Abstract
Objective
To determine whether daily measurement of Model for End-Stage Liver Disease (MELD) score adds prognostic value to the initial MELD score in predicting mortality among cirrhotic patients admitted to the intensive care unit (ICU).
Methods
We included 830 consecutive cirrhotic patients admitted to a tertiary care ICU between January 1, 2003 and December 31, 2013 who had MELD scores on admission day 1 (MELD-D1). Daily MELD score during the first seven days of ICU admission were retrospectively abstracted. The performances of MELD-D1 to MELD-D7 and changes in MELD score on consecutive days (Δ-MELD) in predicting 90-day mortality were determined using logistic regression.
Results
MELD-D1 was an independent predictor of mortality (adjusted OR 1.07, 95% CI, 1.05-1.10; P<.001), with an area under receiver operating characteristic curve (AUC) 0.72. MELD-D2 to MELD-D7 yielded comparable performance to MELD-D1 with a ∼10% increase in risk of death per each incremental unit of MELD score (range of ORs 1.09-1.11, P<.001, AUCs 0.68-0.72). Δ-MELD-D2 to Δ-MELD-D7 were not independently associated with mortality (P>.05 for all) and did not increase the predictive performance (AUCs) when combined with MELD-D2 to MELD-D7.
Conclusions
Repeating MELD score assessment during the first seven days after ICU admission does not improve the ability of the initial MELD score for predicting 90-day mortality among cirrhotic patients. Our finding does not support the practice of routine daily measurement of the MELD score.
Keywords: serial MELD scores, delta MELD score, cirrhosis, prognostic performance
Introduction
Approximately $3 billion is spent annually for 26,000 intensive care unit (ICU) admissions secondary to cirrhosis. 1 Despite high hospital expense, cirrhotic patients have poor outcomes with a 3-fold increased risk of in-hospital mortality compared to non-cirrhotic patients. 2 Thus outcome prediction is crucial in assisting clinician's decision making as well as allocating limited medical resources. Many prognostic scores obtained on the day of ICU admission - or initial scores - have been evaluated for predicting outcomes of cirrhotic patients. 3 However, the conditions of critically ill patients may rapidly change, therefore the ideal predictors of outcomes should promptly reflect change in illness severity when measured on a daily or more frequent basis.
The Model for End-Stage Liver Disease (MELD) is a measure of the severity of liver dysfunction that is well established among clinicians caring cirrhotic patients. It is not uncommon to observe the score being obtained frequently as a prognostic tool in the ICU setting. Furthermore, the MELD score has appeal as a sequential predictor because its components, the serum creatinine, international normalized ratio (INR) and total bilirubin, fluctuate from day to day in ill patients. 4-6 Obtaining the MELD score on a daily basis may reflect the severity of the patient's clinical picture.
To our knowledge, only three studies have examined the use of serial MELD scores in the ICU setting. The study results were conflicting and none examined the use of the serial MELD score obtained on a daily basis. Cholongitas et al. 7 reported that the MELD score on the second day of ICU admission outperformed the initial score. In contrast, McPhail et al. 8 and Das et al. 9 found that the initial score had a higher performance in predicting mortality compared to subsequent MELD scores obtained on the third and seventh days after ICU admission.
Consequently, conclusive data on the utility of a daily MELD score and changes in the MELD score - or Δ-MELD - is lacking. We hypothesized that obtaining MELD measurements on a daily basis during ICU admission would provide a meaningful predictor in patients with cirrhosis. Our aims were 1) to assess the performance of the MELD score calculated on each of the first seven days after ICU admission in predicting 90-day mortality; and 2) to determine whether the Δ-MELD score increased the predictive ability of the MELD score during the first week after ICU admission.
Methods
In this retrospective study, we utilized data from the Mayo Clinic Life Sciences System (MCLSS) with an automated query-building tool called Data Discovery and Query Builder (DDQB). MCLSS is an exhaustive clinical research database that stores patient information gathered from various hospital source systems for both in and outpatients at Mayo Clinic, Rochester, MN. 10 The ICU settings for this cohort included medical, surgical, and mixed medical-surgical ICUs. The study was approved by the Institutional Review Board with a waiver of informed consent.
Eligible criteria were as follows:
Admitted to the ICU between January 1, 2003 and December 31, 2013
Aged ≥18 on the ICU admission date
Had a previous diagnosis of cirrhosis identified using the DDQB query based on a text search algorithm. This algorithm searches for the key word “cirrhosis” in the past medical history section of physician notes. Exclusion terms including “no”, “none”, “do not”, “denies”, or “family history” were applied in the same sentence as “cirrhosis” to increase specificity of the search algorithm. This search method has been validated with a sensitivity of 100% and specificity of 99% for identifying patients diagnosed with cirrhosis at our institution (Supplemental Methods). 11 Additionally, 15% of patients were randomly selected for manual chart review to verify the diagnosis of cirrhosis obtained by DDQB search algorithm. Cirrhosis was defined by a physician note, histology and/or by clinical features, including portal hypertension, radiologic characteristics of cirrhosis in cross-sectional images (small sized nodular liver and/or caudate lobe hypertrophy, portal hypertension indicated by the presence of collateral vessels, varices, and/or splenomegaly), and/or platelet count <150 × 103/μL. The kappa statistics was 1.0, indicating the perfect agreement of diagnosis of cirrhosis obtained DDQB search algorithm with diagnosis of cirrhosis directly abstracted from the physician's note in the medical record. 12
Had available laboratory data to calculate the MELD score on the day of ICU admission. The rationale of including only patients with available MELD score was that the focus of this investigation was to evaluate the prognostic utility of daily MELD score during the admission.
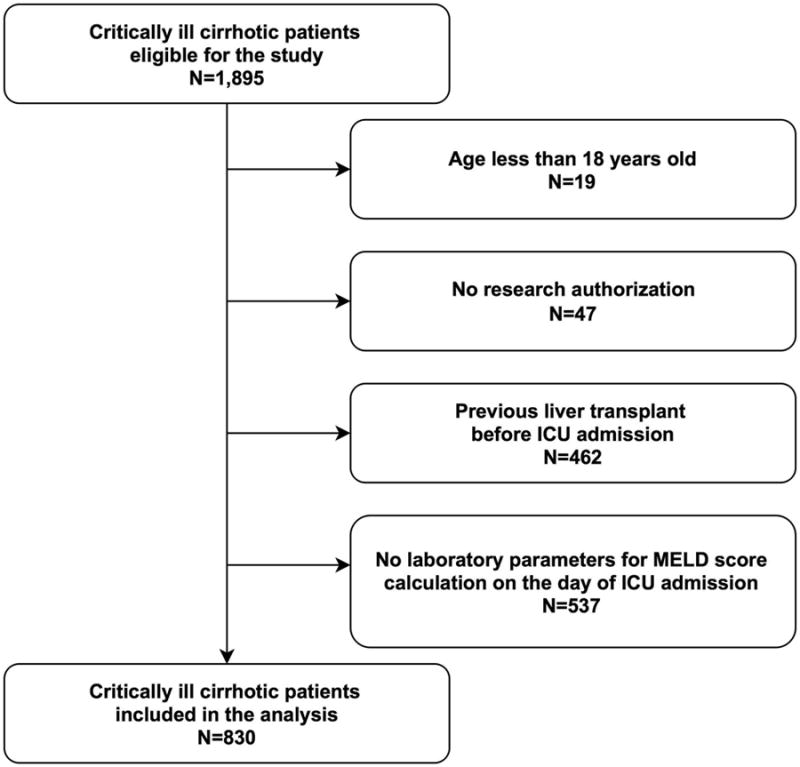
Patients with age less than 18 years old (n=19), those without research authorization (n=47), those who had previously received a liver transplant before the index ICU admission (n=462), and those without laboratory parameters for MELD score calculation on the day of admission (n=537) were excluded (Figure 1). For patients with multiple ICU admissions, only the first admission during the study period was included in the analysis. The final cohort comprised 830 consecutive cirrhotic patients.
Figure 1. Enrollment and exclusion of the study patients.
Data collection
Demographic, comorbidity, medications, and laboratory data were abstracted from the electronic medical record. DDQB was used to identify preexisting comorbid conditions according to the Charlson comorbidity index. This method was previously validated with a sensitivity and specificity of more than 90%. 11 Etiology of cirrhosis and other diagnoses during the admission were identified using the ICD-9-CM code (Supplemental Table 1) or a text search. Five percent of medical records were randomly selected and manually reviewed. Kappa statistics were calculated to determine the agreement of data identified using the ICD-9-CM code or a text search with data directly abstracted from the medical record, with kappa statistics of > 0.9 for all variables (substantial to almost perfect observer agreement). 12
The MELD score for each day was calculated by applying the United Network for Organ Sharing (UNOS) formula: 9.57 × loge[creatinine (mg/dl)] + 3.78 × loge[total bilirubin (mg/dl)] + 11.20 × loge(INR) + 6.43. 13 Per convention, creatinine, bilirubin and INR values of <1 were rounded up to 1. Patients with end-stage kidney disease or acute kidney injury requiring renal replacement therapy within a week prior to the date of assessment, or those with creatinine >4.0 mg/dl, were automatically assigned a creatinine of 4.0 mg/dl as per the UNOS modification. 14 If there was more than one instance of a MELD laboratory value obtained within a 24 hour period, the maximum value for the day was used. In case of missing data on any given day, the value for a single MELD variable on the immediately previous day was allowed to be used as a replacement. However, MELD score was not calculated if two or three variables were missing. From the expected 2,490 variables (3 variables for each of 830 patients) for initial MELD score calculation, 150 (6.0%) were missing and were replaced (92 bilirubin, 13 creatinine, and 45 INR values). The MELD score for each patient was assessed daily from the index ICU admission to day 7 after ICU admission or until hospital discharge, whichever occurred earlier. The Δ-MELD score was calculated by subtraction of the MELD score of the current day from the most recent prior MELD score. Other ICU-specific scores including APACHE III, 15 SOFA 16 and CLIF-SOFA scores 17 were also obtained on the day of ICU admission for comparison of predictive performance with initial MELD score (Supplemental Methods).
The primary outcome was 90-day mortality after the ICU admission. Vital status was abstracted from the electronic medical record. For patients who had less than 90 days follow up, the vital status at the end of the study period was obtained from the United States Social Security Index and LexisNexis® Accurint® (Dayton, Ohio), a public records search tool, on August 7, 2014.
Statistical analysis
Continuous variables were reported as mean (±standard deviation, SD) or median (interquartile range, IQR) and compared using the Student's t-test or the Wilcoxon-rank sum test as appropriate. Categorical variables were reported as counts with percentages and compared using the Chi-Square test.
The association between the initial MELD score and baseline characteristics and risk of 90-day mortality was determined using univariate logistic regression analysis. Variables with P<.05 in the univariate analysis, except for APACHE III, SOFA, CLIF-SOFA scores, were included in the multivariate model to identify independent predictors of death. The model also included age and sex as potential confounders. Creatinine, total bilirubin and INR were not included in the multivariable model as they are components of the MELD score.
To assess the predictive performance of daily MELD score and Δ-MELD score during the 7 days after ICU admission, the cohort of patients who remained hospitalized on each subsequent day were created using landmark analysis, i.e. patients who were discharged or died before the day of the analysis were excluded from the analysis. The reason for using landmark analysis (and not including only the patients who survived to day seven after ICU admission) was to avoid possible bias from including only the patients who survived long enough to have the last MELD determination, which could potentially bias the study towards a more important prognostic role of subsequent MELD and Δ-MELD, a point well illustrated by D' Amico in 2005. 18
The association between daily MELD score and 90-day mortality was determined using univariate logistic regression analysis. To evaluate whether Δ-MELD score enhanced the prognostic ability of the MELD score, both daily MELD score and Δ-MELD score were included in the multivariate logistic regression model. The AUCs of daily MELD and Δ-MELD score were calculated. An AUC of ≥0.7 was considered acceptable. 19 All statistical analyses were performed using JMP statistical software (version 9.0, SAS Institute Inc., Cary, NC) and MedCalc (version 12.5, Medcalc Software, Ostend, Belgium). All comparisons were two sided. P<.05 was considered statistically significant.
Results
Study cohort
Table 1 displays baseline characteristics of the 830 study patients (mean age 60.0±12.4 years and 59.9% were male). The most common etiologies of cirrhosis were alcohol (34.2%), viral hepatitis C (24.8%), and nonalcoholic fatty liver disease (14.1%). The patients were in hospital for 0.9 (± 3.1) days prior to medical (51.8%), surgical (19.3%), and mixed medical-surgical ICUs (28.9%) admission. There were 50.8% of patients admitted for cirrhotic-related conditions including acute variceal bleeding, hepatic encephalopathy, hepatorenal syndrome, or spontaneous bacterial peritonitis and 51.0% required mechanical ventilation, vasopressor support and/or renal replacement therapy. The median [IQR] length of ICU and hospital stay were 2 [2,4] days and 7 [5,14] days, respectively.
Table 1. Baseline characteristics of 830 study patients.
| Total patients (N=830) | Survivor (N=549) | Non-Survivor (N=281) | P value | |
|---|---|---|---|---|
| Age, years (mean±SD) | 60.0±12.4 | 59.7±12.6 | 60.4±12.1 | .42 |
| Male, n (%) | 497 (59.9) | 323 (58.8) | 174 (61.9) | .39 |
| White, n (%) | 764 (92.1) | 506 (92.2) | 258 (91.8) | .86 |
| Body mass index, kg/m2 (mean±SD) | 29.4±7.6 | 29.5±7.5 | 29.3±7.8 | .79 |
| Obesity, n (%) | 316 (39.0) | 212 (39.7) | 104 (37.7) | .58 |
| Diabetes, n (%) | 305 (36.8) | 198 (36.1) | 107 (38.1) | .57 |
| Chronic kidney disease, n (%) | 185 (22.3) | 112 (20.4) | 73 (26.0) | .07 |
| Charlson comorbidity index (median [IQR]) | 4 [2,6] | 4 [2,6] | 5 [2,7] | .12 |
| Cause of cirrhosis | .45 | |||
| Alcohol, n (%) | 284 (34.2) | 189 (34.4) | 95 (33.8) | |
| Viral hepatitis Cc, n (%) | 206 (24.8) | 132 (24.0) | 74 (26.3) | |
| Nonalcoholic fatty liver disease, n (%) | 117 (14.1) | 80 (14.6) | 37 (13.2) | |
| Cryptogenic, n (%) | 66 (8.0) | 40 (7.3) | 26 (9.3) | |
| Primary biliary cirrhosis, n (%) | 48 (5.8) | 38 (6.9) | 10 (3.6) | |
| Cardiac cirrhosis, n (%) | 26 (3.1) | 19 (3.5) | 7 (2.5) | |
| Primary sclerosing cholangitis, n (%) | 21 (2.5) | 14 (2.6) | 7 (2.5) | |
| Autoimmune hepatitis, n (%) | 19 (2.3) | 13 (2.4) | 6 (2.1) | |
| Otherd, n (%) | 43 (5.2) | 24 (4.4) | 19 (6.8) | |
| Diagnosis during ICU stay | ||||
| Acute kidney injury, n (%) | 312 (37.6) | 147 (26.8) | 165 (58.7) | <.001 |
| Hepatic encephalopathy, n (%) | 293 (35.3) | 160 (29.1) | 133 (47.3) | <.001 |
| Acute variceal bleeding, n (%) | 128 (15.4) | 93 (16.9) | 35 (12.5) | .09 |
| Septic shock, n (%) | 108 (13.0) | 41 (7.5) | 67 (23.8) | <.001 |
| Hepatorenal syndrome, n (%) | 85 (10.2) | 35 (6.4) | 50 (17.8) | <.001 |
| Spontaneous bacterial peritonitis, n (%) | 68 (8.2) | 28 (5.1) | 40 (14.2) | <.001 |
| Cardiogenic shock, n (%) | 19 (2.3) | 5 (0.09) | 14 (5.0) | <.001 |
| Organ support during ICU stay | ||||
| Mechanical ventilation, n (%) | 297 (35.8) | 169 (30.8) | 128 (45.6) | <.001 |
| Vasopressor support, n (%) | 264 (31.8) | 121 (22.0) | 143 (50.9) | <.001 |
| Renal replacement therapy, n (%) | 118 (14.2) | 39 (7.1) | 78 (28.1) | <.001 |
| Prognostic scores at ICU admission | ||||
| MELD score (mean±SD) | 19±9 | 17±7 | 24±9 | <.001 |
| APACHE III score (mean±SD) | 81 ±29 | 73±23 | 96±32 | <.001 |
| SOFA score (mean±SD) | 8±4 | 7±3 | 10±5 | <.001 |
| CLIF-SOFA score (mean±SD) | 8±4 | 6±3 | 10±4 | <.001 |
| Laboratory parameters at ICU admission | ||||
| Sodium, mEq/L (mean±SD) | 134±6 | 135±6 | 133±7 | <.001 |
| Creatinine, mg/dL (median [IQR]) | 1.2 [0.9,2.1] | 1.1 [0.8,1.7] | 1.7 [1.1,2.8] | <.001 |
| Total Bilirubin, mg/dL (median [IQR]) | 2.3 [1.0,5.0] | 1.9 [0.9,4.0] | 3.8 [1.7,8.7] | <.001 |
| INR (mean±SD) | 1.7±0.9 | 1.5±0.7 | 2.0±1.1 | <.001 |
| Albumin, g/dL (mean±SD) | 3.0±0.7 | 3.1±0.7 | 2.9±0.6 | <.001 |
APACHE III = Acute Physiology and Chronic Health Evaluation III; CLIF-SOFA = Chronic Liver Failure-Sequential Organ Failure Assessment; ICU = intensive care unit; INR = international normalized ratio; IQR = interquartile range; MELD = Model for End-Stage Liver Disease; SD = standard deviation; SOFA = Sequential Organ Failure Assessment
SI conversion factors: To convert sodium to mmol/L, multiply values by 1. To convert creatinine to μmol/L, multiply values by 88.4. To convert total bilirubin to μmol/L, multiply values by 17.104. To convert albumin to g/L, multiple values by 10.
included 14.8% of total patients who had both alcohol and viral hepatitis C infection as cause of cirrhosis
included viral hepatitis B, cardiac, alpha-1 antitrypsin deficiency, hemochromatosis, and Wilson disease.
At 90-days after the ICU admission, 281 patients (33.9%) had died. These patients were more likely to have hepatic encephalopathy, hepatorenal syndrome, spontaneous bacterial peritonitis, acute kidney injury, cardiogenic shock and septic shock, compared to those who remained alive (P<.05 for all) (Table 1).
Initial MELD, APACHE III, SOFA, and CLIF-SOFA scores and 90-day mortality
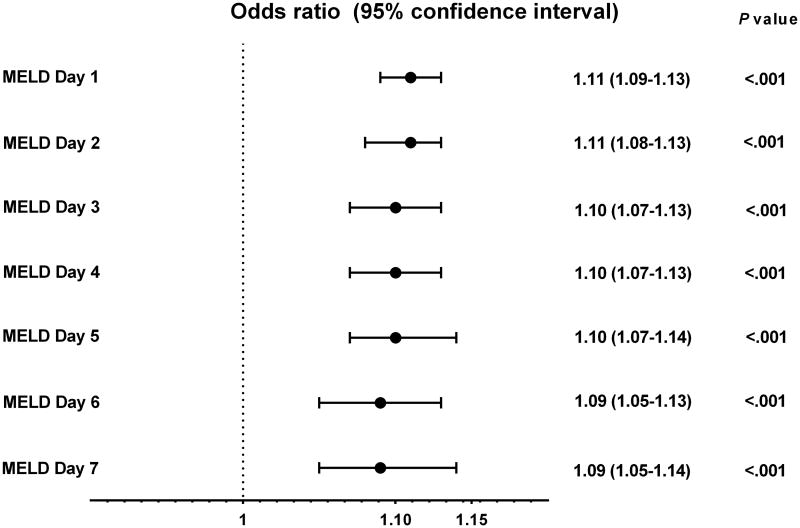
As anticipated, the initial MELD (24±9 vs. 17±7), APACHE III (96±32 vs. 73±23), SOFA (10±5 vs. 7±3) and CLIF-SOFA (10±4 vs. 6±3) scores were higher among non-survivors compared to survivors (P<.001 for all) (Table 1). By univariate analysis, each one unit increase in the initial MELD score was associated with an 11.0% increase in 90-day mortality (OR 1.11 95% CI, 1.09-1.13, P<.001) (Table 2).
Table 2. Univariate and multivariate logistic regression analysis of potential predictors for 90-day mortality after ICU admission.
| OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
|---|---|---|---|---|
| Age, per 10 years | 1.05 (0.93-1.18) | .42 | 1.20 (1.03-1.39) | .02 |
| Male | 1.14 (0.85-1.53) | .39 | 1.16 (0.82-1.64) | .42 |
| White | 0.95 (0.86-1.64) | .86 | -- | |
| Body mass index | 1.00 (0.98-1.02) | .78 | -- | |
| Obesity | 0.92 (0.68-1.24) | .58 | -- | |
| Diabetes | 1.09 (0.81-1.47) | .57 | -- | |
| Chronic kidney disease | 1.37 (0.97-1.92) | .07 | -- | |
| Charlson comorbidity index | 1.06 (1.01-1.11) | .03 | 1.05 (0.99-1.12) | .13 |
| Cause of cirrhosis | .45 | -- | ||
| Alcohol | 1 (reference) | |||
| Viral hepatitis Cb | 1.12 (0.76-1.62) | |||
| Nonalcoholic fatty liver disease | 0.92 (0.58-1.45) | |||
| Cryptogenic | 1.29 (0.74-2.24) | |||
| Primary biliary cirrhosis | 0.52 (0.24-1.06) | |||
| Cardiac cirrhosis | 0.73 (0.28-1.73) | |||
| Primary sclerosing cholangitis | 0.99 (0.37-2.48) | |||
| Autoimmune hepatitis | 0.92 (0.31-2.40) | |||
| Other | 1.58 (0.81-3.01) | |||
| Diagnosis during ICU stay | ||||
| Acute kidney injury | 3.89 (2.88-5.28) | <.001 | 1.67 (1.15-2.41) | .007 |
| Hepatic encephalopathy | 2.18 (1.62-2.95) | <.001 | 1.55 (1.08-2.21) | .02 |
| Acute variceal bleeding | 0.70 (0.45-1.05) | .09 | -- | |
| Septic shock | 3.88 (2.56-5.94) | <.001 | 1.13 (0.66-1.93) | .66 |
| Hepatorenal syndrome | 3.18 (2.02-5.06) | <.001 | 0.94 (0.52-1.72) | .85 |
| Spontaneous bacterial peritonitis | 3.09 (1.87-5.17) | <.001 | 1.58 (0.88-2.88) | .13 |
| Cardiogenic shock | 5.70 (2.16-17.80) | <.001 | 3.81 (1.25-13.37) | .02 |
| Organ support during ICU stay | ||||
| Mechanical ventilation | 1.88 (1.40-2.53) | <.001 | 1.23 (0.84-1.79) | .29 |
| Vasopressor support | 3.67 (2.70-5.00) | <.001 | 2.04 (1.33-3.13) | .001 |
| Renal replacement therapy | 5.11 (3.39-7.82) | <.001 | 1.69 (1.01-2.83) | .04 |
| Prognostic scores at ICU admission | ||||
| MELD score | 1.11 (1.09-1.13) | <.001 | 1.07 (1.05-1.10) | <.001 |
| APACHE III score | 1.03 (1.03-1.04) | <.001 | -- | |
| SOFA score | 1.21 (1.16-1.26) | <.001 | -- | |
| CLIF-SOFA score | 1.25 (1.20-1.30) | <.001 | -- | |
| Laboratory parameters at ICU admission | ||||
| Sodium | 0.95 (0.93-0.97) | <.001 | 0.99 (0.96-1.02) | .50 |
| Creatinine | 1.40 (1.26-1.57) | <.001 | -- | |
| Total Bilirubin | 1.10 (1.07-1.13) | <.001 | -- | |
| INR | 1.99 (1.62-2.49) | <.001 | -- | |
| Albumin | 0.66 (0.53-0.83) | <.001 | 0.66 (0.51-0.86) | .002 |
APACHE III = Acute Physiology and Chronic Health Evaluation III; CLIF-SOFA = Chronic Liver Failure-Sequential Organ Failure Assessment; INR = international normalized ratio; MELD = Model for End-Stage Liver Disease; SOFA = Sequential Organ Failure Assessment
included 14.8% of total patients who had both alcohol and viral hepatitis C infection as cause of cirrhosis
Additionally, APACHE III, SOFA, CLIF-SOFA scores were significantly associated with mortality (Table 2). After adjusting for age, sex, and variables significant in the univariate model, the initial MELD score remained significantly associated with 90-day mortality (adjusted OR 1.07 95% CI, 1.05-1.10; P<.001) (Table 2). The AUC of the initial MELD (0.72) was not different from that of APACHE III (0.72), SOFA (0.70) and CLIF-SOFA scores (0.72) (P>.05 for all) (Supplemental Figure 1).
Performance of daily MELD score assessment in predicting 90-day mortality
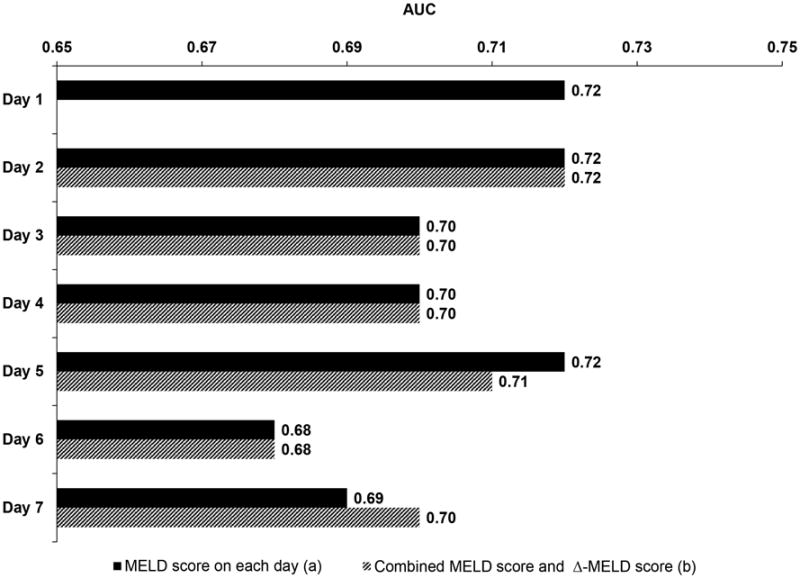
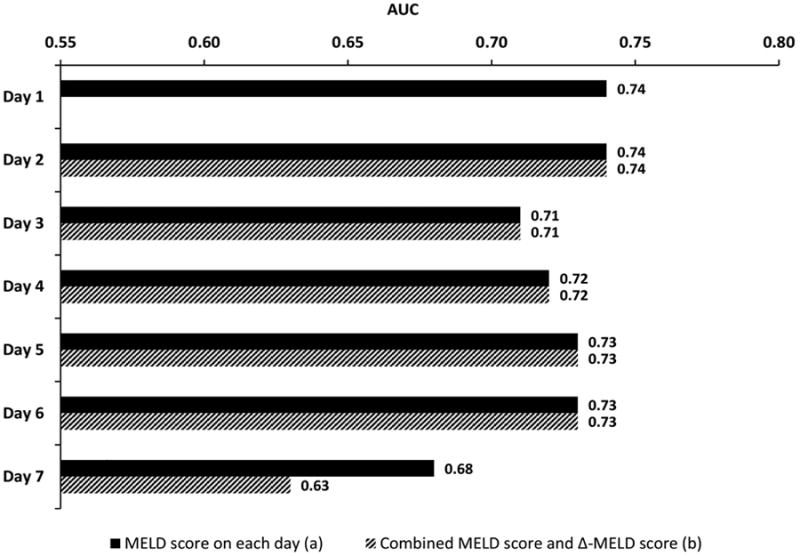
The non-survivor group had a higher mean MELD score than the survivor group during each of the first seven days after ICU admission (P<.001 for each day) (data not shown). The MELD-D2 to MELD-D7 were associated with 90-day mortality with estimated odds ratios of 1.09-1.11 (P<.001 for each day) (Figure 2). Among the AUC values calculated from MELD-D1 to MELDD7, the highest AUC of 0.72 was achieved on the first day of ICU admission (i.e. MELD-D1). Subsequent MELD assessment during the next six days did not have better predictive performance than the initial MELD score. Overall, the AUCs using the MELD score for predicting 90-day mortality in each of the first seven days ranged from 0.68 to 0.72, with a nadir on day 6 (Figure 3).
Figure 2. Association between MELD score on day 1 – 7 after ICU admission and 90-day mortality.
Figure 3. Performance of daily MELD score (a) and combined MELD score and Δ-MELD score (b) for predicting 90-day mortality.
Utility of Δ-MELD score assessment for predicting 90-day mortality
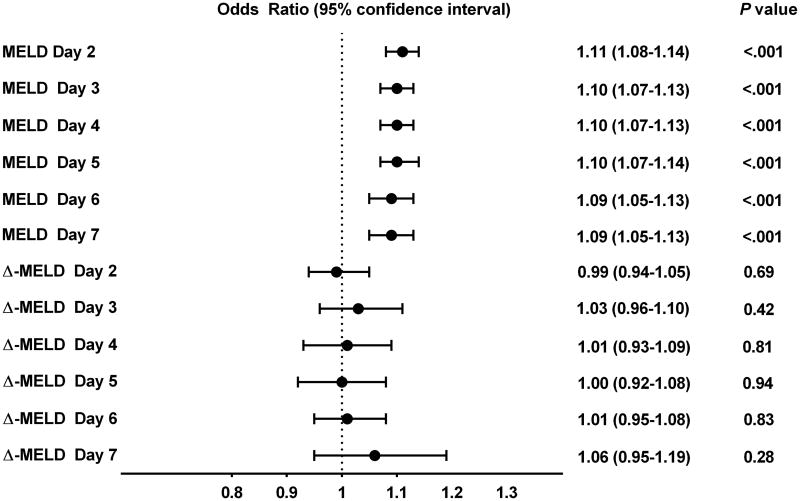
The Δ-MELD-D2 to Δ-MELD-D7 score was not independently associated with 90-day mortality (P≥.05 for all) (Figure 4). The AUCs were 0.68-0.72 when the MELD and Δ-MELD scores were combined, and these AUC values were very close to the AUCs of the MELD score alone, suggesting that Δ-MELD score did not add discriminative ability to the MELD score (Figure 3).
Figure 4. Multivariate analysis of association between MELD score or Δ-MELD score and 90-day mortality.
We further performed five sensitivity analyses to evaluate whether different means of Δ-MELD score calculation would yield consistent result. First, we applied two different definitions of Δ-MELD score, including (a) the subtraction of the current MELD score from the MELD-D1 score, and (b) subtraction of the current MELD score from the MELD score for the immediately previous day. Second, three additional analyses were performed which were similar to the prior three analyses, except for treating the direction of change of Δ-MELD score as a categorical variable (i.e., increase in MELD [coded as 1] vs. no change or decrease in MELD [coded as 0]). The results of these analyses were similar to the initial result using the original definition of Δ-MELD score (Supplemental Table 2-3).
Subgroup analyses
We performed subgroup analysis of the 423 patients who required mechanical ventilation, vasopressor support and/or renal replacement therapy. The median [IQR] length of ICU stay was 4 [2,7] days in this subgroup. The results were consistent with the main findings of the study (Figure 5). Further subgroup analyses were performed to assess whether daily MELD score would be more prognostic in particular subgroups of patients including (a) initial MELD score ≥15, (b) patients allocated only to the medical ICU, (c) patients allocated only to the surgical ICU, (d) those with septic shock, and (e) patients admitted primarily for cirrhosis-related conditions. The results of all subgroup analyses were consistent with the findings for the entire cohort (data not shown).
Figure 5.
Performance of daily MELD score (a) and combined MELD score and Δ-MELD score (b) for predicting 90-day mortality in the subgroup of 423 patients who required mechanical ventilation, vasopressor support and/or renal replacement therapy.
Discussion
The present study suggests that in cirrhotic patients admitted to the ICU, daily assessment of the MELD score does not provide additional prognostic value beyond the information provided by the initial MELD score obtained at the time of ICU admission. The differences in subsequent scores, or Δ-MELD scores, were not prognostic and did not enhance outcome prediction when they were combined with the daily MELD score. Thus our findings do not support the clinical practice of obtaining MELD score as a daily routine.
This study fills the knowledge gap by answering the question whether daily MELD or Δ-MELD scores have better predictive performance compared to the initial MELD score alone. To our knowledge, this is the first study to extensively evaluate the utilization of MELD and Δ-MELD score on every single day during the first week of ICU admission. The strengths of this study are that our cohort population was large, heterogeneous and included all types of ICUs. Furthermore, results from comprehensive sensitivity and subgroup analyses were consistent with the main findings.
Prior to this investigation, few studies had evaluated the utility of serial MELD scores in the ICU setting. Cholongitas et al. 7 found that MELD scores obtained on day 1 and day 2 after admission were predictive of outcome, however the change in MELD score between day 1 and day 2 (or Δ-MELD) was not predictive of mortality. Two other studies concurred that MELD scores obtained on the third and seventh days had similar or reduced predictive ability compared to the initial MELD score. 8, 9 We extended these prior investigations by examining the value of the daily MELD score and the changes in MELD score on the subsequent 7 days after ICU admission.
There are a number of possible explanations for this finding. First, the MELD score was initially developed for predicting short-term mortality in cirrhotic patients who received elective transjugular intrahepatic portosystemic shunt (or TIPS) procedures, and was not specifically designed for critically ill patients. 20 Moreover, the MELD score does not include variables reflective of requirement for mechanical ventilation, vasopressor support, hepatic encephalopathy, acute variceal bleeding or the albumin level, all of which were independent factors of mortality in our cohort. Second, total bilirubin may include a delta bilirubin fraction that is covalently bonded to albumin and has a prolonged half-life of up to 14 days. 21 Consequently, the total bilirubin may not change acutely on a daily basis during severe illness. Last, the value of each component of MELD score can be influenced by conditions other than liver impairment. For instance, INR alterations can be due to non-hepatic conditions such as administration of anticoagulant medication, vitamin K or fresh frozen plasma.
As the focus of health care shifts towards quality and value-based care, our results highlight the importance of rigorous evaluation of the value of routine testing, in order to reduce the use of potentially unnecessary laboratory tests. Although daily MELD score measurement is not useful for prognostication, specific measurements of the total bilirubin, INR or creatinine may be necessary for monitoring or following treatment for specific organs. Moreover, repeated MELD score measurements may still be beneficial for patients on the liver transplant list who require updated MELD status.
The search for the ideal prognostic model for ICU patients with cirrhosis remains challenging. Although our study showed that the ICU-specific scores performed similarly to the MELD score on the day of ICU admission, there is evidence from other studies that the ICU-specific scores (SOFA and CLIF-SOFA score) outperform the liver-specific score (MELD score) in predicting mortality when measured on the day of ICU admission. 7, 9, 22-26 For instance, in a prospective study of 377 cirrhotic patients, Levesque et al. reported that the SOFA score obtained on the day of ICU admission predicted ICU mortality better than the MELD score (AUC 0.92 vs. 0.82). 24 The discrepancies between our results and previous studies may be due to differences in the study populations.
It is also important to note that although daily assessment of MELD score may not provide additional prognostic value beyond the information provided by the initial MELD score at the time of ICU admission, the sequential assessment of ICU-specific scores was found to provide more prognostic information in previous studies. McPhail et al. observed that the SOFA and CLIF-SOFA scores obtained on the third and seventh days of ICU admission had a better predictive value for in-hospital mortality than the scores obtained on the day of ICU admission among patients admitted in the ICU. 8 Additionally, Jalan et al also reported that a novel ICU score, CLIF consortium acute-on-chronic liver failure (CLIF-C ACLF) measured on the second, third and eighth days of admission had incremental prognostic value in predicting 90-day mortality among patients with ACLF. 27 However, changes in scores over time were not examined in these two studies. In our study, we were not able to determine the prognostic utility of serial ICU-specific scores (SOFA and CLIF-SOFA scores) because the arterial partial pressure of oxygen/fraction of inspired oxygen (PaO2/FIO2) was not available for all patients and the grade of hepatic encephalopathy was not routinely documented.
Some limitations in this study merit comment. First, the retrospective study design was potentially subject to bias and confounders. Although consecutive cirrhotic patients admitted to the ICU were enrolled in this study, not all patients had laboratory values available for MELD score calculation on consecutive days (Supplemental Figure 2). However, the results are likely to be reliable given the large number of patients included in the landmark analysis (i.e. 180–500 cases on each day during the study period). Second, patients with available MELD scores may have been sicker, prompting requests for additional lab tests, and introducing an element of selection bias. Third, our search method might have missed cirrhotic patients who were admitted to the ICU at other institutions during the study period or those who were admitted to the ICU before the study period. Unfortunately, it was not feasible to enroll cirrhotic patients who were admitted to ICUs at other institutions due to unavailability of the data. Fourth, as cirrhosis is a progressive disease, including only the first ICU admission in patients with multiple admissions might have limited the number of patients in the cohort with advanced cirrhosis. However, if all readmissions were included in the analysis, the study might be biased toward a sicker population because of their higher readmission rate. To confirm our findings in patients with advanced cirrhosis, we performed multiple subgroup analyses e.g. patients who required organ support, those with initial MELD score ≥15, and those who were admitted primarily for cirrhosis-related conditions. All the results were consistent with the main findings of the study. Fifth, the level of severity reflected by the mean MELD score and the median length of ICU stay of our cohort was lower than those of previous reports. 3-8 In the subgroup of 407 patients who did not require any organ support, the median length of ICU stay was 2 [2,3] days and the main indications for ICU admission were suspected variceal bleeding with either unstable hemodynamic status or potential requirement for intubation during upper endoscopy, altered mental status with potential requirement for intubation, and sepsis with unstable hemodynamic status. This may explain why our overall ICU patient population was less sick compared to those in other studies, as our practice appears to proactively admit patients at high risk for imminent decompensation to the ICU. The fact that the study results limited to the sicker subgroups are essentially the same as the results for the entire cohort is reassuring. Lastly, this study was conducted in a single center and it will be prudent to validate this finding in future cohorts.
Conclusions
This study found that neither daily MELD score measurement nor Δ-MELD on the first week of admission increase outcome prediction among ICU patients with cirrhosis. Thus routinely obtaining serial MELD scores in cirrhotic patients in the ICU setting does not appear to be of prognostic benefit.
Supplementary Material
Supplemental Figure 1. Performance of initial MELD, APACHE III, SOFA and CLIF-SOFA scores
Supplemental Figure 2. The total number of patients on day 1 – 7 after ICU admission (a), the number of patients with available MELD score (b), and the number of patients who had died within 90 days after ICU admission (c)
Supplemental Table 1. ICD-9-CM code of diagnosis and cause of cirrhosis
Supplemental Table 2. Odds ratio and performance of combined MELD score and Δ-MELD score for predicting 90-day mortality after ICU admission using different definitions of Δ-MELD score
Supplemental Table 3. Odds ratio and performance of combined MELD score and Δ-MELD score for predicting 90-day mortality after ICU admission using different definitions of Δ-MELD score coded as categorical variable
Acknowledgments
Financial disclosures: This work was supported by Grants CA100882 and CA128633 from the National Institutes of Health; the Mayo Clinic Center for Cell Signaling in Gastroenterology (NIDDK P30DK084567); the Mayo Clinic Cancer Center, and the Mayo Foundation (to LRR); the Mayo Clinic Center for Clinical and Translational Science (NCATS UL1 TR000135).
Abbreviation List
- APACHE III
Acute Physiologic and Chronic Health Evaluation III
- AUC
Area under the receiver operating characteristic curve
- CLIF-SOFA
Chronic Liver Failure-Sequential Organ Failure Assessment
- ICU
Intensive care unit
- INR
International normalized ratio
- MELD
Model for End-Stage Liver Disease
- SOFA
Sequential Organ Failure Assessment
- UNOS
United Network for Organ Sharing
Footnotes
Authors' contributions: Thoetchai Peeraphatdit: had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; Study concept and design; Acquisition of data; Analysis and interpretation of data; Statistical analysis; Drafting of the manuscript.
Niyada Naksuk: Study concept and design; Analysis and interpretation of data; Statistical analysis; Critical revision of the manuscript for important intellectual content.
Charat Thongprayoon: Study concept and design; Acquisition of data; Analysis and interpretation of data; Statistical analysis; Critical revision of the manuscript for important intellectual content.
William S. Harmsen: Study concept and design; Analysis and interpretation of data; Statistical analysis
Terry M. Therneau: Study concept and design; Analysis and interpretation of data; Statistical analysis
Paola Ricci: Critical revision of the manuscript for important intellectual content
Lewis R. Roberts: Study concept and design; Analysis and interpretation of data; Critical revision of the manuscript for important intellectual content.
Roongruedee Chaiteerakij: had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; Study concept and design; Acquisition of data; Analysis and interpretation of data; Statistical analysis; Drafting of the manuscript
Potential competing interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Thoetchai Peeraphatdit, Email: peera004@umn.edu, peeraphatdit.thoetchai@mayo.edu.
Niyada Naksuk, Email: naksuk.niyada@mayo.edu.
Charat Thongprayoon, Email: thongprayoon.charat@mayo.edu.
William S. Harmsen, Email: harmsen.william@mayo.edu.
Terry M. Therneau, Email: therneau@mayo.edu.
Paola Ricci, Email: paola.ricci@va.gov.
Roongruedee Chaiteerakij, Email: chaiteerakij.roongruedee@mayo.edu.
References
- 1.Olson JC, Wendon JA, Kramer DJ, et al. Intensive care of the patient with cirrhosis. Hepatology. 2011;54(5):1864–1872. doi: 10.1002/hep.24622. [DOI] [PubMed] [Google Scholar]
- 2.Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: analysis of the National Hospital Discharge Survey. Chest. 2003;124(3):1016–1020. doi: 10.1378/chest.124.3.1016. [DOI] [PubMed] [Google Scholar]
- 3.Karvellas CJ, Bagshaw SM. Advances in management and prognostication in critically ill cirrhotic patients. Curr Opin Crit Care. 2014;20(2):210–217. doi: 10.1097/MCC.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 4.Munoz SJ, Stravitz RT, Gabriel DA. Coagulopathy of acute liver failure. Clin Liver Dis. 2009;13(1):95–107. doi: 10.1016/j.cld.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Chiou WL, Hsu FH. Pharmacokinetics of creatinine in man and its implications in the monitoring of renal function and in dosage regimen modifications in patients with renal insufficiency. J Clin Pharmacol. 1975;15(5-6):427–434. doi: 10.1002/j.1552-4604.1975.tb02364.x. [DOI] [PubMed] [Google Scholar]
- 6.Bloomer JR, Berk PD, Howe RB, Berlin NI. Interpretation of plasma bilirubin levels based on studies with radioactive bilirubin. JAMA. 1971;218(2):216–220. [PubMed] [Google Scholar]
- 7.Cholongitas E, Betrosian A, Senzolo M, et al. Prognostic models in cirrhotics admitted to intensive care units better predict outcome when assessed at 48 h after admission. J Gastroenterol Hepatol. 2008;23(8 Pt 1):1223–1227. doi: 10.1111/j.1440-1746.2007.05269.x. [DOI] [PubMed] [Google Scholar]
- 8.McPhail MJ, Shawcross DL, Abeles RD, et al. Increased Survival for Patients With Cirrhosis and Organ Failure in Liver Intensive Care and Validation of the Chronic Liver Failure-Sequential Organ Failure Scoring System. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.08.041. published online ahead of print September 21, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Das V, Boelle PY, Galbois A, et al. Cirrhotic patients in the medical intensive care unit: early prognosis and long-term survival. Crit Care Med. 2010;38(11):2108–2116. doi: 10.1097/CCM.0b013e3181f3dea9. [DOI] [PubMed] [Google Scholar]
- 10.Alsara A, Warner DO, Li G, Herasevich V, Gajic O, Kor DJ. Derivation and validation of automated electronic search strategies to identify pertinent risk factors for postoperative acute lung injury. Mayo Clin Proc. 2011;86(5):382–388. doi: 10.4065/mcp.2010.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh B, Singh A, Ahmed A, et al. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc. 2012;87(9):817–824. doi: 10.1016/j.mayocp.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 13.Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 14.Kamath PS, Kim WR Advanced Liver Disease Study G. The model for end-stage liver disease (MELD) Hepatology. 2007;45(3):797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 15.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 16.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 17.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426–1437. 1437 e1421–1429. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 18.D'Amico G. Developing concepts on MELD: delta and cutoffs. J Hepatol. 2005;42(6):790–792. doi: 10.1016/j.jhep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Hosmer DW, Lemeshow S. Applied logistic regression. Second. New York: Wiley; 2000. p. 162. [Google Scholar]
- 20.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 21.Weiss JS, Gautam A, Lauff JJ, et al. The clinical importance of a protein-bound fraction of serum bilirubin in patients with hyperbilirubinemia. N Engl J Med. 1983;309(3):147–150. doi: 10.1056/NEJM198307213090305. [DOI] [PubMed] [Google Scholar]
- 22.Cholongitas E, Senzolo M, Patch D, et al. Risk factors, sequential organ failure assessment and model for end-stage liver disease scores for predicting short term mortality in cirrhotic patients admitted to intensive care unit. Aliment Pharmacol Ther. 2006;23(7):883–893. doi: 10.1111/j.1365-2036.2006.02842.x. [DOI] [PubMed] [Google Scholar]
- 23.Filloux B, Chagneau-Derrode C, Ragot S, et al. Short-term and long-term vital outcomes of cirrhotic patients admitted to an intensive care unit. Eur J Gastroenterol Hepatol. 2010;22(12):1474–1480. doi: 10.1097/MEG.0b013e32834059cd. [DOI] [PubMed] [Google Scholar]
- 24.Levesque E, Hoti E, Azoulay D, et al. Prospective evaluation of the prognostic scores for cirrhotic patients admitted to an intensive care unit. J Hepatol. 2012;56(1):95–102. doi: 10.1016/j.jhep.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 25.Pan HC, Jenq CC, Tsai MH, et al. Scoring systems for 6-month mortality in critically ill cirrhotic patients: a prospective analysis of chronic liver failure - sequential organ failure assessment score (CLIF-SOFA) Aliment Pharmacol Ther. 2014;40(9):1056–1065. doi: 10.1111/apt.12953. [DOI] [PubMed] [Google Scholar]
- 26.Theocharidou E, Pieri G, Mohammad AO, et al. The Royal Free Hospital score: a calibrated prognostic model for patients with cirrhosis admitted to intensive care unit. Comparison with current models and CLIF-SOFA score. Am J Gastroenterol. 2014;109(4):554–562. doi: 10.1038/ajg.2013.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jalan R, Saliba F, Pavesi M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61(5):1038–1047. doi: 10.1016/j.jhep.2014.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Performance of initial MELD, APACHE III, SOFA and CLIF-SOFA scores
Supplemental Figure 2. The total number of patients on day 1 – 7 after ICU admission (a), the number of patients with available MELD score (b), and the number of patients who had died within 90 days after ICU admission (c)
Supplemental Table 1. ICD-9-CM code of diagnosis and cause of cirrhosis
Supplemental Table 2. Odds ratio and performance of combined MELD score and Δ-MELD score for predicting 90-day mortality after ICU admission using different definitions of Δ-MELD score
Supplemental Table 3. Odds ratio and performance of combined MELD score and Δ-MELD score for predicting 90-day mortality after ICU admission using different definitions of Δ-MELD score coded as categorical variable