Abstract
Sadness is an emotion universally recognized across cultures, suggesting it plays an important functional role in regulating human behavior. Numerous adaptive explanations of persistent sadness interfering with daily functioning (hereafter “depression”) have been proposed, but most do not explain frequent bidirectional associations between depression and greater immune activation. Here we test several predictions of the host defense hypothesis, which posits that depression is part of a broader coordinated evolved response to infection or tissue injury (i.e. “sickness behavior”) that promotes energy conservation and reallocation to facilitate immune activation. In a high pathogen population of lean and relatively egalitarian Bolivian foragerhorticulturalists, we test whether depression and its symptoms are associated with greater baseline concentration of immune biomarkers reliably associated with depression in Western populations (i.e. tumor necrosis factor alpha [TNF-α], interleukin-1 beta [IL-1β], interleukin-6 [IL-6], and C-reactive protein [CRP]). We also test whether greater pro-inflammatory cytokine responses to ex vivo antigen stimulation are associated with depression and its symptoms, which is expected if depression facilitates immune activation. These predictions are largely supported in a sample of older adult Tsimane (mean±SD age=53.2±11.0, range=34-85, n=649) after adjusting for potential confounders. Emotional, cognitive and somatic symptoms of depression are each associated with greater immune activation, both at baseline and in response to ex vivo stimulation. The association between depression and greater immune activation is therefore not unique to Western populations. While our findings are not predicted by other adaptive hypotheses of depression, they are not incompatible with those hypotheses and future research is necessary to isolate and test competing predictions.
Keywords: depression, sickness behavior, immune activation, host defense, evolution, Tsimane
1. Introduction
Sadness is an emotion universally recognized across cultures (1), suggesting it plays an important functional role in regulating human behavior. Darwin hypothesized that emotion and mood evolved to motivate responses to recurring adaptive problems (2). Positive valence motivates continuation of prior behavior associated with its occurrence, while negative valence motivates disengagement and pursuit of alternative strategies (3). Numerous adaptive explanations of persistent sadness interfering with daily functioning (hereafter “depression”) have been proposed that build on Darwin's insights, yet the high prevalence of depressogenic risk alleles remains an evolutionary conundrum given the economic and social costs of depression worldwide (4). Many adaptive explanations posit social benefits to depression, including improving one's ability to solve social problems through rumination (5), compelling the loser of a conflict to avoid greater costs by signaling submission (6), avoiding exclusion from vital social relationships (7), and soliciting greater investment from a social partner by imposing costs on that partner (the “labor strike” hypothesis) (8). According to these explanations vegetative symptoms often associated with depression (e.g. tiredness, anhedonia, feelings of uselessness) facilitate energy conservation and reallocation to solve fitness-relevant social problems. Depression may also disengage motivation from unreachable goals (social or other), perhaps explaining its occurrence when achievements fail to meet expectations (9, 10).
Another adaptive explanation, the “host defense hypothesis” (11-13), similarly argues that depression functions to conserve and reallocate energy, but does not posit a social benefit or direct role in regulating nonsocial goal pursuits. According to the host defense hypothesis depression is a part of “sickness behavior”, i.e., one component of a broader coordinated adaptive response to infection or tissue injury (14). Innate immune cells produce pro-inflammatory cytokines that act on the brain via endocrine, circulatory and nervous system pathways to promote sickness behaviors (e.g. anorexia). It is hypothesized that infection or injury induces depression, and that depression promotes energy conservation and reallocation to facilitate innate or adaptive immune activation in response. Secondary proposed benefits are that depression reduces risk of transmitting infection to kin and valued conspecifics via social withdrawal, and that depression reduces risk of acquiring novel infection or sustaining injury during periods of greater immune activation via risk aversion. It is also proposed that psychosocial stressors – many of which involve social rejection, interpersonal loss or conflict and strongly increase risk of depression throughout life – up-regulate components of the innate immune system involved in inflammation because over evolutionary history these stressors reliably portended increased risk of pathogen exposure (e.g. via wounding or ostracism) (12, 15). This helps provide a host defense explanation for why psychosocial stress (real, anticipated or imagined) is inflammatory. Unlike the other adaptive hypotheses described above, the host defense hypothesis uniquely predicts that depression is associated with indicators of morbidity (e.g. inflammation), and that depressed individuals exhibit greater immune responses to challenge than non-depressed individuals (all else equal).
Several indirect lines of evidence support these predictions. Depressive symptoms are consistently associated with inflammation (and vice versa) in nonhuman animal models, human clinical samples, experiments among healthy volunteers, and large-scale naturalistic studies in Western populations (16-18). Mice or rats administered high loads of certain pro-inflammatory cytokines (e.g. interferon alpha [IFN-α]) present depressive-like symptoms such as decreased motor activity and reduced interest in eating or sex in a dose- and time-dependent manner, and these symptoms are attenuated with repeated anti-depressant treatment (19, 20). Among healthy men, typhoid but not placebo injection yields an inflammatory response indicated by greater circulating interleukin-6 (IL-6), and mood reduction at three hours post-injection (21). This mood reduction is not associated with increased salivary cortisol or self-reported illness symptoms, suggesting a direct link to IL-6 response; mood reduction is also correlated with enhanced activity in brain regions implicated in the etiology of depression. Similarly, among healthy men and women, Escherichia coli-derived endotoxin but not placebo administration yields greater IL-6 and tumor necrosis factor alpha (TNF-α) concentrations, and greater feelings of depression and social disconnection (22). Among hepatitis C patients receiving IFN-α immunotherapy, depressive symptoms develop even in the absence of a history of mental disorder (23). Moreover, depression risk alleles identified by candidate gene and genome-wide association studies are associated with alleles promoting innate and adaptive immunity (12). The types of pro-inflammatory genotypes that are over-represented in depressed samples may, in fact, confer enhanced survivorship in high pathogen environments while reducing survivorship when pathogenic exposures are minimized (24, 25). Although direct evidence that depression induces greater immune activation in response to infection or injury is scant, immune profiles of clinically depressed individuals show strong similarities to the acute phase response that is characterized by an activated innate immune system including elevated blood concentrations of inflammatory biomarkers (18, 26).
The acute phase response is a highly conserved part of innate immunity that initiates inflammation, clears pathogens and facilitates recovery from infection or injury. Local innate immune cells including monocytes and macrophages produce pro-inflammatory cytokines (e.g. IL-6, TNF-α, interleukin-1 beta [IL-1β]) that exert local and systemic effects such as recruiting leukocytes and modifying acute phase protein (e.g. C-reactive protein [CRP]) production in the liver. Fever is common within a few hours, as it can inhibit pathogen replication, conserve body heat, and potentiate innate or adaptive immunity (13). Lethargy and reduced interest in performing daily activities (e.g. eating, grooming) are common behavioral correlates of sickness. This coordinated adaptive response is costly: among adults with febrile illnesses (e.g. pulmonary tuberculosis, arthritis), resting metabolic rate (RMR) increases by 7-15% per 1°C rise in body temperature under febrile versus afebrile conditions (13, 27). Even in the absence of fever and chronic disease RMR increases by 8-14% during respiratory infection (28). In addition to energetic costs, other potential costs of sickness behavior include by-products of prolonged immune activation (e.g. tissue catabolism), and opportunity costs of foregone productivity or sociality due to lethargy or social withdrawal. Given these costs sickness behavior usually dissipates once the infection is cleared or injury healed.
However, unlike most sickness behaviors depression is not necessarily reversible once a pathogen clears or injury heals. In addition, certain depressive symptoms including suicidal ideation are not easily explained by the notion that depression is an adaptation to protect the host from infection or injury (29). Other lines of evidence are not consistent with the host defense hypothesis, and suggest that depression may be a maladaptive consequence of cytokine-induced sickness (30). Depression is often associated with immune dysregulation such as delayed mucosal wound healing (31) and reduced cellular immunity (32). The “immune dysregulation hypothesis” posits that reduced infectious microbial exposure during development in Western populations contributes to insufficient anti-inflammatory signaling, which increases baseline levels of depressogenic cytokines, promotes hyper-inflammatory responses to psychosocial or other stressors, and induces depression (33). In the Philippines, where infections are more common than in Western populations, depression is not associated with IL-6 or CRP among younger or older adults (34). Infectious exposures during sensitive periods of immune development may promote efficient immune responses, which might preclude a persistent association between depression and greater immune activation in non-Western populations with heavier infectious burden. This logic and evidence do not support the hypothesis that depression serves an adaptive immunomodulatory role that is precipitated by infection or injury. Rather, any prolonged pro-inflammatory state characteristic of immune dysregulation might lead to a greater incidence of depression (35). Moreover, among healthy US adults, positive affect prospectively predicts higher helper T lymphocyte counts and greater natural killer cell cytotoxicity after controlling for potential confounders (36). Yet all else equal, the host defense hypothesis predicts that negative affect potentiates immunity because over evolutionary history the types of immune responses associated with depression should have provided, on average, enhanced immune protection against the pathogens most likely to have reduced survival and reproduction. It therefore remains unclear whether depression is associated with greater immune activation in highly pathogenic environments, and whether depression is an adaptive component of sickness behavior.
1.1. Study goals and predictions
Here we test the host defense hypothesis of depression in a high pathogen population of Bolivian forager-horticulturalists. Studying mood variation in small-scale societies, which possess similar socio-ecological features typical of the vast majority of human evolutionary history (see below, this paragraph), provides unique insight into the costs and benefits implied by the different adaptive models of depression. Among Tsimane Amerindians, the population studied here, comorbidity with infections from multiple sources (e.g. bacterial, viral) is prevalent and levels of immune activation are high throughout life relative to Westerners (37-41). Thus we can examine whether links between depression and immune activation exist in a population with sufficient “priming” of the immune system during development. Aside from inadequate pathogenic exposure in development, other features of Western lifestyles – low physical activity level, high prevalence of metabolic disorder, and high levels of psychosocial stress from economic inequality, intense social competition and/or residential isolation from kin – have led researchers to posit that depression may be a maladaptive by-product of modernity (42-46). If depression is not a “disease of civilization” but instead an adaptive response to conditions regularly experienced over human evolutionary history, then it should be readily observable in small-scale subsistence societies like the Tsimane, which are physically active (47), lean (37), relatively egalitarian given a lack of defensible material possessions (48), and kin-oriented given high fertility, kin-based residence, and frequent resource transfers across households (49).
We test whether depression in adulthood is associated with greater baseline immune activation (P1), indicated by serum concentrations of TNF-α, IL-1β, IL-6 and CRP, as meta-analyses report reliable associations between depression and these biomarkers in Western populations (50, 51). We examine whether energetic status moderates the relationship between depression and immune activation, as individuals with fewer energy stores may especially benefit from depression-induced energy conservation and reallocation (P2). On the other hand, reduced energetic status among Tsimane may co-occur with other stressors (e.g. food insecurity, physical limitations impeding subsistence work), which may result in depression regardless of infectious status (52, 53).
We also explore whether individual depressive symptoms are associated with greater baseline immune activation. Depression is a collection of different types of symptoms which may vary in their functions (e.g. tiredness directly promotes energy conservation; pessimism reduces motivation to pursue an unreachable goal). Symptom profiles may differ depending upon the kind of situation that precipitated depression (54, 55), and whether emotional, cognitive, and somatic symptoms are each associated with greater immune activation is unclear. Symptoms directly promoting energy conservation (e.g. tiredness) may be more strongly linked to immune activation than symptoms that are indirectly or not implicated in down-regulating activity levels (e.g. suicidal ideation, nervousness). On the other hand, if symptoms co-occur as part of a coordinated adaptive response across multiple inter-related physiological systems, then consistent links between individual symptoms and immune activation may not be so apparent. We therefore adopt an exploratory approach to analysing links between individual symptoms and immune activation, as the host defense hypothesis makes no explicit predictions.
Finally, we test whether greater cytokine (TNF-α, IL-1β, IL-6) responses to ex vivo whole blood antigen stimulation with the antigens lipopolysaccharides (LPS) and phytohaemagglutinin (PHA) are associated with depression in adulthood (P3). If depression facilitates greater immune activation as part of the host defense response to infection or injury, then depressed individuals should show greater cytokine responses to immune challenges compared to non-depressed individuals (all else equal). LPS is a cell wall component of gram negative bacteria that induces a B-cell response similar to that generated by gram negative bacteria, while PHA initiates a T-cell mediated response similar to that generated by viral infection (56-58). This quasi-experimental approach has several advantages, including the ability to examine whether depression may affect host responses to multiple pathogens. More importantly, by stimulating cytokine responses ex vivo we are able to make inter-individual comparisons in immune activation which would otherwise be impossible or unethical while controlling for potential confounders (e.g. baseline immune activation). We also explore whether greater cytokine responses to antigen stimulation are associated with individual depressive symptoms. If depression is indeed part of a broader coordinated adaptive reaction to infection or injury, then greater immune response to challenge may be associated with emotional, cognitive and somatic symptoms.
2. Materials and methods
2.1. Study population
Tsimane (population ~15,000) inhabit 90+ villages that vary in proximity to the market town of San Borja (population ~25,000) in the Beni Department of lowland Bolivia. Related families co-reside in clusters of closely-spaced houses, and jointly produce and consume food. Most of the diet comes from horticulture (66% of calories), hunting (17%), fishing (7%), and fruits and nuts gathered from the forest (6%) (59). Tsimane have relatively short life expectancy (e0=42 years, e15=57, e45=66), high fertility (total fertility rate=9 births per woman) and physical work load (~5-6 hours/day spent in lifestyle-moderate activity), and minimal access to modern healthcare, sanitation and electricity. Tsimane recognize a mood state that approximates depression (yoquedye’) and often attribute its onset to musing over chronic illness (self or kin), death of a loved one, or extreme poverty compared to market-integrated Bolivian nationals (napo'in). Suicide associated with persistent sadness has been documented. To the best of our knowledge no participant has ever taken prescription medication to treat depression.
2.2. Depression interview
We developed an interview based on focus groups, 10+ years of ethnographic experience, and a review of validated depression scales used among diverse samples with good test-retest reliability (Beck's Depression Inventory, Hamilton Depression Rating Scale, and Center for Epidemiologic Studies Depression Scale) (52, 53). The 18-item interview contains most or all of the symptoms contained in previous scales, although two items (irritability and indecision) were omitted from analyses because neither was strongly correlated with any other item and mean item scores were low. The interview was translated from Spanish into Tsimane by two bilingual Tsimane research assistants. To test translation accuracy the Tsimane interview was back-translated into Spanish by a different Tsimane researcher, and discussions among the three Tsimane ensued until a translation was found that captured the essence of each item. To minimize recall bias, participants were queried about prevalence of symptoms over the past month. Responses were given on an anchored scale where 1 corresponds to “rarely,” 2 to “occasionally,” 3 to “often,” and 4 to “always” experiences symptom. Symptoms contain either emotional (two symptoms), somatic (three), cognitive (eight), or both cognitive and somatic (three) components.
Few participants were new to formal interviews because of their continuous participation in the Tsimane Health and Life History Project (THLHP) since 2002. Our long-term presence has helped establish trusting and collaborative relationships with Tsimane. Interviews were conducted in a private location in the Tsimane language by a Tsimane researcher with multiple years of relevant experience on the THLHP. Internal consistency of the 16-items surpasses the standard benchmark of 0.7 (Cronbach's α=0.72). The 16 items were summed to create a “depression score” (mean±SD=38.7±6.6, range: 22-59), and scores are normally distributed for each sex (53). After the interview, the interviewer used the same 4-point anchored scale to rate a subset of respondents based on his observations of the extent to which the respondent was laughing and smiling (jovial) while participating in other THLHP interviews (~45 minutes in total). This was done to assess external validity of the interview, as joviality can reflect mood. Depression scores were correlated with joviality in the expected negative direction (Spearman's =−0.42, p=0.003, n=48).
Adults were recruited regardless of their health status. Older adults are over-represented given the THLHP's focus on aging. Mean±SD age in the sample is 53.2±11.0 (range=34-85). Interviews were conducted from 2011-2012 at the THLHP's San Borja health clinic. Adults were transported from their villages (n=649 adults across 60 villages) to the clinic for a three-day stay to participate in this and other studies requiring more controlled conditions (see next section). All methods were approved by Institutional Review Boards at UNM and UCSB, and by the Tsimane government, village leaders and study participants.
2.3. Biomarkers of immune activation
Morning fasting blood specimens were collected by venipuncture during medical exams conducted at the THLHP's clinic during each participant's three-day stay. Samples were collected and prepared by a trained THLHP biotechnician with multiple years of relevant experience on the THLHP. After sample collection leukocyte counts were conducted manually. Two 100 μL aliquots of heparinized whole blood were added to separate round bottom microtiter wells in a sterile 96-well plate. The first aliquot received 100 μL of LPS (Sigma-Aldrich cat. L2630) diluted in RPMI (RPMI-1640 with 100 U/mL penicillin, 100 μg/mL streptomycin, 1mM pyruvate, and 2mM L-glutamate) to 10 μg/mL. The second aliquot received 100 μL of PHA (Sigma-Aldrich cat. 61764) diluted in RPMI to 10 ng/mL. In the absence of a CO2 incubator, the microtiter plate was sealed in a glass Tupperware with a lit candle, which burned the O2 in the container, enriching the CO2 concentration (60). The remaining unstimulated portion of whole blood was allowed to clot, and then serum was separated via centrifugation (1500 g × 10 minutes) and frozen in liquid nitrogen. The sealed plate was incubated for 72 hours at 37°C. Afterward, the supernatant was aliquoted into cryovials and frozen in liquid nitrogen for transport to UNM. Specimens were transported on dry ice and stored at −80°C for up to two years before assay.
At UNM's Hominoid Reproductive Ecology Lab, TNF-α, IL-1β and IL-6 were analyzed using the Milliplex MAP High-Sensitivity 13-plex Human Cytokine Panel (Millipore, Billerica, MA, HSCYTO-60) on the Luminex MagPix Instrument and Millipore Analyst Software. The limit of detection was at least 0.05 pg/mL for TNF-α, 0.06 pg/mL for IL-1β, and 0.10 pg/mL for IL-6. Standards were prepared with a serum matrix for analysis of unstimulated samples, and with RPMI for antigen-stimulated samples. Quality control samples were within expected ranges provided by the manufacturer (CVs are not reported for each cytokine but are available upon request). Due to a combination of logistical difficulties associated with obtaining reagents, cost, and sample volume constraints, stimulations were run on a random subset of specimens (see Table 1 for sample sizes).
Table 1.
Bivariate associations between study variables and depression as a dichotomous variable (≥48 indicates a mean item score ≥3, i.e. “often” experiencing symptom).
| Immune activation biomarkers | ≥48 (High-scorers, 10%a) | <48 (Lower-scorers, 90%) | N |
|---|---|---|---|
| Cytokine or CRP concentration | |||
| TNF-α (pg/mL) (median) | 10.95*** | 8.06 | 634 |
| IL-1β (pg/mL) (median) | 0.07^ | 0.06 | 636 |
| IL-6 (pg/mL) (median) | 0.82** | 0.52 | 649 |
| CRP (mg/L) (median) | 5.49** | 1.80 | 496 |
| Cytokine response to ex vivo antigen stimulation | |||
| TNF-α (pg/mL) | |||
| LPS-stimulated (median) | 143 | 149 | 117 |
| PHA-stimulated (median) | 62^ | 38 | 121 |
| IL-1β (pg/mL) | |||
| LPS-stimulated (median) | 540 | 468 | 119 |
| PHA-stimulated (median) | 239* | 98 | 123 |
| IL-6 (pg/mL) | |||
| LPS-stimulated (median) | 3,719* | 2,589 | 118 |
| PHA-stimulated (median) | 3,461* | 2,053 | 124 |
| Controls | |||
| Age (years) (mean±SD) | 55.4^ ± 11.9 | 53.0 ± 10.8 | 649 |
| % female | 72*** | 46 | 649 |
| BMI (kg/m2) (mean±SD) | 23.5^ ± 3.9 | 24.1 ± 3.3 | 649 |
p≤0.001
p≤0.01
p≤0.05
p≤0.1 (compared to lower-scorers; Mann-Whitney U or χ2 test)
Risk set includes interviewees that provided a morning fasting blood sample (n=649)
CRP was measured at UCSB's Human Biodemography Lab with an enzyme immunoassay described elsewhere (61). The limit of detection was 0.07 mg/L and the CVs were 7.3% and 10.2% for the high and 5.3% and 9.2% for the low controls. Due to resource constraints CRP assays were run on a random subset of specimens that was larger than the antigen stimulation subset.
2.4. Demographics and anthropometrics
Birth years were assigned based on a combination of methods including using known ages from written records, relative age lists, dated events, photo comparisons of people with known ages, and cross-validation of information from independent interviews of kin (62). Height and weight were measured during medical exams using a Seca stadiometer (Road Rod 214) and Tanita scale (BF680). Thirteen pregnant women were excluded from analysis due to potential confounding effects of pregnancy on immune activation.
2.5. Data analysis
In bivariate analyses depression is operationalized dichotomously to describe the sample and preliminarily assess potential threshold effects. A depression score cut-off of 48 indicates a mean item score ≥3, i.e. “often” experiencing symptom. Mann-Whitney U and chi-square tests are used to compare immune activation biomarkers, demographics, anthropometrics, and depression item scores by depression score cut-off. In multivariate analyses depression is operationalized continuously (summed 16 items). Most individuals contributed one data point but a random subset (<4%, n=23 individuals) contributed two data points to the analyses reported in sections 3.1-3.4 since they received two medical exams during the study period (one per year). These repeated measures are included in analyses to avoid unnecessarily reducing sample sizes, and because parameter estimates from multivariate analyses are directly comparable to those omitting repeated measures. Generalized estimating equations (GEE) analyses are used to model the effect of unstimulated immune activation biomarkers (TNF-α, IL-1β, IL-6 and CRP) on depression score after adjusting for potential confounders (section 3.3). The GEE method accounts for the correlated structure of dependent variables arising from repeated measures over time, controlling for each individual (63). GEE analyses of depression score as an outcome specify an exchangeable correlation structure, and parameter estimates are reported as standardized betas. To model the effect of unstimulated immune biomarkers on depression item score (section 3.4), GEE analyses specify a multinomial distribution. Cumulative logit estimates are converted to odds ratios (ORs), representing change in odds of reporting “always” experiencing a symptom compared to “rarely” (baseline) per SD increase in biomarker concentration. There is no standard absolute goodness-offit measure with the GEE method. Models of item scores as outcomes were also run using binary logistic regression (modelling the probability of reporting “always” experiencing a symptom or not), although no major differences with the multinomial models were found.
Ordinary least squares (OLS) regression is used to model the effects of depression score (section 3.5) and item scores (section 3.6) on cytokine response to ex vivo antigen stimulation. Depression score (or each item score) is entered as a predictor rather than an outcome (as described above) given the role of depression in regulating responses to immune challenges as specified by the host defense hypothesis. We compared effect sizes using both stimulated cytokine values and change in stimulated values from baseline but no major differences were found (estimates are reported using the former outcome). Stimulated cytokine values were analyzed with and without standardization for leukocyte count (estimates are reported using both outcomes), to account for the fact that individuals with higher leukocyte counts may naturally show greater cytokine responses to antigen stimulation. Stimulations were run in batches collected on a given day, and while there is potential for a “batch effect” (e.g. from non-independence due to plate characteristics), inclusion of a random effect of batch ID in OLS regressions does not change results and is thus omitted. For all regressions, despite conducting multiple tests (given 16 depression items, 4 immune biomarkers, and 2 stimulations per cytokine), p-values are not adjusted using Bonferroni or other correction. This is because tests are not independent (i.e. immune biomarkers positively co-vary), and there is no standard correction involving dependent p-values. Analyses were conducted with IBM SPSS Statistics 22 and R v2.15.2.
3. Results
3.1. Sample characteristics
Table 1 presents bivariate associations between study variables and depression as a dichotomous variable. Compared to individuals scoring <48 (hereafter lower-scorers), those scoring ≥48 (high-scorers) have higher median baseline cytokine and CRP concentrations (ranging from 17-205%), and higher cytokine responses to ex vivo antigen stimulations, particularly with PHA. Median PHA-stimulated responses among high-scorers are 63% (TNF-α), 144% (IL-1β), and 69% (IL-6) higher; median LPS-stimulated response is 44% higher for IL-6. On average, high-scorers are older, more likely female and have lower BMI; subsequent multivariate analyses thus control for these factors.
3.2. Prevalence of emotional, cognitive and somatic symptoms of depression
Emotional symptoms – sadness and crying easily – are experienced habitually by about 90% of high-scorers (Table S1). Symptoms containing somatic or cognitive components are also prevalent among high-scorers: 86% habitually experience irregular sleeping patterns (somatic), 84% feelings of uselessness (cognitive) and 77% persistent negative thoughts (cognitive). Prevalence of other somatic and cognitive symptoms, including those containing both components, is highly variable among high-scorers, ranging from 1.6% (paranoia/distrust) to 73.4% (difficulty concentrating). Feelings of uselessness, which in our interview includes perceptions of greatly reduced functional ability relative to past years, are experienced habitually by 42% of lower-scorers and likely reflects the sample being comprised mostly of older adults. Factor analysis did not justify reducing the 16 depression items into discrete dimensions (e.g. by whether symptoms contain emotional, cognitive or somatic components, or by whether symptoms promote energy conservation), and are thus not presented.
3.3. Is depression associated with greater baseline immune activation?
Among high-scorers, 93% are in the upper quintile of at least one immune activation biomarker (TNF-α: ≥12.68 pg/mL; IL-1β: ≥0.22; IL-6: ≥1.91; CRP: ≥5.75 mg/L), compared to 60% of lower-scorers (χ2=18.48, p<0.001). Thirty-three percent of high-scorers are in the upper quintile of at least two markers compared to 19% of lower-scorers (χ2=4.70, p=0.03).
Depression score is associated with higher TNF-α, IL-6 and CRP, but not IL-1β, after controlling for potential confounders (Table 2). Inclusion of all biomarkers as predictors in the same model does not yield a significant parameter estimate for any biomarker, although in this model depression is most strongly associated with CRP (βlog(CRP)=0.064, p=0.124, using controls in Table 2).
Table 2.
GEE analyses of effects of baseline immune activation biomarkers on depression score (summed 16 items) controlling for age, sex, BMI and regiona. Standardized β's are shown (95% CIs for biomarker β's).
| Cytokine or CRP concentration | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| log(TNF-α) | 0.09** (0.03-0.16) | ----- | ----- | ----- |
| log(IL-1β) | ----- | −0.01 (−0.08-0.06) | ----- | ----- |
| log(IL-6) | ----- | ----- | 0.09* (0.02-0.16) | ----- |
| log(CRP) | ----- | ----- | ----- | 0.07* (0.01-0.14) |
| Controls | ||||
| Age (years) | 0.74* | 0.79* | 0.94** | 1.10*** |
| Age2 | −0.63^ | −0.66^ | −0.81* | −0.93** |
| Sex=female | 0.59*** | 0.56*** | 0.57*** | 0.49*** |
| BMI (kg/m2) | −0.09* | −0.10** | −0.09* | −0.06^ |
| N observations, individuals | 634, 614 | 636, 613 | 649, 626 | 496, 477 |
p≤0.001
p≤0.01
p≤0.05
p≤0.1
Parameter estimates for a categorical region fixed effect (1=interior forest, 2=downriver, 3=upriver, 4=near town) are not shown. Significant differences in depression score are observed across regions, and region is controlled in each model
To account for the high percentage of IL-1β and IL-6 values below the detection limit (41% and 21%, respectively) we regressed each of these cytokines on depression score using Tobit models for left-censored data (64)1. We did not find marked differences between estimates derived from Tobit and GEE models using the same controls (not shown). We also regressed each immune activation biomarker on depression score for each sex to determine whether associations were explained by sex differences (Table S2). Associations between biomarkers and depression score are stronger and more consistent for women, but CRP is associated (p<0.05) with depression in the predicted positive direction for both sexes. In the pooled sample we did not find a significant sex*depression score interaction effect on any biomarker (all p's >0.05).
We found no evidence that the association between depression and immune activation is stronger for individuals with reduced energetic status (i.e. by testing for biomarker*BMI interactions and by analyzing sub-samples of BMI categories separately, not shown). We also tested for sex*biomarker interactions on depression score but found no significant effects.
To examine whether depression is associated with immune activation even after accounting for acute infectious status, we repeated GEE analyses excluding participants in the upper quintile of leukocyte count (≥10,600 cells/mm3), an independent measure of immune activation2. Parameter estimates for all biomarkers are very similar to those reported in Table 2 (not shown).
3.4. Are emotional, cognitive and somatic symptoms of depression each associated with greater baseline immune activation?
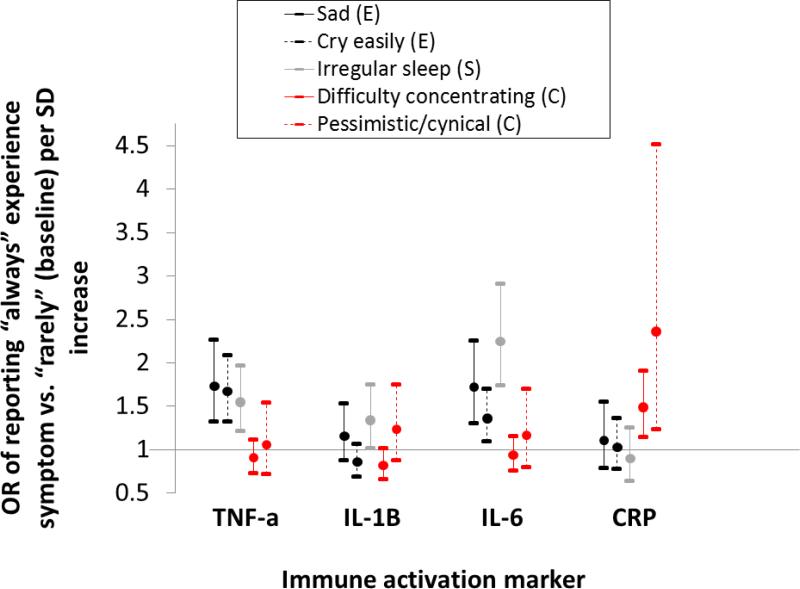
Table S3 presents ORs of habitually experiencing a symptom as a function of cytokine or CRP concentration after controlling for potential confounders. Both emotional symptoms are associated with higher TNF-α (OR of sadness=1.73, 95% CI: 1.32-2.27; OR of crying easily=1.67, 95% CI: 1.33-2.09) and IL-6 (OR of sadness=1.72, 95% CI: 1.31-2.26; OR of crying easily=1.36, 95% CI: 1.09-1.70) (Figure 1). Irregular sleep (somatic) is associated with higher TNF-α (OR=1.55, 95% CI: 1.22-1.97), IL-1β (OR=1.34, 95% CI: 1.02-1.75), and IL-6 (OR=2.25, 95% CI: 1.74-2.91). In addition, three cognitive symptoms – difficulty concentrating, pessimism, and feelings of uselessness – are associated with CRP (p<0.05) in the predicted positive direction (Table S3; Figure 1), with ORs ranging from 1.35-2.36 per SD increase. Feelings of fear/nervousness (somatic-cognitive) are associated with higher TNF-α (OR=1.54, 95% CI: 1.13-2.09) and IL-6 (OR=1.54, 95% CI: 1.14-2.08), although another somatic-cognitive symptom (reduced interest/lethargy) is both positively and negatively associated with a biomarker (CRP and TNF-α, respectively). Two cognitive symptoms, including one positively associated with CRP (feelings of uselessness and self-criticism) are negatively associated with at least one cytokine.
Figure 1.
OR (95% CI) of reporting “always” experiencing a symptom compared to “rarely” per SD increase in cytokine or CRP concentration (logged). Estimates are derived from multinomial logistic regression controlling for age, age2 (if p≤ 0.1), sex, BMI, and region. Symptoms are categorized by whether they include emotional (E, black line), somatic (S, gray), or cognitive (C, red) components.
In total, across 64 tests (16 items*4 biomarkers), 22 significant (p<0.05) associations were found, of which 18 (82%) were in the predicted positive direction. No symptom was associated with significantly higher concentration across all four biomarkers. One somatic symptom (irregular sleep) was positively associated with 3/4 biomarkers, and six symptoms (two emotional, one somatic, two cognitive, and one somatic-cognitive) were positively associated with at least two biomarkers. Two symptoms (one cognitive and one somatic-cognitive) were both positively and negatively associated with a biomarker.
3.5. Are greater cytokine responses to ex vivo antigen stimulations associated with depression?
IL-1β and IL-6 responses to LPS and PHA stimulations are positively associated with depression score, with effect sizes ranging from 0.21-0.41 SDs after controlling for potential confounders (Table 3). Effect sizes are similar after excluding individuals in the upper quintile of leukocyte count (not shown). In this reduced sample, TNF-α response to PHA is also positively associated with depression (βDepression score=0.21, p=0.057, same controls, n=98). In the full sample, however, for each cytokine and stimulation combination, effect sizes are attenuated after standardizing stimulated cytokine response by leukocyte count (defined as stimulated cytokine response/leukocyte count; see Table S4). Standardized IL-1β responses to LPS and PHA remain associated with depression score (for LPS IL-1β: βDepression score=0.25, p=0.010; for PHA IL-1β: βDepression score=0.36, p<0.001), but standardized LPS IL-6 response is no longer significant (βDepression score=0.10, p=0.312) and standardized PHA IL-6 response is of borderline significance (βDepression score=0.17, p=0.069).
Table 3.
OLS regressions of the effect of depression scorea on cytokine response to ex vivo antigen stimulation. Standardized β's are shown (95% CIs for βDepression score) controlling for age, sex, BMI and unstimulated serum cytokine concentrationb,c.
| TNF-α |
IL-1β |
IL-6 |
||||
|---|---|---|---|---|---|---|
| Parameter | LPS-stimulated | PHA-stimulated | LPS-stimulated | PHA-stimulated | LPS-stimulated | PHA-stimulated |
| Depression score | 0.09 (−0.12-0.30) | 0.16^ (−0.04-0.36) | 0.32*** (0.13-0.51) | 0.41*** (0.23-0.60) | 0.21* (0. 01-0.43) | 0.27** (0.07-0.47) |
| Controls | ||||||
| Age (years) | 0.04 | 0.09 | −0.08 | −0.05 | −0.06 | 0.06 |
| Sex=female | −0.20 | −0.23 | −0.74*** | −0.54** | −0.30^ | −0.49** |
| BMI (kg/m2) | 0.01 | 0.06 | 0.08 | 0.13 | 0.05 | 0.02 |
| log(unstimulated serum cytokine concentration) | 0.15 | 0.24* | −0.05 | −0.08 | −0.03 | −0.05 |
| Adjusted R2 | <0.01 | 0.06 | 0.13 | 0.15 | <0.01 | 0.06 |
| N individuals | 117 | 121 | 119 | 123 | 118 | 124 |
p≤0.001
p≤0.01
p≤0.05
p≤0.1
Summed 16 items
95% CIs for controls are not included due to space limitations
Cytokine concentrations (unstimulated and stimulated) are logged to normalize distributions
No association between stimulated cytokine response (standardized by leukocyte count or not) and depression score is in the opposite direction as predicted.
3.6. Are greater cytokine responses to ex vivo antigen stimulation associated with emotional, cognitive and somatic symptoms of depression?
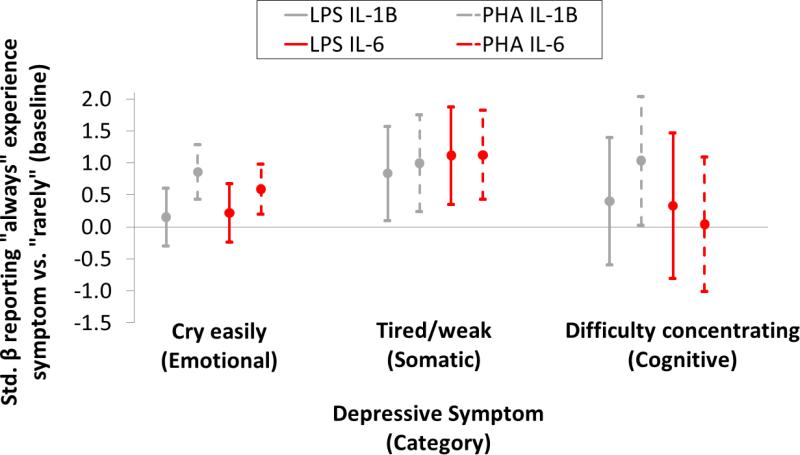
Table S5 presents OLS estimates of the effect of habitually experiencing a symptom on stimulated cytokine responses after controlling for potential confounders (Table S6 presents identical estimates after standardizing stimulated cytokine response by leukocyte count). While greater responses are associated with emotional, somatic and cognitive symptoms (Figure 2), responses are more consistent with the experience of tiredness/weakness (somatic). Crying easily (emotional) is associated with both higher unstimulated IL-6 (Figure 1) and at least one stimulated IL-6 response (Figure 2; Tables S5-S6). For all symptoms effects on IL-1β and IL-6 are more consistent than on TNF-α. In total, across 96 tests (16 items*3 cytokines*2 stimulations) 16 significant associations were found (in Table S5; 9 are reported in Table S6), all of which were in the predicted positive direction. Effect sizes range from 0.48-1.21 SDs.
Figure 2.
Standardized β(95% CI) from OLS regression of the effect of depression item score on cytokine response to ex vivo antigen stimulation. Estimates represent the change in stimulated cytokine response from reporting “always” experiencing a symptom compared to “rarely” controlling for age, sex, BMI, and unstimulated serum cytokine concentration. Stimulated cytokine response is standardized by leukocyte count. Cytokine concentrations (unstimulated and stimulated) are logged to normalize distributions. Gray lines represent IL-1β and red lines IL-6 (solid lines=LPS stimulation, dashed=PHA).
4. Discussion
We tested several predictions of the host defense hypothesis of depression among adults in a high pathogen population. As uniquely predicted by the host defense hypothesis, depression among Tsimane is associated with greater baseline immune activation (P1), particularly TNF-α, IL-6, and CRP. The association between depression and greater immune activation is therefore not unique to Western populations, as has been suggested previously (33, 34, 65). This finding challenges the immune dysregulation hypothesis, which posits that pre-adult priming of the immune system weakens linkages between depression in adulthood and inflammation. Furthermore, the fact that depression among Tsimane co-occurs with greater baseline immune activation even after excluding from analyses adults with elevated leukocyte count (indicative of acute infection) suggests a common physiological mechanism linking reduced psychological well-being to low-grade inflammation across diverse populations. Longitudinal research will be necessary to determine whether greater systemic inflammation due to infection or tissue injury is the mechanism directly leading to depression.
Depression among Tsimane is a collection of emotional, somatic, and cognitive symptoms (Table S1). While depressive symptoms may vary in their functions (8, 54, 55), we find that emotional, somatic, and cognitive symptoms are each associated with greater baseline immune activation (Figure 1; Table S3). This preliminarily supports the notion that depression is one component of a broader coordinated evolved response to infection or injury, i.e. sickness behavior (13), although further research is needed to clarify why certain symptoms are more consistently linked to biomarkers of immune activation. Moreover, alternative hypothesized functions were not tested and cannot be ruled out; alternative functions may complement a host defense function. Empirical research is thus needed to determine the function of individual depressive symptoms, and whether symptoms serve multiple functions simultaneously. Sadness, for example, may help ego identify and withdraw from situations imposing net fitness costs (8, 66), while also promoting energy conservation and reallocation through risk aversion. Pessimism may similarly conserve energy through diminished initiative while facilitating social withdrawal, which may reduce risk of transmitting infection to kin and other valued social partners. These differing hypothesized functions are not mutually exclusive and future research should identify and test competing predictions generated by the alternative adaptive models of depression. Whether depression induces social withdrawal, as predicted by the host defense hypothesis, or facilitates social integration, as predicted by the social risk hypothesis (7), is one instance in which alternative models generate competing predictions.
As uniquely predicted by the host defense hypothesis, greater cytokine responses (particularly IL-1 and IL-6) to ex vivo antigen stimulations are associated with depression among Tsimane adults (P3). Concentrations of both LPS- and PHA-stimulated cytokines positively covary with depression score, perhaps suggesting a general role for depression in potentiating immunity to both bacterial and viral infection. Consistent with our findings linking individual depressive symptoms to baseline immune activation biomarkers (section 3.4) we find that stimulated biomarkers are associated with emotional, somatic, and cognitive symptoms (section 3.6). These associations are evident even for symptoms that do not directly promote energy conservation, as in the case of “crying easily” (Figure 2).
We find no evidence of an association between immuno-suppression – as indicated by a significantly reduced cytokine response to ex vivo stimulation – and depression or any depressive symptom (Tables 3 and S4-6, Figure 2). In Western clinical and community samples both immune activation and suppression are associated with depression (15, 67), and innate and acquired immunity appear to be involved in complex ways (35, 68, 69). Few attempts have been made to categorize immune profile variability among depressed individuals, in Western or non-Western populations, in part because evidence of immune activation and suppression within the same individual is scant. One untested possibility is that more energetically costly aspects of immunity, such as cell-mediated adaptive immunity, are more sensitive to the potentiating effects of depression compared to less costly innate immune responses.
4.1. Limitations
First, the study design limits our ability to test with sufficient power whether immune activation from infection or tissue injury prospectively induces depression, and whether depression potentiates immunity in response. We are thus unable to test whether depression accelerates recovery rate, as uniquely predicted by the host defense hypothesis. The study design also limits our ability to distinguish between host defense responses to acute and chronic infectious diseases. Second, we do not test other predictions unique to the host defense hypothesis, such as whether depression reduces risk of transmitting infection to kin or other valued social partners via social withdrawal. While most of the findings we report are not predicted by other adaptive hypotheses of depression, our findings are not incompatible with those hypotheses and alternative explanations cannot be ruled out. Depression may, for example, function to solicit investment from social partners during periods of greater baseline immune activation. Third, we are unable to determine whether the results reported here generalize to younger adults, as older adults are over-represented in this study. Since selection acts strongly on young adults of reproductive age, if depression is indeed an adaptation then it must provide fitness benefits to younger adults (70). Fourth, despite a subsistence-based economy and lack of significant public health infrastructure, the Tsimane are not pure hunter-gatherers and may differ in important ways from ancestral human populations in terms of residential mobility, diet, disease exposures, and other factors. Yet no population can represent the range of experiences across different environments that shaped the evolution of our species over the millennia in which global climates and ecologies fluctuated.
4.2. Conclusion
The association between depression and greater immune activation is not unique to Western populations. Depression exists in the absence of risk factors found in industrialized societies including low pathogenic exposures in development, high prevalence of metabolic disorder, and high levels of psychosocial stress from rampant economic inequality or social isolation. Depression thus appears to be a response to conditions regularly experienced over human history, and not simply a by-product of modernity. While reduced psychological well-being is associated with poorer health across diverse societies including Tsimane (42, 52, 53, 71), depressed Tsimane adults show greater, not reduced, cytokine responses to ex vivo antigen stimulation compared to non-depressed adults. These results are consistent with the host defense hypothesis of depression. Other adaptive explanations that focus on social benefits or benefits from regulation of energy expenditure for nonsocial goals do not explain consistent bidirectional associations between depression and greater immune activation. In light of our findings, additional longitudinal research will be necessary to isolate and test competing predictions generated by the alternative adaptive scenarios of depression and its symptoms. Mood research in small-scale societies is useful to determine whether mood variation is adaptive, and to advance a more general theoretical framework for understanding susceptibility to mood disorders.
Supplementary Material
HIGHLIGHTS.
Among Bolivian forager-farmers, depression is associated with immune activation
Emotional, somatic and cognitive symptoms are associated with immune activation
Greater immune response to ex vivo blood stimulation is associated with depression
Greater ex vivo response is linked to emotional, somatic and cognitive symptoms
Acknowledgments
We thank the Tsimane for participating and THLHP personnel for collecting and coding data. Ivan Maldonado Suarez carefully conducted antigen stimulation protocols. Ed Hagen and two anonymous reviewers provided important comments that improved the quality of the manuscript. Funding comes from the National Institutes of Health/National Institute on Aging (R01AG024119 and R56AG02411), and from the Agence Nationale de la Recherche (ANR)-Labex IAST. Funding sources had no direct involvement in study design, data collection, analysis, interpretation of data, or manuscript preparation or submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Tobit regression treats observations below the detection limit as censored values lying somewhere between zero and the detection limit, and adjusts the variance accordingly. Lubin et al. (64) argue that Tobit regression produces unbiased variance estimates, unlike several alternatives, when the percentage of measurements below the detection limit is moderate or high.
While this leukocyte count threshold was used because it does not appeal to reference ranges developed in societies experiencing different epidemiological conditions than the Tsimane, it is fairly similar to the NIH-recommended cutoff of 10,000 cells/mm3 (http://www.nlm.nih.gov/medlineplus/ency/article/003643.htm).
References
- 1.Nesse R. Evolutionary explanations of emotions. Human Nature. 1990;1(3):261–289. doi: 10.1007/BF02733986. [DOI] [PubMed] [Google Scholar]
- 2.Darwin C. The expression of the emotions in man and animals. John Murray; London: 1872. [Google Scholar]
- 3.Nesse RM. Is depression an adaptation? Archives of General Psychiatry. 2000;57(1):14. doi: 10.1001/archpsyc.57.1.14. [DOI] [PubMed] [Google Scholar]
- 4.WHO . Global health risks: mortality and burden of disease attributable to selected major risks. WHO; 2009. [Google Scholar]
- 5.Andrews PW, Thomson J JA. The bright side of being blue: Depression as an adaptation for analyzing complex problems. Psychological Review. 2009;116(3):620–654. doi: 10.1037/a0016242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price J, Gardner R, Jr., Erickson M. Can depression, anxiety and somatization be understood as appeasement displays? Journal of Affective Disorders. 2004;79:1–11. doi: 10.1016/S0165-0327(02)00452-4. [DOI] [PubMed] [Google Scholar]
- 7.Allen NB, Badcock PBT. The social risk hypothesis of depressed mood: evolutionary, psychosocial, and neurobiological perspectives. Psychological bulletin. 2003;129(6):887. doi: 10.1037/0033-2909.129.6.887. [DOI] [PubMed] [Google Scholar]
- 8.Hagen EH. The bargaining model of depression. In: Hammerstein P, editor. Genetic and cultural evolution of cooperation. MIT Press; Berlin: 2003. pp. 95–123. [Google Scholar]
- 9.Klinger E. Consequences of commitment to and disengagement from incentives. Psychological Review. 1975;82(1):1–25. [Google Scholar]
- 10.Taylor SE, Brown JD. Illusion and well-being: a social psychological perspective on mental health. Psychol Bull. 1988;103(2):193–210. [PubMed] [Google Scholar]
- 11.Anders S, Tanaka M, Kinney DK. Depression as an evolutionary strategy for defense against infection. Brain Behav Immun. 2013;20(12):00532–00536. doi: 10.1016/j.bbi.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Raison CL, Miller AH. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D). Mol Psychiatry. 2012;18(1):15–37. doi: 10.1038/mp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart BL. Biological basis of the behavior of sick animals. Neuroscience & Biobehavioral Reviews. 1988;12(2):123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 14.Shattuck EC, Muehlenbein MP. Human sickness behavior: Ultimate and proximate explanations. American Journal of Physical Anthropology. 2015:n/a–n/a. doi: 10.1002/ajpa.22698. [DOI] [PubMed] [Google Scholar]
- 15.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140(3):774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Biggelaar AH, et al. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Experimental Gerontology. 2007;42(7):693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. Journal of psychosomatic research. 2002;53(4):873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 18.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayley S, Scharf J, Anisman H. Central administration of murine interferon-α induces depressive-like behavioral, brain cytokine and neurochemical alterations in mice: A mini-review and original experiments. Brain, Behavior, and Immunity. 2013;31(0):115–127. doi: 10.1016/j.bbi.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Yirmiya R, et al. Cytokines, “depression due to a general medical condition,” and antidepressant drugs. Advances in experimental medicine and biology. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [DOI] [PubMed] [Google Scholar]
- 21.Harrison NA, et al. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological psychiatry. 2009;66(5):407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenberger N, Inagaki T, Mashal N, Irwin M. Inflammation and social experience: An inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, Behavior, and Immunity. 2010;24:558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonaccorso S, et al. Immunotherapy with interferon-alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. Psychiatry research. 2001;105(1):45–55. doi: 10.1016/s0165-1781(01)00315-8. [DOI] [PubMed] [Google Scholar]
- 24.Kuningas M, et al. Selection for genetic variation inducing pro-inflammatory responses under adverse environmental conditions in a Ghanaian population. PLOS One. 2009;11(4):e7795. doi: 10.1371/journal.pone.0007795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasunilashorn S, et al. Inflammatory gene variants in the Tsimane, an indigenous Bolivian population with a high infectious load. Biodemography and Social Biology. 2011;57(1):33–52. doi: 10.1080/19485565.2011.564475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maes M. Cytokines, stress, and depression. Springer; 1999. Major depression and activation of the inflammatory response system. pp. 25–46. [DOI] [PubMed] [Google Scholar]
- 27.Baracos VE, Whitmore WT, Gale R. The metabolic cost of fever. Can J Physiol Pharmacol. 1987;65(6):1248–1254. doi: 10.1139/y87-199. [DOI] [PubMed] [Google Scholar]
- 28.Muehlenbein MP, Hirschtick JL, Bonner JZ, Swartz AM. Toward quantifying the usage costs of human immunity: Altered metabolic rates and hormone levels during acute immune activation in men. Am J Hum Biol. 2010;22(4):546–556. doi: 10.1002/ajhb.21045. [DOI] [PubMed] [Google Scholar]
- 29.Syme K, Garfield Z, Hagen E. (Under revision) Testing the costly signaling vs. inclusive fitness models of suicidal behavior against the ethnographic record. Evolution and Human Behavior [Google Scholar]
- 30.Charlton BG. The malaise theory of depression: major depressive disorder is sickness behavior and antidepressants are analgesic. Medical hypotheses. 2000;54(1):126–130. doi: 10.1054/mehy.1999.0986. [DOI] [PubMed] [Google Scholar]
- 31.Bosch JA, Engeland CG, Cacioppo JT, Marucha PT. Depressive symptoms predict mucosal wound healing. Psychosom Med. 2007;69(7):597–605. doi: 10.1097/PSY.0b013e318148c682. [DOI] [PubMed] [Google Scholar]
- 32.Irwin M, et al. Cellular immunity to varicella-zoster virus in patients with major depression. J Infect Dis. 1998;178(1):S104–108. doi: 10.1086/514272. [DOI] [PubMed] [Google Scholar]
- 33.Raison CL, Lowry CA, Rook GA. Inflammation, sanitation, and consternation: loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Archives of General Psychiatry. 2010;67(12):1211–1224. doi: 10.1001/archgenpsychiatry.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDade TW, Borja JB, Adair LS, Kuzawa C. Depressive symptoms are not associated with inflammation in younger and older adults in the Philippines. Evolution, Medicine, and Public Health. 2013;2013(1):18–23. doi: 10.1093/emph/eos004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rook GA, Lowry CA. The hygiene hypothesis and psychiatric disorders. Trends Immunol. 2008;29(4):150–158. doi: 10.1016/j.it.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Segerstrom SC, Taylor SE, Kemeny ME, Fahey JL. Optimism is associated with mood, coping, and immune change in response to stress. J Pers Soc Psychol. 1998;74(6):1646–1655. doi: 10.1037//0022-3514.74.6.1646. [DOI] [PubMed] [Google Scholar]
- 37.Gurven M, et al. Inflammation and infection do not promote arterial aging and cardiovascular disease risk factors among lean horticulturalists. PLoS ONE. 2009;4:1–12. doi: 10.1371/journal.pone.0006590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurven M, Stieglitz J, Hooper PL, Gomes C, Kaplan H. From the womb to the tomb: The role of transfers in shaping the evolved human life history. Experimental Gerontology. 2012;47:807–813. doi: 10.1016/j.exger.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasunilashorn S, et al. Blood lipids, infection, and inflammatory markers in the Tsimane of Bolivia. American Journal of Human Biology. 2010;22(6):731–740. doi: 10.1002/ajhb.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackwell AD, Martin M, Kaplan H, Gurven M. Antagonism between two intestinal parasites in humans: the importance of co-infection for infection risk and recovery dynamics. Proceedings of the Royal Society B: Biological Sciences. 2013;280(1769):20131671. doi: 10.1098/rspb.2013.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blackwell A, et al. Evidence for a peak shift in a humoral response to helminths: Age profiles of IgE in the Shuar of Ecuador, the Tsimane of Bolivia, and the U.S. NHANES. PLoS Neglected Tropical Diseases. 2011;5(6):e1218. doi: 10.1371/journal.pntd.0001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain, behavior, and immunity. 2007;21(7):901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Miller G, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro-and anti-inflammatory signaling pathways six months later. Psychosomatic Medicine. 2009;71(1):57. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon GE, et al. Association between obesity and psychiatric disorders in the US adult population. Archives of general psychiatry. 2006;63(7):824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspectives in biology and medicine. 2003;46(3):S39–S52. [PubMed] [Google Scholar]
- 46.Miller GE, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gurven M, Jaeggi AV, Kaplan H, Cummings D. Physical Activity and Modernization among Bolivian Amerindians. Plos One. 2013;8(1):e55679. doi: 10.1371/journal.pone.0055679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gurven M, et al. Domestication alone does not lead to inequality. Current Anthropology. 2010;51(1):49–64. [Google Scholar]
- 49.Walker RS, et al. Living with kin in lowland horticultural societies. Current Anthropology. 2013;54(1):96–103. [Google Scholar]
- 50.Dowlati Y, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 51.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 52.Stieglitz J, et al. Work to live and live to work: Productivity, transfers, and psychological well-being in adulthood and old age. In: Weinstein M, Lane M, editors. Sociality, Hierarchy, and Health: comaprative biodemography. Committee on Population of the National Research Council, National Academies Press; Washington DC: 2014. pp. 197–221. [PubMed] [Google Scholar]
- 53.Stieglitz J, Schniter E, von Rueden C, Kaplan H, Gurven M. Functional Disability and Social Conflict Increase Risk of Depression in Older Adulthood Among Bolivian Forager-Farmers. Journal of Gerontology Series B: Psychological and Social Sciences. 2014 doi: 10.1093/geronb/gbu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keller MC, Neale MC, Kendler KS. Association of different adverse life events with distinct patterns of depressive symptoms. Am J Psychiatry. 2007;164(10):1521–1529. doi: 10.1176/appi.ajp.2007.06091564. quiz 1622. [DOI] [PubMed] [Google Scholar]
- 55.Keller MC, Nesse RM. Is low mood an adaptation? Evidence for subtypes with symptoms that match precipitants. J Affect Disord. 2005;86(1):27–35. doi: 10.1016/j.jad.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Wheelock EF. Interferon-Like Virus-Inhibitor Induced in Human Leukocytes by Phytohemagglutinin. Science. 1965;149(3681):310–311. doi: 10.1126/science.149.3681.310. [DOI] [PubMed] [Google Scholar]
- 57.Heumann D, Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clinica chimica acta; international journal of clinical chemistry. 2002;323(1-2):59–72. doi: 10.1016/s0009-8981(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 58.Ulevitch RJ, Tobias PS. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Current opinion in immunology. 1999;11(1):19–22. doi: 10.1016/s0952-7915(99)80004-1. [DOI] [PubMed] [Google Scholar]
- 59.Martin MA, et al. Fatty acid composition in the mature milk of Bolivian forager-horticulturalists: controlled comparisons with a US sample. Maternal & child nutrition. 2012;8(3):404–418. doi: 10.1111/j.1740-8709.2012.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.May L, et al. Performance of the whole-blood stimulation assay for assessing innate immune activation under field conditions. Cytokine. 2009;45(3):184–189. doi: 10.1016/j.cyto.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 61.Brindle E, Fujita M, Shofer J, O'Connor KA. Serum, plasma, and dried blood spot high-sensitivity C-reactive protein enzyme immunoassay for population research. Journal of immunological methods. 2010;362(1):112–120. doi: 10.1016/j.jim.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gurven M, Kaplan H, Zelada Supa A. Mortality experience of Tsimane Amerindians of Bolivia: Regional variation and temporal trends. American Journal of Human Biology. 2007;19:376–398. doi: 10.1002/ajhb.20600. [DOI] [PubMed] [Google Scholar]
- 63.Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 64.Lubin JH, et al. Epidemiologic Evaluation of Measurement Data in the Presence of Detection Limits. Environmental Health Perspectives. 2004;112(17):1691–1696. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rook GAW, Raison CL, Lowry CA. Childhood microbial experience, immunoregulation, inflammation and adult susceptibility to psychosocial stressors and depression in rich and poor countries. Evolution, Medicine, and Public Health. 2013;2013(1):14–17. doi: 10.1093/emph/eos005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nesse RM. Natural selection and the elusiveness of happiness. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2004;359(1449):1333–1347. doi: 10.1098/rstb.2004.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain, Behavior, and Immunity. 2011;25(2):221–229. doi: 10.1016/j.bbi.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):664–675. doi: 10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 69.Miller AH. Depression and immunity: a role for T cells? Brain Behav Immun. 2010;24(1):1–8. doi: 10.1016/j.bbi.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hagen EH. The Functions of Postpartum Depression. Evolution and Human Behavior. 1999;20(5):325–359. [Google Scholar]
- 71.Diener E, Chan MY. Happy People Live Longer: Subjective Well-Being Contributes to Health and Longevity. Applied Psychology: Health and Well-Being. 2011;3(1):1–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.