Abstract
Background
Obesity is associated with systemic and intestine-specific inflammation as well as alterations in gut microbiota, which in turn impact mucosal immunity. Nonetheless, a specific role of obesity and its interaction with genetics in the progression of Crohn's disease (CD) is unclear.
Methods
We conduced a cross-sectional study of CD patients enrolled in Prospective Registry in Inflammatory Bowel Disease Study at Massachusetts General Hospital (PRISM). Information on diagnosis of CD and its complications were collected and confirmed through review of medical records. A genetic risk score was calculated using previously reported single nucleotide polymorphisms (SNPs) associated genome-wide with CD susceptibility. We used logistic regression to estimate the effect of body mass index (BMI) and its interaction with genetic risk on risk of CD complications.
Results
Among 846 CD patients, 350 required surgery, 242 with penetrating disease, 182 with stricturing disease, and 226 with perianal disease. There were no associations between obesity (BMI ≥ 30) and risk of perianal disease, stricturing disease, or surgery. Compared to normal weight individuals with BMI < 25, obesity was associated with lower risk of penetrating disease (OR = 0.56, 95% CI 0.31-0.99). This association persists among a subgroup of participants with available BMI prior to development of penetrating disease (OR = 0.40, 95% CI 0.16-0.88). There were no interactions between BMI and genetic risk score on risk of CD complications (All Pinteraction > 0.28).
Conclusion
Our data suggest that obesity does not negatively impact long-term progression of CD, even after accounting for genetic predisposition.
Keywords: Obesity, Crohn's Disease Complications, Genetic Predisposition
Introduction
Crohn's disease (CD) is a chronic inflammatory disorder of the gastrointestinal tract with a broad spectrum of clinical presentations. Disease phenotype has previously been characterized according to age of diagnosis, disease location, and disease behavior defined by the presence of stricturing, penetrating, or perianal disease1. Few population-based studies have evaluated the natural history of CD with available data suggesting that over 40% of patients require surgery within 10 years of diagnosis and nearly 50% experience stricturing or penetrating disease within 20 years of diagnosis2,3. Because of the significant variability in disease presentation and course, identifying environmental or genetic factors that help stratify high-risk individuals is critical. Few studies have found disease characteristics such as perianal disease or presence of ileal involvement to be associated with development of complications 4-6. However, there is a paucity of data on the role of environmental and genetic factors on progression of CD.
Adiposity, as measured by body mass index (BMI), has been linked to elevated levels of pro-inflammatory markers such as tumor necrosis factor-alpha (TNF-α) and C-reactive protein (CRP)7,8. More specifically, obese individuals (BMI >=30 kg/m2) have been shown to have higher levels of inflammation in the gastrointestinal tract as measured by stool calprotectin9. Despite this link between obesity and systemic and gut inflammation, prior studies on the effect of BMI on risk and progression of CD have yielded conflicting results10-13. These studies have been limited by their sample size and lack of detailed information on important confounders such as smoking. In addition, prior studies have focused on the impact of obesity on disease progression regardless of genetic predisposition. However, considering the importance of genetics on CD pathogenesis14, a more complete assessment of the influence of environmental factors on disease complications may be better characterized in the context of genetic predisposition. We therefore sought to examine the effect of obesity as measured by BMI and its interaction with genetic make up as defined by individual genotypes across the known CD risk variants on risk of disease complications in a large cohort of CD patients enrolled in a tertiary referral center.
Methods
Study Population
Starting in 2004, adult patients, age ≥ 18 years, with a diagnosis of CD, ulcerative colitis, or indeterminate colitis were recruited in the Prospective Registry in IBD Study at Massachusetts General Hospital (PRISM). At the time of recruitment, patients were interviewed by a study coordinator to collect detailed information on their disease characteristics according to the Montreal classification, lifestyle factors including smoking, BMI, and dietary factors, and other comorbid conditions. In addition, approximately 10 cc of blood was drawn from each participant at the time of enrollment for extraction of the buffy coat. For this study 846 CD patients (> 93% of the entire cohort) with available data on BMI were eligible for primary analyses. The institutional review board at the Massachusetts General Hospital (MGH) approved this study.
Assessment of BMI and Other Covariates
Participants reported their height at the time of enrollment while weight was measured at baseline of the study. BMI was calculated from height and weight. BMI categories were selected based on World Health Organization international classifications (BMI < 25 kg/m2, normal; 25 – 29.9 kg/m2, overweight; ≥ 30 kg/m2, obese). As we had few participants with BMI <18.5 kg/m2, we did not create a separate category for underweight participants. We also collected information on sex, race, smoking status at the time of enrollment (never, past, or current), age of diagnosis, disease location (ileal, colonic, ileocolonic, and upper gastrointestinal tract involvement), ever use of CD-related medications (mesalamines, thiopuines, methotrexate, and biologics), primary gastroenterology provider, number of disease-related encounters at MGH prior to enrollment, and laboratory values including inflammatory markers (ESR and CRP). Data on age of diagnosis, disease location, and medications were confirmed by review of medical records and verified by primary gastroenterologists.
Genotyping and Computation of Genetic Risk Score
Genotyping was performed on the Illumina Immunochip at the Broad Institute (Cambridge, MA). Immunochip is an Illumina Infinium genotyping chip, containing 196,524 polymorphisms (718 small insertion deletions, 195,806 SNPs) designed both to perform deep replication of major autoimmune and inflammatory diseases, and fine-mapping of established GWAS significant loci15. Genetic data were available for 751 participants (89% of participants with available data on BMI). The baseline characteristics of the participants with genetic data including age at enrollment, age of diagnosis, BMI, smoking, perianal disease, fistulizing disease, and stricturing disease were similar to those of the entire cohort (All Pcomparisons > 0.10).
We used the most recent meta-analysis of genome-wide association studies to identify 140 single nucleotide polymorphisms (SNPs) associated with risk of CD (Supplementary Table 1)16. Genetic risk score was calculated on the basis of these SNPs by using a previously reported weighted method17,18. Each SNP was recoded as 0, 1, or 2 according to the number of risk alleles (CD increasing alleles), and each SNP was weighted by its relative effect size (β coefficient) derived from the previously reported meta-analysis data. We created the genetic risk score using the equation: genetic risk score = (β1×SNP1 + β2×SNP2 + ... + βn×SNPn) / (sum of the β coefficients), where β is the β coefficient of each individual SNP on CD. This genetic risk score was previously shown to be associated with higher risk of CD complications in PRISM19. In sensitivity analysis, we recalculated the genetic risk score by giving each SNP equal weights. We categorized individuals according to their genetic risk score into high and low risk groups based on the median genetic risk score value of the entire cohort.
Outcome Ascertainment
We defined CD complication as the presence of penetrating disease, stricturing disease, perianal disease, or the need for having a bowel resection. At the time of enrollment, information on disease behavior (penetrating, stricturing, and perianal disease) and previous surgeries were collected and confirmed by review of medical records and further verified by primary gastroenterologists.
Statistical Analysis
For pairwise comparisons, we used Chi-squared test and Wilcoxon signed-rank test to compare categorical and continuous variables, respectively. In our primary analysis, we estimated the odds ratio (OR) and 95% confidence interval (CI) using logistic regression modeling adjusting for potential confounders. In our models, we adjusted for the number of CD-related encounters (endoscopy, clinic visits, and surgery) at MGH prior to enrollment to account for potential differences in the availability of data and differences in practice patterns and therefore threshold for surgery. To account for differences in rates of development of disease complications according to time, we adjusted for duration of disease in our models. As use of anti-TNF therapy has previously been associated with a significant increase in body weight and may also be protective against developing disease complication20,21, we adjusted for ever use of anti-TNF in our models. We also examined the association between BMI and disease complication according to the strata of other potential risk factors. Finally, we explored whether the effect of BMI on disease complications is modified by genetic risk score by entering BMI and the binary genetic risk score (high vs. low) in our models as a multiplicative interaction term. We used SAS version 9.3 (Cary, NC) for these analyses. All P-values were 2-sided and < 0.05 was considered statistically significant.
Results
Among 846 CD patients with available data on BMI, 450 (53%) had a BMI less than 25, 257 (30%) were overweight (BMI between 25 and 29.9) and 16% were obese (BMI ≥ 30) (Table 1). Compared to participants with normal BMI (< 25 kg/m2), obese participants were older at the time of enrollment, less likely to be male, and more likely to be smokers (Table 1).
Table 1.
Characteristics of Participants According to Body Mass Index at Enrollment*
| Body mass index (kg/m2) | |||
|---|---|---|---|
| < 25 N = 450 | 25-29.9 N = 258 | ≥ 30 N = 138 | |
| Age at enrollment, yrs, mean (std)** | 36 (14) | 42 (15) | 45 (14) |
| Sex, female (%)** | 282 (63) | 104 (40) | 79 (57) |
| Age at diagnosis (yrs), n (%)** | |||
| ≤ 16 | 108 (24) | 45 (17) | 10 (7) |
| 17-40 | 288 (64) | 149 (58) | 89 (64) |
| > 40 | 54 (12) | 64 (25) | 39 (28) |
| Duration of disease (yrs), mean (std)** | 11 (11) | 13 (11) | 12 (10) |
| History of smoking, n (%)** | 136 (30) | 101 (39) | 68 (49) |
| Previous and current treatment, n (%) | |||
| Azathioprine | 165 (37) | 112 (43) | 57 (41) |
| Methotrexate | 74 (16) | 45 (17) | 32 (23) |
| Biologic therapy | 261 (58) | 133 (52) | 76 (55) |
| Remicade | 214 (48) | 119 (46) | 62 (45) |
| Humira | 126 (28) | 68 (26) | 35 (25) |
| Cimzia | 53 (12) | 21 (8) | 22 (16) |
| Tysabri | 12 (3) | 5 (2) | 6 (4) |
| Steroid therapy§ | 379 (84) | 205 (79) | 105 (76) |
Abbreviation: number (n), standard deviation (std), years (yrs).
Statistically significant differences across BMI categories (p < 0.05).
Steroid therapy includes a history or current use of prednisone or budesonide.
Compared to participants with normal BMI, obese patients were less likely to have penetrating disease (OR = 0.58, 95% CI 0.35-0.94) (Table 2). The risk was not significantly altered after adjusting for known and potential confounders including age at enrollment, age of diagnosis, sex, duration of disease, number of disease encounters at MGH prior to enrollment, ever use of anti-TNF or steroids, and smoking. Compared to CD patients with normal BMI, the multivariable-adjusted OR of penetrating CD among obese patients was 0.56 (95% CI, 0.31-0.99). In contrast, we did not observe an association between being overweight and risk of penetrating disease (multivariable-adjusted OR = 0.80, 95% CI 0.52-1.22). Similarly, we did not observe an association between obesity and risk of perianal disease, stricturing disease, or the need for surgery. Compared to participants with normal BMI, obese patients had a multivariable-adjusted OR of 1.03 (95% CI, 0.59-1.76) for stricturing disease, 1.17 (95% CI, 0.71, 1.90) for perianal disease, and 0.71 (95% CI, 0.39, 1.28) for the need for surgery.
Table 2.
Body Mass Index and Risk of Crohn's Disease Complications*
| Body Mass Index (kg/m2) | |||
|---|---|---|---|
| < 25 N = 450 | 25-29.9 N = 258 | ≥ 30 N = 138 | |
| Penetrating Disease | |||
| Cases | 139 | 73 | 30 |
| Age-adjusted (95% CI) | 1.00 | 0.77 (0.53-1.11) | 0.58 (0.35-0.94) |
| MV-adjusted (95% CI)¶ | 1.00 | 0.79 (0.51-1.21) | 0.56 (0.31-0.99) |
| Stricturing Disease | |||
| Cases | 99 | 50 | 33 |
| Age-adjusted (95% CI) | 1.00 | 0.71 (0.47-1.08) | 0.84 (0.51-1.38) |
| MV-adjusted (95% CI)¶ | 1.00 | 0.70 (0.44-1.12) | 1.03 (0.59-1.76) |
| Perianal Disease | |||
| Cases | 121 | 69 | 36 |
| Age-adjusted (95% CI) | 1.00 | 1.07 (0.75-1.52) | 1.09 (0.69-1.71) |
| MV-adjusted (95% CI)¶ | 1.00 | 1.00 (0.67-1.48) | 1.17 (0.71-1.90) |
| Surgery | |||
| Cases | 184 | 117 | 49 |
| Age-adjusted (95% CI) | 1.00 | 1.06 (0.77-1.45) | 0.67 (0.44-1.00) |
| MV-adjusted (95% CI)¶ | 1.00 | 1.05 (0.66-1.65) | 0.71 (0.39-1.28) |
Abbreviations: multivariable (MV), confidence interval (CI).
Models are adjusted for age (years), sex, duration of disease (years), age at diagnosis (≤16, 17-40, > 40), number of MGH encounters prior to enrollment, biologic therapy (ever, never), steroid therapy (ever, never), and smoking (ever, never).
We explored the possibility that the protective effect of obesity on risk of penetrating disease may in part be related to significant weight change as a result of active and/or aggressive disease behavior. Thus, we limited our analysis to individuals with normal inflammatory markers (CRP and ESR), surrogates of disease activity, at the time of BMI measurements. Compared to CD patients with normal BMI, the multivariable-adjusted OR of penetrating CD among obese patients in this subgroup was 0.26 (95% CI, 0.06-1.10). In addition, we performed sensitivity analysis limiting the study population to participants with available BMI information prior to development of penetrating disease. Among 93 participants with available BMI information prior to developing penetrating disease, obesity was associated with a reduced risk of penetrating disease (multivariable-adjusted OR = 0.40, 95% CI 0.16-0.88).
As use of anti-TNF therapy has previously been associated with a significant increase in body weight and may also be protective against developing disease complication20,21, we performed sensitivity analyses excluding individuals with prior or current use of anti-TNF therapy and obtained similar results. Compared to participants with normal BMI, the multivariable-adjusted OR of developing penetrating disease among obese participants was 0.58 (95% CI, 0.24-1.33).
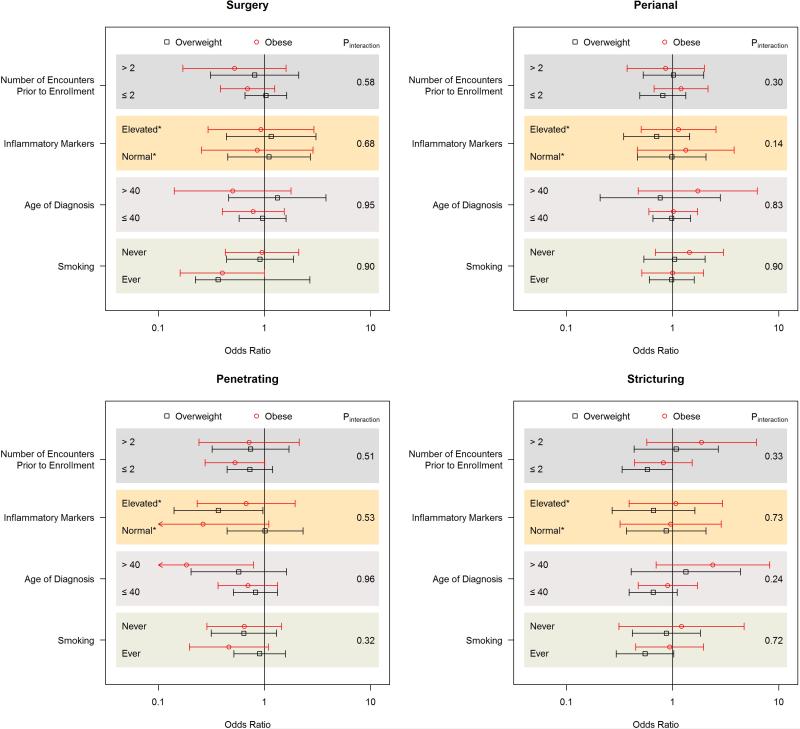
We examined the effect of BMI on CD complications according to strata defined by inflammatory markers, smoking, and age of diagnosis (Figure 1). The effect of BMI on risk of CD complications was not modified by age of diagnosis, inflammatory markers, and smoking (All Pinteraction > 0.10). As the number of disease encounters prior to enrollment may influence the availability of detailed information on disease behavior and complications, we also assessed the impact of BMI on risk of disease complications according to strata defined by the number of disease encounters prior to enrollment and observed no effect modification (All Pinteraction > 0.30) (Figure 1).
Figure 1.
Body Mass Index and Risk of Crohn's Disease Complications According to Selected Strata
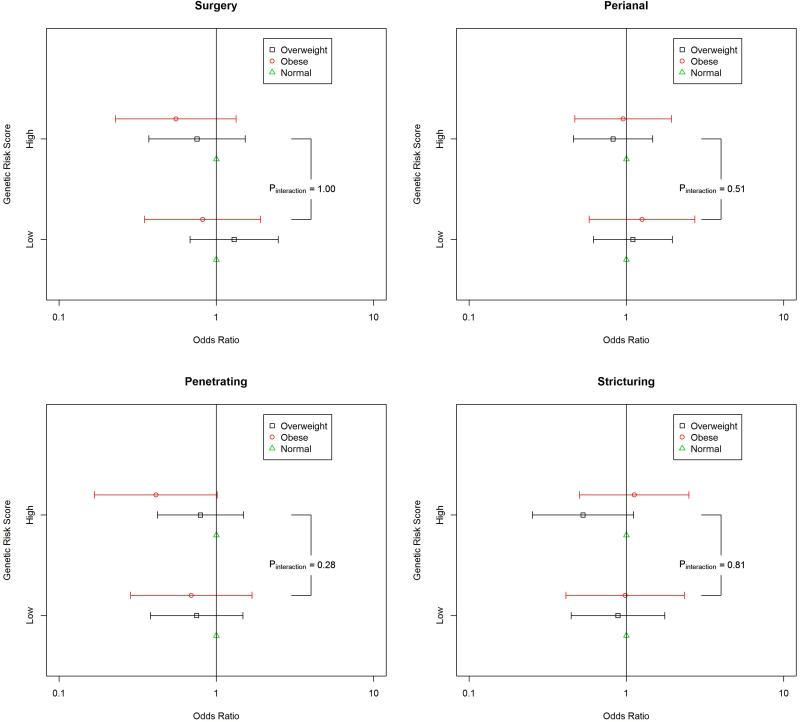
We evaluated potential differences in the influence of BMI on disease complications according to genetic predisposition as defined by genetic risk score (Figure 2). The effect of obesity on risk of penetrating disease did not appear to be modified by genetic predisposition (Pinteraction = 0.28). Similarly, there were no interactions between obesity and genetic risk score on risk of stricturing disease, perianal disease, or the need for surgery (All Pinteraction > 0.51). These results were consistent in analyses in which genetic risk score was treated as a continuous variable (All Pinteraction > 0.40). As SNPs in IL-23 gene have previously been shown to interact with obesity in other chronic inflammatory disorders22, we explored the possibility that the two SNPs in our genetic risk score related to IL-23 gene (rs11209026 and rs6871626) may influence the effect of BMI on risk CD complications and found no effect modification by either SNPs (All Pinteractions > 0.30). Finally, in sensitivity analysis we recalculated genetic risk score by giving equal weight to each SNPs and obtained similar results (data not shown).
Figure 2.
Body Mass Index and Risk of Crohn's Disease Complications According to Genetic Risk Score
Discussion
In a large cohort of CD patients, we show that obesity is not associated with risk of stricturing disease, perianal disease, or the need for surgery. In addition, our data suggest that obesity may be associated with a lower risk of penetrating disease. Finally, genetic predisposition did not appear to modify the effect of obesity on risk of penetrating disease, stricturing disease, perianal disease, or the need for surgery.
Prior studies on the effect of BMI on disease complication have had conflicting results10-13. A small retrospective study of IBD patients at a tertiary referral center found no difference in time to first surgery between the group with a BMI < 25 kg/m2 and those with a BMI ≥ 25 kg/m211. In contrast, a small retrospective study of obese IBD patients compared to matched controls found obese patients to be more likely to have perianal disease, disease flares, and require hospitalizations10. However, obesity in this study was defined as BMI > 25 kg/m2 at the time of disease onset and long-term outcome of disease was not altered by obesity. More recently, two retrospective studies evaluating the impact of obesity in pediatric IBD found no association between obesity and risk of CD complications with the exception of surgery and infections12,13.
Although there is growing evidence suggesting a link between obesity and systemic and luminal inflammation, the exact role of obesity on pathophysiology of CD is largely unknown. Nevertheless, there are distinct features between adipocyte tissue in obesity and CD mesenteric fat depot (e.g. creeping fat). First, while mesenteric fat tissue from patients with CD is a major mesenteric source of TNF-α, subcutaneous fat from CD patients or the mesenteric or subcutaneous fat of healthy controls does not appear to produce TNF-α 23. Second, the production of a key anti-inflammatory adipocyte-derived mediator, adiponectin, is decreased with visceral adiposity but increased with chronic inflammatory disorders including CD24,25. Although in healthy controls adiponectin is negatively correlated with inflammatory markers, in chronic inflammatory disorders there appears to be a positive correlation 25. Proinflammatory effects of adiponectin have also been reported in tissues such as joint synovium and colonic epithelium26-28. Nevertheless, as shown in models of systemic lupus erythematous (SLE), the accepted negative correlation between adiponectin and insulin resistance, a marker of obesity, is likely present, but on the background of increased adiponectin levels with significant systemic inflammation29. This paradoxical link between adiponectin and levels of inflammation may in part explain our findings that obesity is protective against penetrating disease. In support of this hypothesis, Karmiris and colleagues found that only patients with penetrating CD have a trend for higher serum levels of adiponectin30, suggesting that obesity through its effect on adiponectin levels in patients with established CD may have a protective effect on development of penetrating disease.
Our study has several notable strengths. First, to our knowledge this is the first comprehensive examination of an environmental influence on CD complications in the context of genetic predisposition. Second, we confirmed all cases of CD and disease complications through medical record review. This is a significant advantage over studies that rely on self-report or diagnoses codes that may not be accurate. Third, information on weight was measured by clinic staff, an advantage over retrospective studies that rely on self-report of weight that may be affected by recall bias.
We acknowledge several limitations. First, the cross-sectional nature of our study makes it possible that our observed association was primarily derived by significant weight loss among individuals with more severe disease. However, the association between BMI and disease complications appears to be unique to penetrating disease making it less likely that the effect is driven by reverse causation. Moreover, the influence of BMI on risk of penetrating disease did not vary according to levels of inflammatory markers at the time of BMI measurement and remained consistent among participants with BMI measurements prior to developing penetrating disease. Second, as with other cross-sectional studies we cannot rule out the possibility that unmeasured confounders may have accounted for our observed effect estimates. However, adjusting for other known and important confounders such as smoking and duration of disease did not materially alter our effect estimates. Third, it is possible that obesity may have precluded individuals from having surgery, which could have in turn limited our ability to detect an association between obesity and risk of surgery. In addition, we did not systematically screen all participants for disease complications which may have also not allowed us to find as association between obesity and CD complications. Nevertheless, the average duration of follow up for our patients were over 10 years making it less likely that subclinical stricturing or penetrating disease have gone undiagnosed. Fourth, our study is based on an experience of a single tertiary referral center with predominantly white population and therefore it's possible that our findings may not be generalizable to other populations. However, the average age of diagnosis, distribution of smoking and BMI, and rates of complications in our study population is similar to large US population cohorts3,31. Finally, we acknowledge that BMI is a measure of total abdominal obesity and other measurements of adiposity obtained from CT and DEXA scans, which more accurately reflect visceral and mesenteric adiposity, may play a more important role in development and progression of CD.
In conclusion, we show that obesity as measured by BMI is not associated with risk of stricturing disease, perianal disease, or the need for surgery among CD patients. Taken together with our finding that obesity may be inversely associated with risk of penetrating disease, our data suggest that obesity does not negatively impact long-term progression of CD even after accounting for genetic predisposition.
Supplementary Material
Acknowledgments
Grant Support: Dr. Chan is supported by a senior investigator grant from the Crohn's and Colitis Foundation of America (CCFA) and K24 DK098311. Dr. Khalili is supported by a career development award from the American Gastroenterological Association (AGA) and by National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK099681).
Footnotes
Authors Contributions
PLP - Acquisition of data; statistical analysis; interpretation of data; drafting of the manuscript.
KOS - Acquisition of data; statistical analysis; interpretation of data; drafting of the manuscript.
JMP - Acquisition of data; interpretation of data; critical revision of the manuscript.
HCS - Acquisition of data; critical revision of the manuscript.
DN - Acquisition of data; critical revision of the manuscript.
JS - Acquisition of data; critical revision of the manuscript.
JJG - Acquisition of data; critical revision of the manuscript.
VY - Acquisition of data; critical revision of the manuscript.
ANA- Acquisition of data; critical revision of the manuscript.
ATC - Interpretation of data; critical revision of the manuscript.
RJX - Acquisition of data; critical revision of the manuscript.
HK – Study concept and design; acquisition of data; statistical analysis; interpretation of data; drafting of the manuscript.
Financial Disclosures: Dr. Ananthakrishnan is a member of the scientific advisory board for Prometheus Inc, and Janssen, Inc. Dr. Chan has served as a consultant for Bayer Healthcare, Millennium Pharmaceuticals, Pfizer Inc., and Pozen Inc. Other authors have no financial disclosures. Dr. Xavier has served as a consultant for Novartis. Dr. Yajnik has served as a consultant for Biogen, UCB, NPS, Jansen, Abbvie, and Takeda.
Ethical Approval: The institutional review board at the Massachusetts General Hospital approved this study.
References
- 1.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005 Sep;19(Suppl A):5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 2.Solberg IC, Vatn MH, Hoie O, et al. Clinical course in Crohn's disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007 Dec;5(12):1430–1438. doi: 10.1016/j.cgh.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L, Loftus EV, Jr., Colombel JF, Sandborn WJ. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol. 2010 Feb;105(2):289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 4.Tarrant KM, Barclay ML, Frampton CM, Gearry RB. Perianal disease predicts changes in Crohn's disease phenotype-results of a population-based study of inflammatory bowel disease phenotype. Am J Gastroenterol. 2008 Dec;103(12):3082–3093. doi: 10.1111/j.1572-0241.2008.02212.x. [DOI] [PubMed] [Google Scholar]
- 5.Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV., Jr. Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology. 2010 Oct;139(4):1147–1155. doi: 10.1053/j.gastro.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn's disease. Gastroenterology. 2006 Mar;130(3):650–656. doi: 10.1053/j.gastro.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995 May;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenfield JR, Samaras K, Jenkins AB, et al. Obesity is an important determinant of baseline serum C-reactive protein concentration in monozygotic twins, independent of genetic influences. Circulation. 2004 Jun 22;109(24):3022–3028. doi: 10.1161/01.CIR.0000130640.77501.79. [DOI] [PubMed] [Google Scholar]
- 9.Poullis A, Foster R, Shetty A, Fagerhol MK, Mendall MA. Bowel inflammation as measured by fecal calprotectin: a link between lifestyle factors and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2004 Feb;13(2):279–284. doi: 10.1158/1055-9965.epi-03-0160. [DOI] [PubMed] [Google Scholar]
- 10.Blain A, Cattan S, Beaugerie L, Carbonnel F, Gendre JP, Cosnes J. Crohn's disease clinical course and severity in obese patients. Clin Nutr. 2002 Feb;21(1):51–57. doi: 10.1054/clnu.2001.0503. [DOI] [PubMed] [Google Scholar]
- 11.Hass DJ, Brensinger CM, Lewis JD, Lichtenstein GR. The impact of increased body mass index on the clinical course of Crohn's disease. Clin Gastroenterol Hepatol. 2006 Apr;4(4):482–488. doi: 10.1016/j.cgh.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Long MD, Crandall WV, Leibowitz IH, et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm Bowel Dis. 2011 Oct;17(10):2162–2168. doi: 10.1002/ibd.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zwintscher NP, Horton JD, Steele SR. Obesity has minimal impact on clinical outcomes in children with inflammatory bowel disease. J Pediatr Surg. 2014 Feb;49(2):265–268. doi: 10.1016/j.jpedsurg.2013.11.033. discussion 268. [DOI] [PubMed] [Google Scholar]
- 14.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009 Nov 19;361(21):2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortes A, Brown MA. Promise and pitfalls of the Immunochip. Arthritis Res Ther. 2011;13(1):101. doi: 10.1186/ar3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012 Nov 1;491(7422):119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi Q, Chu AY, Kang JH, et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med. 2012 Oct 11;367(15):1387–1396. doi: 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Zhao JH, Luan J, et al. Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from EPIC-Norfolk prospective population study. PLoS Med. 2010;7(8) doi: 10.1371/journal.pmed.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ananthakrishnan AN, Huang H, Nguyen DD, Sauk J, Yajnik V, Xavier RJ. Differential effect of genetic burden on disease phenotypes in Crohn's disease and ulcerative colitis: analysis of a North American cohort. Am J Gastroenterol. 2014 Mar;109(3):395–400. doi: 10.1038/ajg.2013.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renzo LD, Saraceno R, Schipani C, et al. Prospective assessment of body weight and body composition changes in patients with psoriasis receiving anti-TNF-alpha treatment. Dermatol Ther. 2011 Jul-Aug;24(4):446–451. doi: 10.1111/j.1529-8019.2011.01439.x. [DOI] [PubMed] [Google Scholar]
- 21.Hmamouchi I, Roux C, Paternotte S, Kolta S, Dougados M, Briot K. Early increase of abdominal adiposity in patients with spondyloarthritis receiving anti-tumor necrosis factor-alpha treatment. J Rheumatol. 2014 Jun;41(6):1112–1117. doi: 10.3899/jrheum.131150. [DOI] [PubMed] [Google Scholar]
- 22.Li WQ, Han JL, Zhang MF, Qureshi AA. Interactions between adiposity and genetic polymorphisms on the risk of psoriasis. Br J Dermatol. 2013 Mar;168(3):639–642. doi: 10.1111/bjd.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desreumaux P, Ernst O, Geboes K, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology. 1999 Jul;117(1):73–81. doi: 10.1016/s0016-5085(99)70552-4. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto K, Kiyohara T, Murayama Y, et al. Production of adiponectin, an anti- inflammatory protein, in mesenteric adipose tissue in Crohn's disease. Gut. 2005 Jun;54(6):789–796. doi: 10.1136/gut.2004.046516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008 Feb;121(2):326–330. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Ehling A, Schaffler A, Herfarth H, et al. The potential of adiponectin in driving arthritis. J Immunol. 2006 Apr 1;176(7):4468–4478. doi: 10.4049/jimmunol.176.7.4468. [DOI] [PubMed] [Google Scholar]
- 27.Fayad R, Pini M, Sennello JA, et al. Adiponectin deficiency protects mice from chemically induced colonic inflammation. Gastroenterology. 2007 Feb;132(2):601–614. doi: 10.1053/j.gastro.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 28.Ogunwobi OO, Beales IL. Adiponectin stimulates proliferation and cytokine secretion in colonic epithelial cells. Regul Pept. 2006 May 15;134(2-3):105–113. doi: 10.1016/j.regpep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Sada KE, Yamasaki Y, Maruyama M, et al. Altered levels of adipocytokines in association with insulin resistance in patients with systemic lupus erythematosus. J Rheumatol. 2006 Aug;33(8):1545–1552. [PubMed] [Google Scholar]
- 30.Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006 Feb;12(2):100–105. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 31.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010 Dec 2;363(23):2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.