Abstract
Activating autoantibodies to the angiotensin type 1 receptor (AT1R) are associated with hypertensive disorders. The angiotensin type 2 receptor (AT2R) is known to counter-regulate the actions of AT1R. We investigated whether AT2R autoantibodies produced in immunized rabbits will activate AT2R and suppress the vasopressor responses to angiotensin II (Ang II) and AT1R-activating autoantibodies. Five rabbits immunized with a peptide corresponding to the second extracellular loop of AT2R developed high AT2R antibody titers. Rabbit anti-AT2R sera failed to directly dilate isolated rat cremaster arterioles; however, when co-perfused with Ang II or AT1R-activating autoantibodies, the anti-AT2R sera significantly inhibited their contractile effects. Rabbit anti-AT2R sera recognized a predominant sequence near the N-terminus of the AT2R second extracellular loop. A decoy peptide based on this sequence effectively reversed the opposing effect of the anti-AT2R sera on Ang II-induced contraction of rat cremaster arterioles. A similar blockade of the anti-AT2R sera effect was observed with the AT2R antagonist PD 123319 and the guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Rabbit anti-AT2R sera reacted specifically with AT2R. No cross-reactivity with AT1R was observed. Blood pressure did not change in immunized animals. However, the pressor responses to incremental Ang II infusions were blunted in immunized animals. Thirteen subjects with primary aldosteronism demonstrated increased AT2R autoantibody levels compared to normal controls. In conclusion, AT2R autoantibodies produced in immunized rabbits have the ability to activate AT2R and counteract the AT1R-mediated vasoconstriction. These autoantibodies provide useful and selective tools for study of their roles in blood pressure regulation and possible therapeutic intervention.
Keywords: activating autoantibodies, angiotensin II type 1 receptor, angiotensin II type 2 receptor, hypertension, vasoconstriction, rabbit
Introduction
The renin angiotensin system plays a key role in the regulation of blood pressure and cardiovascular function. Angiotensin II (Ang II) binds to two major subtypes of receptor, the angiotensin type 1 receptor (AT1R) and angiotensin type 2 receptor (AT2R). The majority of the actions of Ang II are mediated by AT1R; however, recent evidence suggests that AT2R opposes AT1R by promoting vasodilatation rather than vasoconstriction.1–4 There has been an increasing awareness of the presence of autoantibodies directed toward the extracellular loops of several G protein-coupled receptors and these often express remarkable specificity for a given receptor.5, 6 Autoantibodies that activate the AT1R have been described and appear to be highly selective for their target domain on the second extracellular loop (ECL2) of AT1R.7, 8 These autoantibodies were initially identified in a limited number of subjects, but this list has expanded rapidly in the last few years in several hypertensive-related conditions such as preeclampsia,9 malignant and refractory hypertension,10, 11 renal allograft rejection,12 and more recently with primary aldosteronism.13–15 There is no currently published information as to whether activating autoantibodies to the AT2R exist; and whether they might alter the complex role of Ang II in vascular homeostasis.
We have recently developed a rabbit autoimmune model of AT1R-activating autoantibody (AT1R-AAb)-mediated hypertension.16 These animals were immunized with a peptide containing the known functional AT1R-AAb epitope in the ECL2 of AT1R. After immunization, all animals developed elevated blood pressure. The anti-AT1R sera induced significant AT1R activation and vasoconstriction in vitro. In the present study, we describe our successful efforts to develop AT2R-activating autoantibodies (AT2R-AAbs) in immunized rabbits and to demonstrate their potential impact on Ang II-mediated hypertension and AT1R-AAb-mediated vasoconstriction. We have discovered AT1R-AAbs and AT2R-AAbs maintain a high degree of specificity for their target receptors and produce opposing effects, thus providing a potentially valuable tool for studies of their roles in hypertension.
Methods
This study protocol was approved by the Institutional Animal Care and Use Committee of the Oklahoma City Veterans Affairs Medical Center and Oklahoma University Health Sciences Center, and conforms to international standards for animal safety and comfort.
Animal Model
Five New Zealand white rabbits (2.5–3 kg) were fed on standard rabbit chow and were immunized with 1 mg of a synthetic peptide corresponding to the AT2R ECL2 (YFRDVRTIEYLGVNACIMAFPPEKYAQWS) (GenScript, Piscataway, NJ) in 0.5 ml of complete Freund’s adjuvant. The animals were boosted with the same peptide plus incomplete Freund’s adjuvant (1 mg/0.5 ml) at 2 and 4 weeks. Arterial blood pressure was measured at preimmune and 6 weeks after immunization. Under anesthesia (ketamine/xylazine 35 mg/5 mg/kg), the rabbit central ear artery was cannulated and the catheter connected to a pressure transducer. To determine the acute effect of Ang II on blood pressure before and after immunization, increasing dosages of Ang II (10, 100, and 500 ng/kg) were injected intravenously at 5-minute intervals using an infusion pump, and the blood pressure response at each dose was recorded. Each rabbit served as its own control. Preimmune and postimmune sera were obtained from all animals for ELISA and functional assays of the expected antibodies generated during immunization. AT1R-AAbs were obtained from the rabbits previously immunized with the AT1R ECL2 peptide.16
Human Studies
Sera were available for testing autoantibodies from a group of 13 hypertensive subjects who previously had been diagnosed with primary aldosteronism using an IRB-approved protocol. These subjects for the most part had not had lateralization studies since their hypertension was generally under good control with medications, and the patients had declined to consider surgery irrespective of the testing outcome or there were evident contraindications from the physician’s viewpoint. Each fulfilled criteria for establishing the diagnosis using one of the established methods for confirmation of an abnormal plasma aldosterone/plasma renin activity (PA/PRA) ratio of over 30.17 The two defining tests used included a captopril suppression study (n=9) and saline infusion study (n=1), while others had a PA/PRA ratio of >50 and an absolute PA value over 25 ng/dl (n=3) negating the need for further diagnostic tests.
ELISA
Antibodies produced in the sera were detected by ELISA. Briefly, microtiter plates were coated with the AT2R ECL2 peptide at 10 μg/ml. To determine antibody titer, rabbit sera were diluted 1:10000 and thereafter serially diluted 2-fold. Goat anti-rabbit IgG conjugated with alkaline phosphatase and its substrate para-nitrophenyl-phosphate 104 were used to detect antibody binding. Titers were determined as the highest dilution with an optical density (OD) value of 0.10 at 60 minutes. Human sera were diluted 1:100, and goat anti-human IgG conjugated with alkaline phosphatase was used for the secondary antibody. Their OD values were read at 60 min.
Epitope Mapping
Epitope mapping for rabbit AT2R autoantibodies was performed with multipin peptides (constructed in Dr. Judith James’s laboratory, Oklahoma Medical Research Foundation), which comprised a set of 22 octapeptides, overlapping by 7 amino acids spanning the ECL2 of AT2R. Rabbit anti-AT2R sera (1:200) was added to the wells of 96-well plate along with the multipin peptides and incubated for 2 hours at room temperature. The multipin peptides were washed with PBS-Tween and incubated with anti-rabbit IgG antibody conjugated with horseradish peroxidase. Reactivity of rabbit anti-AT2R sera to the pins was measured by adding tetramethyl benzidine substrate solution in the developing plate. The OD values were read at 405 nm.
Isolated Arteriole Assay
Autoantibody contractile activity was assayed using an isolated rat cremaster arteriole assay as previously described.16, 18 Briefly, arteriolar segments were microdissected from the cremaster muscle of anesthetized rats and cannulated with glass micropipettes. After equilibration and development of steady-state myogenic tone, the arterioles were perifused with rabbit sera. Dosage responses for Ang II (10−10 ~ 10−7 M) and rabbit anti-AT1R sera (1:500 - 1:50) in the absence and presence of rabbit anti-AT2R sera (1:50) were performed to examine the impact of AT2R-AAbs on Ang II and AT1R-AAb-induced contractile response. The AT2R antagonist PD 123319 (10 nM) was used to block AT2R-mediated effect. The potent soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, 1 μM) was also tested for neutralization of the vasodilatory effect of AT2R-AAbs. Measurements of vessel diameter were made using a video edge detector. Data are reported as percentage of baseline diameter to normalize each response.
Statistical Analysis
Data are presented as mean ± SD. Comparison between two groups and multiple group comparisons were performed using the nonparametric Mann-Whitney test and Kruskal-Wallis test followed by Dunn’s multiple comparison test, respectively. Statistical significance was set at P<0.05.
Results
Effect of AT2R-AAbs on Ang II and AT1R-AAb-Induced Contractile Response in Vitro
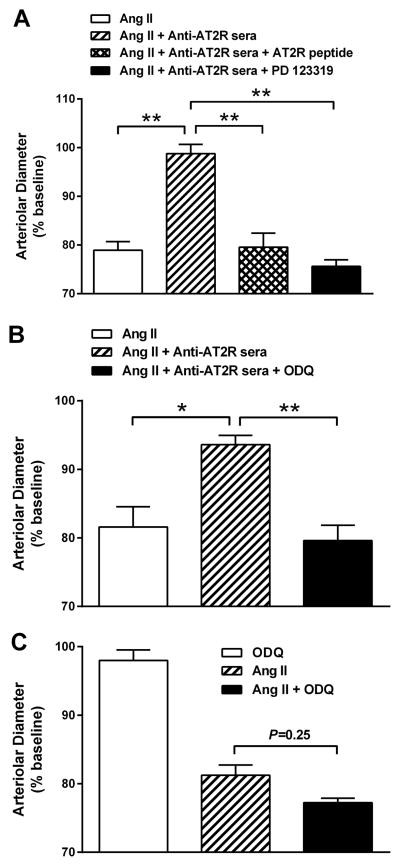
All 5 rabbits developed high antibody titers to AT2R ranging from 1:320,000 to 1:640,000 after peptide immunization. The ability of rabbit antisera to activate AT2R was tested by their effect on counteracting AT1R-mediated vasoconstriction in the rat cremaster arteriole assay. The isolated resistance arterioles were tested with the anti-AT2R sera alone at dilutions from 1:500 to 1:50, and increasing concentrations of Ang II from 10−10 to 10−7 M. Rabbit anti-AT1R sera was examined at equivalent dilutions in the absence and presence of anti-AT2R sera at 1:50 dilution. The contractile responses were recorded and compared. The anti-AT2R sera failed to produce any direct effect on arteriole diameter (Figure 1A). However, when co-perfused with Ang II (Figure 1B) or anti-AT1R sera (Figure 1C), the anti-AT2R sera largely suppressed their contractile responses. Both Ang II and anti-AT1R sera produced a dose-dependent stepwise contraction, which was completely blocked by the anti-AT2R sera.
Figure 1.
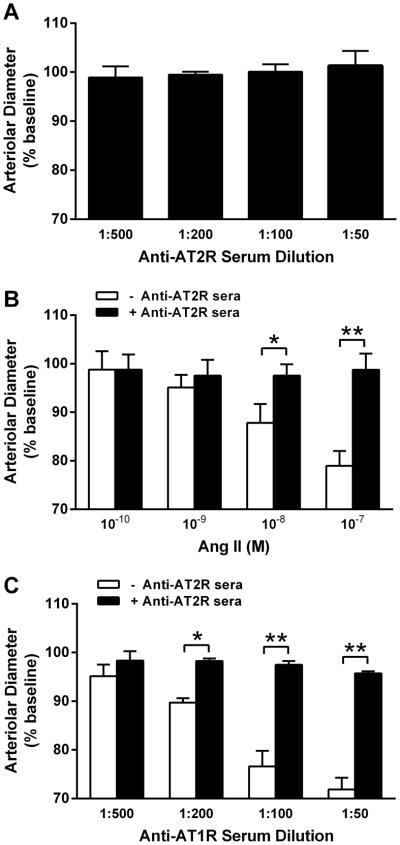
A, Effect of rabbit anti-AT2R sera on contractility in the rat cremaster arteriole assay. No direct effect on the arterial diameter was observed when anti-AT2R sera (1:500 - 1:50) were tested alone (n=5). B, Effect of rabbit anti-AT2R sera on Ang II-induced contraction of isolated rat cremaster arterioles. Ang II (10−10 to 10−7 M) produced a dose-dependent contraction, which was effectively blocked by the anti-AT2R sera (1:50). *P<0.05, **P<0.01, n=3. C, Effect of rabbit anti-AT2R sera on rabbit anti-AT1R sera-induced contraction of isolated rat cremaster arterioles. There was a similar dose-dependent contractile effect for the anti-AT1R sera (1:500 - 1:50), which was also suppressed by the anti-AT2R sera (1:50). *P<0.05, **P<0.01, n=3. Data are expressed as percentage of buffer baseline.
Identification of Functional Epitope and Specificity of AT2R-AAbs
To determine the epitope of AT2R-AAbs, a set of 22 overlapping peptides covering the ECL2 of AT2R were synthesized and tested for their reactivity with the anti-AT2R sera. The predominant sequence recognized by the anti-AT2R sera was DVRTIEYL located near the N-terminus of the AT2R ECL2 (Figure 2).
Figure 2.
Epitope mapping of rabbit anti-AT2R sera. Epitope determination was performed by ELISA using a multipin peptide set of 22 overlapping octapeptides spanning the second extracellular loop (ECL2) of AT2R. The anti-AT2R sera reacted predominantly with the peptide DVRTIEYL near the N-terminus of AT2R ECL2.
An 8-mer peptide containing the epitope sequence DVRTIEYL was then synthesized and tested for its ability to inhibit the functional activity of AT2R-AAbs in the rat cremaster arteriole assay. The contractile responses to Ang II (10−7 M), and Ang II (10−7 M) plus anti-AT2R sera (1:50) in the absence and presence of the AT2R epitope peptide (1 μM) or AT2R antagonist PD 123319 (10 nM) were examined and compared (Figure 3A). Ang II expectedly caused significant vasoconstriction, which was greatly inhibited by the anti-AT2R sera (from 79±3% to 98±3%, P<0.01, n=3). The addition of the decoy peptide effectively reversed the opposing effect of the anti-AT2R sera on Ang II-induced contraction (from 98±3% to 80±5%, P<0.01, n=3). A similar blockade of the AT2R-AAb effect was observed with the AT2R blocker PD 123319 (from 98±3% to 76±2%, P<0.01, n=3), confirming AT2R-mediated effect of the anti-AT2R sera.
Figure 3.
A, Effect of AT2R peptide and AT2R blocker on blocking the activity of rabbit anti-AT2R sera in the rat cremaster arteriole assay. Ang II (10−7 M)-induced vasoconstriction was reversed by the anti-AT2R sera (1:50). The addition of the AT2R epitope peptide DVRTIEYL (1 μM) or AT2R antagonist PD 123319 (10 nM) completely blocked the opposing effect of the anti-AT2R sera on Ang II-induced contraction. **P<0.01, n=3. B, Effect of guanylyl cyclase inhibitor on the vasoactivity of rabbit anti-AT2R sera in the rat cremaster arteriole assay. Ang II (10−8 M)-induced vasoconstriction was similarly reversed by the anti-AT2R sera (1:100). The vasodilatory effect of the rabbit anti-AT2R sera was neutralized by the guanylyl cyclase inhibitor ODQ (1 μM). *P<0.05, **P<0.01, n=5. C, Effect of ODQ on Ang II-induced vasoconstriction in the rat cremaster arteriole assay. ODQ (1 μM) alone had no effect on the basal arteriolar diameter. ODQ given with Ang II (10−8 M) led to a slight but not significant increase in contractility compared to Ang II alone (P=0.25, n=4). Data are expressed as percentage of buffer baseline.
We repeated the Ang II (10−8 M) contractile response before and after addition of the anti-AT2R sera (1:100). This had the expected inhibitor impact on the Ang II response. We then added the guanylyl cyclase inhibitor ODQ (1 μM) and observed a significant increase in arteriole contractility. This value reached the contractility observed with the Ang II alone (Figure 3B). When ODQ (1 μM) was administered alone, there was no significant change in baseline vessel diameter (Figure 3C). There was a slight increase in the Ang II (10−8 M) contractile response when ODQ was added compared to Ang II alone, but this did not reach significance (P=0.25).
To check the specificity of rabbit antisera, the anti-AT2R sera were examined for cross-reactivity with AT1R by ELISA. As shown in Figure 4A, the anti-AT2R sera reacted specifically with the AT2R, and not the AT1R ECL2 peptide. Cross-reactivity was further examined in a cell-based AT1R activation assay.16 The rabbit anti-AT1R sera stimulated significant AT1R activation in AT1R-transfected cells, while the anti-AT2R sera failed to activate AT1R (Figure 4B). In addition, the AT2R ECL2 peptide failed to suppress anti-AT1R sera-induced AT1R activation (data not shown). Unfortunately, there are currently no bioassays available for direct measurement of AT2R activation in transfected cells.
Figure 4.
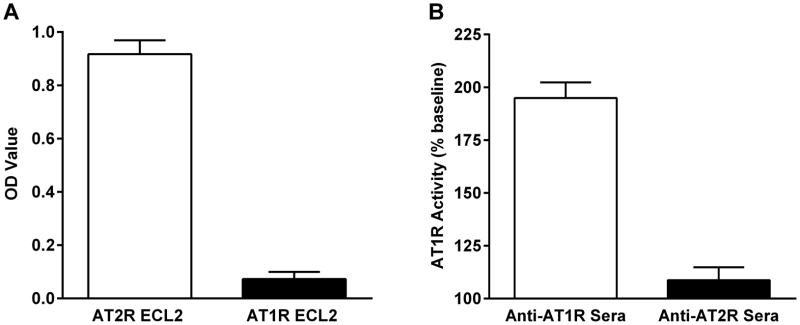
Specificity of rabbit anti-AT2R sera. Rabbit anti-AT2R sera were tested for reactivity with AT2R vs. AT1R ECL2 peptide in ELISA (A). The anti-AT2R sera demonstrated AT2R-specific reactivity. No cross-reactivity with AT1R was observed. Rabbit anti-AT2R sera were also tested for activation of AT1R in AT1R-transfected cells (B). Significant activation of AT1R was observed with rabbit anti-AT1R sera, while there was no AT1R activation by the anti-AT2R sera. Data are expressed as percentage of buffer baseline.
Effect of AT2R-AAbs on Ang II-Induced Pressor Response in Vivo
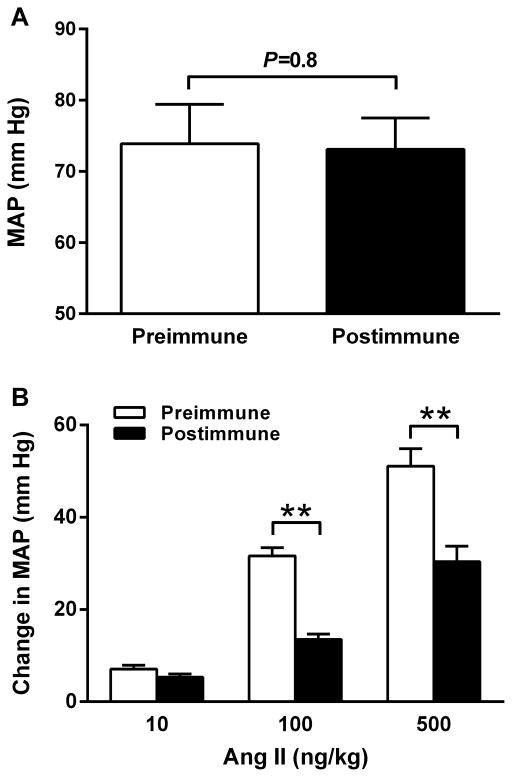
The immunized rabbits remained normotensive at 6 weeks after immunization despite high AT2R-AAb titers. No significant difference in the mean arterial pressure (MAP) was observed between the preimmune and postimmune states (preimmune: 74±6 vs. postimmune: 73±4 mm Hg, P=0.8) (Figure 5A). This was in contrast to our previously reported data in AT1R ECL2 peptide-immunized rabbits who developed significant elevation of blood pressure.16 Their antibody titers were in the similar range.
Figure 5.
Blood pressure (A) and pressor responses to Ang II infusion (B) in AT2R-immunized rabbits. No significant change in the mean arterial pressure (MAP) was observed at 6 weeks after immunization. However, infusion of the rabbits with increasing doses of Ang II (10, 100 and 500 ng/kg) before immunization caused significantly greater increases in MAP than those observed at 6 weeks after immunization. **P<0.01, n=5.
To determine whether AT2R-AAbs would alter the blood pressure response to Ang II injections, we infused increasing dosages (10, 100 and 500 ng/kg) of Ang II into rabbits before and after immunization and compared their MAP values. Ang II infusion caused transient, dose-dependent increases in MAP in both preimmune and postimmune states; however, the magnitude of the pressor response in the postimmune state was significantly lower than that in the preimmune state (Figure 5B). At dosages of 100 and 500 ng/kg, Ang II caused an increase in MAP of 13.5±1.2 and 30.4±3.3 mm Hg respectively in the postimmune state compared to 31.6±1.8 and 51.1±3.8 mm Hg respectively in the preimmune state (P<0.01), which demonstrated a significant opposing effect of AT2R-AAbs on AT1R-mediated pressor response to Ang II.
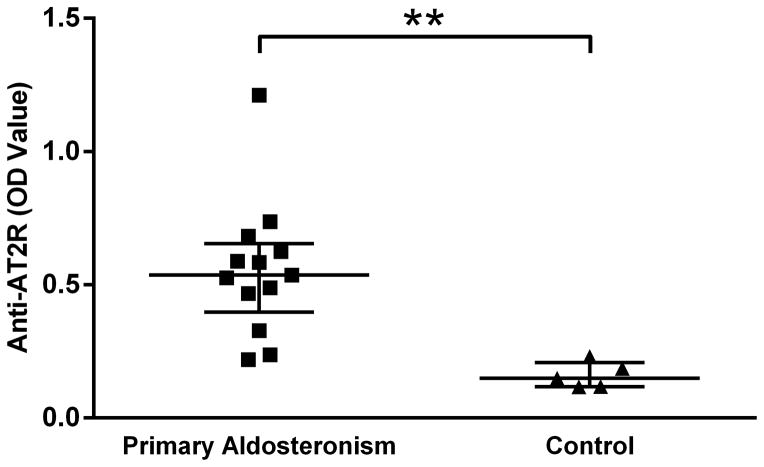
AT2R Autoantibodies in Primary Aldosteronism
We screened 18 human subjects for circulating autoantibodies directed against the same AT2R ECL2 peptide used for the present animal studies. Thirteen subjects had previously been diagnosed with hypertension associated with primary aldosteronism. Five were normal subjects without measured hypertension. The mean ELISA OD values were significantly higher in patients with primary aldosteronism compared to the normal subjects (Figure 6). We did not sample subjects with essential hypertension in this preliminary study.
Figure 6.
ELISA detection of autoantibodies to AT2R in patients with primary aldosteronism (n=13) and healthy control subjects (n=5). Median optical density (OD) values and interquartile ranges are shown. **P<0.01.
Discussion
The classical angiotensin ligand-receptor interaction has been complicated by the discovery of the AT2R with its quite different spectrum of activation and consequences upon activation by Ang II. A focused view of this concurrent orthosteric receptor activation by Ang II includes an inhibitory effect on Ang II-mediated contractility in vascular tissues. There are additional effects on growth responses, again with the impression of the AT2R counteracting the AT1R-mediated actions of Ang II.19 The discovery of AT1R-AAbs in some hypertensive diseases which exert an allosteric impact on AT1R-mediated actions has added an additional level of complexity.7, 8 To date, there has been no report of an AAb directed toward the AT2R. The present study reports our efforts to examine the possibility that such an AAb exists. This is not easily accomplished since direct assays of AT2R activation are not readily available and most studies rely on measurement of secondary responses.
We have hypothesized the presence of AAbs targeting the AT2R ECL2 similar to those observed against other G protein-coupled receptors. A synthetic peptide containing the human AT2R ECL2 sequence was used as immunogen to generate antigen-specific antibodies in rabbits. This model readily produced antibodies to the AT2R ECL2 in a 6 week period. We have subsequently demonstrated these antibodies activate AT2R with indirect but substantial proofs. These antibodies effectively diminished the blood pressure response to Ang II in immunized animals. We have also demonstrated these antibodies are active in vitro. They blocked the contractile effects of Ang II and AT1R-AAbs on cremaster arterioles. These effects were not due to a direct interaction with the AT1R since they were reversed by the AT2R blocker PD 123319 and by the ODQ blockade of guanylyl cyclase known to be activated by AT2R-stimulated nitric oxide production. There were no apparent direct effects of either inhibitor on the vessel contractility when used alone. The AT2R-AAbs did not react with AT1R when studied in vitro. We have also used a decoy peptide, constructed to mimic the potential primary target epitope on the AT2R ECL2, to completely inhibit the activity of the induced AT2R-AAbs.
Based on data from other investigators and our own, we have developed the concept that the orthosteric actions of Ang II on both AT1R and AT2R are of primary importance in the normally balanced Ang II-mediated transduction observed in vivo. The autoimmune production of active autoantibodies to either the AT1R and/or AT2R may superimpose allosteric modulation on the primary orthosteric activity of Ang II and lead to either enhanced or diminished primary effects of the Ang II depending on the receptor and upon the spectrum of AAbs present.
With our present knowledge and technique, it is difficult to ascertain the clinical relevance of AT2R-AAbs since they do not cause a recognizable phenotype in our immunized animals. We do provide evidence that the concurrence of AT2R-AAbs and AT1R-AAbs or Ang II may modulate the hypertensive effects of these agonists. The acute effect of infused Ang II on the systemic blood pressure in the AT2R-immunized rabbits was significantly blunted compared to that observed in their preimmune period.
In order to obtain preliminary information as to whether AT2R autoantibodies are present in human disease, we screened by ELISA a group of subjects with primary aldosteronism who also harbored AT1R-AAbs.13, 14 A significant number of these subjects showed positivity for autoantibodies directed to the AT2R ECL2. We are currently developing selective alternative assays to identify the presence of activity associated with these autoantibodies since they frequently coexist with AT1R-AAbs. ELISA assays using small peptides as the target can be variable in sensitivity and specificity and fail to document activity. The development of other assays to monitor activity without increasing their complexity would permit investigators to determine if the AT2R-AAbs may alter the impact of Ang II or AT1R-AAbs on blood pressure in several forms of hypertension in humans. The use of the selective ECL2 decoy peptide to inhibit activity of the AT2R-AAbs in physiological experiments might also provide an avenue to examine their biological relevance. Their co-presence with elevated Ang II or AT1R-AAbs might also provide the rationale for use of an angiotensin receptor blocker rather than angiotensin converting enzyme inhibitor for some forms of therapy.
There are little clinical data concerning the effects of AT2R in normal and hypertensive subjects. Since excessive stimulation of the AT1R by Ang II is known to cause detrimental effects, activation of the AT2R is generally thought to serve as a protective role in the cardiovascular system by counter-regulating the AT1R function.1–4 Pharmacological activation of AT2R thus provides a new therapeutic strategy that can be used alone or in conjunction with AT1R blockade. A nonpeptide AT2R agonist C21 has been developed with potential therapeutic applications in hypertensive conditions.20, 21 Our discovery of AT2R-AAbs and their high specificity offer another potential approach to therapeutic interventions.
Perspectives
We have developed novel AT2R-AAbs in immunized rabbits and demonstrated these antibodies are able to counteract the pressor responses to Ang II and AT1R-AAbs. These effects are blocked by the AT2R antagonist PD 123319, by an AT2R-derived decoy peptide and by the guanylyl cyclase inhibitor ODQ which blocks AT2R downstream signaling by inhibiting cyclic GMP formation. These data support the concept that AT2R-AAbs activate the AT2R and are capable of modulating Ang II action. These data have important implications since AT2R-AAbs, when present, may alter the roles of Ang II and AT1R-AAbs in blood pressure regulation. The high specificity of our AT2R-AAbs also provides a unique opportunity to develop a new therapeutic strategy for treatment of hypertensive disorders.
Novelty and Significance.
What Is New?
Immunization of rabbits with a peptide from the angiotensin AT2 receptor (AT2R) produced AT2R-activating autoantibodies, which counteracted the pressor effects of angiotensin II and angiotensin AT1 receptor-activating autoantibodies. These autoantibodies were observed in high prevalence when screening hypertensive patients with primary aldosteronism.
What Is Relevant?
AT2R-activating autoantibodies when present may contribute to blood pressure regulation in certain hypertensive disorders.
Summary
This study supports a role of AT2R-activating autoantibodies in blood pressure regulation. Their high specificity may open a new avenue for developing treatment strategies for hypertension.
Acknowledgments
Source of Funding:
This work was supported by funding from a Veterans Affairs Merit Review Award (D.C. Kem and X. Yu), National Heart, Lung, and Blood Institute R01 HL056267 (M.W. Cunningham and D.C. Kem), American Heart Association Postdoctoral Fellowship (H. Li), and individual grant support from Will and Helen Webster. C. Liles was supported by the Oklahoma NARCH Student Development Program funded by NIH grant GM092238/U26IHS300412 through the Oklahoma Native American Research Centers for Health (ONARCH VI).
Footnotes
Presented in part at the 40th International Aldosterone Conference, June 2014, Chicago, IL.
Disclosures: None.
References
- 1.Carey RM. Angiotensin type-2 receptors and cardiovascular function: Are angiotensin type-2 receptors protective? Curr Opin Cardiol. 2005;20:264–269. doi: 10.1097/01.hco.0000166596.44711.b4. [DOI] [PubMed] [Google Scholar]
- 2.Savoia C, D’Agostino M, Lauri F, Volpe M. Angiotensin type 2 receptor in hypertensive cardiovascular disease. Curr Opin Nephrol Hypertens. 2011;20:125–132. doi: 10.1097/MNH.0b013e3283437fcd. [DOI] [PubMed] [Google Scholar]
- 3.Padia SH, Carey RM. At2 receptors: Beneficial counter-regulatory role in cardiovascular and renal function. Pflugers Arch. 2013;465:99–110. doi: 10.1007/s00424-012-1146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones ES, Vinh A, McCarthy CA, Gaspari TA, Widdop RE. At2 receptors: Functional relevance in cardiovascular disease. Pharmacol Ther. 2008;120:292–316. doi: 10.1016/j.pharmthera.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallukat G, Schimke I. Agonistic autoantibodies directed against g-protein-coupled receptors and their relationship to cardiovascular diseases. Semin Immunopathol. 2014;36:351–363. doi: 10.1007/s00281-014-0425-9. [DOI] [PubMed] [Google Scholar]
- 6.Dragun D, Philippe A, Catar R, Hegner B. Autoimmune mediated g-protein receptor activation in cardiovascular and renal pathologies. Thromb Haemost. 2009;101:643–648. [PubMed] [Google Scholar]
- 7.Xia Y, Kellems RE. Angiotensin receptor agonistic autoantibodies and hypertension: Preeclampsia and beyond. Circ Res. 2013;113:78–87. doi: 10.1161/CIRCRESAHA.113.300752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaMarca B, Wallace K, Granger J. Role of angiotensin ii type i receptor agonistic autoantibodies (at1-aa) in preeclampsia. Curr Opin Pharmacol. 2011;11:175–179. doi: 10.1016/j.coph.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin at1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu ML, Herlitz H, Schulze W, Wallukat G, Micke P, Eftekhari P, Sjogren KG, Hjalmarson A, Muller-Esterl W, Hoebeke J. Autoantibodies against the angiotensin receptor (at1) in patients with hypertension. J Hypertens. 2000;18:945–953. doi: 10.1097/00004872-200018070-00017. [DOI] [PubMed] [Google Scholar]
- 11.Liao YH, Wei YM, Wang M, Wang ZH, Yuan HT, Cheng LX. Autoantibodies against at1-receptor and alpha1-adrenergic receptor in patients with hypertension. Hypertens Res. 2002;25:641–646. doi: 10.1291/hypres.25.641. [DOI] [PubMed] [Google Scholar]
- 12.Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kelha M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schonemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G. Angiotensin ii type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 13.Rossitto G, Regolisti G, Rossi E, Negro A, Nicoli D, Casali B, Toniato A, Caroccia B, Seccia TM, Walther T, Rossi GP. Elevation of angiotensin-ii type-1-receptor autoantibodies titer in primary aldosteronism as a result of aldosterone-producing adenoma. Hypertension. 2013;61:526–533. doi: 10.1161/HYPERTENSIONAHA.112.202945. [DOI] [PubMed] [Google Scholar]
- 14.Kem DC, Li H, Velarde-Miranda C, Liles C, Vanderlinde-Wood M, Galloway A, Khan M, Zillner C, Benbrook A, Rao V, Gomez-Sanchez CE, Cunningham MW, Yu X. Autoimmune mechanisms activating the angiotensin at1 receptor in ‘primary’ aldosteronism. J Clin Endocrinol Metab. 2014;99:1790–1797. doi: 10.1210/jc.2013-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Yu X, Cicala MV, Mantero F, Benbrook A, Veitla V, Cunningham MW, Kem DC. Prevalence of angiotensin ii type 1 receptor (at1r)-activating autoantibodies in primary aldosteronism. J Am Soc Hypertens. 2015;9:15–20. doi: 10.1016/j.jash.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Kem DC, Zhang L, Huang B, Liles C, Benbrook A, Gali H, Veitla V, Scherlag BJ, Cunningham MW, Yu X. Novel retro-inverso peptide inhibitor reverses angiotensin receptor autoantibody-induced hypertension in the rabbit. Hypertension. 2015;65:793–799. doi: 10.1161/HYPERTENSIONAHA.114.05037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF, Jr, Montori VM. Case detection, diagnosis, and treatment of patients with primary aldosteronism: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Zuccolo J, Kem DC, Zillner C, Lee J, Smith K, James JA, Cunningham MW, Yu X. Implications of a vasodilatory human monoclonal autoantibody in postural hypotension. J Biol Chem. 2013;288:30734–30741. doi: 10.1074/jbc.M113.477869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T International union of pharmacology. Xxiii. The angiotensin ii receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 20.Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu X, Johansson B, Holm M, Botoros M, Karlen A, Pettersson A, Nyberg F, Fandriks L, Gallo-Payet N, Hallberg A, Alterman M. Design, synthesis, and biological evaluation of the first selective nonpeptide at2 receptor agonist. J Med Chem. 2004;47:5995–6008. doi: 10.1021/jm049715t. [DOI] [PubMed] [Google Scholar]
- 21.Foulquier S, Steckelings UM, Unger T. Impact of the at(2) receptor agonist c21 on blood pressure and beyond. Curr Hypertens Rep. 2012;14:403–409. doi: 10.1007/s11906-012-0291-6. [DOI] [PubMed] [Google Scholar]