related to disease severity in Parkinson’s disease, providing evidence that OCT is a useful biomarker for neurodegenerative diseases (10–12). OCT is also useful in detecting subclinical visual impairment or neuronal loss, as thinning of the RNFL and macula can even be seen in patients without afferent visual defects (10,13).
Thus far, to our knowledge, there has been no investigation into the utility of OCT measurements as a biomarker for NPSLE. Although kerato-conjunctivitis sicca is the most common ocular symptom among SLE patients, retinal disease is present in 10% of patients and the optic nerve is affected in about 1% of patients (14). Additionally, a postmortem histopathological study of the eyes of a 26-year-old SLE patient with peripheral neuropathy, but no diagnosis of optic neuritis and no significant ocular problems prior to the onset of sudden, repeated attacks of visual loss three weeks prior to her death, showed lesions with hemorrhage and axonal degeneration in the temporal RNFL and neuronal loss and degeneration in the inner nuclear layer and ganglion cell layer (15).
Given the not uncommon involvement of the retina and optic nerve in SLE, and the utility of OCT measurements as a biomarker for neurodegeneration in other disorders, we hypothesized that compared to healthy controls and lupus patients without NP involvement, patients with NPSLE would show thinning in their RNFL and macula, as measured by OCT. Additionally, we hypothesized that the thickness of the RNFL and macula would show a positive correlation with performance on cognitive tests.
Methods
Study Design
Patients with a history of neuropsychiatric syndromes that are determined by a rheumatologist to be primary manifestations of SLE (NPSLE) and patients with a diagnosis of SLE but with no history of NP involvement (non-NP SLE) were recruited from the University of Chicago Medical Center. Healthy volunteer controls were recruited from the University of Chicago employee and student population. Subjects and controls were excluded if over the age of 70 or under the age of 18; if they had any history of optic neuritis or ophthalmic disease other than refractive error (subjects with correction of >8.00D excluded), astigmatism or cataracts; or if they had a history of neurological disease not attributable to SLE. This was an observational study conducted at the University of Chicago and was approved by the University of Chicago Medical Center Institutional Review Board. All subjects and controls provided written informed consent prior to the collection of data.
Data Collection
Spectral domain optical coherence tomography (SD-OCT) examination (Spectralis OCT, software version 5.6.4.0; Heidelberg Engineering, Vista, CA) was performed on all subjects to measure RNFL thickness, macular thickness, and the volume of the macula.
RNFL scans were performed using a preset scan protocol activated by the OCT user interface. The eye being examined was fixated on an internal light and the eye was actively tracked while a high-speed circle scan of 6-mm diameter centered on the optic nerve head was performed. The RNFL thickness measurements used were taken as the average thickness across six segments around the 360° circle: temporal (315° to 45 °), temporal superior (45 ° to 90 °), nasal superior (90 ° to 135 °), nasal (135 ° to 225 °), nasal inferior (225 ° to 270 °), and temporal inferior (270 ° to 315°). The average RNFL thickness was also calculated for the papillomacular bundle region and globally around the entire 6-mm diameter circle. The computer software maps the two borders of the RNFL, the vitreo-retinal interface and the anterior boundary of retinal pigment epithelium/Bruch’s membrane complex, and automatically calculates the distance between the two borders.
Macular thickness and volume were measured using the preset ‘posterior pole’ scan on the Spectralis OCT which centers on the fovea and takes 73 horizontal B-scans covering a superior-to-inferior distance of 6 mm, 25 frames per eye, 20° × 20° scans, and an automatic real-time mean value set at 9. Any scans with a quality score of less than 20 dB were excluded.
The inner retinal complex, ganglion cell complex (GCC) and inner nuclear layer (INL) in the area of the macula were manually segmented by taking a cross-sectional image from the ‘posterior pole’ scan which ran across fovea and visually delineating the borders of each layer (i.e. the inner limiting membrane and inner edge of the INL for the inner retina, the outer edge of the RNFL and inner edge of the INL for the GCC, and the edges of the inner and outer plexiform layers for the INL). Once the borders were manually delineated, the OCT software automatically calculated the average thickness across each layer.
Patient information collected included age, history of SLE, medication history, and history of neuropsychiatric disease or symptoms. Global disease activity at time of exam was measured using the SLE Disease Activity Index (SELENA-SLEDAI). History of SLE manifestations and major organ damage was collected from patient rheumatology records. Control patients were screened for absence of ophthalmologic or neurologic involvement, and were age-matched. Cognitive testing was performed as part of the clinical evaluation of patients and control subjects. The Symbol Digit Modalities Test (SDMT) and Trail Making Test B (TMT-B) were performed on subjects and scores were compared to normative values according to age and education (16). The SDMT and TMT-B were selected in order to measure psychomotor speed and complex attention, respectively. NPSLE patients were found in a previous study to perform significantly worse than non-NP SLE patients and controls in these two cognitive domains specifically (17). All OCT scans, manual segmentation of GCC and INL, and administration of cognitive tests were performed by the same trained investigator. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Chicago.
Statistical analysis
OCT measurements were averaged between the right and left eye of each subject. There were three NPSLE patients where measurements for the GCC and INL were only taken from one eye due to the poor quality of the scan from the excluded eye. Independent-sample t-test was used to assess the differences in OCT measurements between NPSLE vs. non-NP SLE and NPSLE vs. controls. Independent-sample t-test was used to compare all SLE patients together and controls, and to compare SLE patients not taking hydroxychloroquine with SLE patients on hydroxychloroquine and healthy controls. The relationship between OCT measurements and performance on cognitive tests was analyzed using Pearson’s correlation (SPSS V. 21; Chicago, IL USA).
Results
Patient and Control Characteristics and Demographics
Investigators succeeded in recruiting 15 patients with neuropsychiatric lupus (NPSLE), 16 patients with SLE with no history of NP symptoms (non-NP SLE), and 16 healthy controls. The NPSLE patients recruited all had CNS involvement. The most common primary NPSLE syndrome present in patients was cognitive dysfunction (seven patients), followed by seizure disorder (five patients), psychosis (three patients), movement disorder (two patients), transverse myelitis (one patient), CNS vasculitis (one patient), and infarction (one patient). Three patients had lupus-related headaches in addition to their primary NPSLE syndrome. Of the patients with NPSLE, seven had active neuropsychiatric symptoms within two months prior to exam. Eleven out of 15 NPSLE patients had brain MRI records and the most common finding was nonspecific T2 hyperintensities (seen in 6 patients). In addition, one MRI showed evidence of posterior reversible encephalopathy syndrome (PRES); one showed periventricular white matter hyperintensities suggesting demyelination; three were unremarkable. One out of the 16 non-NPSLE patients had brain MRI records, which showed no abnormalities.
Table 1 lists the age, gender, and race of all patients and controls, as well as SLEDAI score, major organ involvement, years since diagnosis of SLE, hydroxychloroquine use and corticosteroid use and dosage (prednisone or methylprednisolone) for SLE patient groups.
TABLE 1.
Patient and normal control characteristics
| Characteristic | NPSLE (n=15) | Non-NP SLE (n=16) | Controls (n=16) |
|---|---|---|---|
| Age at exam—mean (range) | 43 (20–62) | 42 (19–60) | 40 (22–63) |
| Male/female | 0/15 | 1/15 | 1/15 |
| Years since SLE diagnosis at time of exam –mean | 14 | 13 | n/a |
| Race | |||
| African-American | 6 (40%) | 6 (37.5%) | 6 (37.5%) |
| White | 9 (60%) | 8 (50%) | 9 (56.25%) |
| Asian | 0 (0%) | 2 (12.5%) | 1 (6.25%) |
| Hydroxychloroquine use (percent of subjects) | 93% | 75% | n/a |
| Corticosteroid use (percent of subjects) | 80% | 50% | n/a |
| Dosage—mean (range) | 14.1 mg (2.5–60 mg) | 6.3 mg (1–40 mg) | |
| SLEDAI score—mean (range) | 8 (1–30) | 5 (0–10) | n/a |
| SLE Major Organ Involvement/Manifestation | n/a | ||
| Immunologic Disorder (positive serologies) | 7 (47%) | 13 (81%) | |
| Dermatologic Disorder | 11 (73%) | 11 (69%) | |
| Inflammatory Arthritis | 13 (87%) | 11 (69%) | |
| Hematologic Disorder | 5 (33%) | 9 (56%) | |
| Pericarditis or Pleurisy | 7 (47%) | 6 (37.5%) | |
| Lupus Nephritis | 5 (33%) | 4 (25%) | |
Demographic comparison between patients with neuropsychiatric systemic lupus erythematosus (NPSLE), SLE patients without NP symptoms (Non-NP SLE), and controls. SLEDAI=systemic lupus erythematosus disease activity index. SLEDAI measures SLE symptoms from within 10 days of OCT exam. Immunologic disorder defined as history of anti-double stranded DNA or anti-Smith antibodies. Dermatologic disorder includes history of malar rash, photosensitive rash, or discoid rash. Hematologic Disorder includes history of hemolytic anemia, leukopenia, or thrombocytopenia. Lupus nephritis diagnosed by renal biopsy.
Retinal Nerve Fiber Layer and Macular Thickness
There was no significant difference in RNFL or macular thickness between NPSLE patients and non-NP SLE patients. There was a significant difference (p<0.05) when comparing NPSLE patients with healthy controls in macular volume and thickness of the global RNFL, temporal superior RNFL, nasal RNFL, inner retina and ganglion cell complex. (Table 2) When all patients with SLE were analyzed together, the central macula, inner retina, GCC, INL, global RNFL, temporal superior RNFL and nasal RNFL were all found to be significantly thinner in SLE patients than controls. Macular volume was also significantly less in SLE patients than controls. (Table 3)
TABLE 2.
Macular volume, and retinal thickness comparing neuropsychiatric lupus (NPSLE) patients with SLE patients without NP symptoms (Non-NP SLE) and healthy controls.
| Region | NPSLE
|
Non-NP SLE
|
Controls
|
||
|---|---|---|---|---|---|
| T (s.d) | T (s.d.) | p1 | T (s.d.) | p2 | |
| Macular Volume (mm3) | 8.30 (0.33) | 8.35 (0.30) | ns | 8.63 (0.39) | p=0.017 |
| Central Macula | 266 (24) | 261 (21) | ns | 279 (21) | ns |
| Inner Retina | 88 (7) | 86 (7) | ns | 93 (6) | p=0.047 |
| Ganglion Cell Complex | 63 (5) | 62 (6) | ns | 67 (6) | p=0.035 |
| Inner Nuclear Layer | 34 (2) | 33 (2) | ns | 36 (3) | ns |
| Global RNFL | 93 (9) | 93 (10) | ns | 101 (10) | p=0.029 |
| Temporal RNFL | 67 (10) | 66 (12) | ns | 69 (8) | ns |
| Temporal Superior RNFL | 123 (16) | 126 (22) | ns | 137 (14) | p=0.016 |
| Nasal Superior RNFL | 111 (28) | 100 (26) | ns | 112 (25) | ns |
| Nasal RNFL | 68 (10) | 71 (14) | ns | 80 (14) | p=0.016 |
| Nasal Inferior RNFL | 107 (18) | 103 (20) | ns | 112 (24) | ns |
| Temporal Inferior RNFL | 136 (18) | 144 (20) | ns | 147 (16) | ns |
| Papillomacular Bundle RNFL | 52 (7) | 51 (9) | ns | 53 (6) | ns |
Spectral domain optical coherence tomography (SD-OCT) measurements of macular volume in mm3 (with standard deviation), full retinal thickness in the central macula, and thickness of inner retina, ganglion cell complex and inner nuclear layer of central macula in μm (s.d), and RNFL thickness taken in 6 regions around a 6 mm circle centered on optic nerve in μm (s.d). T= thickness in μm. s.d.= standard deviation. ns= not significant
p-value: non-NP SLE vs. NPSLE
p-value: controls vs. NPSLE
TABLE 3.
Macular volume and retinal thickness of all systemic lupus erythematosus (SLE) patients vs. controls and SLE patients not taking hydroxychloroquine (HCQ) vs. controls
| Region | All SLE, n=31
|
SLE off HCQ, n=5
|
Healthy Controls, n=16
|
||
|---|---|---|---|---|---|
| T (s.d) | p1 | T (s.d) | p2 | T (s.d.) | |
| Macular Volume (mm3) | 8.33 (0.34) | p=0.008* | 8.33 (0.52) | p=0.171 | 8.63 (0.39) |
| Central Macula | 264 (22) | p=0.029* | 273 (18) | p=0.566 | 279 (21) |
| Inner Retina | 87 (7) | p=0.008* | 85 (6) | p=0.033* | 93 (6) |
| Ganglion Cell Complex | 63 (6) | p=0.011* | 58 (4) | p=0.010* | 67 (6) |
| Inner Nuclear Layer | 34 (2) | p=0.029* | 33 (2) | p=0.208 | 36 (3) |
| Global RNFL | 93 (9) | p=0.014* | 92 (8) | p=0.092 | 101 (10) |
| Temporal Superior RNFL | 124 (19) | p=0.028* | 128 (10) | p=0.196 | 137 (14) |
| Nasal Superior RNFL | 105 (27) | p=0.438 | 84 (8) | p=0.026* | 112 (25) |
| Nasal RNFL | 70 (12) | p=0.013* | 74 (14) | p=0.398 | 80 (14) |
A comparison between controls, all SLE patients, and SLE patients not taking hydroxychloroquine, of spectral domain optical coherence tomography (SD-OCT) measurements of macular volume in mm3 (with standard deviation), full retinal thickness in the central macula and thickness of inner retina, ganglion cell complex and inner nuclear layer of central macula in μm (s.d), and RNFL thickness taken in 6 regions around a 6 mm circle centered on optic nerve in μm (s.d). HCQ= hydroxychloroquine. T= thickness in μm. s.d = standard deviation.
p-value: All SLE vs. controls
p-value: SLE patients not taking hydroxychloroquine vs. controls
statistically significant
Effects of Hydroxychloroquine on RNFL and Macular Thickness
26 patients enrolled in this study were taking hydroxychloroquine at the time of exam, and daily dosage ranged from 200 to 400 mg. Five patients enrolled in this study had taken hydroxychloroquine in the past but were not currently taking the drug due to various adverse reactions and allergies, not including documented retinal toxicity, and did not show any signs of retinal toxicity. There was no significant difference in RNFL or macular thickness between these patients and the SLE patients taking hydroxychloroquine. Additionally, when compared to controls, these patients showed decreased macular volume and thinning in the same domains as the larger SLE group—central macula, inner retina, GCC, INL, global RNFL, temporal superior RNFL and nasal RNFL—which trended toward significance. (Table 3)
Cognitive Testing and RNFL Thickness
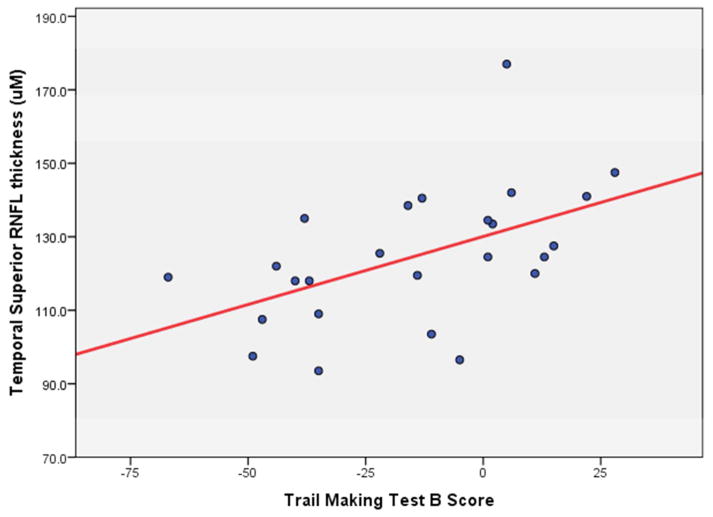
Performance on the Trail Making Test B was found to correlate significantly with the thickness of temporal superior RNFL (p=0.009, Pearson correlation coefficient=0.509) and temporal inferior RNFL (p=0.010, Pearson correlation coefficient=0.507). (Figure 1) Correlation between TMT-B scores and thickness of the temporal RNFL approached significance (p=0.071, Pearson correlation coefficient=0.367). Performance on the written and oral Symbol Digits Modalities Test (SDMT) did not correlate significantly with RNFL thickness, macular thickness, or macular volume.
FIGURE 1.
Correlation between temporal superior retinal nerve fiber layer (RNFL) thickness and score on the Trail Making Test B, with scores ranging from a low of −67 to a high or+28. (Pearson correlation coefficient= 0.509, p=0.009)
Discussion
The search for a reliable biomarker for NPSLE has been a longstanding challenge for clinicians and researchers (5). To our knowledge, this was the first study to examine the utility of OCT measurements of RNFL and macular thickness in diagnosing and tracking neuropsychiatric involvement in SLE. Our results supported our hypothesis that there was a significant difference in RNFL and macular thickness between NPSLE patients and healthy controls; although it also revealed that there was no significant difference in RNFL or macular thickness between SLE patients with or without a history of NP involvement. Our results also supported our hypothesis of a positive correlation between cognitive functioning (as measured by the Trail Making Test B) and temporal RNFL thickness.
Our finding that both NPSLE and non-NPSLE patients differed significantly from normal controls in terms of RNFL and macular thickness is consistent with previous studies that examined brain imaging from NP and non-NPSLE patients and found that both groups had higher rates of abnormal brain scans than healthy controls. The molecular mechanisms behind these differences deserve further study; however, it is generally believed that small vessel vasculitis may be responsible for retinal pathology in SLE. The immunopathologic studies by Karpik et al. of ocular changes in SLE found IgG immune complexes deposited in the walls of retinal vessels which were associated with RNFL infarcts and ganglion cell atrophy (18). Thus it is possible that vasculitis leading to retinal microinfarcts is responsible for the RNFL and macular thinning found in our study. Given that NMDA receptors are present on retinal ganglion cells, it is also possible that cross-reactive anti-DNA, anti-NMDA receptor antibodies which have been found to lead to neuronal death in the hippocampus and amygdala of SLE mouse models may also be responsible for ganglion cell death and subsequent RNFL thinning (19).
The significant thinning of the temporal superior RNFL in patients with SLE compared to controls is consistent with the predominance of temporal RNFL thinning found in MS, Parkinson’s disease, and other neurodegenerative disorders (13,20,21). This also agrees with Nag and Wadhwa’s findings of temporal RNFL degeneration in the retina of a 26 year old SLE patient with peripheral neuropathy (15). The finding that Trail Making Test B scores correlated significantly with temporal RNFL thickness is consistent with a previous study which found that NPSLE patients performed significantly worse than controls and non-NP SLE patients in tests of complex attention, as measured by the Trail Making Test B (17). This finding also agrees with the study by van Koolwijk et al which showed a positive correlation between scores of cognitive tests and RNFL thickness in young to middle-age individuals (9).
The results of this study suggest that OCT measurements of RNFL and macular thickness may be a useful biomarker of early retinal and/or CNS involvement in SLE. The significant thinning found in the temporal superior RNFL of SLE patients, and the significant correlation between temporal superior, and temporal inferior RNFL thickness and measurements of cognitive functioning suggest that CNS involvement and/or mild cognitive impairment may be more prevalent in SLE patients than previously thought.
We do not believe that the results of this study are due to effects of hydroxychloroquine on the retina, as all SLE patients included in this study who were taking hydroxychloroquine were dosed according to current dosing guidelines, received yearly eye exams and were excluded if they had documented retinal toxicity, which is estimated to occur in only 0.5–1% of patients who take the drug, Additionally, none of the individual OCT scans in this study demonstrated bulls-eye maculopathy, or loss of the photoreceptor inner/outer segment junction typically associated with early hydroxychloroquine toxicity (22). Although the sample size of SLE patients not taking hydroxychloroquine (five patients) was too small to have enough power to detect a difference between this group and controls, the fact that this group of patients also had RNFL and macular thinning approaching significance when compared to healthy controls supports our hypothesis that the effects seen in this study are due to the disease rather than drug.
One limitation of this study is the wide range of syndromes that fall under ‘NPSLE,’ as different syndromes may affect RNFL and macular thickness to differing degrees, or not at all. The small sample size of this study is another limitation, and thus further research with a larger cohort is warranted given the significant results seen in this study. Further research with a larger cohort of NPSLE patients to compare RNFL and macular thickness between patients with different neuropsychiatric syndromes would be useful to determine whether OCT measurements of retinal thickness could be a biomarker for specific NPSLE syndromes. Additionally, we did not do a comprehensive assessment of visual function or perform extensive neuropsychological testing, and thus additional study which compares RNFL and macular thickness to these parameters would further elucidate the implications and pathophysiology of the retinal thinning found in this study.
In conclusion, while the results of this study suggest that RNFL and macular thinning are associated with SLE generally rather than NPSLE specifically, further research must be done in this area to determine whether this effect is limited to the retina or whether this implies widespread but subclinical CNS involvement as a feature of SLE.
Acknowledgments
Funding
Funding received from NIH Training Grant through the National Institute of Diabetes and Digestive and Kidney Diseases. Grant 2T35DK062719-26.
We would like to thank Brian Fernandez, M.D. from Heidelberg Engineering, Christina Sprayberry and Ashley Finch for their technical support.
Footnotes
Supplementary Material
Please direct any questions, comments or request for supplementary materials to: jbernard@neurology.bsd.uchicago.edu
Conflict of Interest
No commercial sources have provided any financial support for the work reported in this manuscript. Dr. Bernard has received IIS funds and consultant fees from Biogen-Idec as well as speaker fees from Bayer; Dr. Bernard has received IIT support from Biogen-Idec.
References
- 1.Ainiala H, Loukkola J, Peltola J, Korpela M, Hietaharju A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology. 2001;57:496–500. doi: 10.1212/wnl.57.3.496. [DOI] [PubMed] [Google Scholar]
- 2.Muscal E, Brey RL. Neurologic manifestations of systemic lupus erythematosus in children and adults. Neurol Clin. 2010;28:61–73. doi: 10.1016/j.ncl.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unterman A, Nolte JES, Boaz M, Abady M, Shoenfeld Y, Zandman-Goddard G. Neuropsychiatric syndromes in systemic lupus erythematosus: a meta-analysis. Semin Arthritis Rheum. 2011;41:1–11. doi: 10.1016/j.semarthrit.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Hanly JG, McCurdy G, Fougere L, Douglas JA, Thompson K. Neuropsychiatric events in systemic lupus erythematosus: attribution and clinical significance. J Rheumatol. 2004;31:2156–62. [PubMed] [Google Scholar]
- 5.Bertsias GK, Boumpas DT. Pathogenesis, diagnosis and management of neuropsychiatric SLE manifestations. Nat Rev Rheumatol. 2010;6:358–67. doi: 10.1038/nrrheum.2010.62. [DOI] [PubMed] [Google Scholar]
- 6.Sabbadini MG, Manfredi AA, Bozzolo E, et al. Central nervous system involvement in systemic lupus erythematosus patients without overt neuropsychiatric manifestations. Lupus. 1999;8:11–9. doi: 10.1191/096120399678847344. [DOI] [PubMed] [Google Scholar]
- 7.Ramage AE, Fox PT, Brey RL, et al. Neuroimaging evidence of white matter inflammation in newly diagnosed systemic lupus erythematosus. Arthritis Rheum. 2011;63:3048–57. doi: 10.1002/art.30458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ. Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol. 2008;4:664–75. doi: 10.1038/ncpneuro0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Koolwijk LME, Despriet DDG, Van Duijn CMV, et al. Association of cognitive functioning with retinal nerve fiber layer thickness. Invest Ophthalmol Vis Sci. 2009;50:4576–80. doi: 10.1167/iovs.08-3181. [DOI] [PubMed] [Google Scholar]
- 10.Pula JH, Towle VL, Staszak VM, Cao D, Bernard JT, Gomez CM. Retinal nerve fibre layer and macular thinning in spinocerebellar ataxia and cerebellar multisystem atrophy. Neuro-Ophthalmology. 2011;35:108–14. doi: 10.3109/01658107.2011.580898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altintaş Ö, Işeri P, Özkan B, Çağlar Y. Correlation between retinal morphological and functional findings and clinical severity in Parkinson’s disease. Doc Ophthalmol. 2007;116:137–46. doi: 10.1007/s10633-007-9091-8. [DOI] [PubMed] [Google Scholar]
- 12.Gordon-Lipkin E, Chodkowski B, Reich DS, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology. 2007;69:1603–9. doi: 10.1212/01.wnl.0000295995.46586.ae. [DOI] [PubMed] [Google Scholar]
- 13.Moschos MM, Tagaris G, Markopoulos I, et al. Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss. Eur J Ophthalmol. 2010;21:24–9. doi: 10.5301/ejo.2010.1318. [DOI] [PubMed] [Google Scholar]
- 14.Sivaraj RR, Durrani OM, Denniston AK, Murray PI, Gordon C. Ocular manifestations of systemic lupus erythematosus. Rheumatology. 2007;46:1757–62. doi: 10.1093/rheumatology/kem173. [DOI] [PubMed] [Google Scholar]
- 15.Nag TC, Wadhwa S. Histopathological changes in the eyes in systemic lupus erythematosus: an electron microscope and immunohistochemical study. Histol Histopathol. 2005;20:373–82. doi: 10.14670/HH-20.373. [DOI] [PubMed] [Google Scholar]
- 16.Tombaugh TN. Trail Making Test A and B: Normative data stratified by age and education. Arch Clin Neuropsychol. 2004;9:203–14. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 17.Loukkola J, Laine M, Ainiala H, et al. Cognitive impairment in systemic lupus erythematosus and neuropsychiatric systemic lupus erythematosus: a population-based neuropsychological study. J Clin Exp Neuropsychol. 2003;25:145–51. doi: 10.1076/jcen.25.1.145.13621. [DOI] [PubMed] [Google Scholar]
- 18.Karpik AG, Schwartz MM, Dickey LE, Streeten BW, Roberts JL. Ocular immune reactants in patients dying with systemic lupus erythematosus. Clin Immunol Immunopathol. 1985;35:295–312. doi: 10.1016/0090-1229(85)90091-1. [DOI] [PubMed] [Google Scholar]
- 19.Diamond B. Antibodies and the brain: lessons from lupus. J Immunol. 2010;185:2637–40. doi: 10.4049/jimmunol.1090080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider E, Zimmermann H, Oberwahrenbrock T, et al. Optical coherence tomography reveals distinct patterns of retinal damage in neuromyelitis optica and multiple sclerosis. PLoS One. 2013;8:e66151. doi: 10.1371/journal.pone.0066151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stricker S, Oberwahrenbrock T, Zimmermann H, et al. Temporal retinal nerve fiber loss in patients with spinocerebellar ataxia type 1. PLoS One. 2011;6:e23024. doi: 10.1371/journal.pone.0023024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen E, Brown DM, Benz MS, et al. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopahty (the “flying saucer” sign) Clin Ophthalmol. 2010;4:1151–8. doi: 10.2147/OPTH.S14257. [DOI] [PMC free article] [PubMed] [Google Scholar]