Abstract
Background
Doppler tissue imaging (DTI) has been used to evaluate myocardial velocity during ventricular filling, a means of characterizing diastolic function. Previous studies in older children have shown an age-related increase in early diastolic tissue velocities, but there are limited data in preterm infants. The aim of this study was to prospectively determine maturational changes in diastolic tissue velocities at two points in time: 1) 7 days of age, and 2) 36 weeks post-menstrual age (PMA). We further determined whether DTI measures were altered in infants who developed bronchopulmonary dysplasia (BPD) with or without pulmonary hypertension (PH).
Methods
277 preterm infants, born at less than 34 weeks PMA, with a birth weight between 500-1250g, were prospectively enrolled. Echocardiograms were performed at 7 days of age and repeated at 36 weeks PMA. Measurements included DTI assessment of early (E’) and late (A’) annular velocities of the left ventricular (LV) free wall septum, and the right ventricular (RV) free wall. Statistical analysis included the Wilcoxon Rank Sum test, simple linear regression, and Chi-square test.
Results
At 7 days of age, there was a statistically significant increase in E’/A’ ratio as a function of gestational age at birth. At 36 weeks PMA, E’/A’ increased but there was no association with gestational age. DTI measures were not different between infants who did or did not develop BPD or PH at either time point.
Conclusions
We found a gestational age related increase in the early diastolic tissue velocity of preterm infants. At a gestational age equivalent to near-term, we observed no difference in diastolic tissue velocities regardless of gestational age at birth. Our findings suggest that maturational changes in diastolic function occur relatively independent of the timing of birth.
Keywords: tissue Doppler, preterm, diastolic function
Introduction
Doppler tissue imaging (DTI) has been used extensively in adults to evaluate myocardial velocity as a means of characterizing diastolic function1-3. This technique has also been applied to neonates and children4-7, leading to the observation that myocardial tissue velocities change with somatic growth. In infants, these changes appear to be closely related to developmental maturation that occurs during the adaptation from fetal to ex utero life8.
Early diastolic filling coincides with longitudinal motion of the atrioventricular valve annuli toward the base of the heart, which is characterized by a distinguishable deflection (E’) in DTI spectral Doppler patterns, followed by late diastolic filling that results from atrial contraction (A’). The ratio of E’/A’ is a useful indicator of the degree to which ventricular filling depends on atrial systole, or rather how easily blood fills the ventricle as a function of properties of the ventricle itself, relative to filling related to atrial contraction.
The ovine fetal myocardium is characterized by a larger non-contractile mass, as only 30% of the fetal myocardium contains contractile mass in comparison to 60% in the adult9. Others have postulated that this structural difference results in lower ventricular compliance and higher end-diastolic pressure, suggesting suboptimal diastolic function. Postnatally, both right and left ventricles remodel to reflect changes in afterload10,11; during the normal transition, the right ventricle undergoes a reduction in mass related to a decrease in pulmonary vascular resistance, and the left ventricle increases wall thickness in response to its new role as the lone systemic ventricle12.
The interruption of normal fetal cardiopulmonary developmental by preterm birth has poorly understood consequences with regard to diastolic function. Small studies in preterm infants have shown that early diastolic tissue velocities are lower than in term infants5,13,14; however, this finding has not been validated in a large, prospective cohort, and the developmental changes in diastolic tissue velocities are poorly understood. Similarly, the prognostic value of DTI measurements with regard to the development of pulmonary hypertension (PH) and bronchopulmonary dysplasia (BPD) has not been explored in a systematic and prospective fashion. The impact of a patent ductus arteriosus on tissue velocities in preterm infants has been evaluated in small cohorts, and appears to decrease diastolic tissue velocity relative to infants with a hemodynamically insignificant ductus15.
The aim of this study was to describe the impact of gestational age on diastolic tissue velocities in preterm infants at two points in time: 1) 7 days of age, and 2) 36 weeks post-menstrual age (PMA). Associations between DTI measurements and the development of PH and BPD were evaluated, along with the impact of a patent ductus arteriosus. We hypothesized that diastolic myocardial tissue velocity in preterm infants will increase with gestational age as a function of structural changes of the myocardium that are inherent to the normal developmental pattern seen in neonates. We also hypothesized that this developmental pattern is altered in diseases such as BPD and PH, as reflected by differences in DTI measurements.
Materials and Methods
All data were obtained as part of a prospective observational research protocol performed at hospitals affiliated with two academic institutions (University of Colorado Denver and Indiana University), which took place between July 2006 to March 2012. The protocol was approved by the Institutional Review Boards of each of the participating sites. Written informed consent was received from the parents/guardians of all participants. Assent was not obtained because all participants were infants.
Criteria for enrollment included a gestational age of 34 weeks or less at birth, a birth weight of 500 to 1250 g, and subjects were required to be enrolled before 7 days of age. Gestational age refers to the period prior to birth, where post-menstrual age refers to pre- and post-natal age. Exclusion criteria included clinical evidence of congenital heart disease (except patent ductus arteriosus [PDA], atrial septal defect [ASD] < 1cm, or ventricular septal defect [VSD] < 2mm if known prior to enrollment); lethal congenital abnormality; and futile cases (anticipated death prior to hospital discharge). Infants who died, withdrew, or were transferred prior to undergoing echocardiogram assessment at 36 weeks’ PMA were excluded from analysis (n = 39).
BPD status and severity was assessed at 36 weeks PMA using a modification of the NIH workshop definition16, with application of the oxygen reduction test as described by Walsh17. Infants were assigned to a BPD status according to the following criteria: neonates on positive pressure support or receiving ≥30% supplemental oxygen (≥35% at Denver altitude) were assigned the outcome severe BPD. Patients who received < 28 days oxygen and were on room air at 36 weeks PMA were assigned to the outcome of No BPD. Patients receiving > 28 days of supplemental oxygen but were on room at 36 weeks PMA were assigned to the outcome of Mild BPD. Those receiving <30% supplemental oxygen (<35% at Denver altitude) at 36 weeks PMA underwent oxygen reduction testing17 to differentiate between No BPD (required < 28 days of oxygen and passed reduction test), Mild BPD (required > 28 days of oxygen and passed oxygen reduction test), or Moderate BPD (required > 28 days of oxygen and did not pass the reduction test). Disease severity categories were adjusted for altitude (applied to the result of the oxygen reduction test for those who performed the test) for subjects enrolled in Denver, Colorado (1600 m)18. All data was prospectively collected and managed using REDCap (Research Electronic Data Capture)19 database hosted at the University of Colorado Denver Development and Informatics Service Center.
Echocardiogram Screening
Research echocardiograms were performed at 7 days of age and at 36 weeks’ PMA20. Echocardiograms done in the first days of life have the limitation of coinciding with rapidly changing hemodynamics during the transition from fetal to ex utero life, therefore initial echocardiograms were performed at 1 week of age. Echocardiography was performed using a Vivid 7 (GE Healthcare, Milwaukee, WI) or iE33 (Philips Medical Systems, Andover, MA) platform, with probe frequencies appropriate for body habitus. Echocardiographic images were reviewed offline, using commercially available software (AGFA Healthcare Cardiovascular Review Station version 2.14.03, Mortsel, Belgium). Measurements included tricuspid regurgitant jet velocity (TRJV), measurable dimensions of heart chambers, any detectable shunt lesions, and the direction of flow for any shunt lesion. Shunt lesions were defined as any cardiac level shunt (patent foramen ovale [PFO], atrial septal defect [ASD], and ventricular septal defect [VSD]) or patent ductus arteriosus (PDA). A PDA equal in diameter or larger than the PA was deemed large. Differentiating between moderate and small PDA’s involved subjective assessment of the 2D appearance of the PDA, the appearance of the shunt vena contracta by color Doppler, and the size of the left heart from multiple views. Systemic systolic blood pressure (sBP) was recorded at the time of echocardiogram via blood pressure cuff unless the patient had an existing arterial catheter. The myocardial performance index (MPI) was obtained by summing the ventricular isovolumic contraction time and relaxation time and dividing by right ventricular ejection time21,22. Peak early diastolic tissue velocity (E’) and late diastolic tissue velocity (A’) were measured at the atrioventricular valve annular level from the apical window.
Qualitative echocardiographic measures of pulmonary hypertension, such as right atrial (RA) enlargement, right ventricular (RV) dilation, right ventricular hypertrophy (RVH), ventricular septal wall flattening, and PA dilation, were noted. The TRJV measurements had to meet predefined quality criteria in order to be used to estimate right ventricular systolic pressure (RVSP). The criteria required the spectral Doppler pattern to consist of a clearly defined envelope with a discernable peak velocity; incomplete or poorly demarcated envelopes were not used to estimate RVSP. Estimates of RVSP were calculated with no allowance for the right atrial pressure, by using the modified Bernoulli equation (TRJV2 × 4).
All echocardiograms were interpreted by a single cardiologist (AKY) blinded to subjects’ clinical status in order to provide consistent evaluation of findings. Criteria for PH were met by any of the following findings: an estimated RVSP >40 mm Hg, or RVSP/sBP >0.5, or any cardiac shunt with bidirectional or right-to-left flow, or any degree of ventricular septal wall flattening. To determine whether DTI measures of myocardial tissue velocity were associated with the development of PH or BPD, we compared measurements between infants who were or were not subsequently diagnosed with PH or BPD. Similarly, subjects were grouped by the presence of a moderate-large PDA in order to determine the impact of a PDA on diastolic tissue velocities.
All research echocardiograms were also separately interpreted by each institution’s clinical cardiologists in order to provide real-time results to the caring physicians as required by the Institutional Review Boards. However, these interpretations were not utilized in the analyses. Management decisions based on the echocardiogram results, including the use of vasodilators and the timing of additional clinical echocardiograms, were at the discretion of the caring physicians.
Statistics
Continuous data are presented as mean ± standard deviation or median (interquartile range [IQR]), and categorical data are presented as proportions (%). Distributions for all continuous variables were assessed using the Shapiro-Wilk test for normality. All outcome variables with skewed distributions were analyzed in simple comparisons using Wilcoxon Rank Sum Tests. Simple linear regression fit DTI outcomes by gestational age at birth and tested whether the slopes were significantly different from zero. Chi-square test was used to assess the association between categorical variables. Statistical significance was assessed using an alpha level of 0.05. All statistics were computed using SAS v 9.4 (Cary, NC).
Results
Two-hundred and seventy seven subjects were enrolled and survived to have a 36 week PMA echocardiogram performed. All but 3 of the 277 infants had an echocardiogram performed at 7 days of age. Baseline characteristics are summarized in table 1. The median gestational age (IQR) of the cohort was 27 weeks (25-28), with a median birth weight of 909 grams (750-1075), and there was no gender predominance. Two hundred and seventeen (78.3%) of the subjects received antenatal corticosteroids.
Table 1.
Sample Characteristics
| Variable | N (%) or Median (IQR) |
|---|---|
| Birth Weight (g) | 909 (750 - 1075) |
| Birth Weight Z-Score | −0.27 (−0.82 - 0.29) |
| Birth Weight Strata (g) | |
| 500-749 (n= 67) | 660 (600 - 695) |
| 750-999 (n= 116) | 870 (810 - 940) |
| 1000-1250 (n= 94) | 1129 (1070 - 1190) |
| Gestational Age at birth (weeks) | 27 (25-28) |
| 23-26 weeks | 129 (46.6%) |
| 27-29 weeks | 114 (41.2%) |
| 30-33 weeks | 34 (12.3%) |
| Gender (Male) | 135 (48.7) |
| Small for Gestational Age | 29 (10.5) |
| Maternal Smoking | 38 (13.7) |
| Antenatal Corticosteroids | 217 (78.3) |
| Cesarean Section | 210 (75.8) |
| Pneumonia | 35 (12.6) |
| Necrotizing Enterocolitis | 45 (16.3) |
| Sepsis | 59 (21.3) |
| PDA - Indocin Treatment | 118 (42.6) |
| PDA - Surgical Ligation | 44 (15.9) |
| Days of CPAP | 12 (6-25) |
| Required CPAP at 36 Weeks | 15 (5.4) |
| Days of MV | 16 (5-41) |
| Required MV at 36 Weeks | 20 (7.2) |
| Length of stay (NICU) | 90 (74-114) |
| Discharged on Oxygen | 166 (59.9) |
PDA = patent ductus arteriosus, CPAP = continuous positive airway pressure, MV = mechanical ventilation, NICU = neonatal intensive care unit
Anatomic, volumetric, and functional echocardiographic data appear in table 2A & 2B, at 7 days of age and 36 weeks PMA, respectively. In table 2A, as expected, PDA was more common in the early preterm infants, becoming less frequent in the more advanced GA cohorts. The incidence of other shunt lesions did not change significantly. LV diameter and mass increased, while ejection fraction and shortening fraction decreased, remaining within normal limits. LV MPI was not different, but RV MPI was progressively lower in the more advanced GA cohorts. Table 2B, data obtained at 36 weeks PMA regardless of GA at birth, showed no difference in any of the above parameters, apart from RV end-diastolic dimension; there was a small but statistically significant decrease RV diameter when the early and late preterm cohorts are compared.
Table 2A.
Anatomic, Volumetric, and Functional Echocardiographic Data at 7 Days of Age
| Post-Menstrual Age at Birth (Weeks) | p-value* | ||||||
|---|---|---|---|---|---|---|---|
| n | 23 to 26 (N=126) |
n | 27 to 29 (N=114) |
n | 30 to 33 (N=34) |
||
| Med(IQR) or n(%) | Med(IQR) or n(%) | Med (IQR) or n (%) | |||||
| PDA | 110 | 55 (43.7%) | 93 | 35 (30.7%) | 28 | 4 (11.8%) | 0.002 |
| ASD/PFO | 108 | 108 (85.7%) | 89 | 86 (75.4%) | 27 | 27 (79.4%) | 0.158 |
| VSD | 108 | 6 (4.8%) | 94 | 1 (0.9%) | 27 | 1 (2.9%) | 0.199 |
| RVDd | 104 | 0.54 (0.44-0.64) | 91 | 0.59 (0.47-0.70) | 25 | 0.61 (0.49-0.73) | 0.05 |
| LVDd | 107 | 1.12 (1.04-1.29) | 93 | 1.23 (1.12-1.38) | 29 | 1.35 (1.26-1.41) | <0.0001 |
| LVEF | 106 | 69.9 (65.5-76.6) | 91 | 66.4 (60.3-73.1) | 29 | 63.3 (60.5-69.0) | 0.0006 |
| FS (%) | 107 | 36.8 (33.4-42.2) | 92 | 34.5 (30.2-39.4) | 29 | 31.6 (29.7-36.0) | 0.0005 |
| LV mass (g) | 106 | 1.71 (1.41-2.23) | 92 | 2.20 (1.82-2.60) | 29 | 2.36 (2.14-2.66) | <0.0001 |
| LVMPI | 113 | 0.33 (0.28-0.44) | 107 | 0.38 (0.29-0.47) | 31 | 0.37 (0.29-0.47) | 0.416 |
| RV MPI | 112 | 0.30 (0.22-0.42) | 98 | 0.26 (0.17-0.39) | 29 | 0.17 (0.05-0.31) | 0.0007 |
| TR jet peak velocity | 14 | 2.52 (2.42-2.61) | 15 | 2.41 (1.95-2.91) | 1 | 2.19 (2.19-2.19) | 0.56 |
| RV pressure estimate | 13 | 26 (24.4-27.2) | 14 | 24.1 (17.3-33.8) | 1 | 19.8 (19.8-19.8) | 0.449 |
| AT/ET | 111 | 0.38 (0.32-0.47) | 99 | 0.39 (0.32-0.45) | 31 | 0.39 (0.36-0.47) | 0.377 |
| Mitral Valve E | 90 | 0.44 (0.38-0.53) | 91 | 0.48 (0.40-0.54) | 24 | 0.48 (0.41-0.57) | 0.329 |
| Tricuspid Valve E | 89 | 0.38 (0.33-0.47) | 90 | 0.42 (0.36-0.50) | 23 | 0.39 (0.33-0.47) | 0.116 |
PDA = patent ductus arteriosus, ASD = atrial septal defect, PFO = patent foramen ovale, VSD = ventricular septal defect, RVDd = right ventricular end-diastolic dimension in diastole, LVDd = left ventricular end-diastolic dimension in diastole, LVEF = left ventricular ejection fraction, FS = fractional shortening, LV = left ventricular, MPI = myocardial performance index, TR tricuspid regurgitant, AT = acceleration time, ET = ejection time
P-value was obtained using a non-parametric Wilcoxon Signed Rank Test for paired data.
Table 2B.
Anatomic, Volumetric, and Functional Echocardiographic Data at 36 Weeks PMA
| Post-Menstrual Age at Birth (Weeks) | p-value* | ||||||
|---|---|---|---|---|---|---|---|
| n | 23 to 26 (N=126) |
n | 27 to 29 (N=114) |
n | 30 to 33 (N=34) |
||
| Med(IQR) or n(%) | Med(IQR) or n(%) | Med (IQR) or n (%) | |||||
| PDA | 129 | 20 (15.5%) | 114 | 12 (10.5%) | 34 | 2 (5.9%) | 0.3 |
| ASD/PFO | 129 | 100 (77.5%) | 114 | 87 (76.3%) | 34 | 28 (82.4%) | 0.7 |
| VSD | 129 | 4 (3.1%) | 114 | 2 (1.8%) | 34 | 1 (2.9%) | 0.7 |
| RVDd | 114 | 0.84 (0.64-1.04) | 101 | 0.81 (0.66-0.98) | 30 | 0.71 (0.58-0.83) | 0.03 |
| LVDd | 115 | 1.77 (1.59-1.91) | 106 | 1.72 (1.62-1.87) | 31 | 1.72 (1.53-1.84) | 0.6 |
| LVEF | 114 | 68 (63.3-73.0) | 105 | 66.5 (62.4-71.8) | 31 | 64.6 (62.6-69.3) | 0.1 |
| FS (%) | 115 | 35.3 (31.7-39.2) | 106 | 33.9 (31.0-38.2) | 31 | 32.6 (31.2-36.2) | 0.08 |
| LV mass (g) | 114 | 5.36 (4.33-6.91) | 104 | 5.77 (4.54-7.02) | 31 | 4.72 (4.14-5.85) | 0.1 |
| LVMPI | 116 | 0.35 (0.28-0.44) | 108 | 0.33 (0.27-0.41) | 31 | 0.32 (0.19-0.41) | 0.08 |
| RVMPI | 111 | 0.25 (0.18-0.35) | 101 | 0.25 (0.17-0.35) | 29 | 0.20 (0.11-0.30) | 0.09 |
| TR jet peak velocity | 13 | 2.84 (2.71-3.00) | 4 | 2.76 (2.47-3.05) | 1 | 2.43 (2.43-2.43) | 0.3 |
| RV pressure estimate | 14 | 31.9 (27.0-36.0) | 4 | 30.5 (24.4-37.1) | 2 | 12.06 (0.50-23.62) | 0.2 |
| AT/ET | 115 | 0.31 (0.25-0.39) | 104 | 0.33 (0.27-0.43) | 31 | 0.76 (0.64-1.02) | 0.2 |
| Mitral Valve E | 65 | 0.86 (0.71-0.93) | 67 | 0.80 (0.68-0.92) | 19 | 0.61 (0.50-0.72) | 0.5 |
| Tricuspid Valve E | 59 | 0.65 (0.56-0.74) | 66 | 0.62 (0.54-0.70) | 20 | 0.6 | |
PDA = patent ductus arteriosus, ASD = atrial septal defect, PFO = patent foramen ovale, VSD = ventricular septal defect, RVDd = right ventricular end-diastolic dimension in diastole, LVDd = left ventricular end-diastolic dimension in diastole, LVEF = left ventricular ejection fraction, FS = fractional shortening, LV = left ventricular, MPI = myocardial performance index, TR tricuspid regurgitant, AT = acceleration time, ET = ejection time
P-value was obtained using a non-parametric Wilcoxon Signed Rank Test for paired data.
Comparing data obtained at 7 days of age and 36 weeks PMA (not presented in table form), LV MPI was significantly lower when measured at 36 weeks PMA, compared to 7 days of age (p = 0.006). RV MPI was lower, but not statistically different (p = 0.06). The ratio of right ventricular outflow acceleration time to ejection time was significantly lower at 36 weeks PMA (p < 0.0001). Both mitral and tricuspid valve inflow peak velocity were significantly increased at 36 weeks PMA (p <0.0001 for both tests).
DTI measurements of LV lateral wall, septal, and RV free wall velocities are presented in table 3A and 3B, at 7 days of age and 36 weeks PMA, respectively. The subjects are grouped by PMA at delivery for the purposes of illustrating the evolution of diastolic myocardial tissue velocity during post-natal development. As shown in table 3A, when studied at 7 days of age, there was a statistically significant increase in LV lateral wall diastolic tissue velocity (E’), as a function of increasing gestational age (GA). LV lateral wall A’ decreased with GA, and as such, the ratio of lateral wall E’/A’ increases significantly with GA. Median septal and RV free wall E’ and A’ increase with GA, but are not statistically different. The ratio of septal E’/A’ was found to be significantly different between the two time points. RV free wall E’/A’ increase, without achieving statistical significance. The ratio of mitral inflow peak velocity (E) to peak early diastolic tissue velocity (E’) decreased significantly related to GA. Table 3B shows data collected at 36 weeks PMA, also grouped by GA at birth. The LV or RV free wall diastolic myocardial velocities were not different by the time infants reached 36 weeks PMA, regardless of GA at birth.
Table 3A.
DTI Measurements of the LV Wall, Septum, and RV Wall at 7 Days of Age
| Post Menstrual Age at Blrth (Weeks) | p-value* | ||||||
|---|---|---|---|---|---|---|---|
| n | 23 to 26 (N=126) | n | 27 to 29 (N=114) | n | 30 to 33 (N=34) | ||
| Med (IQR) or n (%) | Med (IQR) | Med (IQR) | |||||
| PDA | 110 | 55 (43.7%) | 93 | 35 (30.7%) | 28 | 4 (11.8%) | 0.002 |
| LV Lateral Wall - E' (cm/sec) | 93 | 4 (4 - 5) | 81 | 5 (4 - 7) | 21 | 5 (5 - 6) | 0.003 |
| LV Lateral Wall - A' (cm/sec) | 85 | 7 (6 - 8) | 80 | 6 (5 - 8) | 21 | 5 (4 - 7) | 0.019 |
| LV Lateral Wall E'/A' | 85 | 0.60 (0.50 - 0.80) | 80 | 0.75 (0.60 - 1.00) | 21 | 1.00 (0.67 - 1.20) | <.0001 |
| Septum – E' (cm/sec) | 97 | 4 (3 - 5) | 86 | 4 (4 - 5) | 23 | 4 (3 - 5) | 0.065 |
| Septum – A' (cm/sec) | 88 | 6 (5 - 7) | 85 | 6 (5 - 7) | 22 | 6 (5 - 7) | 0.679 |
| Septum E'/A' | 88 | 0.63 (0.50 - 0.75) | 85 | 0.71 (0.60 - 0.83) | 22 | 0.67 (0.60 - 0.80) | 0.037 |
| RV Free Wall - E' (cm/sec) | 88 | 5 (4 - 6) | 76 | 6 (5 - 7) | 22 | 5.5 (5 - 7) | 0.445 |
| RV Free Wall - A' (cm/sec) | 81 | 9 (8 - 10) | 75 | 9 (8 - 10) | 21 | 8 (7 - 9) | 0.158 |
| RV Free Wall E'/A' | 81 | 0.63 (0.50 - 0.70) | 75 | 0.64 (0.56 - 0.78) | 21 | 0.67 (0.63 - 0.86) | 0.055 |
| MVE/E' | 81 | 10.67 (8.17 - 13.00) | 81 | 9.14 (6.33 - 11.75) | 19 | 9.20 (6.60 - 11.60) | 0.026 |
| TVE/E' | 76 | 0.07 (0.06 - 0.09) | 70 | 0.07 (0.06 - 0.09) | 16 | 0.07 (0.06 - 0.08) | 0.913 |
LV = left ventricular, RV = right ventricular, MV = mitral valve, TV = tricuspid valve, E' = early diastolic tissue velocity, A' = late diastolic tissue velocity.
P-value was obtained using a non-parametric Kruskal-Wallis Test.
Table 3B.
DTI Measurements of the LV Wall, Septum, and RV Wall at 36 Weeks PMA
| Post Menstrual Age at Blrth (Weeks) | p-value* | ||||||
|---|---|---|---|---|---|---|---|
| n | 23 to 26 (N=126) | n | 27 to 29 (N=114) | n | 30 to 33 (N=34) | ||
| Med (IQR) | Med (IQR) | Med (IQR) | |||||
| PDA | 129 | 20 (15.5%) | 114 | 12 (10.5%) | 34 | 2 (5.9%) | 0.268 |
| LV Lateral Wall - E' (cm/sec) | 83 | 7 (6 - 9) | 67 | 7 (6 - 9) | 23 | 8 (6-10) | 0.589 |
| LV Lateral Wall – A' (cm/sec) | 76 | 8 (6 - 10) | 66 | 9 (7 - 11) | 23 | 7 (6 - 9) | 0.113 |
| LV Lateral Wall E'/A' | 75 | 0.82 (0.64 - 1.43) | 66 | 0.76 (0.63 - 1.13) | 23 | 0.89 (0.75 - 1.43) | 0.169 |
| Septum – E' (cm/sec) | 82 | 6 (5 - 7) | 68 | 6 (5 - 7) | 21 | 6 (6 - 7) | 0.358 |
| Septum – A' (cm/sec) | 75 | 8 (6 - 9) | 67 | 8 (7 - 10) | 21 | 7 (6 - 7) | 0.009 |
| Septum E'/A' | 75 | 0.71 (0.60 - 0.88) | 67 | 0.71 (0.57 - 0.86) | 21 | 0.86 (0.67 - 1.00) | 0.031 |
| RV Free Wall – E' (cm/sec) | 66 | 9 (7 - 11) | 57 | 9 (7 - 11) | 13 | 7 (5 - 8) | 0.177 |
| RV Free Wall – A' (cm/sec) | 61 | 11 (9 - 13) | 56 | 11 (9 - 13) | 13 | 9 (8 - 11) | 0.07 |
| RV Free Wall E'/A' | 61 | 0.78 (0.62 - 0.91) | 56 | 0.79 (0.61 - 1.15) | 13 | 0.80 (0.64 - 1.20) | 0.674 |
| MV E/E' | 60 | 12.07 (9.00 - 14.10) | 55 | 11.17 (9.29 - 14.60) | 15 | 8.11 (5.83 - 12.67) | 0.092 |
| TV E/E' | 41 | 0.06 (.05 - .09) | 44 | 0.07 (0.05 - 0.09) | 10 | 0.08 (0.06 - 0.13) | 0.334 |
LV = left ventricular, RV = right ventricular, MV = mitral valve, TV = tricuspid valve, E' = early diastolic tissue velocity, A' = late diastolic tissue velocity.
P-value was obtained using a non-parametric Kruskal-Wallis Test.
Figure 1 depicts E’/A’ versus PMA at delivery for each location that was interrogated at 7 days of age, the RV free wall (A), septum (B), and LV lateral wall (C) as well as at 36 weeks PMA. The right ventricular E’/A’ ratio at 7 days of age showed a trend toward increase with PMA at delivery that did not reach statistical significance (p = 0.053). However, both the LV free wall and septum showed a statistically significant PMA-related increase in E’/A’ ratio. Furthermore, the ratio of E’/A’ at 36 weeks PMA was not related to GA at birth.
Figure 1. Myocardial E’/A’ Ratio versus Gestation Age (Weeks).
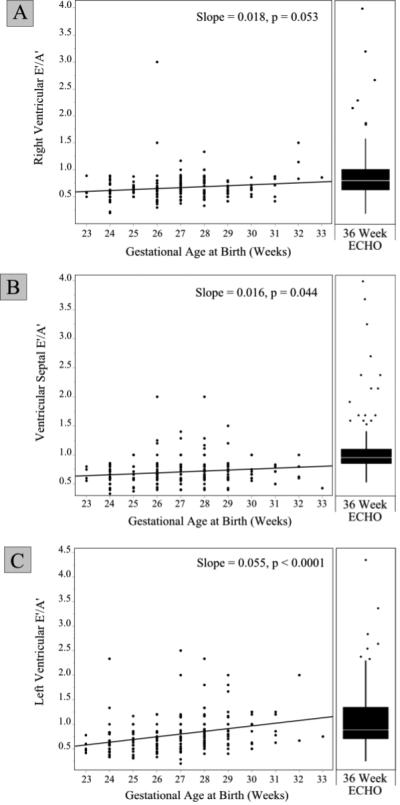
Legend: A scatter plot for each site of interrogation is presented, RV free wall (A), septum (B), and LV free wall (C). Based on data obtained at 7 days of age, the ratio of E’/A’ is plotted vs. gestational age for each site. A statistically significant increase in E’/A’ was present for the septum (p = 0.04) and the LV free wall (p <0.001). A trend toward increasing E’/A’ was present for the RV free wall that did not reach statistical significance (p = 0.05).
When the subjects were grouped by BPD or PH status, no significant association between DTI measures and the presence or absence of PH or BPD was present. Similarly, DTI measurements were not significantly different between infants who were or were not treated with mechanical ventilation. Table 4 also groups subjects by PMA at delivery, but further divides them into those without a PDA or a small PDA vs. a moderate or large PDA. Diastolic tissue velocity of these subgroups was compared to explore the impact of a moderate to large PDA on these measurements. There was no difference in diastolic tissue velocity of the LV lateral wall, septum or RV free wall as a function of PDA size. The only statistically significant finding was an increase in RV free wall E’/A’ in the 27-29 week cohort where a moderate or large PDA was present.
Table 4.
DTI Measurements of the LV Wall, Septum, and RV Wall Grouped by Hemodynamically Significant Patent Ductus Arteriosus at 7 Days of Age
| Gestational Age at Birth (Weeks) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 23 to 26 (N = 126) |
p* | 27 to 29 (N = 114) |
p* | 30 to 33 (N = 34) |
p* | ||||
| PDA Size | PDA Size | PDA Size | |||||||
| None/Small (N = 104) |
Moderate/ Large (N = 22) |
None/Small (N = 98) |
Moderate/Large (N = 16) |
None/Small (N = 31) |
Moderate/Large (N = 3) |
||||
| Med(IQR) | Med(IQR) | Med (IQR) | |||||||
| LV Lateral Wall – E'(cm/sec) | 4 (3-5) | 4.5 (4-6) | 0.39 | 5 (4-7) | 5.5 (3-7) | 0.67 | 5 (5-6) | 2 (2-2) | 0.17 |
| LV Lateral Wall – A' (cm/sec) | 66-8) | 7.5 (5.5-10) | 0.27 | 6 (5-8) | 6 (5-7) | 0.71 | 5 (4-7.5) | 4 (4-4) | 0.45 |
| LV Lateral Wall E'/A' | 0.60 (0.50-0.80) | 0.52 (0.42-0.82) | 0.35 | 0.75 (0.61-1) | 0.79 (0.44-1.08) | 0.84 | 1 (0.71-1.2) | 0.5 (0.5-0.5) | 0.13 |
| Septum – E' (cm/sec) | 4 (3-5) | 4 (3-5) | 0.17 | 4 (4-5) | 4 (3-5) | 0.65 | 4 (3-5) | 3 (3-3) | 0.27 |
| Septum – A (cm/sec) | 6 (5-7) | 6 (5-7) | 0.98 | 6 (5-7) | 5 (5-6) | 0.07 | 6 (5-7) | 5 (5-5) | 0.41 |
| Septum E'/A | 0.61 (0.50-0.75) | 0.67 (0.60-0.75) | 0.19 | 0.71 (0.57-0.83) | 0.75 (0.67-0.83) | 0.42 | 0.67 (0.60-0.80) | 0.60 (0.60-0.60) | 0.43 |
| RV Free Wall – E' (cm/sec) | 5 (4-6) | 5 (4-8) | 0.57 | 6 (4.5-7) | 6 (5.5-7.0) | 0.24 | 5 (5-7) | 6 (6-6) | 0.74 |
| RV Free Wall – A (cm/sec) | 9 (8-10) | 9 (7-11) | 1 | 9 (8-10) | 8 (7-9) | 0.22 | 8 (7-9) | 11 (11-11) | 0.18 |
| RV Free Wall E'/A | 0.60 (0.50-0.67) | 0.70 (0.50-0.80) | 0.17 | 0.63 (0.50-0.78) | 0.75 (0.64-0.83) | 0.04 | 0.69 (0.63-0.86) | 0.55 (0.55-0.55) | 0.36 |
| MV E/E' | 10.6 (8.0-12.3) | 13.3 (9.4-19) | 0.08 | 9.2 (6.3-11.4) | 7.8 (6-21.5) | 0.74 | 9.2 (6.6-11.6) | ||
| TV E/E' | 0.07 (0.06-0.09) | 0.08 (0.05-0.09) | 0.9 | 0.07 (0.06-0.09) | 0.07 (0.06-0.08) | 0.55 | 0.07 (0.06-0.08) | ||
LV = left ventricular, RV = right ventricular, MV = mitral valve, TV = tricuspid valve, E' = early diastolic tissue velocity. A' = late diastolic tissue velocity.
Discussion
This is the first prospective study in preterm infants to show developmental changes in measurements of diastolic function. We found a progressive increase in the ratio of early to late diastolic tissue velocity (E’/A’) as a function of gestational age at birth. However, by 36 weeks PMA, infants have similar diastolic tissue velocities independent of gestation age at birth. We also found that these echocardiographic measurements do not differ between preterm infants who develop BPD or PH and those who do not. This is the first such study in preterm infants to show developmental changes in measurements of diastolic function.
Our results suggest that, despite preterm birth, the development of the ventricular myocardium follows a trajectory that ultimately arrives at a state comparable to term infants by 36 weeks PMA5. This suggests that disruption of normal cardiopulmonary development by preterm birth does not drastically alter the evolution of ventricular diastolic function over time during early infancy. Additionally, ventricular filling in preterm infants has a greater dependence on atrial systole, which has clinical implications for optimizing cardiac output in this population. In support of this observation, we see a statistically significant decrease in LV free wall A’ and an increase in E’ with GA. Specifically, maintaining atrioventricular synchrony in order to maintain preload would appear to be especially important in order to maintain cardiac output. The change in LV free wall E’ observed in the present study, while statistically significant, is small; the clinical significance of this finding is unknown. It is also important to note that adults with a history of preterm birth may have abnormalities in diastolic function when compared to controls born at term23.
Other investigators have demonstrated findings similar to ours in preterm infants13,14,24. Previous studies in older children have also shown significant correlations between cardiac growth and DTI parameters which was most evident within the first year of age, when somatic growth velocity is at its peak6,25. These authors hypothesized that this relatively rapid increase in myocardial velocity is related to maturation of the myocardium during this period of development. The same study evaluated the effect of heart rate on DTI velocities. This has been identified as a potential source of error in DTI measurements26. Notably, heart rate had a negligible clinical effect on DTI velocities of the LV free wall and septum, and little or no association with RV free wall tissue velocity. With regard to MPI, our data indicate that there is a statistically significant GA-related decrease in LV MPI, though the median value is within the normal range for neonates6. Of note, others have observed a GA-related decrease in MPI during fetal development, which is consistent with our findings in preterm infants27.
Interestingly, we did not find an association between DTI measures of diastolic function and BPD or PH in this cohort of preterm infants. In older children with PH, tricuspid valve E’ velocity has been associated with WHO functional class28. An association between reduced tricuspid valve E’ velocity and PH status has also been shown in adults29. Prior studies have examined the relationship between estimated PA pressure30 and RV MPI31, suggesting that these measures were predictive of BPD in neonates. Our data do not support this, which may be related to the fact that measurements of tissue velocity may not detect more subtle changes in ventricular compliance related to elevated PA pressures in preterm neonates. There may also be a fundamental difference in the physiology of a preterm infant undergoing ‘physiologic atrophy’ of a right ventricle that was a systemic ventricle in utero and a child or adult who develops RVH in the setting of prolonged elevation in PVR. In addition, the small number of subjects with PH in this current study may limit our ability to discern a clear association. Further large, prospective studies of premature infants may be useful in revealing the relative contributions of prematurity and other factors to the development of BPD and PH.
The effect of deviation from the normal post-natal pattern of decreasing PVR on the development of the right ventricle is of particular interest in preterm neonates. In the present study, measurements of RV free wall E’/A’ at 7 days of age tended to increase with PMA but this did not achieve statistical significance. In comparison, left ventricular free wall and septal velocities increased significantly with respect to PMA. Other investigators using DTI as a means of evaluating diastolic function have also found that the progression of post-natal RV adaptation proceeds more slowly than the LV, termed ‘delayed relaxation’24. In another study of term neonates, tricuspid annular velocities were lower than those measured at the mitral annulus and did not evolve during the first week of life32.
The impact of a hemodynamically significant PDA on diastolic tissue velocities has been assessed in 72 pre-term infants (<30 weeks GA), and a decrease in both RV and LV free wall velocity was found15. Because our cohort with the lowest PMA had lower myocardial velocities and more prevalent PDA, we compared tissue velocities within PMA cohorts to assess whether the differences in DTI measurements we found were being confounded by the presence of PDA. As shown in table 4, the presence of a moderate-large PDA did not result in decreased tissue velocities. Therefore, our data showing a gradual increase in tissue velocity as function of PMA at delivery (Figure 1) are unlikely to be solely due to the presence of a PDA.
Limitations
This study population does not represent a consecutive birth cohort because enrollment in this study required informed consent and not all eligible patients participated. One potential limitation to our study is that this cohort of infants were cared for at tertiary care referral centers, suggesting that our enrollment may have some selection bias based on disease severity or other factors. However, our cohort is large and heterogeneous and is likely representative of preterm infants in other settings as well. In addition, we were not able to obtain adequate DTI measurements in all patients. If the ability to obtain reliable measurements was not random, the results of our study may be skewed. The use of a single vendor echocardiogram platform was not feasible, and we were not able to perform inter-vendor comparison as part of the present study. If there are significant differences in DTI measurements between vendors, this may impact our results. PH was not confirmed by right heart catheterizations, which is the gold standard for diagnosis, because performing these invasive evaluations was not practical in this population.
Conclusions
Overall, we conclude that diastolic myocardial tissue velocity increased in premature infants as a function of GA, and there was no significant difference in diastolic myocardial tissue velocity at 36 weeks PMA regardless of GA at birth. These findings suggest that developmental changes in myocardial structure and function proceed in spite of preterm birth in most infants.
Highlights.
Tissue Doppler was used to assess diastolic function in preterm infants.
An age related increase in the early diastolic tissue velocity was found.
At a gestational age equivalent to near-term, we observed tissue velocities that compare favorably to other term infants.
Tissue Doppler measurements were not different between infants who did or did not develop brochopulmonary dysplasia or pulmonary hypertension.
Acknowledgements
This publication was made possible by through support from the Thrasher Foundation and National Institutes of Health, including by grant number 5 K23 RR02102 (PMM), HL085703(SHA, PMM, BBP), HL68702 (SHA); and NCATS Colorado CTSI Grant Number UL1 TR001082. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Abbreviations
- DTI
Doppler Tissue Imaging
- PH
Pulmonary hypertension
- BPD
Bronchopulmonary dysplasia
- PMA
Post-menstrual age
- PDA
Patent ductus arteriosus
- ASD
Atrial septal defect
- VSD
Ventricular septal defect
- TRJV
Tricuspid regurgitant jet velocity
- RVSP
Right ventricular systolic pressure
- PFO
Patent foramen ovale
- sBP
Systemic systolic blood pressure
- MPI
Myocardial performance index
- RA
Right atrium
- RV
Right ventricle
- V
Left ventricle
- RVH
Right ventricular hypertrophy
- PA
Pulmonary arterial
- PASP
Pulmonary arterial systolic pressure
- IQR
Interquartile range1
- GA
Gestational age
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oki T, Tabata T, Yamada H, Wakatsuki T, Shinohara H, Nishikado A, et al. Clinical Application of Pulsed Doppler Tissue Imaging for Assessing Abnormal Left Ventricular Relaxation. Am J Cardiol. 1997;79:921–8. doi: 10.1016/s0002-9149(97)00015-5. [DOI] [PubMed] [Google Scholar]
- 2.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–80. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 3.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 4.Alp H, Karaarslan S, Baysal T, Çimen D, Örs R, Oran B. Normal values of left and right ventricular function measured by M-mode, pulsed doppler and Doppler tissue imaging in healthy term neonates during a 1-year period. Early Hum Dev. 2012;88:853–9. doi: 10.1016/j.earlhumdev.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Negrine RJS, Chikermane A, Wright JGC, Ewer AK. Assessment of myocardial function in neonates using tissue Doppler imaging. Arch Dis Child. 2012;97:F304–6. doi: 10.1136/adc.2009.175109. [DOI] [PubMed] [Google Scholar]
- 6.Eidem BW, McMahon CJ, Cohen RR, Wu J, Finkelshteyn I, Kovalchin JP, et al. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr. 2004;17:212–21. doi: 10.1016/j.echo.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Mori K, Hayabuchi Y, Kuroda Y, Nii M, Manabe T. Left ventricular wall motion velocities in healthy children measured by pulsed wave Doppler tissue echocardiography: Normal values and relation to age and heart rate. J Am Soc Echocardiogr. 2000;13:1002–11. doi: 10.1067/mje.2000.108131. [DOI] [PubMed] [Google Scholar]
- 8.O'Leary PW. Pediatric diastology: use and limitations of Doppler echocardiography in the evaluation of ventricular diastolic function in children. Prog Pediatr Cardiol. 1999;10:83–93. [Google Scholar]
- 9.Friedman WF. The intrinsic physiologic properties of the developing heart. Prog Cardiovasc Dis. 1972;15:87–111. doi: 10.1016/0033-0620(72)90006-0. [DOI] [PubMed] [Google Scholar]
- 10.Joyce JJ, Dickson PI, Qi N, Noble JE, Raj JU, Baylen BG. Normal right and left ventricular mass development during early infancy. Am J Cardiol. 2004;93:797–801. doi: 10.1016/j.amjcard.2003.11.063. [DOI] [PubMed] [Google Scholar]
- 11.Smolich JJ, Walker AM, Campbell GR. Left and right ventricular myocardial morphometry in fetal, neonatal, and adult sheep. Am J Physiol. 1989;257:H1–9. doi: 10.1152/ajpheart.1989.257.1.H1. [DOI] [PubMed] [Google Scholar]
- 12.Rudolph AM. The Changes in the Circulation After Birth Their Importance in Congenital Heart Disease. Circ. 1970;41:343–359. doi: 10.1161/01.cir.41.2.343. [DOI] [PubMed] [Google Scholar]
- 13.Kozák-Bárány A, Jokinen E, Saraste M, Tuominen J, Välimäki I. Development of left ventricular systolic and diastolic function in preterm infants during the first month of life: a prospective follow-up study. J Pediatr. 2001;139:539–45. doi: 10.1067/mpd.2001.118199. [DOI] [PubMed] [Google Scholar]
- 14.Lee A, Nestaas E, Liestøl K, Brunvand L, Lindemann R, Fugelseth D. Tissue Doppler imaging in very preterm infants during the first 24 h of life: an observational study. Arch Dis Child. 2014;99:F64–9. doi: 10.1136/archdischild-2013-304197. [DOI] [PubMed] [Google Scholar]
- 15.Parikh R, Negrine RJS, Chikermane A, Rasiah SV, Ewer AK. Assessment of myocardial function in preterm infants with patent ductus arteriosus using tissue Doppler imaging. Cardiol Young. 2015;25:70–5. doi: 10.1017/S1047951113001595. [DOI] [PubMed] [Google Scholar]
- 16.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 17.Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. 2003;23:451–6. doi: 10.1038/sj.jp.7210963. [DOI] [PubMed] [Google Scholar]
- 18.Baker CD, Balasubramaniam V, Mourani PM, Sontag MK, Black CP, Ryan SL, et al. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Euro Resp J. 2012;40:1516–22. doi: 10.1183/09031936.00017312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mertens L, Seri I, Marek J, Arlettaz R, Barker P, McNamara P, et al. Targeted Neonatal Echocardiography in the Neonatal Intensive Care Unit: practice guidelines and recommendations for training. Writing Group of the American Society of Echocardiography (ASE) in collaboration with the European Association of Echocardiography (EAE) and the Association for European Pediatric Cardiologists (AEPC) J Am Soc Echocardiogr. 2011;24:1057–78. doi: 10.1016/j.echo.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9:838–47. doi: 10.1016/s0894-7317(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 22.Roberson DA, Cui W. Right ventricular Tei index in children: effect of method, age, body surface area, and heart rate. J Am Soc Echocardiogr. 2007;20:764–70. doi: 10.1016/j.echo.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Lewandowski AJ, Augustine D, Lamata P, Davis EF, Lazdam M, Francis J, et al. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circ. 2013;127:197–206. doi: 10.1161/CIRCULATIONAHA.112.126920. [DOI] [PubMed] [Google Scholar]
- 24.Frommelt PC, Ballweg JA, Whitstone BN, Frommelt MA. Usefulness of Doppler tissue imaging analysis of tricuspid annular motion for determination of right ventricular function in normal infants and children. Am J Cardiol. 2002;89:610–3. doi: 10.1016/s0002-9149(01)02308-6. [DOI] [PubMed] [Google Scholar]
- 25.Roberson D, Cui W, Chen Z, Madronero L, Cuneu B. Annular and septal Doppler tissue imaging in children: normal z-score tables and effects of age, heart rate, and body surface area. J Am Soc Echocardiogr. 2007;20:1276–84. doi: 10.1016/j.echo.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Cantinotti M, Lopez L. Nomograms for blood flow and tissue Doppler velocities to evaluate diastolic function in children: a critical review. J Am Soc Echocardiogr. 2013;26:126–41. doi: 10.1016/j.echo.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Bhorat I, Bagratee J, Reddy T. Gestational age-adjusted trends and reference intervals of the Modified Myocardial Performance Index (Mod-MPI) and its components, with its interpretation in the context of established cardiac physiological principles. Prenat Diagn. 2014;34:1031–1036. doi: 10.1002/pd.4414. [DOI] [PubMed] [Google Scholar]
- 28.Takatsuki S, Nakayama T, Jone P-N, Wagner BD, Naoi K, Ivy DD, et al. Tissue Doppler imaging predicts adverse outcome in children with idiopathic pulmonary arterial hypertension. J Pediatr. 2012;161:1126–31. doi: 10.1016/j.jpeds.2012.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruan Q, Nagueh SF. Clinical Application of Tissue Doppler Imaging in Patients With Idiopathic Pulmonary Hypertension. Chest. 2007;131:395–401. doi: 10.1378/chest.06-1556. [DOI] [PubMed] [Google Scholar]
- 30.Skinner JR, Boys RJ, Hunter S, Hey EN. Pulmonary and systemic arterial pressure in hyaline membrane disease. Arch Dis Child. 1992;67:366–73. doi: 10.1136/adc.67.4_spec_no.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czernik C, Rhode S, Metze B, Schmalisch G, Bührer C. Persistently elevated right ventricular index of myocardial performance in preterm infants with incipient bronchopulmonary dysplasia. PLoS ONE. 2012;7:e38352. doi: 10.1371/journal.pone.0038352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori K. Pulsed wave Doppler tissue echocardiography assessment of the long axis function of the right and left ventricles during the early neonatal period. Heart. 2004;90:175–80. doi: 10.1136/hrt.2002.008110. [DOI] [PMC free article] [PubMed] [Google Scholar]


