Abstract
Background
Intima-media thickness (IMT) measured on ultrasound images of the common carotid artery (CCA) is associated with cardiovascular risk factors and events. Based on the physics of ultrasound, CCA far wall IMT measurements are favored over near wall measurements but this theoretical advantage is not well studied.
Methods
We studied 6606 members of the Multi-Ethnic Study of Atherosclerosis (MESA), a longitudinal cohort study (mean age 62.1 years; 52.7% female) who had near wall and far wall CCA IMT measurements. Multivariable linear regression models were used to estimate model goodness-of-fit of Framingham risk factors (FRF) with near wall IMT, far wall IMT, and combined mean IMT. Multivariable Cox proportional hazards models were used to estimate hazard ratios for incident coronary heart disease (CHD) events for each IMT variable. Change in Harrell’s C-statistic was used to compare the incremental value of each IMT variable when added to FRF.
Results
Mean IMT had the strongest association with risk factors (R2 = 0.31), followed by the near wall (R2 = 0.26) and far wall IMT (R2 = 0.22). Far wall IMT improved the prediction of coronary artery disease events over the FRF (change in C-statistic of 0.012; 95% confidence intervals: 0.006, 0.017; p < 0.001) as did mean IMT (p = 0.004) but near wall IMT did not.
Conclusions
Far wall CCA IMT showed the strongest association with incident CHD whereas mean IMT had the strongest associations with risk factors. This difference might affect the selection of appropriate IMT variables in different studies.
Keywords: risk factors, common carotid artery, ultrasound, coronary heart disease, intima media thickness, carotid artery bifurcation
Introduction
Carotid intima-media thickness (IMT) has been defined as the distance between the lumen-intima and the media-adventitia interfaces seen on ultrasound images of the common carotid artery (CCA) wall 1.
The IMT measurement can be made on the wall nearest to the ultrasound transducer (near wall) as well as on the wall that is furthest (far wall). It has been argued that the near wall IMT measurement might not be reliable due to the physics of ultrasound imaging 2. While it is true that cardiovascular disease outcomes are associated with far wall IMT measurements 3, it is also true that this association holds for measurements combining the near and far wall IMT 4. With respect to cardiovascular outcomes, there is no clear cut-evidence of an advantage to either approach.
Intervention trials that use carotid IMT as an outcome tend to combine near and far wall measurements 5. This seems to improve the reliability of the measurements 6, 7. At least one epidemiologic study has suggested that this approach strengthens the association between risk factors and IMT 8.
We hypothesized that combining near and far wall IMT of the common carotid artery would improve the associations of IMT with risk factors and with coronary artery disease outcomes. We study these two hypotheses in the Multi-Ethnic Study of Atherosclerosis (MESA), a multi-ethnic cohort.
Materials and Methods
Population
The MESA (Multi-Ethnic Study of Atherosclerosis) study is a multiethnic population of 6814 men and women aged 45–84 years enrolled between July 2000 and August 2002 at six sites in the United States9. The MESA cohort includes whites, African-American, Hispanic, and Chinese participants. Participants were excluded if they had physician diagnosis of heart attack, stroke, transient ischemic attack, heart failure, angina, atrial fibrillation, a history of any cardiovascular procedure, a weight above 300 lbs, pregnancy, or any medical conditions that would prevent long-term participation. MESA protocols and all studies described herein have been approved by the Institutional Review Boards of all collaborating institutions and all participants gave informed consent.
Risk factors and anthropomorphic variables
Age, sex, race/ethnicity, and medical history as well as medication use were self-reported. Current smoking was defined as self-report of one or more cigarettes in the last 30 days. Resting systolic and diastolic blood pressures (BP) were measured three times in the seated position using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, Florida); the average of the last two measurements was used in these analyses Glucose and lipids were measured after a twelve-hour fast. Serum glucose was measured by rate reflectance spectrophotometry on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, NY). Diabetes mellitus was determined according to the 2003 ADA fasting criteria10. Total cholesterol was measured using a cholesterol oxidase method (Roche Diagnostics), as was HDL after precipitation of non-HDL cholesterol with magnesium/dextran.
The Framingham risk factors used in the analyses are the original risk factors determined for coronary heart disease events (including angina) as reported by Wilson et al.11: age, systolic blood pressure, total cholesterol, HDL-cholesterol, smoking history and diabetes to which we added sex and race/ethnicity. We used the risk factors rather than calculated risk scores in order to circumvent calibration problems due to the diverse ethnic composition of our cohort12. We also added use of blood pressure lowering medication as part of the augmented Framingham risk score for cardiovascular disease proposed by D’Agostino et al.13 in a sensitivity analysis.
Carotid artery measures
The results of the common carotid artery measurements are part of a comprehensive protocol that acquired videotaped images from both sides of the neck and included imaging of the distal common carotid artery (1 view on each side) and the proximal internal carotid artery/bulb (3 projections on each side). Details of the carotid artery evaluation have previously been described 14 and the acquisition protocol is the same one used in the Cardiovascular Health Study (CHS)15. The participants were imaged in the supine position with their head rotated 45° away from the side being imaged. The common carotid artery was imaged with the beginning of the bulb shown on the image (to the left). A matrix array probe (M12L, General Electric, Waukesha, WI) was used with the frequency set at 13 MHz with two focal zones and at a frame rate of 32 frames-per-second.
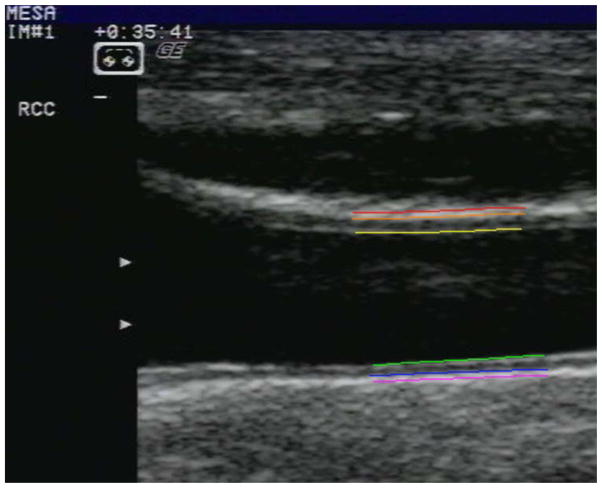
All carotid artery measurements were blinded and made at the ultrasound reading center at Tufts Medical Center in Boston, MA. Videotapes were reviewed and images were selected from a short cine recording lasting a few seconds. The readers were instructed to capture (digitize) a common carotid artery image for IMT measurement such that the artery lumen had the smallest diameter during the cardiac cycle, the near and far walls had clear interfaces, and there was minimal degradation due to motion. Near and far wall common carotid IMT measurements were made of each common carotid artery (1 projection) using hand drawn continuous tracings of the intima-lumen and media-adventitia interfaces (Figure 1) that were processed with a previously described algorithm16.
Figure 1.
Representative image of the right common carotid artery. A reader has identified the near and far wall interfaces. The reader has then identified the beginning of the bulb (divergence of the outer wall of the artery) and drawn the interfaces to the right. The lines are color coded and, from the top of the image, are the near wall interfaces: peri-adventitia/adventitia (red), adventitia/media (orange) and intima/lumen (yellow). The far wall interfaces are the lumen/intima (green), media/adventitia (blue), and adventitia/peri-adventitia (pink) interfaces.
Far wall IMT was the mean of the far wall IMT of both the right and left common carotid arteries. Similarly, near wall IMT was the mean of the near wall IMT of both the right and left common carotid arteries. The mean IMT was the mean of all four common carotid artery IMT values.
Outcomes
Events were identified during follow-up examinations and by telephone interview conducted every 9 to 12 months to inquire about all interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. Copies were obtained of all death certificates and of all medical records for hospitalizations and outpatient cardiovascular diagnoses. The review process included all generated ICD-9 definitions but the final adjudication of MESA endpoints was based on specific criteria applied to data obtained from medical records by two committee members or by the whole study events committee in case of disagreement.
Coronary heart disease (CHD) events included myocardial infarction (MI), death due to CHD, resuscitated cardiac arrest, definite or probable angina followed by coronary revascularization, and definite angina not followed by coronary revascularization. Cases of coronary artery revascularization that did not have a concurrent diagnosis of angina were not included in order to minimize possible referral bias. “Hard” cardiovascular disease (CVD) events included myocardial infarction, resuscitated cardiac event, stroke, and death due to CHD or stroke.
The diagnosis of myocardial infarction was based on a combination of symptoms, electrocardiographic findings, and circulating cardiac biomarkers. Death was considered related to CHD if it occurred within 28 days after a myocardial infarct, if the participant had experienced chest pain within the 72 hours preceding death, or if the participant had a history of CHD and died without documentation of any other cause of death. Resuscitated cardiac arrest included participants who successfully recovered from full cardiac arrest through cardiopulmonary resuscitation. Adjudicators graded the presence of angina based on the following criteria: definite or probable angina required clear and definite documentation of symptoms without the development of MI. Definite angina also required objective evidence of reversible myocardial ischemia or obstructive coronary artery disease. Stroke was defined as a documented new onset neurological event lasting more than 24 hours or until death if death occurred in the first 24 hours.
Statistical analyses
The mean (and standard deviation) and median (and inter-quartile range) values of continuous variables and the percent distribution of dichotomous variables as a percentage in each group are shown for the participants. We calculated the individual near and far wall IMT measurements for each individual and reported the difference as a mean and standard deviation as well as median and inter-quartile range.
Reproducibility was assessed in two ways: 1) repeat image acquisitions and 2) the re-reading of already acquired imaging studies. Blinded replicate scans were performed on 150 participants. Because of blinding, the same readers read most of the 150 repeat studies (144 of the 150 studies). Blinded re-reads of 155 studies were also performed. The same readers (intra-reader variability) read 78 of these cases and different readers (inter-reader variability) the remaining 77 cases. Reproducibility was calculated as Pearson correlation coefficients and 95% confidence intervals.
Multivariable linear regression models were created with three separate outcomes: near wall IMT, far wall IMT, and mean IMT. The original Framingham risk factors were used as independent variables with the addition of race/ethnicity.
The main outcome was time to first CHD event. A baseline multivariable Cox proportional hazards model using robust error handling was created with the components of the Framingham risk score: age, systolic blood pressure, diabetes, HDL-cholesterol, total cholesterol, and smoking to which were added sex and race/ethnicity. To this model was added each of the three IMT metrics as continuous variables in order to create three new models for the estimation of hazard ratios. We used standardized values for systolic blood pressure, total and HDL cholesterol as well as the IMT metrics in order to facilitate comparisons of the hazards ratios. Harrell’s C-statistics were obtained for all of the Cox proportional hazards models. The predictive value of each model with a given IMT measurement was compared to the baseline model using the differences in Harrell’s C-statistic. The proportional hazards assumption was verified using Schoenfeld’s residuals. Net Reclassification Improvement and Incremental Discrimination Improvement were calculated as described by Pencina et al. 17, 18. Net reclassification improvement was calculated form the Framingham cut-points of 6% and 20% at 10 years4, 17.
Kaplan-Meier curves were generated for illustrative purposes using unadjusted quartiles of near wall, far wall, and combined near and far wall IMT.
We performed a sensitivity analysis by using the difference between near wall and far wall IMT as a variable in the multivariable Cox proportional hazards models adjusted for risk factors, sex, and race/ethnicity. We also evaluated the predictive power of IMT measurements for incident “hard” CVD events in a sensitivity analysis.
Statistical analyses were performed using STATA 11.2 (StataCorp, College Station, Texas). Level of statistical significance was set at p = 0.05. Net reclassification improvement and incremental discrimination improvement were calculated with the help of a STATA add-on from the Uppsala Clinical Research Center: (http://www.ucr.uu.se/en/index.php/epistat/program-code/306-nri-and-idi).
Results
Of the original 6814 MESA participants, 6739 had an ultrasound examination at baseline. Near wall measurements were obtained in 6667 individuals, far wall IMT in 6722. Complete far wall and near wall IMT measurements were made on 6663 individuals. We excluded another 52 individuals due to missing risk factors and 5 with prevalent CHD not detected at the first examination for a final study population of 6606.
There were 484 coronary heart disease events, of which 209 were angina events, during a median 11.2 years (IQR: 10.6, 11.7) of follow-up. Table 1 summarizes the characteristics of the population studied. Average age was 62.1 years and 47.3% of the population was male. There were 38.8% whites, 12.0% Chinese, 27.2% blacks and 22.0% Hispanics. Mean systolic blood pressure was 126.5 mmHg (+/− 21.5 mmHg SD), mean HDL cholesterol 50.9 mg/dL (+/− 14.8 mg/dL SD), and total cholesterol 194.1 mg/dL (35.6 mg/dL SD). The prevalence of diabetes was 14.0% and 12.9% of participants were current smokers. The mean difference between near and far wall IMT values was 0.047 mm ± 0.197 mm, the near wall IMT being larger (p < 0.0001).
Table 1.
Key variables for the population studied
| Variable | Value * | Median and inter-quartile ranges |
|---|---|---|
| Age (years) | 62.1 (± 10.2) | 62 (53, 70) |
| Sex (men) | 3123 / 6606 (47.3%) | - |
| Race-Ethnicity | ||
| White | 2564 / 6606 (38.8%) | - |
| Chinese | 792 / 6606 (12.0%) | - |
| African American | 1795 / 6606 (27.2%) | - |
| Hispanic | 1455 / 6606 (22.0%) | - |
| Diabetes (yes) | 923 / 6606 (14.0%) | - |
| Smoker (yes) | 855 / 6606 (12.9%) | - |
| Blood pressure lowering medication (yes) | 2424/6606 (36.7%) | |
| Systolic blood pressure (mmHg) | 126.5 (± 21.5) | 123.5(111, 139.5) |
| HDL cholesterol (mg/dL) | 50.9 (± 14.8) | 48.0 (40, 59) |
| Total cholesterol (mg/dL) | 194.1 (± 35.6) | 192 (170, 215) |
| Mean near wall intima-media thickness (mm) | 0.79 (± 0.19) | 0.76 (0.66, 1.03) |
| Mean far wall intima-media thickness (mm) | 0.74 (± 0.21) | 0.71 (0.60, 0.84) |
| Mean near and far wall intima-media thickness (mm) | 0.76 (± 0.18) | 0.74 (0.64, 0.86) |
| Difference between near and far wall intima-media thickness (mm) | 0.047 (± 0.197) | 0.06 (−0.05, 0.15) |
| Number of coronary heart disease events | 484 / 6606 (7.3 %) | - |
| Number of “hard” cardiovascular disease events | 477 / 6606 (7.2 %) | - |
Total population n = 6606.
The values ± standard deviations (between parentheses) are given if the variable is continuous or the percentage if the variables are ordinal;
For replicate image acquisitions, correlation coefficients were stronger for far wall IMT (r = 0.92; 95% C.I.: 0.88, 0.94) and mean IMT (r = 0.91; 95% C.I.: 0.87, 0.93) than for near wall IMT (r = 0.80; 95% C.I.: 0.73, 0.85). Intra-reader variability was high and similar for far wall IMT (r = 0.96; 95% C.I.: 0.94, 0.98), near wall IMT (r = 0.97; 95% C.I.: 0.94, 0.98) and mean IMT (r = 0.97; 95% C.I.: 0.96, 0.98). Inter-reader variability was consistently lower than intra-reader variability at r = 0.79 (95% C.I.: 0.69, 0.86) for the far wall IMT, r = 0.84 (95% C.I.: 0.75, 0.89) for the near wall IMT and r = 0.82 (95% C.I.: 0.73, 0.88) for the mean IMT.
Cross-sectional associations of risk factors with intima-media thickness
Results of the multivariable linear regression models are shown in Table 2. All risk factors were significantly associated with the individual IMT measurements with the exception of current smoking. The respective coefficients for age, systolic blood pressure, HDL cholesterol, and diabetes in the three models were similar. Despite remaining significant in all cases, the standardized coefficients for total cholesterol varied from 0.037 for each change in 1 mm of near wall IMT to 0.055 for the far wall IMT. Differences in the coefficients were also notable for sex, increasing from 0.03 for a change in 1 mm of near wall IMT to 0.045 for the far wall IMT. Race/ethnic differences were present with Chinese having lower IMT values than whites and blacks larger IMT values than whites. Overall, the best goodness of fit for risk factors was for mean IMT (R2 = 0.31), followed by near wall IMT (R2 = 0.26) and then far wall IMT (R2 = 0.22).
Table 2.
Results of multivariable regression models (beta coefficients) with each common carotid artery intima-media thickness (IMT) variable as a separate outcome and the Framingham risk factors as predictors.
| Mean near wall IMT* | Mean far wall IMT* | Mean of near and far wall IMT* | ||||
|---|---|---|---|---|---|---|
| Value | p-value | Value | p-value | Value | p-value | |
| Age (years) | 0.007 | < 0.001 | 0.007 | < 0.001 | 0.007 | < 0.001 |
| Sex (men) | 0.030 | < 0.001 | 0.045 | < 0.001 | 0.038 | < 0.001 |
| Race-Ethnicity | ||||||
| White (referent) | ||||||
| Chinese | −0.044 | < 0.001 | −0.021 | 0.004 | −0.033 | < 0.001 |
| African American | 0.055 | < 0.001 | 0.024 | < 0.001 | 0.039 | < 0.001 |
| Hispanic | −0.002 | 0.69 | −0.008 | 0.19 | −0.006 | 0.25 |
| Diabetes (yes) | 0.036 | < 0.001 | 0.034 | < 0.001 | 0.035 | < 0.001 |
| Smoker (yes) | 0.011 | 0.07 | 0.0003 | 0.97 | 0.006 | 0.30 |
| Systolic Pressure† | 0.134 | < 0.001 | 0.161 | < 0.001 | 0.170 | < 0.001 |
| HDL cholesterol† | −0.075 | < 0.001 | −0.084 | < 0.001 | −0.090 | < 0.001 |
| Total cholesterol† | 0.037 | < 0.001 | 0.055 | < 0.001 | 0.054 | < 0.001 |
| Model goodness-of-fit (R2) | 0.26 | < 0.0001 | 0.22 | < 0.0001 | 0.31 | < 0.0001 |
intima-media thickness
standardized values for coefficients: units of 21.5 mmHg for systolic pressure, 35.6 mg/dL for total cholesterol and 14.8 mg/dL for HDL cholesterol
Longitudinal prediction of coronary heart disease events by intima-media thickness
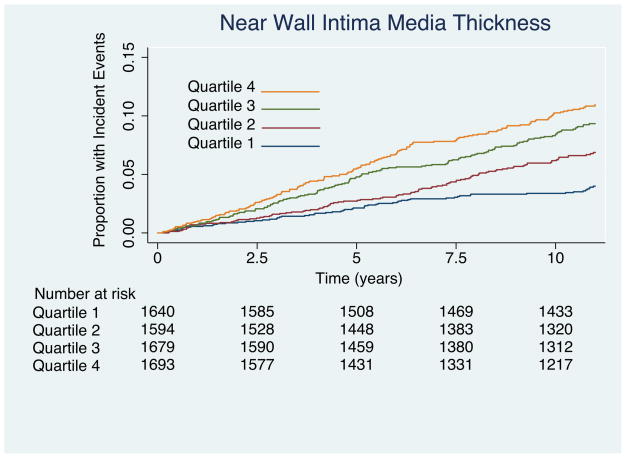
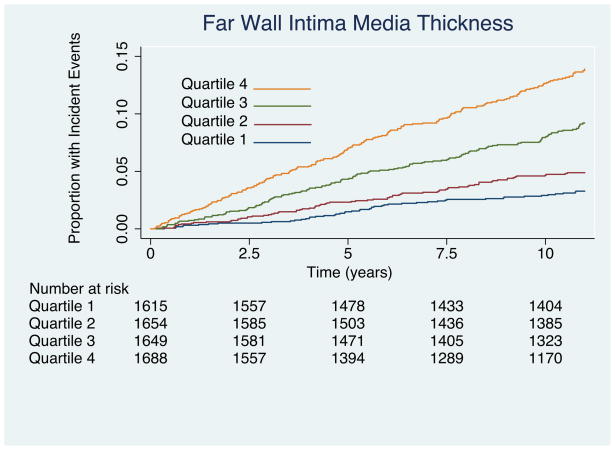
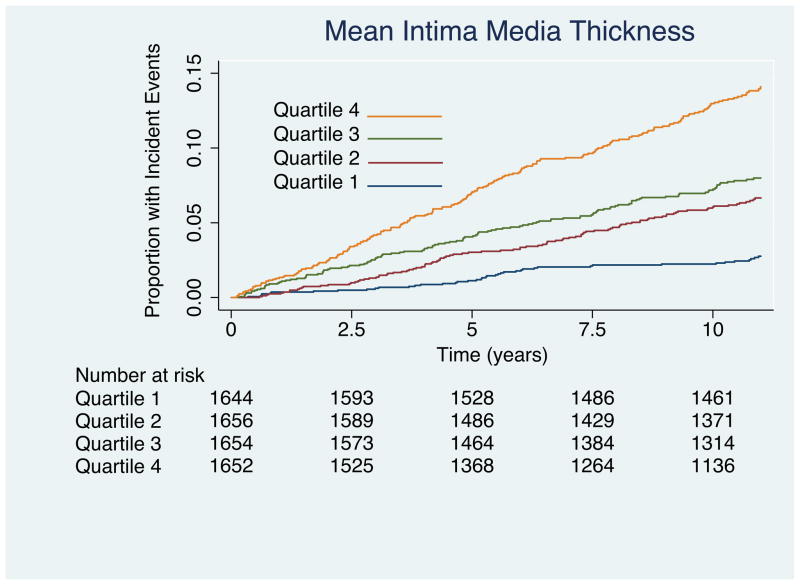
Results of Kaplan-Meir failure curves are plotted by quartile of IMT values as figure 2a for near wall IMT, Figure 2b for far wall IMT, and figure 2c for mean IMT. Results for the multivariable Cox proportional hazards model that includes all risk factors are shown to the left of Table 3. Important predictors of cardiovascular disease were increasing age, male sex, current smoking, increasing systolic blood pressure, presence of diabetes, lower HDL cholesterol, and elevated total cholesterol. There was a tendency for whites to have stronger associations with CHD than other ethnicities. The hazard ratio (HR) of near wall IMT was not significant (1.01; 95% confidence intervals: 0.92, 1.11) for each increase of 0.19 mm whereas the HR of mean IMT (1.17; 95% confidence intervals: 1.08, 1.28) and far wall IMT (HR = 1.21; 95% confidence intervals: 1.13, 1.30) were significant for respective increases of 0.21 mm and 0.18 mm.
Figure 2.
(A) Kaplan-Meier curves showing incident coronary heart disease events for the four quartiles of near wall intima-media thickness. Associations are significant (Log-rank = 58; p < 0.0001). Values for the near wall quartiles are < 0.65 mm. 0.65 to 0.76 mm, 0.76 to 0.89 mm and > 0.89 mm. (B) Kaplan-Meier curves for far wall intima-media thickness. Note that the curves diverge earlier than for the near wall measurements in (A) and that the associations are stronger (Log-rank = 140; p < 0.0001). Values for the far wall quartiles are < 0.6 mm; 0.6 to 0.71mm, 0.71 to 0.84 mm and > 0.84 mm. (C) Kaplan-Meier curves showing incident coronary heart disease events for the four quartiles of mean near and far wall intima-media thickness. Associations are significant (Log-rank = 140; p < 0.0001). Values for the IMT quartiles are < 0.64 mm. 0.64 to 0.74 mm, 0.74 to 0.858 mm and > 0.858 mm.
Table 3.
Results of multivariable Cox proportional hazards models with time to first coronary heart disease event as outcome. The hazards ratios (HR) and the 95% confidence intervals (Lower 95% and Upper 95%) of the basic model or to the left and for the addition of common carotid artery intima-media thickness variables to the right.
| Variables | Risk factors | Risk factors and near wall IMT* | Risk factors and far wall IMT* | Risk factors and mean of the far wall and near wall IMT* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | Lower 95% | Upper 95% | HR | Lower 95% | Upper 95% | HR | Lower 95% | Upper 95% | HR | Lower 95% | Upper 95% | |
| Age (years) | 1.05 | 1.04 | 1.06 | 1.05 | 1.04 | 1.06 | 1.04 | 1.03 | 1.05 | 1.04 | 1.03 | 1.06 |
| Sex (men) | 2.06 | 1.68 | 2.54 | 2.06 | 1.67 | 2.54 | 1.97 | 1.60 | 2.43 | 1.98 | 1.61 | 2.45 |
| Race-Ethnicity | ||||||||||||
| White (referent) | ----- | ----- | ----- | ----- | ----- | ----- | ----- | ----- | ----- | ----- | ----- | ----- |
| Chinese | 0.58 | 0.41 | 0.80 | 0.58 | 0.41 | 0.80 | 0.59 | 0.42 | 0.82 | 0.60 | 0.43 | 0.83 |
| African American | 0.78 | 0.62 | 0.99 | 0.78 | 0.62 | 0.99 | 0.78 | 0.62 | 0.98 | 0.76 | 0.61 | 0.96 |
| Hispanic | 0.77 | 0.61 | 0.98 | 0.77 | 0.61 | 0.98 | 0.79 | 0.62 | 1.00 | 0.78 | 0.61 | 0.99 |
| Diabetes (yes) | 1.85 | 1.49 | 2.30 | 1.85 | 1.49 | 2.30 | 1.79 | 1.44 | 2.22 | 1.79 | 1.44 | 2.22 |
| Smoker (yes) | 1.66 | 1.29 | 2.14 | 1.66 | 1.28 | 2.14 | 1.65 | 1.28 | 2.12 | 1.64 | 1.27 | 2.11 |
| Systolic blood pressure* | 1.29 | 1.18 | 1.41 | 1.29 | 1.18 | 1.41 | 1.24 | 1.14 | 1.36 | 1.25 | 1.14 | 1.37 |
| HDL cholesterol* | 0.85 | 0.76 | 0.95 | 0.85 | 0.76 | 0.95 | 0.86 | 0.76 | 0.96 | 0.86 | 0.76 | 0.96 |
| Total cholesterol* | 1.14 | 1.04 | 1.25 | 1.14 | 1.04 | 1.25 | 1.13 | 1.03 | 1.25 | 1.14 | 1.03 | 1.25 |
| Intima-media thickness* | 1.01† | 0.92 | 1.11 | 1.21 | 1.13 | 1.30 | 1.17 | 1.08 | 1.28 | |||
standardized values: units of 21.5 mmHg for systolic pressure, 35.6 mg/dL for total cholesterol, 14.8 mg/dL for HDL cholesterol. 0.19 mm for near wall intima media thickness, 0.21 mm for far wall intima media thickness, and 0.18 mm for mean of near and far wall intima media thickness
All variables are significantly associated with coronary heart disease outcomes except for the near wall intima media thickness.
The multivariable proportional hazards model with risk factors, sex and race/ethnicity had a C-statistic of 0.729 (95% confidence intervals: 0.708, 0.749). The C-statistic was essentially the same (p=0.55) with near wall IMT added to the model at 0.729 (95% confidence intervals: 0.708, 0.749). The C-statistic for the models with far wall IMT and mean IMT added increased significantly (p < 0.001 and 0.004 respectively) to 0.740 (95% confidence intervals: 0.720, 0.761) and 0.735 (95% confidence intervals: 0.715, 0.755). Far wall IMT increased the C-statistic by 0.012 (95% confidence intervals 0.006, 0.017) and mean IMT modestly improved the C-statistic by 0.006 (95% confidence intervals 0.002, 0.011).
We found only a mild increase in the net reclassification improvement for mean far wall IMT (3.8%; p = 0.02) but not for mean IMT or near wall IMT (Table 4). There was so significant change the incremental discrimination improvement for near wall IMT, but a significant improvement for both far wall IMT (p = 0.007) and overall mean IMT (p = 0.03).
Table 4.
Summary of risk category reclassification of MESA participants with the addition of common carotid mean far wall intima-media thickness (IMT) to the Framingham risk factors*, sex, and race/ethnicity
| RISK FACTORS WITH FAR WALL IMT ADDED | |||||
|---|---|---|---|---|---|
| R | < 6 % | 6–20 % | > 20 % | Total | |
| I | < 6 % | 91 | 20 | 0 | 111 |
| S | 6 – 20% | 10 | 292 | 16 | 318 |
| K | > 20 % | 0 | 12 | 43 | 55 |
| F | Total Events | 101 | 324 | 59 | 484 |
| A | |||||
| C | < 6 % | 3170 | 153 | 0 | 3323 |
| T | 6 – 20% | 233 | 2297 | 66 | 2,596 |
| O | > 20 % | 0 | 43 | 160 | 203 |
| R | |||||
| S | Total Non-Events | 3,403 | 2493 | 226 | 6122 |
age, total-cholesterol, HDL-cholesterol, systolic blood pressure, smoking, and diabetes.
The top half of the table shows an increase in participants “up” classified to a higher risk category to be 14/484 (2.9%). The bottom half of the table represents the non-events with 57 individuals “down” classified (0.93%). The net reclassification improvement is 3.8 % (0.93% + 2.9 %)
The difference between near wall and far wall IMT entered as a variable in the multivariable Cox proportional hazards models adjusted for risk factors, sex, and race/ethnicity gave a significant (p < 0.001) hazard ratio of 0.43 (95% confidence intervals: 0.30, 0.61).
Longitudinal prediction of “hard” cardiovascular events by intima-media thickness
There were 477 “hard” cardiovascular events during the follow-up period. In models with time to “hard” cardiovascular events as outcome, only far wall IMT was a significant predictor (Table 5). . The baseline model without far wall intima-media thickness had a C-statistic of 0.7447 (95% confidence intervals: 0.7245, 0.7649) increasing by a non-significant 0.0029 (p = 0.07; 95% confidence intervals: − 0.0002, 0.0060) with the addition of far wall IMT.
Table 5.
Results of multivariable Cox proportional hazards models with time to first cardiovascular disease event* as outcome.
| Variable | Hazard ratio | Lower 95% confidence interval | Upper 95% confidence interval | p-value |
|---|---|---|---|---|
| Age (years) | 1.05 | 1.04 | 1.06 | < 0.001 |
| Sex (men) | 1.40 | 1.14 | 1.72 | 0.001 |
| Race-Ethnicity | ||||
| White(referent) | ||||
| Chinese | 0.52 | 0.36 | 0.76 | 0.001 |
| African-American | 0.83 | 0.66 | 1.05 | 0.117 |
| Hispanic | 1.01 | 0.80 | 1.28 | 0.951 |
| Diabetes (yes) | 1.59 | 1.27 | 2.00 | < 0.001 |
| Smoker (yes) | 2.33 | 1.83 | 2.96 | < 0.001 |
| Blood pressure lowering therapy (yes) | 1.27 | 1.04 | 1.55 | 0.017 |
| Systolic blood pressure† | 1.36 | 1.25 | 1.49 | < 0.001 |
| HDL cholesterol† | 0.85 | 0.76 | 0.96 | 0.007 |
| Total cholesterol† | 1.11 | 1.01 | 1.21 | 0.03 |
| Far wall intima-media thickness† | 1.13 | 1.05 | 1.22 | 0.002 |
Cardiovascular disease defined as myocardial infarction (MI), death due to coronary heart disease, resuscitated cardiac arrest, confirmed stroke, and death due to stroke.
standardized values: units of 21.5 mmHg for systolic pressure, 35.6 mg/dL for total cholesterol, 14.8 mg/dL for HDL cholesterol, and 0.21 mm for far wall intima media thickness.
Discussion
We have found that there are differences in the way that near wall and far wall IMT measurements are associated with coronary heart disease risk factors and with coronary heart disease outcomes. Based on the goodness-of-fit of multivariable regression models, the near wall IMT and the combined near wall and far wall IMT had stronger associations with risk factors than did far wall IMT. For coronary heart disease outcomes, the far wall IMT had the strongest association since it significantly improved the C-statistic of multivariable Cox proportional hazards models that already included Framingham risk factors.
The stronger association of risk factors with the combined near and far wall IMT measurements than with far wall IMT confirms the observations made in the Rotterdam study8. It plausibly supports the belief that combining both measurements might improve statistical power in intervention trials where IMT serves as outcome6, 7, 19. This strategy was applied to the METEOR (Measuring Effects on Intima-Media Thickness: an Evaluation of Rosuvastatin) study, a recent trial that detected the effects of even modest decreases in serum lipids on the progression of IMT5. The data generated from METEOR showed that near wall as well as far wall IMT measurements detected differences between the treated and non-treated arms but also that the combined near and far wall IMT measurements had slightly higher discriminative power6, 7. While our findings suggest that combined IMT measurements might better reflect changes in risk factors than either the near or far wall, it would be important to confirm this finding by examining change in IMT in a longitudinal manner.
The strongest association between coronary heart disease outcomes and the three IMT variables was with far wall IMT. Outcomes studies where IMT showed positive associations with cardiovascular events had measurements made of the far wall3 while others had combined near wall and far wall IMT as predictors4, 15. We noted an increase in the net reclassification improvement only for the far wall mean IMT (Table 4). However, the significance of this finding was uncertain given that the incremental discrimination improvement was not significantly altered. Conversely, the fact that the C-statistic significantly increased for both mean and far wall IMT indicated that both IMT measurements added to the predictive power for coronary artery disease events17, 20, 21. To give perspective, we noted an increment of 0.012 in the C-statistic for far wall IMT over risk factors for predicting CHD events. Melander et al22 evaluated multiple novel risk factors such as CRP, Cystatin C and n-BNP. The change in C-statistic when each risk factor was added to models with risk factors was non-significant and the increases were 0.003, 0.004, and 0.006 respectively.
A previous publication from MESA used mean of the maxima near and far wall of both common carotid artery IMT23 as a predictor variable. We opted to use the mean of the mean common carotid IMT, the variable used in the Framingham Offspring Study4. The MESA common carotid IMT measurements take into consideration the distance from the common carotid artery bulb i.e. the reference point was the divergence of the outside wall of the artery (Figure 1), not of the lumen24. The results we report also have the advantage of a larger population than the previous report from MESA since the study by Yeboah et al23 is restricted to 1330 MESA participants and our results have greater statistical power since we include 6606 MESA participants.
A major strength of our study is the absence of prevalent cardiovascular disease at baseline. As such, our results might, on the long term, be applicable to the primary prevention of cardiovascular disease.
A limitation to the general applicability of our findings is the ability to acquire and measure IMT in the general population. The data completeness we report for the near wall common carotid artery IMT was 98.9 % (6667/6739 examinations), slightly lower than the 99.7% observed for the far wall (6722/6739 examinations). Data that are available for intervention trials suggest that IMT measurements can be made on almost all participants. However, such evaluations may be biased since one of the selection criteria is good ultrasound visualization of the carotid artery walls. Our participant population was not selected on the basis of the a-priori technical adequacy of ultrasound imaging. Despite this, we have demonstrated a high enough degree of complete IMT data and, thus, we believe that our imaging protocol is likely applicable to the general population if IMT is to be used for cardiovascular risk assessment. It is not clear to what extent the type of ultrasound device and sonographer technical skills might have contributed to our results.
The magnitude of the difference between near wall and far wall IMT is small, 0.047 mm, close to that seen during the cardiac cycle25, 26. Taking into consideration the results of the cross-sectional associations with risk factors, it might be that the greater variability in mean near wall IMT explained by risk factors blunts it’s predictive value when compared to that of far wall IMT. It is possible that the difference in “explained” variability for both measurements holds the predictive power for coronary heart disease events. The positive association we show between the difference in near and far wall IMT and CHD events in our statistical model suggests this possibility. A study looking at the difference between near and far wall noted no difference between near and far wall IMT before ultrasound contrast administration but a significant difference after ultrasound contrast27. The significance of this finding is not clear.
We were not able to directly compare our results to those of other outcome studies since most studies have either focused only on the far wall IMT3 or analyses have not separately been done for near and far wall IMT measurements4, 15. The emphasis of our investigation was to compare the applicability of different IMT measurements as a means of predicting overall future cardiovascular events. As such, we did not focus in the role of any particular risk factor but rather on the incremental change in performance of models when an IMT measurement was added. It is possible that the addition of removal of additional factors, such as socioeconomic status or metabolic syndrome components, might affect the ultimate association of IMT with coronary heart disease events.
Finally, recent guidelines for the assessment of cardiovascular risk were proposed while this manuscript was being completed28. These guidelines suggest that carotid artery IMT has limited value based on one published meta-analysis29. This meta-analysis did however show that IMT was a significant independent predictor of myocardial infarction and stroke. It is important to consider that of the 12 papers included in the analysis, only 5 studies showed a positive association between common carotid artery IMT and this combined end-point of stroke and myocardial infarction29. It is not clear if these studies were underpowered or simply failed to detect this association because of methodology or because the association between common carotid artery IMT and stroke is blunted30, 31. In support of the latter possibility, a recent study from MESA did not show any predictive power of common carotid IMT for stroke outcomes32. The findings from MESA32 and the findings of the Tromso study30 suggest that the common carotid artery IMT phenotype is loosing some predictive power for stroke. The reasons for this weakened association are unclear and this issue needs further study. It does appear that internal carotid artery IMT has an association with stroke when combined with common carotid artery IMT 33 or when estimated as plaque30. Nevertheless, we did find that far wall IMT is a significant independent predictor of ‘hard” cardiovascular events, an outcome that included stroke as well as events related to coronary heart disease.
In summary, we have observed that far wall common carotid artery IMT measurements are superior to combining near wall and far wall IMT measurements for the prediction of coronary heart disease events. However, combined near wall and far wall IMT measurements are more strongly associated with coronary heart disease risk factors. These findings suggest that mean far wall IMT of the common carotid artery is the best predictor of events whereas combined far and near wall IMT measurements might better track changes in risk factors. Both hypotheses would need to be tested in other cohorts.
Acknowledgments
This research was supported by contracts N01-HC-95159 through N01-HC-95167 from the National Heart, Lung, and Blood Institute as well as R01 HL069003 and R01 HL081352 and by grants UL1-RR-024156 and UL1-RR-025005 from NCRR. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Disclosures: Daniel H. O’Leary owns stock in Medpace, Inc. and is a vice-president.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74:1399–1406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 2.Wendelhag I, Gustavsson T, Suurkula M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles and description of a computerized analysing system. Clin Physiol. 1991;11:565–577. doi: 10.1111/j.1475-097x.1991.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 3.Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, et al. Carotid Intima-Media Thickness and Presence or Absence of Plaque Improves Prediction of Coronary Heart Disease. The ARIC (Atherosclerosis Risk in Communities) Study. JACC. 2010;55:1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB., Sr Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–221. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crouse JR, 3rd, Raichlen JS, Riley WA, Evans GW, Palmer MK, O'Leary DH, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297:1344–1353. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 6.Dogan S, Plantinga Y, Crouse JR, 3rd, Evans GW, Raichlen JS, O'Leary DH, et al. Algorithms to measure carotid intima-media thickness in trials: a comparison of reproducibility, rate of progression and treatment effect. J Hypertens. 2011;29:2181–2193. doi: 10.1097/HJH.0b013e32834b0eba. [DOI] [PubMed] [Google Scholar]
- 7.Peters SAE, den Ruijter HM, Palmer MK, Grobbee DE, Crouse JR, 3rd, O'Leary DH, et al. Extensive or restricted ultrasound protocols to measure carotid intima-media thickness: analysis of completeness rates and impact on observed rates of change over time. J Am Soc Echocardiogr. 2012;25:91–100. doi: 10.1016/j.echo.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Bots ML, de Jong PT, Hofman A, Grobbee DE. Left, right, near or far wall common carotid intima-media thickness measurements: associations with cardiovascular disease and lower extremity arterial atherosclerosis. J Clin Epidemiol. 1997;50:801–807. doi: 10.1016/s0895-4356(97)00059-0. [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10.Genuth S, Alberti KGMM, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 11.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 12.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P Group CHDRP. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 13.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 14.Polak JF, Szklo M, Kronmal RA, Burke GL, Shea S, Zavodni AEH, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. JAHA. 2013;2:e000087. doi: 10.1161/JAHA.113.000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 16.Polak JF, Pencina MJ, Herrington D, O'Leary DH. Associations of edge-detected and manual-traced common carotid intima-media thickness measurements with Framingham risk factors: the Multi-Ethnic Study of Atherosclerosis. Stroke. 2011;42:1912–1916. doi: 10.1161/STROKEAHA.110.603449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 18.Sundstrom J, Byberg L, Gedeborg R, Michaelsson K, Berglund L. Useful tests of usefulness of new risk factors: tools for assessing reclassification and discrimination. Scand J Public Health. 2011;39:439–441. doi: 10.1177/1403494810396556. [DOI] [PubMed] [Google Scholar]
- 19.Dogan S, Plantinga Y, Evans GW, Meijer R, Grobbee DE, Bots ML, et al. Ultrasound protocols to measure carotid intima-media thickness: a post-hoc analysis of the OPAL study. Curr Med Res Opin. 2009;25:109–122. doi: 10.1185/03007990802589727. [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 21.Cook NR, Paynter NP. Performance of reclassification statistics in comparing risk prediction models. Biom J. 2011;53:237–258. doi: 10.1002/bimj.201000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melander O, Newton-Cheh C, Almgren P, Hedblad B, Berglund G, Engstrom G, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polak JF, Post WS, Carr JJ, Szklo M, O'Leary DH. Associations of common carotid intima-media thickness with coronary heart disease risk factors and events vary with distance from the carotid bulb. J Am Soc Echocardiogr. 2014;27:991–997. doi: 10.1016/j.echo.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polak JF, Johnson C, Harrington A, Wong Q, O'Leary DH, Burke G, et al. Changes in carotid intima-media thickness during the cardiac cycle: the Multi-Ethnic Study of Atherosclerosis. JAHA. 2012;1:e001420. doi: 10.1161/JAHA.112.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polak JF, Meisner A, Pencina MJ, Wolf PA, D'Agostino RB. Variations in common carotid artery intima-media thickness during the cardiac cycle: implications for cardiovascular risk assessment. J Am Soc Echocardiogr. 2012;25:1023–1028. doi: 10.1016/j.echo.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macioch JE, Katsamakis CD, Robin J, Liebson PR, Meyer PM, Geohas C, et al. Effect of contrast enhancement on measurement of carotid artery intimal medial thickness. Vasc Med. 2004;9:7–12. doi: 10.1191/1358863x04vm522oa. [DOI] [PubMed] [Google Scholar]
- 28.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Sr, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. JACC. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Den Ruijter HM, Peters SAE, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 30.Mathiesen EB, Johnsen SH, Wilsgaard T, Bonaa KH, Lochen M-L, Njolstad I. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: a 10-year follow-up of 6584 men and women: the Tromso study. Stroke. 2011;42:972–978. doi: 10.1161/STROKEAHA.110.589754. [DOI] [PubMed] [Google Scholar]
- 31.Ziegelbauer K, Schaefer C, Steinmetz H, Sitzer M, Lorenz MW. Clinical usefulness of carotid ultrasound to improve stroke risk assessment: ten-year results from the Carotid Atherosclerosis Progression Study (CAPS) Eur J Prevent Cardiol. 2013;20:837–843. doi: 10.1177/2047487312449589. [DOI] [PubMed] [Google Scholar]
- 32.Polak JF, Sacco RL, Post WS, Vaidya D, Arnan MK, O'Leary DH. Incident stroke is associated with common carotid artery diameter and not common carotid artery intima-media thickness. Stroke. 2014;45:1442–1446. doi: 10.1161/STROKEAHA.114.004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardin JM, Bartz TM, Polak JF, O'Leary DH, Wong ND. What do carotid intima-media thickness and plaque add to the prediction of stroke and cardiovascular disease risk in older adults? The Cardiovascular Health Study. J Am Soc Echocardiogr. 2014;27:998–1005. e1002. doi: 10.1016/j.echo.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]