Abstract
STEP into Action assessed the efficacy of a peer-based HIV prevention intervention in reducing HIV risk behaviors among people who inject drugs (PWIDs) in Baltimore. This analysis examined the effect of the intervention on the change in frequency of conversation about HIV prevention topics over time. 114 participants were randomized into an experimental and 113 into a control group. Data was collected prospectively at 6, 12, and 18 months. The experimental group talked more frequently about HIV prevention topics compared to the control group at 6-month visit. At 18 months relative risk ratios (RRR) remained statistically significant for conversation about the danger of needle sharing (RRR = 3.21) and condom use (RRR = 2.81). The intervention resulted in an increased conversation about HIV prevention among PWIDs, but the sustainability past 6 months remained a challenge; suggesting that interventions should be designed to constantly reinforce communication about HIV prevention among PWIDs.
Keywords: peer-based education, HIV prevention, people who inject drugs, Communication, Baltimore
Introduction
In the United States, HIV and drug abuse are major public health problems in the urban setting (1). Since the mid-1980s, injection drug use has been one of the main driving forces of the HIV epidemic in the United States (2–4).
Baltimore has a large population of people who inject drugs (PWIDs). The 2011 National Survey on Drug Use and Health estimates that 10.21% of Baltimore City residents 18 and older have abused drugs or alcohol within the past year (5). This amounts to approximately 63,400 individuals based on 2010 Census data (5). In 2007 there were 59,113 PWIDs in Baltimore according to Tempalski et al. (6). HIV incidence (accounting for 36.5% of all new cases) and prevalence (12%) among PWIDs in Baltimore remain high (7). In 2011 there were 4,159 PWIDs living with HIV accounting for 42.7% of all the HIV cases in Baltimore (7).
Fundamental to HIV transmission among PWIDs are their risky behaviors related to preparation, splitting of drugs and sharing of injection equipment (8). Splitting drugs often involves using a cooker to dissolve the drugs and removing liquids from the cooker by syringes, which can lead to HIV transmission. Needle sharing has declined over the years (9), but risky drug splitting practices still prevail and contribute to the perpetuation of HIV (8, 10).
Numerous public health interventions among PWIDs such as needle exchange programs (NEPs), drug treatment, and peer-based outreach have resulted in a decrease in HIV transmission among PWIDs (1, 11, 12). As mentioned above, in addition to NEPs and drug treatment programs, peer-based education is another method to prevent HIV transmission among PWIDs. These interventions are based on various social influence theories such as the ‘risk environment’ framework (13), diffusion of innovation theory (14), social learning (15), and social identity (16).
Peer-based interventions for HIV prevention have shown mixed results. A review by Simoni et al. (17) concludes that there is support for the effectiveness of peer-based interventions but more research is needed on moderators as well as utilizing rigorous evaluation methods. A systematic review by Ye et al. (18) on the impact of peer-based interventions for men who have sex with men (MSM) found that the effectiveness varied by study and emphasized the need for rigorous study design. These reviews highlight the conclusions of the meta-review conducted by Johnson and colleagues (19) that emphasized the importance of HIV prevention interventions documenting the approaches to behavioral change employed in interventions.
Although the literature on the peer-based interventions is mixed, there are studies which have shown that training PWIDs to be peer leaders in promoting HIV risk reduction has had a positive influence on both them and their community. Peer leaders have reported significant increases in condom use and cleaning of used needles with bleach (20). Their risk networks compared to controls’ risk network members were also more likely to report used needle cleaning (20). Nevertheless, risky injecting behaviors persist within this population and thus HIV prevalence among PWIDs in Baltimore remains high, 12% in 2011 (7).
Peer-based education is effective in reducing risk behaviors among PWIDs (17, 20, 21, 22). One of the key elements of the peer-based education is conversation about HIV prevention (23). Verbal communication plays an important role in the success of these interventions since it is the main agent for establishing, altering, and maintaining social norms (23). Thus far, cross-sectional studies have shown positive association between conversation about HIV prevention methods among PWIDs and reduction in their risk behaviors (24, 25). Nevertheless, little is known about the patterns of conversation about HIV-related topics among PWIDs and how peer-based interventions influence HIV prevention conversation in this population over time. This study aims to determine the effect of a peer-based educational intervention on change in frequency of conversation and sustainability of conversation about HIV prevention topics over time among PWIDs in Baltimore, Maryland.
Methods
Study design and study population
This was a randomized controlled trial of PWIDs with baseline information and prospective data collected at 6, 12 and 18 months. The inclusion criteria for the participants were: age 18 and older, reported injection drug use in the past 6 months, residency in Baltimore, MD, and willingness to have HIV prevention conversations and invite their risk network members into the study. In the study, participants trained to be peer educators are referred to as Index participants and their recruited risk network members are referred to as RNMs. Indexes were randomized to experimental and control groups. Signed consent forms were obtained from the participants at their baseline visit prior to the baseline interview and randomization.
Recruitment and Randomization
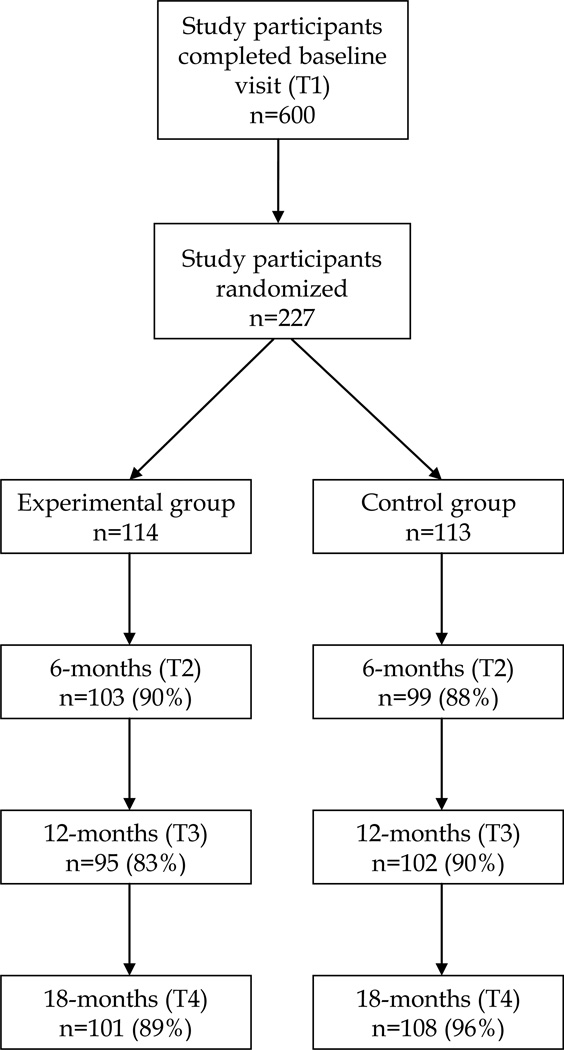
Study participants were recruited from March 2004 to March 2006 through street-based outreach, word of mouth, and advertisements posted throughout the community. 600 Index participants were enrolled into the study, 297 of them were able to recruit at least one risk network member and finally, 227 were randomized into experimental (n=114) and control (n=113) groups (Figure 1). Participants were stratified by gender and then randomized using standard computerized program designed in MS Access and blocking method (size of each block was four). The Johns Hopkins Bloomberg School of Public Health Institutional Review Board approved the study (26).
Figure 1.
Flowchart of study participants in the STEP into Action randomized trial, Baltimore, Maryland
Experimental condition
The intervention consisted of information about HIV prevention and teaching participants the skills needed to promote risk reduction within their personal risk networks. It was composed of seven sessions, five of which were group-based (26). The topics discussed at five group sessions were: introduction to the health educator role and communication, reduction of injection and drug splitting risk behavior (such as promotion of using syringes without needles to split liquid drugs and laminated sheets for dry drugs), sex risk reduction and use of condoms, credibility as a health educator, graduation and sustainability of skills (26). One of seven sessions was an individual session with an Index participant, which included goal-setting for the individual HIV risk reduction and outreach work. In the last one of seven session both Index and their RNMs participated (dyad session) which allowed Indexes to teach the HIV risk reduction methods and to set goals for HIV risk reduction with their RNMs (26).
The training that focused on teaching Indexes how to talk to their RNMs about HIV prevention and reduction in risk behaviors incorporated: different communication tools, numerous role-playing activities, homework assignments and dyad session. Attendance for the intervention sessions was high: 87% of the participants attended at least four of seven intervention sessions, 36% attended all sessions, and 64% completed the dyad session (26).
Control condition
The control condition consisted of five-group sessions (HIV 101 and testing, hepatitis 101, drug treatment, overdose risk factors, and overdose prevention) during which study participants received information on injection-drug use topics, but were not taught skills for HIV risk reduction. 85% of the study participants attended 3 out of 5 sessions.
Follow-up data collection
Participants were followed for 18 months. Data was collected at 6, 12, and 18 months (T2, T3, and T4) since the last session. Interviewers were blinded to the study condition of the participants. Participants were compensated $35 for every follow-up visit. More than 85% of study participants were retained in each study visit, Figure 1.
Outcome measures
One of the goals of the intervention was to increase the frequency of communication about HIV prevention topics between study participants and their “drug buddies” (individuals who study participants inject drugs with). In this analysis we focused on communication between study participants and their drug buddies about five HIV prevention topics: HIV testing, HIV transmission, needle cleaning with bleach, dangers of sharing needles with other people, and use of condoms. All of the outcomes had 8 ordered categories (talking: never; once or twice a year; once a month; a few times a month; once a week; a few times a week; once a day; and more than once a day). Based on the distribution of the outcomes, which was very similar for all the outcome variables at the baseline and not normally distributed, we grouped 8 categories into 3 categories (never = talking never or once or twice a year; at least once a month = once a month or a few times a month or once a week; at least few times a week = a few times a week or once a day or more than once a day).
The section of the questionnaire that contained the outcome variables of interest was skipped if participants reported not using heroin, cocaine or crack within past year. All the randomized participants had a response for the outcome variables at the baseline. At the 6-month visit, a response for the outcome variables was recorded for 80% of the participants. At the 12-month and 18-month visits, responses were recorded for 77% and 67% of the participants, respectively. However, experimental and control groups remained comparable throughout follow-up visits based on their baseline characteristics.
Potential covariates
Socio-demographic characteristics included gender, age, race, education and homelessness in the past 6 months from the baseline visit. Risk behaviors that were considered potential covariates were: exchanging sex in the past 90 days (from the baseline visit) for money, food, dugs or shelter and daily injecting of drugs in the past 6 months (from the baseline visit).
Statistical analysis
The sample for the analysis consisted of 227 randomized participants. Missing data was handled using model-wise deletion. A Chi-square test for categorical and a t-test for continuous variables were used to compare baseline demographic characteristics for the study participants by intervention assignment. Comparability between the two groups at each visit was explored with univariate logistic regression based on the baseline values for demographic characteristics. Univariate multinomial logistic regression was used to assess the association between the outcome and participants’ demographic characteristics. Patterns of missing data were examined with univariate logistic regression to explore whether missingness of the outcome was informative based on the outcome reported in previous visits and based on the baseline covariates.
To examine the intervention effect on participants’ behavior over the 18-month period we used multinomial logistic regression accounting for clustering by individual (27). In the model an independent correlation structure was assumed and standard error was calculated using the Huber-White sandwich estimator. An indicator variable for the four visits was included as a covariate in the model. Interaction between the time and intervention status was significant thus analysis was done stratifying by intervention status and by time. Comparability between the experimental and control groups across time was assessed based on the potential confounders (baseline characteristics) including them in the models as time-varying covariates when appropriate. This analysis suggested that it was not necessary to include covariates in the final models since the randomization was preserved across the visits. Data were analyzed based on the intent-to-treat assumption. Analysis was conducted using STATA, version 13.0 (28).
Results
The study enrolled 227 participants of which 114 were randomized into an experimental and 113 into a control group (Figure 1). Demographic characteristics and selected risk behaviors of the study participants are presented in Table 1. PWIDs in the two groups were comparable based on their baseline characteristics. 55% of the study participants were male. The average age of the participants was 43 years. Most of the participants, 85%, were African American. 45% of the participants completed 12th grade or higher education. 25 out of 203 participants who agreed to HIV testing were HIV positive. Unemployment in the past 6 months from baseline visit was very high, 92%. About one-fourth of participants were in prison in the past 6 months from the baseline visit. A large number of participants engaged in risky injecting and sex behaviors, 48% daily injected in past 6 months from baseline visit, 41% used unclean needles, and 65% used unclean cottons or cookers. 66% of the participants reported having one main sex partner, 34% had two or more sex partners. Among those who reported having sex in the prior 90 days from the initial visit 23% were exchanging sex for food, shelter, drugs or money.
Table 1.
Baseline population characteristics of randomized study participants, n (%).
| Experimental group (n=114) |
Control group (n=113) |
|
|---|---|---|
| Male | 63 (55) | 61 (54) |
| Age | 43.9 (7.8) | 43.0 (7.4) |
| African American | 96 (84) | 98 (87) |
| Orasure seropositive HIV status | 12 (12) out of 102* | 16 (16) out of 101* |
| Education | ||
| Grade 1–11th | 70 (61) | 55 (49) |
| 12th grade/High school diploma | 33 (29) | 41 (36) |
| Some college/college degree | 11 (10) | 17 (15) |
| Homeless in the past 6 months | 43 (38) | 42 (37) |
| Prison in the past 6 months | 27 (24) | 31 (27) |
| Unemployed in the past 6 months | 106 (93) | 101 (89) |
| Daily injection in past 6 months | 58 (51) | 51 (46) |
| Using an unclean needle | 45 (40) | 49 (43) |
| Using an unclean cotton or cooker | 74 (65) | 73 (65) |
| Exchange sex in past 90 days for food, shelter, drugs or money | 20 (22) out of 92** | 24 (25) out of 96** |
Out of 227 participants 203 agreed to take HIV test.
Among those who reported having sex in past 90 days.
The missing data pattern was the same for all five outcome variables. The questions about HIV prevention communication between study participants and their drug buddies were asked only if participants reported injecting in the prior 6 months. Thus, there were more responses missing for the HIV prevention conversation among PWIDs as compared to overall missing of the participants in the follow-up visits shown in Figure 1. All 227 participants had data for 5 outcome variables at the baseline visit whereas 20% of the data was missing at the 6-month visit, 23% at the 12-month visit and 33% at the 18-month visit. However, univariate analysis of missing response in the follow-up visits, based on the baseline covariates and on the outcomes in the prior visits, revealed that there was no difference between participants who responded to the questions and those who were missing the response or were not asked the questions because they did not inject drugs in the past year.
In the final model the interaction between intervention assignment and time was significant for each of the five outcomes, which suggested that the change in the relative risk of talking about HIV prevention topics over time differed across the intervention groups. Thus, we ran stratified analysis by visits (Table 2). Table 2 shows the change in frequency of conversation between the experimental and control group over time. Significantly higher frequency of conversation was observed for most of the HIV prevention topics, with the exception of HIV testing, comparing experimental to control group at the 6-month visit. At the 6-month visit the relative risk of talking ‘at least once a month’ compared to ‘never’ about HIV testing (relative risk ratio (RRR) = 1.80; 95% CI = 0.88 – 3.66), HIV transmission (RRR = 2.07; 95% CI = 1.00 – 4.31), needle cleaning (RRR = 4.52; 95% CI = 2.07 – 9.89), needle sharing (RRR = 4.24; 95% CI = 1.74 – 10.37), and condom use (RRR = 2.10; 95% CI = 0.97 – 4.57) were higher in the experimental compared to the control group although not all of them were statistically significant. The same pattern was observed for the relative risk of talking ‘at least a few times a week’ compared to ‘never’ at 6-month about HIV testing (RRR = 1.86; 95% CI = 0.87 – 3.95), HIV transmission (RRR = 3.22; 95% CI = 1.39 – 7.46), needle cleaning (RRR = 4.35; 95% CI = 1.88 – 10.07), needle sharing (RRR = 4.35; 95% CI = 1.80 – 10.54), and condom use (RRR = 2.25; 95% CI = 1.05 – 4.84).
Table 2.
Time-specific unadjusted relative risk ratios for change in frequency of conversation about HIV prevention topics comparing experimental to control group.
| Time (T1) RRR (95% CI) |
Time (T2) RRR (95% CI) |
Time (T3) RRR (95% CI) |
Time (T4) RRR (95% CI) |
||
|---|---|---|---|---|---|
| HIV testing | |||||
| At least once a month vs. Never | 1.00 (0.54 – 1.84) | 1.80 (0.88 – 3.66) | 1.48 (0.76 – 2.87) | 2.11 (0.99 – 4.48) | |
| At least few times a week vs. Never | 0.59 (0.30 – 1.14) | 1.86 (0.87 – 3.95) | 1.45 (0.62 – 3.37) | 1.77 (0.77 – 4.05) | |
| HIV transmission | |||||
| At least once a month vs. Never | 1.14 (0.63 – 2.03) | 2.07 (1.00 – 4.31) | 1.68 (0.86 – 3.26) | 1.90 (0.94 – 3.83) | |
| At least few times a week vs. Never | 0.82 (0.39 – 1.69) | 3.22 (1.39 – 7.46)** | 1.75 (0.71 – 4.31) | 4.57 (1.56 – 13.43)** | |
| Needle cleaning | |||||
| At least once a month vs. Never | 1.25 (0.66 – 2.36) | 4.52 (2.07 – 9.89)*** | 2.61 (1.29 – 5.25)** | 1.62 (0.77 – 3.41) | |
| At least few times a week vs. Never | 1.28 (0.69 – 2.40) | 4.35 (1.88 – 10.07)** | 2.41 (1.06 – 5.49)* | 2.03 (0.88 – 4.71) | |
| Needle sharing | |||||
| At least once a month vs. Never | 0.90 (0.46 – 1.76) | 4.24 (1.74 – 10.37)** | 2.50 (1.18 – 5.32)* | 3.21 (1.45 – 7.14)** | |
| At least few times a week vs. Never | 1.18 (0.64 – 2.17) | 4.35 (1.80 – 10.54)** | 2.31 (1.06 – 5.00)* | 1.68 (0.75 – 3.76) | |
| Condom use | |||||
| At least once a month vs. Never | 0.88 (0.46 – 1.69) | 2.10 (0.97 – 4.57) | 1.96 (0.95 – 4.03) | 2.81 (1.28 – 6.17)* | |
| At least few times a week vs. Never | 0.56 (0.30 – 1.04) | 2.25 (1.05 – 4.84)* | 1.93 (0.91 – 4.07) | 3.06 (1.35 – 6.95)** | |
P<0.05;
P<0.01;
P<0.001;
CI: confidence interval; RRR: relative risk ratio; T1: baseline; T2: 6-month; T3: 12-month; T4: 18-month visits
The observed differences between experimental and control groups decreased in magnitude over time. Nevertheless, there are number of relative risk ratios that are higher in magnitude at T4 compare to T3. Among those the significant ones were HIV transmission (RRR = 4.57; 95% CI = 1.56 – 13.43), the danger of needle sharing (RRR = 3.21; 95% CI = 1.45 – 7.14) and condom use (RRR = 2.81; 95% CI = 1.28 – 6.17 and RRR = 3.06; 95% CI = 1.35 – 6.95). The frequency of conversation about HIV prevention topics seems to have decreased more drastically over time among controls (Figure 2.) compare to experimental group, which could have lead to higher relative risk ratios at T4 compare to T3. This trend fits with what we have observed in this study for risk behaviors (26); both groups showed reduced risky injecting behaviors at the 6-month visit with the experimental group continuing to reduce risk at subsequent follow-up visits, while the control group tapered off after the 6-month visit (26).
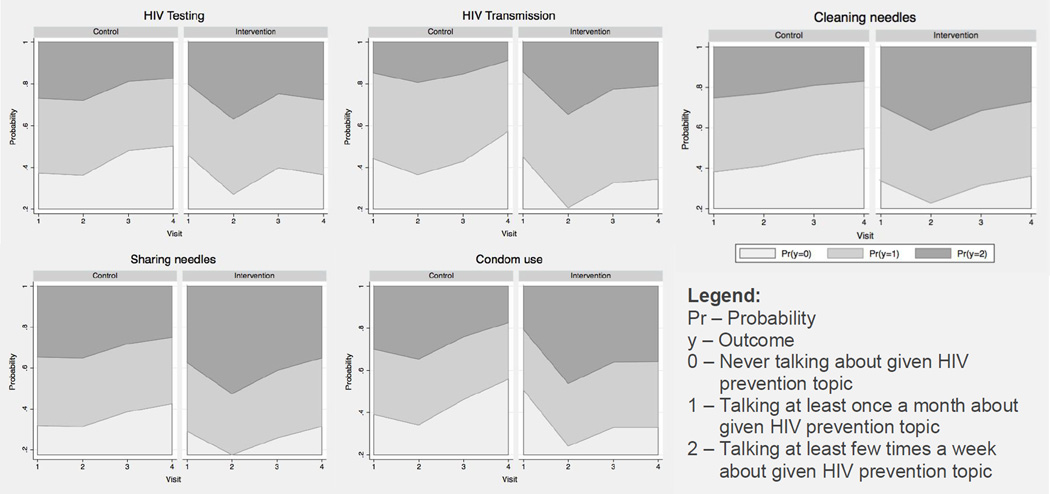
Figure 2.
Over time change in the probability of the conversation frequency about HIV prevention topics stratified by intervention assignment.
Pr – Probability
y – Outcome
0 – Never talking about given HIV prevention topic
1 – Talking at least once a month about given HIV prevention topic
2 – Talking at least few times a week about given HIV prevention topic
Figure 2 represents the cumulative probabilities for different outcomes over time stratified by the intervention assignment. Overall we observed that the probability of talking more than ‘never’ about any of the HIV prevention topics decreased over time among the participants in the control group. In the experimental group the probability of talking more than ‘never’ about HIV testing and HIV transmission increased over time and it remained slightly high at the 18-month visit compared to the baseline visit. The probability of talking about cleaning needles with bleach and danger of sharing needles with other people initially increased at the 6-month visit and decreased by the 12-month visit, continuing to decrease at the 18-month visit to a probability comparable to the baseline. Among the HIV prevention topics, the probability of talking about condom use is the only one that remained higher in the experimental group at 18-months compared to the baseline.
Discussion
This study examined the effect of a peer-based, personal network-focused, educational intervention on the frequency of conversation about HIV prevention topics among PWIDs in Baltimore, Maryland. The objective of the study was to assess the differences in the frequency of conversation between the control and experimental groups over time. In addition to the difference in frequency of conversation, the sustainability of talking about HIV prevention topics over time was examined as well as the type of topics that PWIDs persistently talked about. Understanding how the frequency of communication about HIV prevention changes over time is important in helping design, evaluate, and improve peer-based interventions among PWIDs.
There are a few overall observations that can be drawn from the analysis. First, peer-based education had a significant positive effect on the frequency of conversation about any of the HIV prevention topics examined in this study at 6-months. Secondly, over time the effect of the intervention decayed and the frequency of conversation about any of the topics decreased. Finally, study participants discussed certain topics with their drug buddies more often than others and for some topics PWIDs retained a significantly high level of conversation by the 18-month visit compared to the baseline. An increase in the conversation about any HIV prevention topic at 6-month visit and sustained significantly high level of conversation for some topics at 18 months, suggest that the intervention was effective at teaching the PWIDs skills they needed to promote HIV prevention. Thus, future interventions should be designed to constantly reinforce communication about HIV prevention among PWIDs in order to assure that they continue to disseminate the message.
Comparing the experimental to the control group during different visits we observed that at the 6-month visit those in the experimental group talked significantly more about all HIV prevention topics with the exception of HIV testing. By the 18-month visit the differences that remained statistically significant between the experimental and control groups were for part of conversation about HIV transmission, danger of needle sharing and condom use. Thus, one possible explanation is that study participants may be more comfortable with some conversation topics about HIV prevention and less comfortable with others.
Conversation about HIV transmission and in particular about HIV testing for most part did not persist among PWIDs, which could be due to the stigma associated with HIV and potential discrimination (from society and family) that one might experience if tested HIV positive. Research by Parsons et al. (29) showed several adverse effects for HIV-positive PWIDs whose status was revealed in the community, such as rejection, loss of intimacy and material resources. Some studies have also found that, for example, sex partners have a violent reaction to HIV-related communications (30). In addition, as the topic of HIV is stigmatized, simply talking about HIV prevention may be uncomfortable for some individuals. They may be concerned that others will think that they are infected or that it connotes that they are members of a stigmatized risk group. Thus, communication about HIV prevention among PWIDs requires more education, encouragement and mentoring in order for them to feel confortable sharing the prevention message. A qualitative study from a multisite intervention to increase testing found that the intervention increased personal conversations regarding HIV testing and decreased HIV stigma (31).
Sharing needles (31% prevalence in this study) and use of dirty needles (41% prevalence in this study) is prevalent among PWIDs and it may be hard to alter this behavior, even if trained at safe injecting, when one really needs a ‘fix’. Thus, the decrease in frequency of conversation about those topics could be due to the ‘guilt feeling’ among study participants of not consistently practicing what they are encouraging others to do. At the same time they may also think that it’s not necessary to repeat the information over and over again to the same individuals (at 6, 12, and 18-month study visits, 23%, 40–53%, and 45–48%, respectively, of the same drug buddies were named compared to previous visit). Thus, future research should examine why the conversation about needle sharing and cleaning needles decreases over time among PWIDs.
Finally, the high frequency of conversation about condom use in the experimental group (significantly more frequent conversation compared to controls even at the 18-month visit) could be due to the wide acceptance of condom use. In addition, talking about use of condoms does not necessarily imply prevention of HIV transmission; it could mean prevention of other less stigmatizing sexually transmitted infections (STIs) or prevention of pregnancy.
Nevertheless, as a result of this intervention the frequency of conversation significantly increased by the 6-month visit for most HIV prevention topics with the exception of HIV testing. But a decrease was observed over time, and for most of the conversation topics there was no statistically significant difference between experimental and control groups at the 18-month visit. The fact that the frequency of conversation increased initially and decreased as the study progressed suggests a need to constantly reinforce positive behavior among PWIDs, which could potentially be achieved through booster sessions.
Booster sessions showed association with reduction in risky sex and injecting behaviors among PWIDs. A randomized controlled trial evaluating the effect of a peer-based behavioral intervention among PWIDs in Thai Nguyen, Vietnam reported that those who attended booster sessions and/or support person sessions were more likely to decrease sexual risk behavior (21). Another study among PWIDs in Haryana, India examined the association between the level of exposure to peer-based education sessions and needle sharing practices. These studies showed that the proportion of PWIDs who shared needles substantially decreased among those who attended three or more peer-based education sessions (49% vs. 11%, p<0.001) in a month (22). Thus, the studies suggest that repeated exposure to peer-based educational sessions is more effective in reducing risk behavior among PWIDs than one time exposure. HIV prevention programs have to constantly reinforce the reduction in the risk behavior among PWIDs by promoting repeated, monthly interaction with peer health educators.
One of the limitations of this analysis is the potential contamination of the information received in the control group by information from the experimental group. Increases in the frequency of conversation about HIV prevention topics at the 6-month visit among controls could have been due to spillover of the information from the experimental group. More precisely, it is possible that some of the Indexes from experimental group shared what they learned with those in the control group, which encouraged controls to talk to others about HIV prevention. It is also possible that controls on their own, even though they were not encouraged to do so, shared the content of their sessions with their drug buddies. Further, the outcome was self-reported by the participants. This could have resulted in reporting bias in the experimental group, since at the group sessions they were encouraged to talk to others about HIV prevention. Thus, experimental group participants might have felt compelled to report talking frequently to others about HIV prevention. In addition, participants were recruited through street-based outreach, word of mouth and posted advertisements throughout the community, which may not result in a representative study sample of the target population.
Conclusion
In conclusion, it is challenging to develop behavioral interventions among PWIDs that would assure sustainability of communication about HIV prevention topics over time. We have shown that our intervention had a positive impact on the conversation about HIV prevention among PWIDs, but sustainability past 6 months was challenging for most of the conversation topics. Nevertheless, increase in the conversation is possible for all of the topics since we observed a significant increase in conversation by the 6-month visit. Based on these findings and since conversation plays an important role in the success of peer-based interventions, it would be important to explore the options of using booster sessions to continuously encourage conversation among PWIDs. For example, PWIDs might get bored talking about the same issues over and over again and sharing the information with the same people, since we saw that between 23%-53% of their injecting network is composed of the same individuals over time. They also might run into resistance from their drug buddies, which may affect their social standing negatively. Booster sessions could be used to problem solve as well as discuss methods to keep the conversations fresh and interesting, so the peer educators would continue to share HIV prevention message with the same and new drug buddies. Finally, exploring ways in which conversation about stigmatized topics such as HIV testing could occur in a more effective way, such that individuals do not feel accused or uncomfortable, might result in sustained sharing of HIV prevention message among PWIDs over time.
Acknowledgments
This research was supported by the National Institutes of Health/ National Institute on Drug Abuse.
Contributor Information
Aleksandra Mihailovic, Wilmer Eye Institute/Glaucoma Division, Johns Hopkins University, 1800 Orleans Street, Baltimore, MD 21287.
Karin Tobin, Department of Health Behavior and Society, Johns Hopkins Bloomberg School of Public Health, 2213 McElderry Street, Baltimore, MD 21205.
Carl Latkin, Department of Health Behavior and Society, Johns Hopkins Bloomberg School of Public Health, 624 N. Broadway, Hampton House 737, Baltimore, Maryland 21205, Phone: 410-955-3972, Fax: 410-955-7241, clatkin@jhsph.edu.
References
- 1.Latkin CA, Davey MA, Hua W. Needle exchange program utilization and entry into drug user treatment: is there a long-term connection in Baltimore, Maryland? Subst Use Misuse. 2006;41(14):1991–2001. doi: 10.1080/10826080601026027. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph AE, Crawford ND, Ompad DC, Benjamin EO, Stern RJ, Fuller CM. Comparison of injection drug users accessing syringes from pharmacies, syringe exchange programs, and other syringe sources to inform targeted HIV prevention and intervention strategies. J Am Pharm Assoc (2003) 2010;50(2):140–147. doi: 10.1331/JAPhA.2010.09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report. 2012 Dec;17(4) [Google Scholar]
- 4.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 U.S. dependent areas—2011. HIV Surveillance Supplemental Report. 2013 Oct;18(5) [Google Scholar]
- 5.Baltimore City Health Department. [accessed 9 March 2014];Need Exchange Program. 2012 Available at: http://www.baltimorehealth.org/needle-exchange-info.html. [Google Scholar]
- 6.Tempalski B, Pouget ER, Cleland CM, Brady JE, Cooper HL, Hall HI, Lansky A, West BS, Friedman SR. Trends in the population prevalence of people who inject drugs in US metropolitan areas 1992–2007. PLoS One. 2013 Jun 5;8(6):e64789. doi: 10.1371/journal.pone.0064789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Center for HIV Surveillance, Epidemiology and Evaluation. Maryland Department of Health and Mental Hygiene; [accessed 15 February 2014]. Baltimore City HIV/AIDS Epidemiological Profile Fourth Quarter 2012. Available at: http://phpa.dhmh.maryland.gov/OIDEOR/CHSE/Shared%20Documents/Baltimore-City.pdf. [Google Scholar]
- 8.Koester S, Glanz J, Baron A. Drug sharing among heroin networks: implications for HIV and hepatitis B and C prevention. AIDS Behav. 2005;9(1):27–39. doi: 10.1007/s10461-005-1679-y. [DOI] [PubMed] [Google Scholar]
- 9.Mehta SH, Galai N, Astemborski J, et al. HIV incidence among injection drug users in Baltimore, Maryland (1988–2004) J Acquir Immune Defic Syndr. 2006;43(3):368–372. doi: 10.1097/01.qai.0000243050.27580.1a. [DOI] [PubMed] [Google Scholar]
- 10.De P, Cox J, Boivin JF, Platt RW, Jolly AM, Alexander PE. HIV and HCV discordant injecting partners and their association to drug equipment sharing. Scand J Infect Dis. 2009;41(3):206–214. doi: 10.1080/00365540902721376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlahov D, Des Jarlais DC, Goosby E, et al. Needle exchange programs for the prevention of human immunodeficiency virus infection: epidemiology and policy. Am J Epidemiol. 2001;154(12 Suppl):S70–S77. doi: 10.1093/aje/154.12.s70. [DOI] [PubMed] [Google Scholar]
- 12.Metzger DS, Navaline H, Woody GE. Drug abuse treatment as AIDS prevention. Public Health Rep. 1998;113(Suppl 1):97–106. [PMC free article] [PubMed] [Google Scholar]
- 13.Rhodes T. Risk environments and drug harms: a social science for harm reduction approach. Int J Drug Policy. 2009;20(3):193–201. doi: 10.1016/j.drugpo.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Rogers E. Diffusion of innovations. 5th ed. New York, NY: Free Press; 2003. [Google Scholar]
- 15.Bandura A. Social learning theory. Englewood Cliffs, NJ: Prentice Hall; 1977. [Google Scholar]
- 16.Turner JC. Social comparison and social identity: some perspectives for intergroup behavior. European Journal of Social Psychology. 1978;5:5–34. [Google Scholar]
- 17.Simoni JM, Nelson KM, Franks JC, Yard SS, Lehavot K. Are peer interventions for HIV efficacious? A systematic review. AIDS Behav. 2011 Nov;15(8):1589–1595. doi: 10.1007/s10461-011-9963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye S, Yin L, Amico KR, Simoni JM, Vermund SH, Ruan Y, Shao Y, Qian HZ. Efficacy of peer-led interventions to reduce unprotected anal intercourse among men who have sex with men: A meta-analysis. PLoS One. 2014 Mar 10;9(3):e90788. doi: 10.1371/journal.pone.0090788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson BT, Michie S, Snyder LB. Effects of behavioral intervention content on HIV prevention outcomes: A meta-review of meta-analyses. J Acquir Immune Defic Syndr. 2014 Aug 15;66(Suppl 3):S259–S270. doi: 10.1097/QAI.0000000000000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latkin CA. Outreach in natural settings: the use of peer leaders for HIV prevention among injecting drug users' networks. Public Health Rep. 1998;113(Suppl 1):151–159. [PMC free article] [PubMed] [Google Scholar]
- 21.Go VF, Frangakis C, Le Minh N, et al. Effects of an HIV peer prevention intervention on sexual and injecting risk behaviors among injecting drug users and their risk partners in Thai Nguyen, Vietnam: a randomized controlled trial. Soc Sci Med. 2013;96:154–164. doi: 10.1016/j.socscimed.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain B, Krishnan S, Ramesh S, Sabarwal S, Garg V, Dhingra N. Effect of peer-led outreach activities on injecting risk behavior among male drug users in Haryana, India. Harm Reduct J. 2014;11 doi: 10.1186/1477-7517-11-3. 3-7517-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davey-Rothwell MA, Latkin CA. HIV-related communication and perceived norms: an analysis of the connection among injection drug users. AIDS Educ Prev. 2007;19(4):298–309. doi: 10.1521/aeap.2007.19.4.298. [DOI] [PubMed] [Google Scholar]
- 24.Des Jarlais DC, Friedman SR, Friedmann P, et al. HIV/AIDS-related behavior change among injecting drug users in different national settings. AIDS. 1995;9(6):611–617. doi: 10.1097/00002030-199506000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Gibson DR, Choi KH, Catania JA, Sorensen JL, Kegeles S. Psychosocial predictors of needle sharing among intravenous drug users. Int J Addict. 1993;28(10):973–981. doi: 10.3109/10826089309062177. [DOI] [PubMed] [Google Scholar]
- 26.Tobin KE, Kuramoto SJ, Davey-Rothwell MA, Latkin CA. The STEP into Action study: a peer-based, personal risk network-focused HIV prevention intervention with injection drug users in Baltimore, Maryland. Addiction. 2011;106(2):366–375. doi: 10.1111/j.1360-0443.2010.03146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata, Volume II: Categorical Responses, Counts, and Survival. Third Edition. Taylor & Francis: 2012. [Google Scholar]
- 28.StataCorp LP. Stata data analysis and statistical software: release 13.0. College Station, TX: Stata Corporation; 2013. [Google Scholar]
- 29.Parsons JT, VanOra J, Missildine W, Purcell DW, Gomez CA. Positive and negative consequences of HIV disclosure among seropositive injection drug users. AIDS Educ Prev. 2004;16(5):459–475. doi: 10.1521/aeap.16.5.459.48741. [DOI] [PubMed] [Google Scholar]
- 30.El-Bassel N, Gilbert L, Rajah V, Foleno A, Frye V. Fear and violence: raising the HIV stakes. AIDS Educ Prev. 2000;12(2):154–170. [PubMed] [Google Scholar]
- 31.Maman S, van Rooyen H, Stankard P, Chingono A, Muravha T, Ntogwisangu J, Phakathi Z, Srirak N, F Morin S NIMH Project Accept (HPTN 043) study team. NIMH Project Accept (HPTN 043): results from in-depth interviews with a longitudinal cohort of community members. PLoS One. 2014 Jan 29;9(1):e87091. doi: 10.1371/journal.pone.0087091. [DOI] [PMC free article] [PubMed] [Google Scholar]