Abstract
Type 1 Narcolepsy is caused by a loss of hypocretin (orexin) signaling in the brain. Genetic data suggests the disorder is caused by an autoimmune attack on hypocretin producing neurons in hypothalamus. This hypothesis has however not yet been confirmed by consistent findings of autoreactive antibodies or T-cells in patient samples. One explanation for these negative results may be that the autoimmune process is no longer active when patients present to the clinic. With increasing awareness in recent years, more and more patients have been diagnosed closer and closer to disease onset. In this study, we tested whether an active immune process in the brain could be detected in these patients, as reflected by increased cytokine levels in the cerebrospinal fluid (CSF). Using multiplex analysis, we measured the levels of 51 cytokines and chemokines in the CSF of 40 Type 1 Narcolepsy patients having varying disease duration. For comparison, we used samples from 9 healthy controls and 9 patients with other central hypersomnia. Cytokine levels did not differ significantly between controls and patients, even in 5 patients with disease onset less than a month prior to CSF sampling.
Introduction
The sleep disorder Type 1 Narcolepsy is caused by a loss of hypocretin (hcrt, also known as orexin) in the brain (ISCD-3, 2014). In addition most of the patients also suffer from the pathognomonic symptom cataplexy (muscle atonia triggered by emotions).
Autoimmunity due to environmental triggers is considered the most likely pathogenesis (Partinen 2014), an hypothesis strengthened by the report of increased numbers of cases after H1N1-vaccinations or infections (Dauvilliers et al., 2010; Han et al., 2011; Partinen et al., 2012). Despite this, experimental proof of autoimmunity in narcolepsy is still missing. One reason for this lack of evidence may be that the autoimmune process is no longer active when patients present to the clinic. Several observations suggest that this could indeed be the case. First, type 1 Narcolepsy patients typically have low or even undetectable levels of hypocretin-1 (hcrt-1) neuropeptide in cerebrospinal fluid (CSF) suggesting that the autoimmune destruction of the neurons is nearly complete already at the time of diagnosis (Nishino et al., 2000). Second, cases with intermediate levels are rarely seen (Andlauer et al., 2012; Bourgin et al., 2008; Knudsen et al., 2010). Finally, observations of onset a few weeks to months following H1N1 Pandemrix vaccinations, suggest that the disease process can occur in weeks to a few months (Knudsen et al., 2012; Partinen et al., 2012). In the case of immune responses towards viral infections, the virus is typically cleared within few weeks (Carrat et al., 2008; Ennis et al., 1981), thus immune factors resulting from the autoimmune process may decrease rapidly.
In the past, diagnostic delay for narcolepsy was very long, over ten years in most cases (Thorpy and Krieger, 2014). Thanks to increasing awareness, more and more patients are now coming to the attention of clinicians closer and closer to disease onset, offering new lines of investigations. In this study, we hypothesized that an active immune process could still be present in some patients close to onset, and we focused our attention on immune markers as detected in the CSF. Prior studies have found oligoclonal bands in only a small fraction of patients and normal CSF white blood cell counts (Fredrikson et al., 1990; Schuld et al., 2004) thus we focused on CSF cytokine and chemokine levels. Dauviliers et al. (2014) examined 12 cytokines and chemokines in the CSF of narcolepsy type 1 patients, finding increased levels of IL-4. To complement this study, we extended on this observation by including patients with short (<1 year) disease duration, and by including 51 cytokines and chemokines.
Potentially, CSF cytokines could be used as a biomarker for selecting patients for studies of autoimmune mediators such as autoantibodies and autoreactive T-cells. More importantly, this could also be used as selection criteria for immunomodulatory treatment, if one day the disease could be stopped before hypocretin cell destruction is complete.
Methods
Samples
After ethical approval and informed consent, CSF samples were collected in a clinical setting at three different centers. CSF was kept cold after sampling, and transferred to −80°C for storage. Samples from Bologna, Italy and Glostrup, Denmark were shipped to Stanford on dry-ice and stored at −80°C until analysis. The healthy volunteers were recruited under protocol #13366 at the Stanford Sleep Clinic where the lumbar puncture was performed. None of the volunteers had any sleep disorder complaints.
CSF Hcrt-1 levels and HLA-DQB1*06:02 status were determined as described before (Kornum et al., 2011). Detection limit in the hcrt-1 assay is 10 pg/ml and for all samples with undetectable levels of hcrt-1 the level was set at 10 pg/ml for data analysis.
Cohorts
Cohort 1: 9 typical Type 1 Narcolepsy patients seen in the Stanford Sleep Center clinic within 20 months of disease onset. All patient samples were collected before 2009. Disease onset was defined as onset of cataplexy, as this is the most specific marker of Type 1 Narcolepsy. All patients had clear cut cataplexy, were HLA-DQB1*06:02 positive and had a CSF hcrt-1 level <110 pg/ml. Details can be found in table 1.
Table 1.
Clinical characteristics of patients and controls
| Cohort 1 | Cohort 2 | Cohort 3 | |||||
|---|---|---|---|---|---|---|---|
| Group |
Narcolepsy
type 1 |
Healthy
controls |
Narcolepsy
<1 year |
Narcolepsy
>1 year |
Other
hypersomnia |
Narcolepsy
<1 month |
Narcolepsy
3 mth −3 |
| N | 9 | 9 | 16 | 15 | 9 | 5 | 12 |
| 0602 positive | 9 | 5 | 16 | 15 | 9 | 5 | 12 |
| Age | 22.4 [6-62] | 34.3 [18-42] | 10.2 ± 0.3 | 11.2 ± 2.0 | 10.9 ± 2.6 | 10.4 ± 2.7 | 10.2 ± 2.6 |
| BMI * | 23.1 [18-31] | 23.2 [20-27] | 22.2 ± 6.2* | 24.4 ± 5.3* | 20.4 ± 5.7* | 19.6 ± 3.1 | 19.7 ± 2.5 |
| Sex (M/F) | 3/6 | 3/6 | 7/9 | 7/8 | 5/4 | 4/1 | 6/6 |
|
Months from
onset |
10.2 [2-20] | - | 3.5 ± 2.5 | 23.8 ± 7.7 | 31.4 ± 16.8 | 0.6 ± 0.2 | 14.5 ± 9.7 |
| CSF Hcrt-1 | 47.3 ± 33.9 | 249.9 ± 28.7 | 25 ± 29 | 21 ± 26 | 364 ± 93 | 33 ± 23 | 33 ± 26 |
Data from 1 patient is missing in the three marked groups.
Cohort 2 and 3: 16 typical Type 1 Narcolepsy patients within one year from onset of cataplexy and 15 typical patients more than 1 year from onset. 14 of these were collected after the winter of 2009-10, none were vaccinated with the Pandemrix H1N1 vaccine. All had clear cut cataplexy, CSF hcrt-1 levels < 110 pg/ml, and where DQB1*06:02 positive. 9 patients with other central hypersomnias (Type 2 Narcolepsy and idiopathic hypersomnia) were also included, all had hcrt-1 levels > 250 pg/ml (normal levels). None of these had cataplexy and all were DQB1*06:02 positive. For this control group, disease onset was determined as onset of subjective excessive daytime sleepiness.
5 narcolepsy patients from cohort 2 had been lumbar punctured very close to an abrupt, well defined onset, and these were used in a subanalysis comparing to carefully matched patients. The five patients close to onset had an age span of 7.5-14.4 years and a BMI span of 15.6-23.3. For the comparison we therefore included all Type 1 Narcolepsy patients in cohort 2 within the age interval 5-15 years and BMI interval 15-23.5. Using these criteria we got a very well matched control group (table 1).
Luminex assay for cerebrospinal cytokines
CSF samples were processed by the Stanford core facility: Human Immune Monitoring Center. CSF samples were spun 10 min. at 300 g, and run undiluted in technical duplicates using human 51-plex Luminex kits (Luminex 200 IS System, Affymetrix). Briefly, beads conjugated with capture antibodies for each cytokine, CSF, and cytokine standards were added to pre-wetted 96-well filter-bottom plates and incubated two hours at room temperature plus 18 hours at 4°C. After washing, the plates were incubated with a biotin-labeled detector antibody cocktail for two hours at room temperature, washed again, incubated with streptavidin-PE for 40 minutes, and washed. Samples were acquired in reading buffer on the Luminex MAP200 instrument, with collection criteria set for 100 beads per analyte (2000 beads total). Data were analyzed using MasterPlex software (Hitachi Software Engineering America Ltd., MiraiBio Group).
Concentrations of the following cytokines were determined: CD40 ligand, CXCL5/ENA78, CCL11/Eotaxin, FGFβ, CSF3/G-CSF, CSF2/GM-CSF, CXCL1/GRO alpha, HGF, sICAM-1, IFNα, IFNβ, IFNγ, IL-10, IL-12A/IL-12p70, IL-12B/IL-12p40, IL-13, IL-15, IL-17, IL-17F, IL-1A, IL-1B, IL-1Ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8/CXCL8, CXCL10/IP10, Leptin, LIF, CSF1/M-CSF, CCL2/MCP-1, CCL7/MCP-3, CXCL9/MIG, CCL3/MIP-1A, CCL4/MIP-1B, NGF, PAI-1, PDGFB, CCL5/Rantes, Resistin, SCF, TNFL6/sFAS ligand, TGFα, TGFβ, TNF/TNFα, LXα/TNFβ, TNF10/Trail, sVCAM-1, VEGF.
The coefficient of variation (CV) was determined for each measurement (performed in duplicates). Only 4% of 2550 cytokine concentration measurements had CV > 25, most of these were samples where the value fell below the detection limit. Lower limit of detection (LOD) level was set as the value measured in the blank plus 3 times the standard deviation of the blank (table S1 and S2). Detectable samples with CV > 25 were excluded from the data analysis.
Statistical analysis
For all three cohorts, data were analyzed using GraphPad Prism version 6. In samples with undetectable values the value was set at LOD for the statistical analysis. For cohort 2 regression analysis with age, gender, BMI, hcrt-1, and disease duration as covariates were performed using IBM SPSS statistics v.19.
We also analyzed the dataset by agglomerative hierarchical clustering. Similarity between cases or cytokines was determined by squared Euclidean distance using transformed variables, and for combining of clusters we used average linkage between groups. For transformation of variables we subtracted the minimum value from each value and then divided by the range. The cluster analyses were done using SPSS statistics v.19.
Results
Type 1 Narcolepsy compared to healthy controls
We first tested whether there were any differences between cytokine/chemokine levels in CSF from Type 1 Narcolepsy patients and healthy controls. In the analysis we included all cytokines and chemokines detected in more than 38% of the samples (at least 7 out of the 18 subjects included). We did not find any significant differences between levels of HGF, sICAM-1, IL-1A, IL-7, IL-8, CXCL10, Leptin, LIF, CCL2, CSF1, PAI-1, CCL5, Resistin, SCF, TNFL6, TGFα, TGFβ, sVCAM-1. We did see tendencies for higher levels in the patient group in CCL5, LIF, IL-7, CCL4, and Leptin (table S1). Among the 9 patients in this analysis we had two patients with disease onset only 2.5 and 4 months before sampling. We noticed that CCL5 levels were particular high in these two patients. There was a significant correlation between Leptin levels and BMI as expected (R2=0.42, p-value = 0.007). This serves to prove the validity of the method.
Narcolepsy type 1 close to onset compared to patients with a longer disease history
Next we managed to collect CSF samples from a cohort of young patients within a year from onset of disease, and matched these carefully regarding age, sex and BMI with a group of Type 1 Narcolepsy patients with longer disease duration and also with a group of patients with other hypersomnias (table 1). We did not find any significant differences between levels of CXCL5, FGFβ, HGF, sICAM-1, IFNα, IL-12p40, IL-15, IL-17, IL-1A, IL-1RA, IL-6, IL-8, CXCL10, Leptin, LIF, LXα, CCL2, CCL7, CSF1, CXCL9, CCL3, CCL4, PAI-1, Resistin, SCF, sVCAM-1 TNFL6, TGFα, TGFβ, and TNF10 (table 2S). Using hierarchical clustering we tested whether cases clustered together. This was not the case. CCL5 was undetectable or low in all samples except in one patient very close to disease onset. Again we saw a significant correlation between Leptin and BMI (R2=0.50, p-value < 0.001, figure 1). For all narcolepsy patients (n=31) we analyzed whether levels of any cytokine/chemokine correlated with either disease duration or hcrt-1 levels in CSF. The only significant correlation was found with Leptin, which correlated with both disease duration and CSF hcrt-1, such that subjects with long disease duration and low hcrt-1 had higher Leptin. To reduce the data we performed clustering of cytokine/chemokines, but this analysis did not reveal any significant clusters.
Figure 1. CSF leptin levels correlate with BMI.
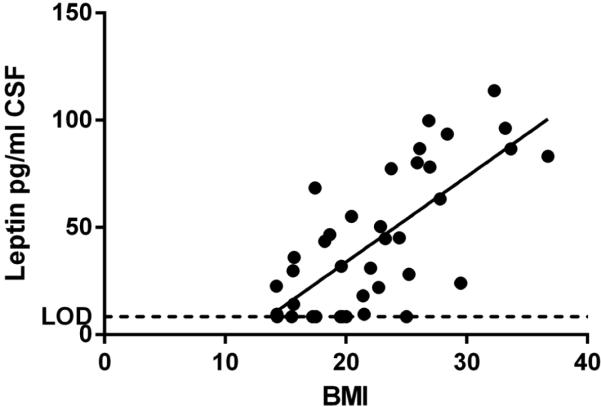
Liniar regression R2=0.50, p-value < 0.001.
Cytokine and chemokine levels in Type 1 Narcolepsy patients very close to onset
In our cohort we had five patients with disease duration less than a month (4 days to 1 month). We compared cytokine levels from these five patients with data from patients with longer disease duration, and found that 11 out of 33 detectable cytokines/chemokines were more than 25% increased close to disease onset. Three were more than 25% decreased close to onset. CXCL10 was significantly higher close to onset (p=0.0066, two-way ANOVA and post hoc Bonferroni corrected multiple comparison). This was however driven by a single outlier. IL1A, IL8, PAI1 and sICAM1 were borderline significant, but not when correcting for multiple t-tests using Bonferroni correction.
T helper cell subtype signatures
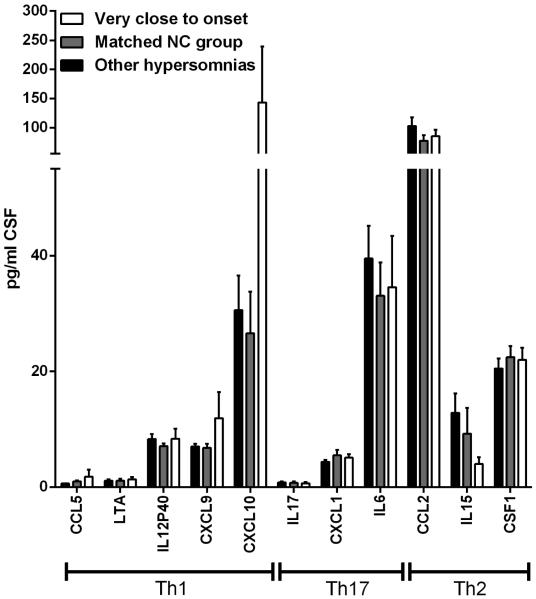
Even though a single cytokine or chemokine did not significantly differ between samples taken close to onset from samples taken further away, the data might reveal overall increases in cytokines/chemokines related to a specific T helper subset signature. The following cytokines/chemokines are related to different T helper responses: Th1: IFNγ, IL12p40, LTα, CCL5, CXCL9, CXCL10, CXCL11. Th17: IL17A, IL17F, CXCL1, CXCL2, and to a lesser extend CCL11, CSF2, and CSF3. Th2: IL-4, IL-5, IL-10, IL-13, CCL2, CCL21, CCL22 (Damsker et al., 2010; Kroenke et al., 2008; Lebre et al., 2005). Of these we have reliably measured IL12p40, LTα, CCL5, CXCL9, CXCL10, IL17A, CXCL1, CCL2 and IL15 (Figure 2). Even though none of these were significantly altered in the group of patients very close to onset, there is was clear tendency for an increase in the Th1 related cytokines and this was not seen for Th17 and Th2 associated cytokines.
Figure 2. Cytokine and chemokine levels in cerebrospinal fluid of Type 1 Narcolepsy patients the first month after onset.
Levels (Mean and SEM) of selected cytokines and chemokines associated with a Th1, Th17, and Th2 response in CSF from Type 1 narcolepsy patients less than a month from disease onset compared to controls.
Discussion
To test the autoimmune hypothesis, we studied inflammatory markers in the CSF of Type 1 Narcolepsy patients, with focus on recent onset cases. Even in patients very close to onset, however, we do not see any marked changes in the levels of any CSF cytokines or chemokines.
We observe in this study a tendency for increased levels in the Th1 associated cytokines/chemokines CCL5, LTα, CXCL9, CXCL10, but not for substances normally associated with a Th17 or Th2 response. This could imply that Type 1 narcolepsy is driven by a Th1 response, consistent with a viral infection as the triggering mechanism. Importantly, however, none of the cytokines/chemokines were significantly increased, and further studies are needed to substantiate this.
The only other study of cytokines in CSF of Type 1 Narcoleptic patients reported an increase in IL4 levels in patients (Dauvilliers et al., 2014). In our dataset only two of the 59 samples had detectable levels of IL-4, despite a similar detection level.
There are some difficulties in comparing our dataset to what has been reported by Dauvillers et al. Most importantly, the two studies did not use the same methodology and it is well known that different platforms can show quantitative differences (Chowdhury et al., 2009), while relative levels remain comparable. Even with a single technique, variation can be seen between experimental setups. Relative levels of cytokines between patient and control groups should however remain consistent between studies if they are biologically relevant.
The overall picture emerging from this and the study by Dauvillers et al. is that cytokine levels in the CSF of Type 1 Narcolepsy patients are not dramatically altered when compared to healthy controls or patients with other hypersomnias. It has previously been speculated that the lack of evidence for an inflammatory process in Type 1 narcolepsy was mainly due to limited access to patient material from patients close to onset. We present for the first time cytokine and chemokine data from patients within the first month from cataplexy onset, and even in these patients we do not find clear evidence of an ongoing inflammatory reaction in the brain. In CSF samples from patients with multiple sclerosis or myelitis optica, several studies have reported increased levels of cytokines such as TNF, IL6, CXCL8, CXCL10, and IL17A (Maimone et al., 1991; Matsushita et al., 2013; Mellergård et al., 2010), showing that CSF cytokine levels can indeed reflect an ongoing disease process in the brain. It is possible that for Type 1 Narcolepsy the autoinflammatory reaction is brief and very confined to the lateral hypothalamus. In this case only small amounts of inflammatory markers will diffuse to the CSF being too little to cause a detectable increase in concentration. A further consequence of this hypothesis is that any changes in biomarker levels in serum, plasma or CSF found in Type 1 Narcolepsy will be reflecting down-stream consequences of the loss of hypocretin rather than the disease process itself. It is also possible that Type 1 Narcolepsy is caused by an unknown immune mechanism that is not associated with increased levels of cytokines and chemokines in cerebrospinal fluid. Currently no animal models of immune mediated destruction of hypocretin neurons have been published, but such a model could be of high value for addressing this question.
In conclusion, we do not find any robust significant changes in cytokine/chemokine levels in the CSF of Type 1 Narcolepsy patients even very close to symptom onset.
Supplementary Material
Highlights.
The autoimmune hypothesis of type 1 narcolepsy has not yet been confirmed.
We hypothesize that the autoimmune process is only active very close to onset.
We collected CSF from 40 narcolepsy patients 4 days to 40 months from onset.
No significant differences were seen in levels of 51 cytokines and chemokines between patients and controls.
Acknowledgements
We thank the patients and their families for contributing samples. Funded by MH080957-03, NIH23734 and the Danish Medical Council 09-066348/FSS.
Disclosure of financial support:
Funded by MH080957-03, the KLS foundation, and the Danish Medical Council (09-066348/FSS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Institution at which the work was performed:
Center for Sleep Sciences and Medicine, Department of Psychiatry and Behavioral Studies, Stanford School of Medicine, Palo Alto, USA
References
- Andlauer O, Moore H, Hong S-C, Dauvilliers Y, Kanbayashi T, Nishino S, Han F, Silber MH, Rico T, Einen M, Kornum BR, Jennum P, Knudsen S, Nevsimalova S, Poli F, Plazzi G, Mignot E. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep. 2012;35:1247–55F. doi: 10.5665/sleep.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgin P, Zeitzer JM, Mignot E. CSF hypocretin-1 assessment in sleep and neurological disorders. Lancet Neurol. 2008;7:7–649. doi: 10.1016/S1474-4422(08)70140-6. [DOI] [PubMed] [Google Scholar]
- Carrat F, Vergu E, Ferguson NM, Lemaitre M, Cauchemez S, Leach S, Valleron A-J. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am. J. Epidemiol. 2008;167:167–775. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- Chowdhury F, Williams A, Johnson P. Validation and comparison of two multiplex technologies, Luminex and Mesoscale Discovery, for human cytokine profiling. J. Immunol. Methods. 2009;340:340–55. doi: 10.1016/j.jim.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: Adversaries and collaborators. Ann. N. Y. Acad. Sci. 2010;1183:1183–211. [Google Scholar]
- Dauvilliers Y, Jaussent I, Lecendreux M, Scholz S, Bayard S, Cristol JP, Blain H, Dupuy A-M. Cerebrospinal fluid and serum cytokine profiles in narcolepsy with cataplexy: A case-control study. Brain. Behav. Immun. 2014;37:37–260. doi: 10.1016/j.bbi.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y, Montplaisir J, Cochen V, Desautels A, Einen M, Lin L, Kawashima M, Bayard S, Monaca C, Tiberge M, Filipini D, Tripathy A, Nguyen BH, Kotagal S, Mignot E. Post-H1N1 narcolepsy-cataplexy. Sleep. 2010;33:33–1428. doi: 10.1093/sleep/33.11.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis FA, Rook AH, Qi YH, Schild GC, Riley D, Pratt R, Potter CW. HLA restricted virus-specific cytotoxic T-lymphocyte responses to live and inactivated influenza vaccines. Lancet. 1981;2:2–887. doi: 10.1016/s0140-6736(81)91389-1. [DOI] [PubMed] [Google Scholar]
- Fredrikson S, Carlander B, Billiard M, Link H. CSF immune variables in patients with narcolepsy. Acta Neurol. Scand. 1990;81:81–253. doi: 10.1111/j.1600-0404.1990.tb00978.x. [DOI] [PubMed] [Google Scholar]
- Han F, Lin L, Warby SC, Faraco J, Li J, Dong SX, An P, Zhao L, Wang LH, Li QY, Yan H, Gao ZC, Yuan Y, Strohl KP, Mignot E. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in china. Ann. Neurol. 2011;70:70–410. doi: 10.1002/ana.22587. [DOI] [PubMed] [Google Scholar]
- ISCD-3 . International classification of sleep disorders. 3rd. American Academy of Sleep Medicine; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen S, Biering-Sørensen B, Kornum BR, Petersen ER, Ibsen JD, Gammeltoft S, Mignot E, Jennum PJ. Early IVIg treatment has no effect on post-H1N1 narcolepsy phenotype or hypocretin deficiency. Neurology. 2012;79:79–102. doi: 10.1212/WNL.0b013e31825dce03. [DOI] [PubMed] [Google Scholar]
- Knudsen S, Jennum PJ, Alving J, Sheikh SP, Gammeltoft S. Validation of the ICSD-2 criteria for CSF hypocretin-1 measurements in the diagnosis of narcolepsy in the Danish population. Sleep. 2010;33:33–169. doi: 10.1093/sleep/33.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornum BR, Kawashima M, Faraco J, Lin L, Rico TJ, Hesselson S, Axtell RC, Kuipers H, Weiner K, Hamacher A, Kassack MU, Han F, Knudsen S, Li J, Dong X, Winkelmann J, Plazzi G, Nevsimalova S, Hong S-C, Honda Y, Honda M, Högl B, Ton TGN, Montplaisir J, Bourgin P, Kemlink D, Huang Y-S, Warby S, Einen M, Eshragh JL, Miyagawa T, Desautels A, Ruppert E, Hesla PE, Poli F, Pizza F, Frauscher B, Jeong J-H, Lee S-P, Strohl KP, Longstreth WT, Kvale M, Dobrovolna M, Ohayon MM, Nepom GT, Wichmann H-E, Rouleau G. a, Gieger C, Levinson DF, Gejman PV, Meitinger T, Peppard P, Young T, Jennum P, Steinman L, Tokunaga K, Kwok P-Y, Risch N, Hallmayer J, Mignot E. Common variants in P2RY11 are associated with narcolepsy. Nat. Genet. 2011;43:43–66. doi: 10.1038/ng.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke M. a, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 2008;205:205–1535. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebre MC, Burwell T, Vieira PL, Lora J, Coyle AJ, Kapsenberg ML, Clausen BE, De Jong EC. Differential expression of inflammatory chemokines by Th1- and Th2-cell promoting dendritic cells: A role for different mature dendritic cell populations in attracting appropriate effector cells to peripheral sites of inflammation. Immunol. Cell Biol. 2005;83:83–525. doi: 10.1111/j.1440-1711.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- Maimone D, Gregory S, Arnason BG, Reder a T. Cytokine levels in the cerebrospinal fluid and serum of patients with multiple sclerosis. J. Neuroimmunol. 1991;32:32–67. doi: 10.1016/0165-5728(91)90073-g. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Tateishi T, Isobe N, Yonekawa T, Yamasaki R, Matsuse D, Murai H, Kira J-I. Characteristic cerebrospinal fluid cytokine/chemokine profiles in neuromyelitis optica, relapsing remitting or primary progressive multiple sclerosis. PLoS One. 2013;8:e61835. doi: 10.1371/journal.pone.0061835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellergård J, Edström M, Vrethem M, Ernerudh J, Dahle C. Natalizumab treatment in multiple sclerosis: marked decline of chemokines and cytokines in cerebrospinal fluid. Mult. Scler. 2010;16:16–208. doi: 10.1177/1352458509355068. [DOI] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:355–39. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Partinen M, Saarenpää-Heikkilä O, Ilveskoski I, Hublin C, Linna M, Olsén P, Nokelainen P, Alén R, Wallden T, Espo M, Rusanen H, Olme J, Sätilä H, Arikka H, Kaipainen P, Julkunen I, Kirjavainen T. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS One. 2012;7:e33723. doi: 10.1371/journal.pone.0033723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuld A, Uhr M, Pollmächer T. Oligoclonal bands and specific antibody indices in human narcolepsy. Somnologie. 2004;8:8–71. [Google Scholar]
- Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. 2014;15:15–502. doi: 10.1016/j.sleep.2014.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.