Abstract
Severe variants of fibrodysplasia ossificans progressiva (FOP) affect <2% of all FOP patients worldwide but provide an unprecedented opportunity to probe the phenotype-genotype relationships that propel the pathology of this disabling disease. We evaluated two unrelated children who had severe reduction deficits of the hands and feet with absence of nails, progressive heterotopic ossification, hypoplasia of the brain stem, motor and cognitive developmental delays, facial dysmorphology, small malformed teeth, and abnormal hair development. One child had sensorineural hearing loss, microcytic anemia and a tethered spinal cord and the other had a patent ductus arteriosus and gonadal dysgenesis with sex reversal (karyotype 46, XY female). Both children had an identical mutation in ACVR1 c.772A>G; p.Arg258Gly (R258G), not previously described in FOP. Although many, if not most, FOP mutations directly perturb the structure of the GS regulatory subdomain and presumably the adjacent αC helix, substitution with glycine at R258 may directly alter the position of the helix in the kinase domain, eliminating a key aspect of the autoinhibitory mechanism intrinsic to the wild type ACVR1 kinase. The high fidelity phenotype-genotype relationship in these unrelated children with the most severe FOP phenotype reported to date suggests that the shared features are due to the dysregulated activity of the mutant kinase during development and postnatally, and provides vital insight into the structural biology and function of ACVR1 as well as the design of small molecule inhibitors.
Keywords: Fibrodysplasia ossificans progressiva (FOP), FOP variant, heterotopic ossification, bone morphogenetic protein (BMP), BMP receptor, ACVR1, ALK2
INTRODUCTION
Fibrodysplasia ossificans progressiva (FOP; OMIM135100) is a severely disabling autosomal dominant disease that causes endochondral bone formation at extraskeletal (heterotopic) sites. Heterotopic ossification begins in childhood and is induced by trauma or can occur spontaneously. Bone formation is episodic and progressive, and forms in well-defined spatial and temporal patterns that cause ankylosis of the joints, immobilizing the patient in a “second skeleton” of heterotopic bone. In addition to postnatal heterotopic bone formation, embryonic malformations occur in the normotopic skeleton. Characteristic malformations of the great toes are present in 100% of classically affected individuals [Shore and Kaplan, 2010; Pignolo et al., 2011; Pignolo et al., 2013].
FOP is an ultra-rare disorder, affecting approximately one in every two million individuals worldwide. Presently, there are approximately eight hundred known patients with FOP. Most cases arise as spontaneous new autosomal dominant mutations. There are a few affected multigenerational families. Reproductive fitness is low. There is no ethnic, racial, gender, or geographic predilection. The diagnosis of FOP is made on the basis of clinical findings; mutational analysis is confirmatory. Ninety-seven percent of patients worldwide have classic FOP defined by the presence of two classic clinical features: 1) characteristic malformations of the great toes, and 2) onset of episodic soft tissue flare-ups leading to progressive heterotopic ossification in characteristic anatomic patterns. [Shore & Kaplan, 2010; Pignolo et al., 2011; Pignolo et al., 2013].
DNA sequence analysis of ACVR1/ALK2, a bone morphogenetic protein type I receptor, in FOP patients who have classic disease features revealed that the same recurrent single nucleotide change in ACVR1 occurs in nearly every FOP patient [Shore et al., 2006; Kaplan et al., 2009]. The c.617G>A mutation results in the substitution of arginine by histidine at codon 206 (p.R206H) within the GS domain of the receptor [Shore et al., 2006]. Protein structural homology modeling correctly predicted that this amino acid substitution results in a conformational change of the receptor that alters its sensitivity and activity leading to loss of autoinhibition with mild constitutive activity as well as ligand-dependent hyperactivity of downstream bone morphogenetic protein (BMP) signaling [Shore et al., 2006; Shen et al., 2009; Kaplan et al., 2009a; Groppe et al., 2011; Chaikuad et al., 2012].
Among patients with FOP-like heterotopic ossification, occasional cases have also been identified that are associated with clinical features unusual for FOP. These atypical FOP patients have been clinically categorized into two groups. Patients classified as “FOP-plus” have one or more features that are uncommon in FOP patients, along with the classic defining FOP features. Patients classified as “FOP variants” present with significant deviation from the standard clinical presentation of one or both of the two classic defining features of FOP [Kaplan et al., 2009b]. Individuals classified as FOP variants are broadly distributed into two groups: 1) those who have minimal or no obvious malformations of the great toes and/or adult-onset progressive heterotopic ossification (<2% of all FOP patients), and 2) those who have severe malformations of the great toes and/or wide-spread reduction deficits of the digits of the feet and hands (<2%) [Kaplan et al., 2009b]. All individuals classified as FOP variants have germline heterozygous activating mutations of ACVR1/ALK2 that cluster in either GS domain or the downstream kinase domain of the receptor [Kaplan et al., 2009b; Chaikuad et al., 2012; Hüning and Gillessen-Kaesbach, 2014].
Recently, we evaluated two unrelated children who had severe reduction deficits of the hands and feet with absence of nails, progressive heterotopic ossification, hypoplasia of the brain stem, motor and cognitive developmental delays, facial dysmorphology, small malformed teeth, and abnormal hair development. One child had hydrocephalus, sensorineural hearing loss, microcytic anemia and a tethered spinal cord and the other had a patent ductus arteriosis and gonadal dysgenesis with sex reversal (karyotype 46, XY female). Both children had the identical ACVR1 mutation at c.772A>G; p.Arg258Gly (R258G), not previously described in FOP.
Although many, if not most, FOP mutations directly perturb the structure of the helix-loop-helix (GS) regulatory subdomain and presumably the adjacent αC helix, substitution with glycine at R258 may directly alter the position of the helix in the kinase domain, eliminating a key aspect of the autoinhibitory mechanism intrinsic to the wild type ACVR1 kinase. The high fidelity phenotype-genotype relationship in these most severely affected and unrelated children suggests that the shared phenotypes are due to the dysregulated activity of the mutant kinase during development and postnatally, and provides vital insight into the structural biology and function of ACVR1 as well as aiding the design of small molecule inhibitors.
CLNICAL REPORTS
Patient-1
A 16-month-old girl with macrocephaly and shunted hydrocephalus, hypoplasia of the brainstem, tethered spinal cord, dysmorphic facial features, microretrognathia, low-set dysmorphic ears, depressed nasal bridge, sparse hair, small malformed teeth, sensorineural hearing loss, dysconjugate gaze, gross developmental motor delay, four-limb digit reduction anomalies with no nails, decreased range of motion of the shoulders, elbows and hips, and multiple, evanescent soft tissue masses on the neck, back and buttocks was seen for genetic evaluation. A soft tissue mass on the back had been biopsied and exhibited features of an inflammatory fibroproliferative neoplasm. A chromosomal analysis revealed a normal female karyotype 46, XX. Whole exome sequencing identified a de novo heterozygous mutation in the ACVR1/ALK2 gene (c.772A>G; R258G). A variant of fibrodysplasia ossificans progressiva (FOP) was suspected. Further examination revealed a fixed-rigid neck with chin-on-chest deformity, functionally ankylosed shoulders, limited flexion of the elbows, hips and knees, multiple firm soft tissue masses in the neck and back, bilateral proximal medial tibial osteochondromas, and severe reduction deficits of the digits of the hands and feet with absence of nails. Radiographs confirmed orthotopic fusions of the lower cervical vertebra, short broad femoral necks with bilateral dysplasia of the hips, proximal medial tibial osteochondromas, severe reduction deficits of the hands and feet, and widespread areas of heterotopic ossification throughout the neck, back and shoulders (Fig 1). An MRI of the brain showed hypoplasia of the brainstem. The child was the product of in vitro fertilization from parental ovum and sperm and was born at 34 weeks of gestation with noted macrocephaly, dysmorphic facial features and four limb reduction anomalies with no nails. Parents and older siblings were in good health without any family history of congenital anomalies or heterotopic ossification.
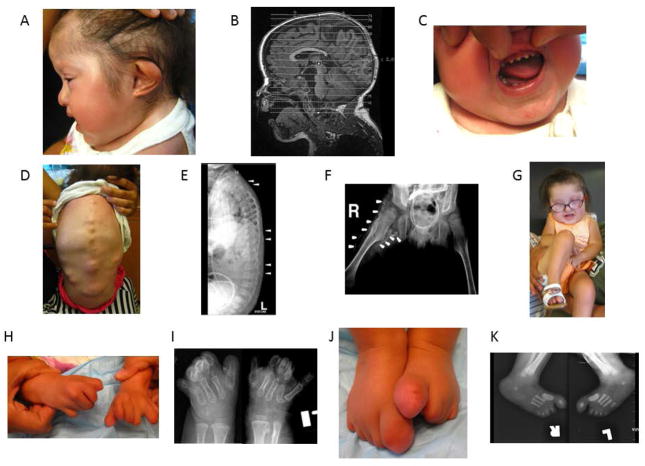
Figure 1.
Clinical and radiographic findings in Patient 1 at 30 months of age (A–F; H–K) and at 42 months of age (G). A. An asymmetric external helix of the left ear, a hypoplastic mandible and generalized alopecia are noted. B. Hypoplasia of the brain stem (including the pons and cerebellum) as well as a ventriculo-peritoneal shunt (for treatment of congenital hydrocephalus) are noted. C. Malformed peg-like teeth are seen. D. Photograph of the back shows multiple FOP flare-ups at various stages of maturation E. Lateral radiograph of the spine shows multiple areas of heterotopic ossification (arrowheads). F. Anteroposterior radiograph of the pelvis reveals short broad femoral necks, bilateral dysplasia of the hips and extensive bridging heterotopic ossification of the right hip (arrows). Also note ventriculo-peritoneal shunt. G. Photograph of child showing severe fixed flexion contracture of right hip. H. Photograph of hands shows severe reduction deficits of all digits and lack of nails. I. Anteroposterior radiographs of hands reveals severe reduction deficits of all digits with multiple malformations. Also note osteochondroma of left distal radius. J. Photograph of feet showing malformed great toes and severe reduction deficits of all digits and lack of nails. K. Radiograph of feet showing malformed great toes, malformed first metatarsals and reduction deficits of all digits.
Patient-2
An 11-month-old girl with trigonocepahly, scaphocephaly, frontal suture fusion, dysmorphic facial features, microretrognathia, low-set dysmorphic ears, hypertelorism, patent ductus arteriosus (repaired surgically at 15 days of age), left renal duplication, four-limb digit reduction anomalies with no nails and absence of middle and distal phalanges of hands and feet, evanescent soft tissue masses on the neck and parietal region which appeared at 25 days of age, and gross motor delay was seen for genetic evaluation. The child had female genitalia with asymmetric labia and hypoplasia of the clitoris. A high resolution karyotype was 46, XY. Ultrasound confirmed the presence of undescended testes in the inguinal canal. The child was diagnosed with gonadal dysgenesis. At 11 months of age, heterotopic ossification was noted on a radiograph of the chest in the area of the previous heart surgery and in the soft tissues of the back where a previous biopsy exhibited features of skeletal muscle atrophy with signs of low-grade fibroblastic proliferation. Plain radiographs of the cervical and thoracic spine revealed malformations of the cervical vertebra and ribs. Further examination revealed a fixed-rigid neck with decreased mobility of the shoulders, elbows, hips and knees, multiple, firm soft tissue masses in the neck and back, bilateral proximal medial tibial osteochondromas, and severe reduction deficits of the digits of the hands and feet with absence of nails. Radiographs confirmed orthotopic fusions of the lower cervical vertebra, malformations of the ribs, short broad femoral necks with bilateral dysplasia of the hips, proximal medial tibial osteochondromas, severe reduction deficits of the hands and feet, and widespread areas of heterotopic ossification throughout the neck, back and shoulders (Fig 2). An MRI of the brain showed agenesis of the corpus callosum. A variant of fibrodysplasia ossificans progressiva (FOP) was suspected. ACVR1/ALK2 gene sequencing showed a de novo heterozygous mutation c.772A>G (R258G). The child was the product of natural fertilization and was born by caesarian at 38 weeks of gestation. Parents were in good health without any family history of congenital anomalies or heterotopic ossification.
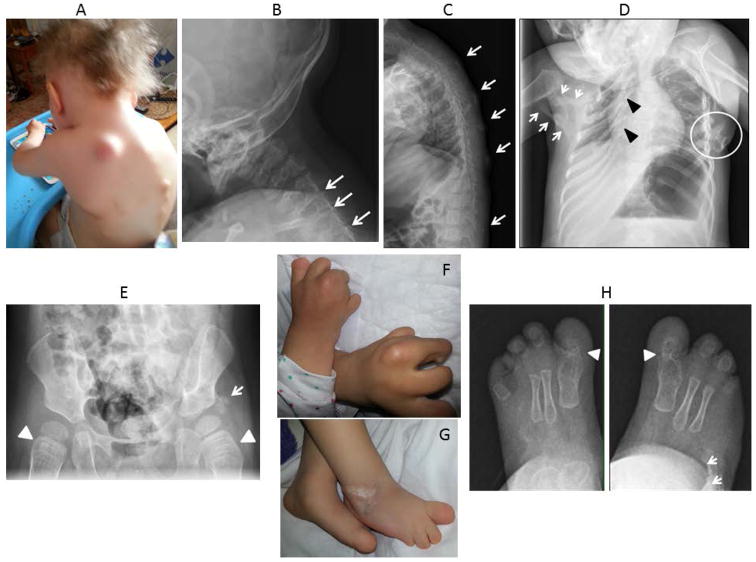
Figure 2.
Clinical and radiographic findings in Patient 2. A. Photograph of the neck and upper back at 30 months of age shows multiple FOP flare-ups as well as sparse scalp hair and severe upper limb malformations. B. Lateral radiograph of cervical spine at 13 months of age reveals orthotopic ankylosis of the posterior elements of C4-C5, C5-C6 and C6-C7 (arrows). C. Lateral radiograph of the thoracic spine at 22 months of age shows multiple areas of heterotopic ossification (arrows). D. Anteroposterior radiograph of the chest at 22 months of age shows severe right rib malformations (black arrowheads), severe restrictive heterotopic ossification of the right chest and shoulder girdle (white arrows) and osteochondromas of the left chest wall (white circle). E. Anteroposterior radiograph of the pelvis at 26 months of age shows short broad femoral necks (arrowheads) and heterotopic ossification adjacent to the left acetabulum (arrow). F. Photograph of hands shows severe malformations of all digits, reduction deficits and lack of nails. G. Photograph of feet shows severe malformations of all digits, reduction deficits and lack of nails. H. Anteroposterior radiographs of the feet at 13 months of age reveal bilateral malformations of the great toes with fusion and malformation of the first metatarso-phalangeal joint (arrowheads), evidence of symmetrical syndactyly, and heterotopic ossification of the right mid foot (arrows).
DISCUSSION
Ultra rare FOP variants harbor a stunning opportunity to probe phenotype-genotype relationships that have revelational implications for deciphering the role of the BMP pathway in normal physiology and for understanding how single amino acid substitutions alter the function of a highly conserved protein kinase receptor. Studies on FOP variants have shown that seemingly small variations in genotype can give rise to large variations in phenotype that provide important insight into the molecular mechanisms of FOP and BMP signaling [Kaplan et al., 2009b]. Additionally, understanding the specific effect of a missense mutation on ACVR1 function could help guide the design of pharmacologic agents that will modify or prevent the post-natal consequences of the disease [Kaplan et al., 2013].
We report here on two unrelated children with the most severe FOP variants ever noted. These two children share the common features of severe reduction deficits of the hands and feet with absence of nails, progressive heterotopic ossification, hypoplasia of the brain stem, motor and cognitive developmental delay, facial dysmorphology, small malformed teeth, and abnormal hair development. All of the affected tissues derive from mesodermal or ectodermal origin, including some from cranial neural crest, and all have been implicated in BMP pathway dysregulation [Kaplan et al., 2009b].
In addition, one child had hydrocephalus, sensorineural hearing loss, microcytic anemia and a tethered spinal cord and the other had a patent ductus arteriosis and gonadal dysgenesis with sex reversal (karyotype 46,XY female). Gonadal dysgenesis has not yet been described in any patient with FOP. However, it is plausible that the ACVR1 (R258G) mutation may be related to the abnormal sexual development observed in Patient 2. In humans, genetic variation within the ACVR1 gene has been associated with anti-Müllerian hormone levels, the testicular glycoprotein involved in regression of the Müllerian ducts in males, and presumed to play a pivotal role in gonadal sex differentiation [Kevenaar et al., 2009]. This finding is consistent with the non-redundant role of ACVR1 in mediating signaling by the Müllerian inhibiting substance (MIS) ligand in complex with the MIS-specific type II receptor and intriguingly suggests that the MIS-Activin pathway and ligands may play a pathophysiologic role in FOP in the context of the mutant receptor [Clarke et al., 2001; Renlund et al., 2007]. Importantly, during embryogenesis, the BMP signaling pathway mediates development in many organ systems, but their roles in sex determination and gonadal differentiation remain largely unknown. Dynamic changes of gonadal BMP7 expression in chick embryos suggest a role in sex-dependent differentiation in avian gonadogenesis [Hoshino et al., 2005]. In mouse embryos, a model has been suggested in which FOXL2 and BMP2 cooperate to ensure correct expression of Follistatin, a secreted glycoprotein required for female sex determination and early ovarian development in the developing ovary [Kashimada et al., 2011]. Whether the R258G mutation affects the development of sexual characteristics is unknown but is plausible. If the loss of auto-inhibition of ACVR1 affects feminization in a female embryo, there would not necessarily be any phenotypic consequences, thus possibly explaining the lack of involvement in one of the children.
At the present time we cannot exclude the possibility of a second mutation in our two patients that may be responsible for their atypical and complex phenotypes beyond their FOP features. Future genome-wide analyses will be conducted to address this question. Importantly, less than 1% of known cases of FOP are the product of in vitro fertilization as in our Patient 1. Although this technology has been associated with an increased risk of birth defects, the mechanism is not understood. The pattern of unshared clinical features in Patient 1 are not typical of those reported associated with in vitro fertilization [Kelley-Quon et al., 2013]. These findings are not likely related but the possibility of an association is not excluded.
All of the mutations in ACVR1 associated with classic and variant forms of FOP reside in or adjacent to the GS regulatory region or active site of the kinase [Kaplan et al., 2009b; Hüning and Gillessen-Kaesbach, 2014]. All of the reported mutations in FOP and its variants are predicted by protein structure homology modeling to activate the ACVR1 protein and enhance receptor signaling [Kaplan et al., 2013]. Importantly, both children described here had an identical mutation in ACVR1 c.772A>G; p.R258G, not previously described in germline mutations causative of FOP. However, a different amino acid substitution has been reported at the same codon position in six unrelated individuals [ACVR1; c.774G>T or G>C; Arg258Ser (R258S)] and leads to a very different phenotype characterized by no or very mild hypoplasia of the great toes and a milder clinical progression than the classic course of the disease [Bocciardi et al., 2009; Morales-Piga et al., 2012; Yazicioğlu et al., 2013; Zhang et al., 2013; Hüning and Gillessen-Kaesbach, 2014].
Relative to other variants, especially R258G, substitution with serine (R258S) produced a milder FOP phenotype in all six patients [Hüning and Gillessen-Kaesbach, 2014]. Based on a crystal structure of the ALK2 receptor kinase, R258 has been hypothesized to restrict the GS loop to a conformation that contributes to autoinhibition of the enzyme via multiple hydrogen bonds formed by the terminal guanidino group of the long aliphatic arm of the sidechain [Chaikuad et al., 2012]. The sidechain of serine, shorter by two methylene carbons and with a single hydroxyl rather than a multivalent guanidino group, would not be able to serve in such a capacity. Hence the sidechain of residue 258 may play a role in regulation of kinase activity, consistent with the milder phenotypes, or alternatively, mediate effects by different means. Because substitution with glycine, like serine, would also be unable to form stabilizing hydrogen bonds, yet produces severe rather than milder phenotypes, the complete lack of a sidechain and/or conformational freedom peculiar to glycine most likely underlies the catastrophically severe phenotype in the two children with the R258G mutation.
In contrast to R206H and many if not most variant FOP mutations which directly perturb the structure of the GS regulatory subdomain (and purportedly the adjacent αC helix, another key element of regulation), substitution with glycine may have direct effects on the position of the helix, which lies just proximal, linked by a three-residue loop segment (Fig. 3). Thus the principle role of the arginine sidechain may be to anchor the polypeptide backbone and confine the N-terminus of the αC helix to an autoinhibitory position, rather than exert indirect effects by stabilizing the GS subdomain. Note that in addition to glycine and serine, single base mutations of codon 258 could result in substitution with four other amino acid residues: tryptophan (772T), lysine (773A), threonine (773C) and methionine (773T). Two mutations would be silent (772C, 774A). Because only serine and glycine mutations have been observed, with serine producing relatively mild effects, perhaps larger and longer sidechains fulfill most of the functional requirements. Substitution with alanine, the only sidechain smaller and shorter than serine, would require two base changes in the codon, but would be predicted to lead to an intermediate phenotype, possibly closer to that of serine than glycine. These structural insights at the molecular level provide important targeting information for the future design of small molecule inhibitors of ACVR1.
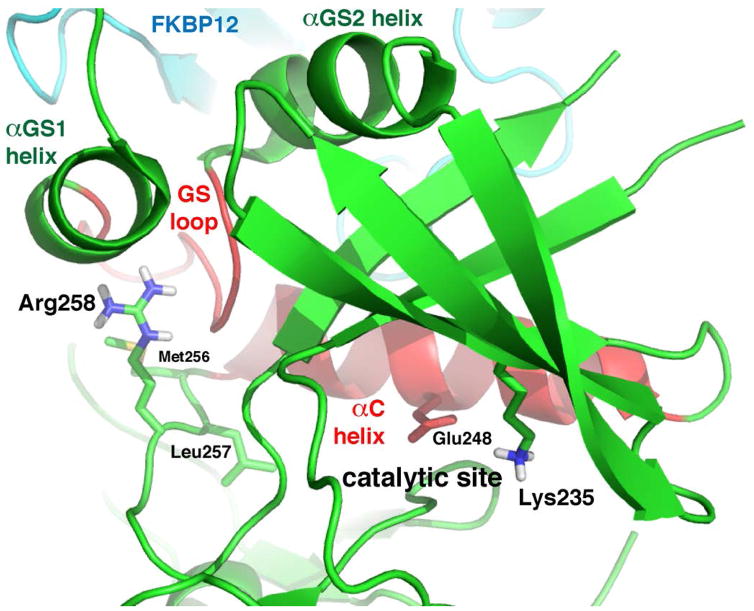
Figure 3.
Structural analysis of effects of glycine substitution at residue 258 in ACVR1. Hydrogen bonds donated by the multivalent guanidino group of Arg258 are predicted to stabilize the regulatory subdomain of ACVR1 via the C-terminus of the αGS1 helix and the GS loop, which impinges on the C-terminus of αC, locking the helix in an inactive position [Chaikuad et al., 2012]. Substitution with glycine at residue 258 is hypothesized to allow for rearrangement and concomitant movement or slippage of the polypeptide backbone, permitting the αC helix, and the key catalytic residue Glu248, to adopt an active conformation. Through ionic interaction, Glu248 properly positions Lys235, which by analogy makes crucial contacts with the α- and β-phosphate groups of the ATP substrate. The ion pair and the mediating role of the αC helix in the mechanism of autoinhibition are common features of protein kinases [Huse et al., 2002]. Graphic model prepared with PyMOL from PDB ID 3H9R.
Mutations at codon 258 are not the only ones that provide parallax on phenotype-genotype features in FOP. Severe FOP variants have been described with codon 328 mutations that are distinct from the less severe mutations that occur at the same codon [Kaplan et al., 2009b; Hüning and Gillessen-Kaesbach, 2014]. ACVR1, therefore, appears to be particularly sensitive to codon 328 and 258 mutations, suggesting importance in regulating receptor function and BMP signaling during embryonic development in many tissues including the central nervous system, specifically the brainstem [Kan et al., 2012; Taylor et al., 2014]. Although both R258S and R258G germline mutations are causative of FOP, only the latter, associated with the more severe phenotype, has been identified as a somatic mutation at residue 258 in diffuse intrinsic pontine gliomas (DIPGs), a rare childhood brainstem tumor [Taylor et al., 2014]. Somatic activating mutations in ACVR1 presumably function as oncogenes in DIPG, consistent with different outcomes of BMP signaling during brain development [Jones & Baker, 2014]. This finding is suggestive of a higher gain-of-function threshold for DIPG, which also requires a second site mutation in a histone gene contributing epigenetic effects [Jones and Baker, 2014; Taylor et al., 2014]. Indeed, the subset of mutations in ACVR1 causative of more severe FOP phenotypes has also been linked to DIPGs, whereas the subset with milder effects has not [Kaplan et al., 2009b; Hüning and Gillessen-Kaesbach, 2014; Taylor et al., 2014]. Hence a mutation in ACVR1 at codon 328 that remains peculiar to DIPGs, G328V, would be expected to impart a more pronounced dysregulating effect than G328R (which appears FOP-specific) and perhaps even G328E and G328W (which are common to both FOP and DIPG).
Our data and those of others support that all of the identified ACVR1 germline missense mutations influence the promiscuous postnatal induction of cartilage and bone cell differentiation [Shore et al., 2006; Kaplan et al., 2009b; Shen et al., 2009; van Dinther et al., 2010; Chaikuad et al., 2012; Culbert et al., 2014]. Progressive postnatal heterotopic ossification is the common feature shared by all patients with FOP. Although the rate of progression and the severity of heterotopic ossification vary among individuals with classic FOP, there appears to be a correlation between the severity of heterotopic ossification and specific mutations among the ACVR1 mutations identified in FOP variant patients [Kaplan et al., 2009b; Hüning and Gillessen-Kaesbach, 2014].
Enhanced expression of BMP transcriptional targets is observed in FOP cells [Shen et al., 2009; Chaikuad et al., 2012]. Overactive BMP signaling in FOP cells may lead paradoxically to orthotopic ankylosis of the joints and early degenerative joint disease as seen in FOP patients and in animal models of promiscuous BMP signaling [Kaplan et al., 2012]. Aberrant ACVR1 signaling may also be relevant to the pathogenesis of degenerative joint disease, as seen in early orthotopic degenerative changes of the great toe, thumb, cervical spine, and in the costovertebral joints before the appearance of FOP flare-ups and subsequent heterotopic ossification [Kaplan et al., 2009b]. All of the classic and common variable features of FOP as well as many, if not all, of the atypical features evaluated in our study could plausibly be ascribed to dysregulation of the BMP signaling pathway [Kaplan et al., 2009a]. Further studies of BMP signaling in animal models of classic and variant FOP will be critical to address these questions. Mouse models containing the Acvr1 R206H mutation successfully mimic the classic FOP phenotype [Chakkalakal et al., 2012] and a knock-in mouse containing the Acvr1 R258G mutation is expected to provide additional insight into the consequences of this mutation; such a model is under development. Identification of disease-causing mutations in ACVR1 has important diagnostic and therapeutic implications. Presently, there is no definitive treatment for patients with FOP or its variants and the identification of heterozygous missense mutations in ACVR1 reveals pharmaceutical targets for the development of signal transduction inhibitors (STIs) as well as other therapeutic strategies [Kaplan et al., 2013].
However, in addition to treating FOP, postnatal inhibition of ACVR1 could have a significant role in treating common acquired disorders of orthotopic and heterotopic ossification and, conversely, the mutation(s) of FOP and its variants could be harnessed for tissue engineering to form new bone for therapeutic applications. Genotype-phenotype correlations of the FOP ACVR1 mutations will help elucidate ACVR1 signaling mechanisms and in vivo functions to further these goals.
Acknowledgments
This work was supported in part by the International Fibrodysplasia Ossificans Progressiva Association (IFOPA), the Center for Research in FOP and Related Disorders, the Ian Cali Endowment for FOP Research, the Whitney Weldon Endowment for FOP Research, the Isaac and Rose Nassau Professorship of Orthopaedic Molecular Medicine (to FSK), the Cali-Weldon Professorship of FOP Research (to EMS), the Penn Center for Musculoskeletal Disorders, and the National Institutes of Health (NIH R01-AR41916). The authors thank Mr. Robert Caron for his invaluable technical assistance.
Footnotes
AUTHOR CONTRIBUTIONS
The report was conceived and initiated by FSK and EMS in August, 2014. Clinical, radiographic and genetic evaluations were provided by FSK, JAK, CO, IC, BL, MR, FM, MX and EMS. Protein homology and structural analysis was provided by JCG. Analysis of the data on the clinical background of fibrodysplasia ossificans progressiva and ACVR1 mutations was provided by FSK, EMS, RJP and JCG. The paper was written by FSK, JAK, CO and JCG. The manuscript was revised by all of the authors and the final manuscript was approved by all of the authors.
The authors indicate no potential conflict of interest.
References
- Bocciardi R, Bordo D, Di Duca M, Di Rocco M, Ravazzolo R. Mutational analysis of the ACVR1 gene in Italian patients affected with fibrodysplasia ossificans progressiva: confirmations and advancements. Eur J Hum Genet. 2009;17:311–318. doi: 10.1038/ejhg.2008.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaikuad A, Alfano I, Kerr G, Sanvitale CE, Boergermann JH, Triffitt JT, von Delft F, Knapp S, Knaus P, Bullock AN. Structure of the bone morphogenetic protein receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J Biol Chem. 2012;287:36990–36998. doi: 10.1074/jbc.M112.365932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TR, Hoshiya Y, Yi SE, Liu X, Lyons KM, Donahoe PK. Müllerian inhibiting substance signaling uses a bone morphogenetic protein (BMP)-like pathway mediated by ALK2 and induces SMAD6 expression. Mol Endocrinol. 2001;15:946–959. doi: 10.1210/mend.15.6.0664. [DOI] [PubMed] [Google Scholar]
- Culbert AL, Chakkalakal SA, Theosmy EG, Brennan TA, Kaplan FS, Shore EM. Alk2 regulates early chondrogenic fate in fibrodysplasia ossificans progressiva heterotopic endochondral ossification. Stem Cells. 2014;232:1289–1300. doi: 10.1002/stem.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe JC, Wu J, Shore EM, Kaplan FS. In vitro analysis of dysregulated R206H ALK2 kinase-FKBP12 interaction associated with heterotopic ossification in FOP. Cells Tissues Organs. 2011;194:291–295. doi: 10.1159/000324230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Koide M, Ono T, Yasugi S. Sex-specific and left-right asymmetric expression pattern of Bmp7 in the gonad of normal and sex-reversed chicken embryos. Dev Growth Differ. 2005;47:65–74. doi: 10.1111/j.1440-169x.2004.00783.x. [DOI] [PubMed] [Google Scholar]
- Hüning I, Gillessen-Kaesbach G. Fibrodysplasia ossificans progressiva: clinical course, genetic mutations and genotype-phenotype correlation. Mol Syndromol. 2014;5:201–211. doi: 10.1159/000365770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M, Kuriyan J. The conformational plasticity of protein kinases. Mol Cell. 2002;109:275–282. doi: 10.1016/s0092-8674(02)00741-9. [DOI] [PubMed] [Google Scholar]
- Jones C, Baker SJ. Unique genetic and epigenetic mechanisms driving pediatric diffuse high-grade gliomas. Nat Rev Cancer. 2014;14:651–661. doi: 10.1038/nrc3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan L, Kitterman JA, Procissi D, Chakkalakal S, Peng CY, McGuire TL, Goldsby RE, Pignolo RJ, Shore EM, Kaplan FS, Kessler JA. CNS demyelination in fibrodysplasia ossificans progressiva. J Neurol. 2012;259:2644–2655. doi: 10.1007/s00415-012-6563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Chakkalakal SA, Shore EM. Fibrodysplasia ossificans progressiva: mechanisms and models of skeletal metamorphosis. Dis Model Mech. 2012;5:756–762. doi: 10.1242/dmm.010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Pignolo RJ, Shore EM. The FOP metamorphogene encodes a novel type I receptor that dysregulates BMP signaling. Cytokine Growth Factor Reviews. 2009a;20:399–407. doi: 10.1016/j.cytogfr.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Pignolo RJ, Shore EM. From mysteries to medicines: drug development for fibrodysplasia ossificans progressiva. Expert Opinion on Orphan Drugs. 2013;1:637–649. doi: 10.1517/21678707.2013.825208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ, Gillessen-Kaesbach G, Hoover-Fong J, Köster B, Pauli RM, Reardon W, Zaidi S-A, Zasloff M, Morhart R, Mundlos S, Groppe J, Shore EM. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009b;30:379–390. doi: 10.1002/humu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashimada K, Pelosi E, Chen H, Schlessinger D, Wilhelm D, Koopman P. FOXL2 and BMP2 Act Cooperatively to Regulate Follistatin Gene Expression during Ovarian Development. Endocrinology. 2011;152:272–280. doi: 10.1210/en.2010-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley-Quon LI, Tseng CH, Janzen C, Shew SB. Congenital malformations associated with assisted reproductive technology: a California statewide analysis. J Pediatr Surg. 2013;48:1218–1224. doi: 10.1016/j.jpedsurg.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Kevenaar ME, Themmen AP, van Kerkwijk AJ, Valkenburg O, Uitterlinden AG, de Jong FH, Laven JS, Visser JA. Variants in the ACVR1 gene are associated with AMH levels in women with polycystic ovary syndrome. Hum Reprod. 2009;24:241–249. doi: 10.1093/humrep/den353. [DOI] [PubMed] [Google Scholar]
- Morales-Piga A, Bachiller-Corral J, Trujillo-Tiebas MJ, Villaverde-Hueso A, Gamir-Gamir ML, Alonso-Ferreira V, Vázquez-Díaz M, Posada de la Paz M, Ayuso-García C. Fibrodysplasia ossificans progressiva in Spain: epidemiological, clinical, and genetic aspects. Bone. 2012;51:748–755. doi: 10.1016/j.bone.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: clinical and genetic aspects. Orphanet J Rare Dis. 2011;6:80. doi: 10.1186/1750-1172-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: diagnosis, management, and therapeutic horizons. In Emerging Concepts in Pediatric Bone Disease. Pediatric Endocrinology Reviews. 2013;10(S-2):437–448. [PMC free article] [PubMed] [Google Scholar]
- Renlund N, O’Neill FH, Zhang L, Sidis Y, Teixeira J. Activin receptor-like kinase-2 inhibits activin signaling by blocking the binding of activin to its type II receptor. J Endocrinol. 2007;195:95–103. doi: 10.1677/JOE-07-0281. [DOI] [PubMed] [Google Scholar]
- Shen Q, Little SC, Xu M, Haupt J, Ast C, Katagiri T, Mundlos S, Seemann P, Kaplan FS, Mullins MC, Shore EM. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest. 2009;119:3462–3472. doi: 10.1172/JCI37412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol. 2010;6:518–527. doi: 10.1038/nrrheum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho T-J, Choi IH, Connor JM, Delai P, Glaser DL, Le Merrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nature Genetics. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Vinci M, Bullock AN, Jones C. ACVR1 mutations in DIPG: lessons learned from FOP. Cancer Res. 2014;74:4565–4570. doi: 10.1158/0008-5472.CAN-14-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dinther M, Visser N, de Gorter DJ, Doorn J, Goumans MJ, de Boer J, ten Dijke P. ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation. J Bone Miner Res. 2010;25:1208–1215. doi: 10.1359/jbmr.091110. [DOI] [PubMed] [Google Scholar]
- Yazicioğlu EC, Karatosun V, Kizildağ S, Ozsoylu D, KavukĢu S. ACVR1 gene mutations in four Turkish patients diagnosed as fibrodysplasia ossificans progressiva. Gene. 2013;515:444–446. doi: 10.1016/j.gene.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang K, Song L, Pang J, Ma H, Shore EM, Kaplan FS, Wang P. The phenotype and genotype of fibrodysplasia ossificans progressiva in China: a report of 72 cases. Bone. 2013;57:386–391. doi: 10.1016/j.bone.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]