Abstract
Objectives
To identify classes of individuals presenting to the ED for suspected ACS who shared similar symptoms and clinical characteristics.
Background
Describing symptom clusters in undiagnosed patients with suspected ACS is a novel and clinically relevant approach, reflecting real-world emergency department evaluation procedures
Methods
Symptoms were measured using a validated 13-item symptom checklist. Latent class analysis was used to describe symptom clusters.
Results
The sample of 874 was 37% female with a mean age of 59.9 years. Four symptom classes were identified: Heavy Symptom Burden (Class 1), Chest Symptoms and Shortness of Breath (Class 2), Chest Symptoms Only (Class 3), and Weary (Class 4). Patients with ACS were more likely to cluster in Classes 2 and 3. Women and younger patients were more likely to group in Class 1.
Conclusions
Further research is needed to determine the value of symptom clusters in the ED triage and management of suspected ACS.
Keywords: Symptom Clusters, Latent Class Analysis, Acute Coronary Syndrome, Age, Sex, Race, Diagnosis
Introduction
Background
Each year in the United States, 5.5 million patients are evaluated for acute coronary syndrome (ACS) in emergency departments (EDs), yet only 13.5% are ultimately ruled in for ACS.1 Triage of these patients has been called one of the most challenging of diagnostic dilemmas.2 Recent advances in rapid diagnosis of ACS include the use of serial measurements of high-sensitivity cardiac troponin,3 computed tomography, angiography, and diagnostic decision tools such as the Chest Pain Choice Decision Aid.4 In addition, many EDs have established chest pain units with protocols for accelerated risk stratification,5 and referral of low risk patients for outpatient stress testing.6 Despite these advances, current approaches still lack adequate sensitivity and specificity given the high costs of evaluation and serious consequences of a missed ACS diagnosis. Most triage protocols use a limited set of symptoms, often focusing on chest related complaints (pain, pressure, discomfort) to evaluate patients for potential ACS. However, patients can present with a variety of symptoms,7 and there is a continuing need to improve ACS risk stratification strategies and protocols.
Reliance on chest pain symptoms alone is inadequate for patients to decide to seek care or for clinicians to determine appropriate diagnostic testing. In addition, chest pain severity is not related to the likelihood of myocardial infarction (MI)8 and women with ACS are more likely to describe non-chest pain symptoms, including shortness of breath, weakness, and fatigue.9 Over 80% of patients report more than one symptom7 with several studies reporting an average of 7 to 8 symptoms.9–11 Consequently, there has been increasing interest in describing symptom clusters in ACS.12–15 Symptom cluster definitions have varied. Miaskowski et al. have defined symptom clusters in cancer as 3 or more symptoms that co-occur and are related to each other.16 Kim et al. defined symptom clusters as 2 or more symptoms.17 Between 3 and 5 different symptom clusters have been described in previous studies of ACS patients.12–15 Prior ACS symptom cluster studies included only patients with diagnosed ACS. In prior work, we found patients clustered in 4 groups which were labeled Heavy Symptom Burden, Chest Pain Only, Sweating and Weak, and Short of Breath and Weak.12 The mean number of symptoms per cluster was six.12 Ryan et al.15 reported that none of the clusters identified in a study of patients with MI included all typical symptoms; however, age, race, and sex were predictors of cluster membership. In the only study analyzing symptom clusters between black and white women, McSweeney et al.13 discovered that younger black women with ACS clustered in the group with the most distressing symptoms. Riegel et al.14 identified four symptom clusters in ACS patients and found that those experiencing a diffuse pattern of symptoms were older and had increased mortality over two years. However, to date no studies have described symptom clusters in patients evaluated for possible ACS in the ED. The current study is novel because it included patients presenting to the ED with symptoms that triggered a cardiac evaluation. The patient’s diagnosis was unknown at the time of enrollment into the study and was subsequently obtained from the medical record following hospital discharge.
The ACS diagnostic dilemma is challenging because of the immense cost of evaluating millions of patients for ACS in EDs annually and the serious consequences of missed ACS for both the patient and the provider. Identification and analysis of symptom clusters in patients who present with potential ACS could assist clinicians in risk stratification, improve rapid evaluation, reduce costs associated with diagnostic testing and hospitalization as well as facilitate patients’ decision-making and treatment seeking behavior. Describing symptom clusters in undifferentiated patients (i.e., those who arrive in the ED without a diagnosis) with suspected ACS is a novel and clinically relevant approach, reflecting the real-world scenario of ED triage and assessment. The majority of patients evaluated in the ED for ACS are undifferentiated on arrival.
Goals of the Investigation
The purpose of this study was to identify classes of individuals presenting to the ED for suspected ACS who shared similar symptoms and clinical characteristics. We hypothesized that subgroups of patients with similar symptom clusters (latent classes) could be identified and that these classes would differ by sex, age and discharge diagnosis.
Methods
Study Design
This analysis is part of a larger prospective, longitudinal study to examine the influence of sex on symptoms during ACS. The study was approved by the institutional review boards at all participating sites. Waiver of consent to complete initial screening from the medical record and to collect symptom data prior to enrollment was obtained. This was necessary given that the parent study’s main aim was to assess symptoms as they were occurring on presentation to the ED.
Sample and Setting
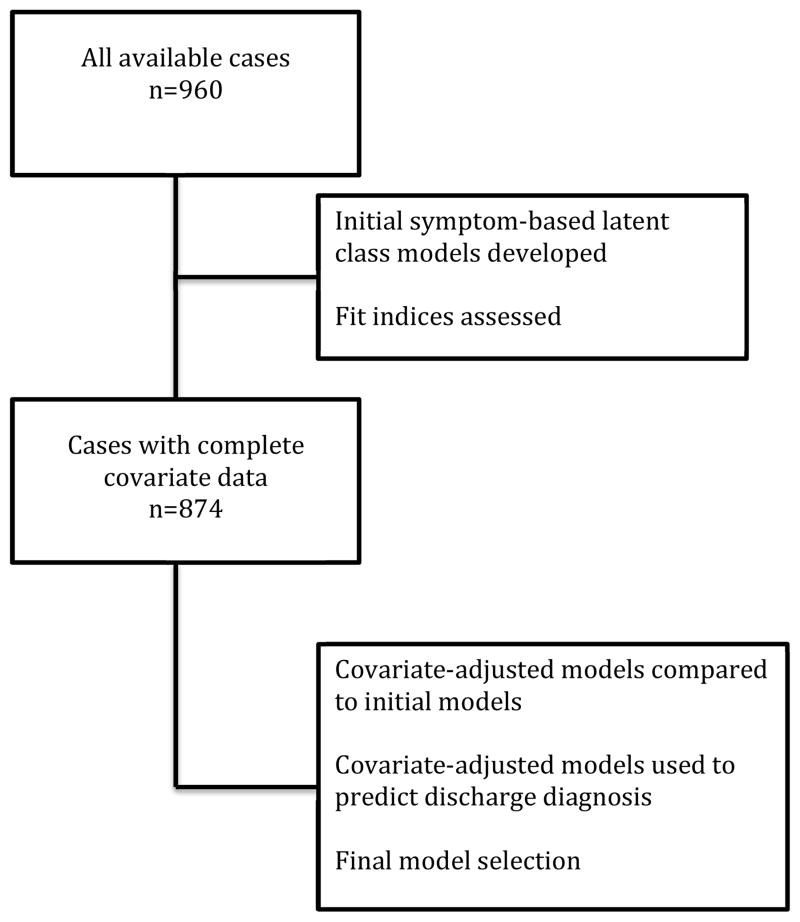
The convenience sample consisted of 960 patients who presented to the ED and were identified by the triage nurse as potentially having ACS; 874 with complete covariate data were included in the final analysis (Figure 1). This protocol was designed to reflect the way patients are actually triaged and evaluated in the ED. Patients were included if they were high risk for ACS (abnormal electrocardiogram (ECG) or positive troponin), ≥ 21 years of age, English speaking, had telephone access, and intact cognition. A positive troponin was defined as a value exceeding the reference norm for the institution. Cognitive capacity was deemed acceptable if the patient was able to understand the purpose of the study and provided written informed consent. Patients were excluded if they had cardiac symptoms in conjunction with an exacerbation of heart failure (B-type natriuretic peptide >500 ng/mL), were referred to the ED from a hemodialysis center or were referred for cardiac dysrhythmia evaluation. The study sites included 4 academic medical centers and a large community hospital located in the Midwest, Southwest, Pacific Northwest, and Western regions of the United States (>250,000 annual ED visits combined). Patients were enrolled from January, 2011 through December, 2013.
Figure 1.
Sample Used in Model Selection
Measures
ACS Symptom Checklist
Symptoms were measured with the validated 13-item ACS symptom checklist.18 Participants indicate whether the symptom is present or absent. Symptoms not listed on the checklist can be recorded in a blank space marked “other”. There is no summary score and each symptom is analyzed separately.
ACS Patient Information Questionnaire
Patient baseline characteristics were collected using the ACS Patient Information Questionnaire. This demographic and clinical questionnaire was designed using the standardized reporting guidelines recommended for studies of ED patients with potential ACS.19
Duke Activity Status Index
Functional status was measured with the Duke Activity Status Index (DASI), a 12-item instrument that measures functional capacity.20 Scores range from 0–58.2, with higher scores representing better physical functioning. Items are weighted to reflect metabolic energy expenditure and correlate highly with peak VO2 (r = .80, p < 0.0001)20 in patients with ACS,21 ischemic heart disease,22 heart failure,23 and revascularization procedures.24
Charlson Comorbidity Index
Comorbid conditions were measured with the Charlson Comorbidity Index (CCI), a 19-item weighted index which has been used extensively to quantify risk associated with comorbid conditions.25,26 Charlson Comorbidity Index scores represent the number and severity of comorbid conditions. Studies have demonstrated that the CCI is a valid measure for predicting disability and death following ischemic stroke and heart disease.27 Correlations with mortality, disability, hospital readmission and length of stay ranged from 0.35–0.93 (p<0.001).28,29
Procedures
The EDs were staffed from 8 to16 hours per day, 7 days per week with trained research assistants (RA) who recorded initial symptoms and clinical characteristics.19 Research Assistants started study procedures after patients were registered and classified by the triage staff as having potential ACS. The ACS Symptom Checklist was then completed by the RA using a face to face interview, usually within 10 minutes of arrival to the ED to assess symptoms present upon arrival. After initial stabilization, patients were screened for eligibility, the study was explained, and written consent was obtained. If patients declined to participate, the symptom checklist was destroyed. The diagnosis at discharge from the hospital, whether from the ED or inpatient setting, was abstracted from the medical record. These discharge diagnoses were clinical judgments made by the attending physician.
Statistical Analysis
Our objective in the present analysis was to identify patient groups (latent classes) with similar symptom patterns based on their responses to the 13 symptoms from the ACS Symptom Checklist. Latent class analysis (LCA), a type of mixture model, was our primary approach. Latent class analysis is used to estimate a categorical latent variable, a model-based variable inferred from measured variables that divides people into subgroups with similar characteristics. Some symptom studies have used a factor analytic method, where the latent variable is a continuous factor score inferred from a group of interrelated symptoms. Either model can be fit to a sample, however, the optimal solution is often a mixture of these two models, called a factor mixture model.30–32 We used the factor mixture model.
Latent Class Analysis modeling is an iterative process, where multiple models are considered (1, 2, 3, up to k classes) until model fit is optimal and conditional independence is met. We had to balance the need to meet model assumptions with a need for useful, interpretable classes. Conditional independence is a strict assumption for the pure LCA model that states that variables within the latent classes must be independent. With an otherwise good fitting, interpretable model, conditional dependence may suggest that more classes are needed because variables within a subgroup show correlation. An alternative solution to adding classes is the factor mixture model, where factors within or across classes are added to model residual correlations between the observed indicators. A factor mixture model can be compared to either LCA or factor analysis with the same relative fit indices since they are all model based.
All LCA analyses were conducted using Mplus Version 7.2.33 Latent class parameters for item response probability (ρs) and probability for class membership (γ) were estimated using maximum likelihood with robust standard errors. Models were further evaluated for conditional independence using bivariate Pearson testing to identify residual correlations within classes; residual associations of the binary indicators were modeled as correlations within classes. The final model was selected based on meaningful interpretability, relative fit indices such as Akaike Information Classification (AIC) and Bayesian Information Classification (BIC), entropy, and associations with covariates and diagnoses. Nested models were compared using Satorra-Bentler scaled chi-square difference tests. In addition to estimating the latent variable, mixture models can be extended to include covariates as predictors of the latent variable or to use the latent variable as a predictor of an outcome like discharge diagnosis. A latent class multinomial regression model was used to assess the influence of covariates including sex, age, body mass index (BMI), functional ability (DASI), comorbid conditions (CCI) and recruitment site on class membership. The recruitment site variable was collapsed into two categories: recruitment site C versus all others. Recruitment site C was compared to all other recruitment sites because site C had a higher proportion of patients ruled-out for ACS compared to all other sites, had a much higher number of patients enrolled, and was the first site to begin enrolling patients. The latent classes were tested as predictors of an ACS diagnosis, including the specific diagnoses of unstable angina, non-ST elevation myocardial infarction (NSTEMI) and ST elevation myocardial infarction (STEMI). A manual 3-step approach that classified individuals based on symptoms and covariates, estimated measurement error, and used class membership adjusted for measurement error as a nominal predictor of diagnosis was used.34 Participants were classified using highest posterior probability for analyses that could not be tested within extended models.
Additional analyses were conducted using SPSS, version 19.0 (IBM Corp, Armonk, NY). Demographic and clinical characteristics were evaluated using descriptive statistics. Chi-square analysis was used to test differences in the occurrence of symptoms by sex and ACS diagnosis. Significance level was set at p<0.05 for all inferential statistics.
Results
Demographic and Clinical Characteristics
Table 1 shows demographic and clinical information for the 874 patients with complete covariate data who were included in the final analysis. Forty-three percent ruled in for ACS, and NSTEMI was the most common form of ACS. The sample was 37% female with a mean age of 59.9 (±14.2) years. The majority of patients had some limitations in physical activity (mean weighted DASI score of 33.7), and major cardiac risk factors (hypertension, diabetes, hypercholesterolemia), but few comorbid conditions as measured by the CCI. The most common comorbid conditions were diabetes (23.0%) and asthma (21.7%).
Table 1.
Demographic and Clinical Characteristics
| Characteristic | n = 874 |
|---|---|
| ACS Diagnosis, (no.;%) | 373 (42.7) |
| Unstable Angina | 87 (10.0) |
| NSTEMI | 195 (22.3) |
| STEMI | 91 (10.4) |
| Women (no., %) | 323 (37.0) |
| Age (mean years, range) | 59.9 (21–94) |
| Body Mass Index (mean, range) | 30.2 (13.4–80.7) |
| Hypertension (no., %) | 572 (65.4) |
| Diabetes (no., %) | 256 (29.3) |
| Hypercholesterolemia (no., %) | 465 (53.2) |
| Duke Status Activity Index (no., %) | |
| 58.2 (no limitations) | 207 (23.7) |
| 30 to < 58.2 | 267 (30.5) |
| < 30 | 400 (45.8) |
| Charlson Comorbidity Index (no., %) | |
| 0 – 1 | 468 (53.5) |
| 2– 3 | 260 (29.7) |
| 4+ | 146 (16.7) |
| Recruitment Site,(no., %) | |
| Site A | 72 (8.2) |
| Site B | 110 (12.6) |
| Site C | 383 (43.8) |
| Site D | 48 (5.5) |
| Site E | 261 (26.9) |
Notes: ACS is acute coronary syndrome. No. is number. sd is standard deviation. NSTEMI is non-ST evelvation myocardial infarction. STEMI is ST elevation myocardial infarction.
Individual Symptoms
The most common symptoms in both women and men were chest discomfort, chest pain, and chest pressure. Fatigue was more common in patients who ruled out for ACS regardless of sex. Among men who ruled out, shortness of breath, lightheadedness, upper back pain, and palpitations were also more common, whereas chest pain was more common in men who ruled in for ACS. No single symptom was more common in women who ruled in for ACS. Chest discomfort was more common among women who ruled out, and women had overall higher rates of non-chest symptoms (Table 2).
Table 2.
Individual Symptoms by ACS Diagnosis
| Symptoms | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| ACS n = 96 | Non-ACS n = 227 | ACS n = 277 | Non-ACS n = 274 | |||||
|
| ||||||||
| n (%) | n (%) | X2 | p-value | n (%) | n (%) | X2 | p-value | |
| Chest Discomfort | 61 (63.5) | 172 (75.8) | 5.0 | 0.025 | 196 (70.8) | 189 (69.0) | 0.2 | 0.649 |
| Chest Pain | 62 (64.6) | 154 (67.8) | 0.3 | 0.570 | 207 (74.7) | 181 (66.1) | 5.0 | 0.026 |
| Chest Pressure | 64 (66.7) | 160 (70.5) | 0.5 | 0.496 | 181 (65.3) | 165 (60.2) | 0.2 | 0.213 |
| Shortness of Breath | 54 (56.3) | 143 (63.0) | 1.3 | 0.256 | 125 (45.1) | 164 (59.9) | 12.0 | 0.001 |
| Unusual Fatigue | 40 (41.7) | 125 (55.1) | 4.8 | 0.028 | 96 (34.7) | 148 (54.0) | 20.9 | <0.0001 |
| Lightheadedness | 41 (42.7) | 123 (54.2) | 3.6 | 0.059 | 95 (34.3) | 122 (44.5) | 6.0 | 0.014 |
| Nausea | 39 (40.6) | 102 (44.9) | 0.5 | 0.475 | 86 (31.0) | 84 (30.7) | 0.0 | 0.921 |
| Arm Pain | 40 (41.7) | 84 (37.0) | 0.6 | 0.431 | 94 (33.9) | 82 (29.9) | 1.0 | 0.313 |
| Sweating | 32 (33.3) | 79 (34.8) | 0.1 | 0.800 | 101 (36.5) | 85 (31.0) | 1.8 | 0.177 |
| Shoulder Pain | 42 (43.8) | 86 (37.9) | 1.0 | 0.325 | 80 (28.9) | 88 (32.1) | 0.7 | 0.409 |
| Upper Back Pain | 32 (33.3) | 93 (41.0) | 1.7 | 0.198 | 45 (16.2) | 73 (26.6) | 8.8 | 0.003 |
| Palpitations | 28 (29.2) | 80 (35.2) | 1.1 | 0.290 | 50 (18.1) | 71 (25.9) | 5.0 | 0.026 |
| Indigestion | 26 (27.1) | 55 (24.2) | 0.3 | 0.589 | 50 (18.1) | 57 (20.8) | 0.7 | 0.414 |
Notes: Bold values represent statistically significant differences.
Latent Class Model Selection
Latent class models were developed using all available cases (n=960) to assess relative fit indices for selecting the number of latent classes (Table 3). Models with 2, 3, 4, and 5 classes were systematically evaluated to determine the best fit, balancing the number of classes and number of correlations allowed within classes. We selected a 4 class factor mixture model that allowed 4 fixed correlations (arm pain and shoulder pain, shoulder pain and upper back pain, nausea and indigestion, and nausea with sweating) for select classes that showed residual correlations. This model showed a significantly better fit to the data than the model assuming conditional independence [χ2(4)=39.96, p<.001,35 and fit indices suggested better fit than the 5 class model assuming conditional independence (Table 3).
Table 3.
Model Selection Indices Based on All Available Cases (n=960)
| Index | 2 Class | 3 Class | 4 Class | 5 Class |
|---|---|---|---|---|
| Akaike Information Classification (AIC) | 14684.09 | 14445.11 | 14370.83 | 14306.06 |
| Bayesian Information Classification (BIC) | 14815.50 | 14644.65 | 14638.52 | 14641.88 |
| Sample-Size Adjusted BIC | 14729.75 | 14514.43 | 14463.84 | 14422.74 |
| Entropy | 0.752 | 0.712 | 0.699 | 0.711 |
| Vuong-Lo-Mendell-Rubin (VLMR) | 0 | 0.027 | 0.085 | 0.015 |
Note: All classes included a correlation between upper back pain and shoulder pain. Class 2 had a correlation between nausea with sweating. Classes 1, 2, and 4 had additional correlations between arm and shoulder pain and nausea and indigestion.
Further analysis compared symptoms only models to models that included all covariates. Covariate-adjusted 4 and 5 class models were also used to predict discharge diagnoses. While the 5 class solution identified separate subgroups for a high rate of chest symptoms with and without arm pain and shoulder pain, these separate classes showed a very similar pattern of diagnoses to the combined class supporting the 4 class model. Finally, we compared the classes to prior findings12 and found that the 4 class model was most consistent and made sense from a clinical perspective. Thus, the 4 class model was selected for additional analyses based on statistical and clinically substantive criteria.
Symptom Clusters
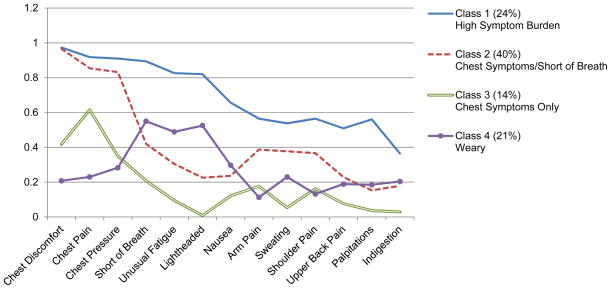
The probability of a specific symptom being present in a cluster was defined as high (≥.60–1.0), moderate (≥.40–<.60), and low (<.40) based on prior symptom cluster studies12,15 and the salience of the information provided in each class. Class 1 was labeled Heavy Symptom Burden as patients in this class had the largest number (7) of high probability symptoms. Class 2 was labeled Chest Symptoms and Shortness of Breath since the only high probability symptoms were chest discomfort, pain, and pressure. Shortness of breath was the only moderate probability symptom in this class. Class 2 had the highest number of patients (n=287). Class 3 contained only one high probability symptom, chest pain, and one moderate probability symptom, chest discomfort, and was thus labeled Chest Symptoms. Class 3 had the fewest number of patients (n=127). Class 4 contained no high probability symptoms and 3 moderate probability symptoms, shortness of breath, unusual fatigue, and lightheadedness, and was labeled Weary (Table 4). The addition of covariates affected the distribution of participants in each class, but had minimal impact on the measurement model, i.e., the item (symptom) response probabilities (Figure 2). The class membership of patients by sex, age, BMI, functional status, comorbid conditions, and recruitment site C are shown in Table 5.
Table 4.
Probability of Symptom Occurrence by Latent Class
| Symptoms | Class 1 Heavy Symptom Burden | Class 2 Chest Symptoms & Shortness of Breath | Class 3 Chest Symptoms Only | Class 4 Weary |
|---|---|---|---|---|
| n=260 (29.7%) | n=287 (32.8%) | n=127 (14.5%) | n=200 (22.9%) | |
| Chest Discomfort | 97.3** | 96.6** | 41.8* | 20.8 |
| Chest Pain | 91.8** | 85.4** | 61.6** | 23.0 |
| Chest Pressure | 91.0** | 83.2** | 34.9 | 28.3 |
| Shortness of Breath | 89.4** | 42.0* | 20.8 | 55.0* |
| Unusual Fatigue | 82.6** | 30.5 | 9.5 | 48.9* |
| Lightheadedness | 82.0** | 22.6 | 0.9 | 52.5* |
| Nausea | 65.6** | 23.7 | 12.2 | 29.7 |
| Arm Pain | 56.5* | 38.7 | 17.7 | 11.3 |
| Sweating | 53.8* | 37.7 | 5.4 | 23.0 |
| Shoulder Pain | 56.5* | 36.7 | 16.2 | 13.2 |
| Upper Back Pain | 50.9* | 22.9 | 7.7 | 18.9 |
| Palpitations | 56.1* | 15.3 | 3.7 | 18.5 |
| Indigestion | 36.4 | 17.9 | 2.9 | 20.4 |
Note:
indicates high probability symptoms (.60–1.0) and
indicate moderate probability symptoms (.40 to <.60).
Models are adjusted for sex, age, BMI, functional status (DASI), comorbidities (CCI), and recruitment site C.
Figure 2.
Symptom Probabilities for Adjusted Latent Classes
Note: Y axis is the probability of the symptom for that Class. Adjusted model includes sex, age, body mass index, Duke Activity Status Index, Charlson Comorbidity Index score, and recruitment site C.
Table 5.
Covariates and Latent Class Membership
| Covariate | Class 1 Heavy Symptom Burden | Class 2 Chest Symptoms & Shortness of Breath | Class 3 Chest Symptoms Only | Class 4 Weary |
|---|---|---|---|---|
| n = 260 (29.7%) | n = 287 (32.8%) | n = 127 (14.5%) | n = 200 (22.9%) | |
| Female (n, %) | 136 (52.3)2,3,4 | 75 (26.1)1 | 29 (22.8)1 | 83 (41.5)1 |
| Age (mean, sd) | 54.8 (14.5)2,3,4 | 59.0 (13.7)1,3 | 65.4 (11.8)1,2 | 64.2 (13.2)1 |
| BMI (mean, sd) | 30.3 (7.6) | 29.8 (6.9) | 29.1 (5.7) | 31.4 (8.9) |
| DASI (mean, sd) | 27.5 (17.9)2,3 | 43.0 (17.2)1,4 | 37.8 (17.3)1,4 | 24.5 (17.1)2,3 |
| CCI (mean, sd) | 2.0 (2.0) | 1.4 (1.6)4 | 2.0 (1.9) | 2.5 (2.3)2 |
| Recruitment Site C (n,%) | 156 (60.0)2,3 | 99 (34.5)1 | 29 (22.8)1 | 99 (49.5) |
Notes: BMI is body mass index. DASI is Duke Activity Status Index. CCI is Charlson Comorbidity Index. n is number. sd is standard deviation.
is a significant difference from Class 1.
is a significant difference from Class 2.
is a significant difference from Class 3.
is a significant difference from Class 4.
Results show that class membership varied by sex; with women most often in Class 1 (Heavy Symptom Burden). Classes also differed by age, functional status, comorbidities, and recruitment site. Patients in Class 1 were younger than those in Classes 2 (Chest Symptoms and Shortness of Breath), 3 (Chest Symptoms), and 4 (Weary). Patients in Classes 1 and 4 had lower DASI scores and Class 4 had higher CCI scores than Class 2 (mean: 2.5 vs. 1.4). Patients from recruitment site C were more likely to be in Class 1 versus Classes 2 and 3.
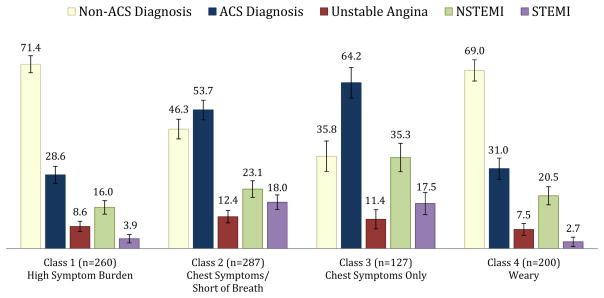
Class membership was hypothesized to differ between patients who ruled-in versus those who ruled-out for ACS and this was confirmed. The majority of patients who ruled-in for ACS clustered in Classes 2 and 3; these classes had a predominance of chest symptoms (Figure 3). In addition, there were significantly more patients with STEMI in Classes 2 and 3. Those diagnosed with NSTEMI were more likely to cluster in Class 3 than in Classes 1 or 4.
Figure 3.
ACS Diagnosis by Latent Class Membership Adjusted for Covariates
Note: The bars represent the predicted percentage of participants with each diagnosis in each Class. Model adjusted for sex, age, body mass index, Duke Activity Status Index score, Charlson Comorbidity Index score, and recruitment site C. NSTEMI is non-ST elevation myocardial infarction. STEMI is ST elevation myocardial infarction.
Discussion
As hypothesized, four subgroups of potential ACS patients with similar symptom clusters (latent classes) were identified and these groups differed by sex, age and discharge diagnosis. These results indicate that symptom clusters could potentially increase precision of current risk stratification models and expedite the ED triage process. The finding that patients with STEMI were most likely to be in the two classes characterized predominately by chest symptoms is also noteworthy. Patients with complete coronary artery occlusion benefit the most from early reperfusion therapies and hence reporting of any chest symptoms at triage requires prompt and expeditious evaluation with an ECG and prompt measurement of cardiac biomarkers.
These findings support the concept that model-based latent classes can be used to identify groups of individuals presenting to the ED with clusters of symptoms suggesting ACS. As depicted in Figure 3, the greatest proportion of patients who ruled-out for ACS were in Class 1, the class with the largest number of high probability symptoms (n=7). Women were more likely to be in Class 1, the class that included multiple non-chest symptoms. These findings discredit the notion that sex differences in symptom clusters are explained by a smaller proportion of women with ACS; the highest proportion of women both with and without ACS were in Class 1. This difference in presentation likely disadvantages women in a clinical presentation model that is overly focused on chest symptoms, in particular pain. Older patients were less likely to be in Class 1 (Heavy Symptom Burden) than Classes 2–4 and less likely to be in Class 2 (Chest Symptoms and Shortness of Breath Only) than Class 3 (Chest Symptoms Only). This finding is consistent with prior literature which demonstrates that older ACS patients often present with fewer symptoms.36,37 Thus, older patients with more common and less specific symptoms such as unusual fatigue or shortness of breath may also be at a disadvantage when seeking care for potential ACS if the symptom model being used is primarily focused on chest pain. Functional status (DASI score) was associated with membership in Classes 1 (Heavy Symptom Burden) and 4 (Weary) as would be expected because of symptoms such as unusual fatigue, lightheadedness and shortness of breath which may limit physical activity.
Class membership varied between site C and the other 3 sites. This may be explained by the higher number of patients recruited at site C and the higher proportion of patients at site C ruled-out for ACS. Site C evaluates a higher proportion of potential cardiac patients compared to the other sites and it is possible that regional, cultural, and health status differences are also reflected in these statistics.
Finally, latent classes of patients in our study showed similarities to prior studies in that class 1 was composed of multiple symptoms and class 3 was composed of chest symptoms12,15. Any differences in our findings compared to previous studies may be partially explained by differences in analytic methods. We chose to use a latent class analysis approach as we aimed to classify clusters of individuals based on symptoms and other characteristics rather than a factor analytic approach (clusters symptoms) which is more suitable for use in evaluating properties of instruments.
Implications for Practice
Classes 2 and 3 best represent the current paradigm of risk for ACS: middle-aged men with chest pain. Chest pain is the predominant symptom of ACS used in clinical presentation models, but is also well-known to the general public. Our findings demonstrate that women and elders are potentially disadvantaged by current symptom models, risk stratification protocols, public health messaging, and patient education that focus on chest pain. Practitioners can use our findings as the basis for assessing the full spectrum of symptoms for suspected ACS patients.
Implications for Research
These findings need to be validated in other studies that also include patients who are undiagnosed on presentation as well as in studies with larger samples of underrepresented racial and ethnic groups. Canto and colleagues38 have stressed the importance of using standardized measures to assess the full range of ACS symptoms experienced by women and men. We recommend use of the 13-item ACS Symptom Checklist in future studies, especially for its ability to capture the symptom experience of women.
Strengths
We enrolled a large, geographically and racially diverse sample of patients presenting to the ED in whom ACS was suspected, representing the actual ED environment. The choice of possible covariates was empirically-based in order to adjust for variables known to affect the symptom experience during ACS (sex, age, BMI, functional status, & comorbid conditions). Additionally, we constructed statistical latent class models based on symptom presentation rather than stratified by discharge diagnosis in order to best represent the clinical environment of the ED. Thus, our findings that patients presenting with symptoms of potential ACS can be classified by sex, age, and discharge diagnosis are applicable to clinical practice and patient education.
Limitations
Selection bias is a limitation to the study since not all patients presenting to the EDs with symptoms suggestive of ACS or receiving an ACS evaluation during the study time period were enrolled. Data collection occurred from 8 to 16 hours per day depending on the availability of research staff. Some eligible patients were missed either because they declined participation or the urgency of the clinical scenario precluded patient enrollment. We purposefully sampled patients at high risk for an ACS diagnosis (abnormal ECG or positive troponin) in order to enroll an adequate number of patients ruled-in for ACS. Without a targeted sampling plan, we would have had to enroll thousands of patients to be adequately powered to detect group differences (n=630; 80% power to detect a small effect, d=02). Recruitment site C enrolled a higher proportion of patients ruled-out for ACS hence recruitment site was included in adjusted latent class models.
The diagnosis of ACS was abstracted from the medical record and was based on the judgment of the attending physician rather than by systematic adjudication by the study team. Although there is the possibility of classification bias, our trained medical record reviewers evaluated troponin results and alerted the site principal investigator to any discrepancies in values and discharge diagnoses. Thus we believe that any misclassifications were rare and random. Our decision to use discharge diagnosis rather than the commonly-used 30-day major adverse cardiac events (MACE) as the clinical endpoint reflects the study focus on triage processes currently used to guide assessment and management of suspected ACS in the ED setting rather than on diagnostic certainty.
Conclusion
Patients presenting to the ED with potential ACS clustered in 4 distinct classes based on their presenting symptoms, and these classes differed by sex, age, discharge diagnosis, functional status, and comorbid conditions. Women who ruled in and ruled out for ACS were more likely to be in the Heavy Symptom Burden class. Elders were less likely to be in the Heavy Symptom Burden class while patients with lower physical functioning were more likely to be in the Heavy Symptom Burden or Weary Classes. Further research is needed to validate the use of symptom clusters in ED triage, treatment and risk stratification of suspected ACS patients, as well as to support their use in patient education and public health messaging.
Acknowledgments
This study is funded by NIH: R01 NR012012 (DeVon, PI).
Abbreviations List
- ACS
acute coronary syndrome
- AIC
Akaike Information Classification
- BIC
Bayesian Information Classification
- BMI
body mass index
- CCI
Charlson Comorbidity Index
- DASI
Duke Activity Status Index
- ECG
electrocardiogram
- ED
emergency department
- MACE
major adverse cardiac events
- MI
myocardial infarction
- No.
number
- NSTEMI
non-ST elevation myocardial infarction
- RA
research assistant
- SD
standard deviation
- STEMI
ST elevation myocardial infarction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anne G. Rosenfeld, Email: anner@email.arizona.edu, University of Arizona College of Nursing, 1305 N. Martin Ave., Tucson, AZ 85721-0203.
Elizabeth P. Knight, Email: eknight@email.arizona.edu, University of Arizona College of Nursing, Tucson, AZ.
Alana Steffen, Email: steffena@uic.edu, University of Illinois at Chicago College of Nursing, 845 S. Damen Ave. #748 MC 802, Chicago, IL 60612.
Larisa Burke, Email: laburke@uic.edu, University of Illinois at Chicago College of Nursing, Chicago, IL.
Mohamud Daya, Email: dayam@ohsu.edu, Oregon Health & Science University, Department of Emergency Medicine, 3181 SW Sam Jackson Park Rd., Portland, OR 97239.
Holli A. DeVon, Email: hdevon1@uic.edu, University of Illinois at Chicago College of Nursing, Chicago, IL.
References
- 1.Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999–2008. NCHS data brief. 2010;(43):1–8. [PubMed] [Google Scholar]
- 2.Newby LK. Myocardial infarction rule-out in the emergency department: are high-sensitivity troponins the answer? Arch Intern Med. 2012;172(16):1218–1219. doi: 10.1001/archinternmed.2012.1808. [DOI] [PubMed] [Google Scholar]
- 3.Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172(16):1211–1218. doi: 10.1001/archinternmed.2012.3698. [DOI] [PubMed] [Google Scholar]
- 4.Hess EP, Knoedler MA, Shah ND, et al. The Chest Pain Choice Decision Aid: a randomized trial. Circ Cardiovasc Qual Outcomes. 2012;5(3):251–259. doi: 10.1161/CIRCOUTCOMES.111.964791. [DOI] [PubMed] [Google Scholar]
- 5.Than M, Cullen L, Aldous S, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol. 2012;59(23):2091–2098. doi: 10.1016/j.jacc.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 6.Scheuermeyer FX, Innes G, Grafstein E, et al. Safety and efficiency of a chest pain diagnostic algorithm with selective outpatient stress testing for emergency department patients with potential ischemic chest pain. Ann Emerg Med. 2012;59(4):256–264. e253. doi: 10.1016/j.annemergmed.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Richards SB, Funk M, Milner KA. Differences between Blacks and Whites with coronary heart disease in initial symptoms and in delay in seeking care. Am J Crit Care. 2000;9(4):237–244. [PubMed] [Google Scholar]
- 8.Edwards M, Chang AM, Matsuura AC, Green M, Robey JM, Hollander JE. Relationship between pain severity and outcomes in patients presenting with potential acute coronary syndromes. Ann Emerg Med. 2011;58(6):501–507. doi: 10.1016/j.annemergmed.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 9.McSweeney JC, Cody M, O'Sullivan P, Elberson K, Moser DK, Garvin BJ. Women's early warning symptoms of acute myocardial infarction. Circulation. 2003;108(21):2619–2623. doi: 10.1161/01.CIR.0000097116.29625.7C. [DOI] [PubMed] [Google Scholar]
- 10.DeVon HA, Ryan CJ, Ochs AL, Shapiro M. Symptoms across the continuum of acute coronary syndromes: differences between women and men. Am J Crit Care. 2008;17(1):14–24. quiz 25. [PMC free article] [PubMed] [Google Scholar]
- 11.Zerwic JJ, Ryan CJ, DeVon HA, Drell MJ. Treatment seeking for acute myocardial infarction symptoms: differences in delay across sex and race. Nurs Res. 2003;52(3):159–167. doi: 10.1097/00006199-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 12.DeVon HA, Ryan CJ, Rankin SH, Cooper BA. Classifying subgroups of patients with symptoms of acute coronary syndromes: A cluster analysis. Res Nurs Health. 2010;33(5):386–397. doi: 10.1002/nur.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McSweeney JC, Cleves MA, Zhao W, Lefler LL, Yang S. Cluster analysis of women's prodromal and acute myocardial infarction symptoms by race and other characteristics. J Cardiovasc Nurs. 2010;25(4):311–322. doi: 10.1097/JCN.0b013e3181cfba15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riegel B, Hanlon AL, McKinley S, et al. Differences in mortality in acute coronary syndrome symptom clusters. Am Heart J. 2010;159(3):392–398. doi: 10.1016/j.ahj.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan CJ, DeVon HA, Horne R, et al. Symptom clusters in acute myocardial infarction: a secondary data analysis. Nurs Res. 2007;56(2):72–81. doi: 10.1097/01.NNR.0000263968.01254.d6. [DOI] [PubMed] [Google Scholar]
- 16.Dodd MJ, Miaskowski C, Lee KA. Occurrence of symptom clusters. J Natl Cancer Inst. 2004;(32):76–78. doi: 10.1093/jncimonographs/lgh008. Monographs. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;28(4):270–282. doi: 10.1097/00002820-200507000-00005. quiz 283–274. [DOI] [PubMed] [Google Scholar]
- 18.Devon HA, Rosenfeld A, Steffen AD, Daya M. Sensitivity, specificity, and sex differences in symptoms reported on the 13-item Acute Coronary Syndrome Checklist. J Am Heart Assoc. 2014;3(2):e000586. doi: 10.1161/JAHA.113.000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollander JE, Blomkalns AL, Brogan GX, et al. Standardized reporting guidelines for studies evaluating risk stratification of ED patients with potential acute coronary syndromes. Acad Emerg Med. 2004;11(12):1331–1340. doi: 10.1197/j.aem.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64(10):651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 21.Katz DA, Aufderheide TP, Bogner M, Rahko PS, Hillis SL, Selker HP. Do emergency department patients with possible acute coronary syndrome have better outcomes when admitted to cardiology versus other services? Ann Emerg Med. 2008;51(5):561–570. 570 e561. doi: 10.1016/j.annemergmed.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krantz DS, Olson MB, Francis JL, et al. Anger, hostility, and cardiac symptoms in women with suspected coronary artery disease: the Women's Ischemia Syndrome Evaluation (WISE) Study. J Womens Health (Larchmt) 2006;15(10):1214–1223. doi: 10.1089/jwh.2006.15.1214. [DOI] [PubMed] [Google Scholar]
- 23.Oka RK, Gortner SR, Stotts NA, Haskell WL. Predictors of physical activity in patients with chronic heart failure secondary to either ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1996;77(2):159–163. doi: 10.1016/s0002-9149(96)90588-3. [DOI] [PubMed] [Google Scholar]
- 24.Bourassa MG, Brooks MM, Mark DB, et al. Quality of life after coronary revascularization in the United States and Canada. Am J Cardiol. 2000;85(5):548–553. doi: 10.1016/s0002-9149(99)90809-3. [DOI] [PubMed] [Google Scholar]
- 25.De Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol. 2003;56(3):221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein LB, Samsa GP, Matchar DB, Horner RD. Charlson Index comorbidity adjustment for ischemic stroke outcome studies. Stroke. 2004;35(8):1941–1945. doi: 10.1161/01.STR.0000135225.80898.1c. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein JA, Chinnaiyan KM, Abidov A, et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) Trial. J Am Coll Cardiol. 2011;58(14):1414–1422. doi: 10.1016/j.jacc.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 28.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 29.Tessier A, Finch L, Daskalopoulou SS, Mayo NE. Validation of the Charlson Comorbidity Index for predicting functional outcome of stroke. Arch Phys Med Rehabil. 2008;89(7):1276–1283. doi: 10.1016/j.apmr.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 30.Muthén B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55(2):463–469. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 31.Vermunt J, Magidson J. Latent class cluster analysis. In: Hagenaars JA, McCutcheon AL, editors. Applied latent class analysis. New York: Cambridge University Press; 2002. pp. 89–106. [Google Scholar]
- 32.Lubke GH, Muthén B. Investigating population heterogeneity with factor mixture models. Psychol Methods. 2005;10(1):21. doi: 10.1037/1082-989X.10.1.21. [DOI] [PubMed] [Google Scholar]
- 33.Muthén LK, Muthén BO. Mplus User's Guide. 7. Los Angeles, CA: Muthen & Muthen; 1998–2012. [Google Scholar]
- 34.Asparouhov T, Muthén B. Exploratory structural equation modeling. Structural Equation Modeling. 2009;16(3):397–438. [Google Scholar]
- 35.Satorra A. Scaled and adjusted restricted tests in multi-sample analysis of moment structures. In: Heijmans RDH, Pollock DSG, Satorra A, editors. Innovations in multivariate statistical analysis. A Festschrift for Heinz Neudecker. London: Kluwer Academic Publishers; 2000. pp. 233–247. [Google Scholar]
- 36.Culic V, Eterovic D, Miric D, Silic N. Symptom presentation of acute myocardial infarction: influence of sex, age, and risk factors. Am Heart J. 2002;144(6):1012–1017. doi: 10.1067/mhj.2002.125625. [DOI] [PubMed] [Google Scholar]
- 37.Schoenenberger AW, Radovanovic D, Stauffer JC, et al. Age-related differences in the use of guideline-recommended medical and interventional therapies for acute coronary syndromes: a cohort study. J Am Geriatr Soc. 2008;56(3):510–516. doi: 10.1111/j.1532-5415.2007.01589.x. [DOI] [PubMed] [Google Scholar]
- 38.Canto JG, Canto EA, Goldberg RJ. Time to standardize and broaden the criteria of acute coronary syndrome symptom presentations in women. Can J Cardiol. 2014;30(7):721–728. doi: 10.1016/j.cjca.2013.10.015. [DOI] [PubMed] [Google Scholar]