Abstract
Objective:
We performed a systematic review and meta-analysis to assess whether the presence of cerebral microbleeds (CMBs) on pretreatment MRI scans of patients with acute ischemic stroke treated with thrombolysis is associated with an increased risk of symptomatic intracerebral hemorrhage (ICH).
Methods:
We searched PubMed for relevant studies and calculated pooled odds ratios (ORs) for symptomatic ICH, using the Mantel–Haenszel fixed-effects method, among individuals with vs without CMBs on pretreatment MRI scans. To minimize potential bias, sensitivity analysis was performed including studies providing data on patients treated only with IV thrombolysis.
Results:
Ten eligible studies including 2,028 patients were pooled in meta-analysis. The overall prevalence of CMBs was 23.3%. Among patients with CMBs, 40 of 472 (8.5%; 95% confidence interval [CI]: 6.1%–11.4%) experienced a symptomatic ICH after thrombolysis compared with 61 of 1,556 patients (3.9%; 95% CI: 3%–5%) without CMBs. The pooled OR of ICH across all studies was 2.26 (95% CI: 1.46–3.49; p < 0.0001). Eight studies, including 1,704 patients (n = 401 with CMBs), provided data on patients treated with IV thrombolysis only; OR for the presence of CMBs and the development of symptomatic ICH was 2.87 (95% CI: 1.76–4.69; p < 0.0001).
Conclusions:
Our meta-analysis of the available published data demonstrates an increased risk of symptomatic ICH after thrombolysis for acute ischemic stroke in patients with CMBs. However, we cannot fully exclude bias or confounding, so our results should be considered hypothesis-generating. Detecting CMBs should not prevent thrombolytic treatment based on present evidence. Further analyses, taking into account CMB number and location, as well as measures of functional outcome, are needed.
IV thrombolysis with recombinant tissue plasminogen activator (rtPA) remains the cornerstone of acute ischemic stroke treatment.1 Early intracerebral hemorrhage (ICH) is the most serious yet unpredictable complication of thrombolysis.2,3 Emerging evidence suggests that neuroimaging markers of cerebral small vessel disease (e.g., leukoaraiosis) might be a risk factor for thrombolysis-related ICH, together with age, early ischemic CT changes, high blood pressure, hyperglycemia, clinical stroke severity, and large infarct volume.2,4
Cerebral microbleeds (CMBs), detected as small, rounded, hypointense lesions on blood-sensitive MRI sequences, including T2*-weighted gradient-recalled echo and susceptibility-weighted imaging (SWI), are commonly found in stroke patients. CMBs are small perivascular hemosiderin deposits usually attributed to leakage through pathologically fragile hemorrhage-prone small vessels.5,6 In the setting of acute ischemic stroke thrombolysis, previous studies have given conflicting results regarding the possible risk of ICH in patients with CMBs.7,8 In 2012, meta-analyses pooling data from a total of 790 patients from 5 studies demonstrated a trend toward increased risk of postthrombolysis ICH in patients with CMBs (relative risk: 1.90; 95% confidence interval [CI]: 0.92–3.93; p = 0.082).9,10 Of note, none of the 5 included studies reached statistical significance; however, all studies, including the meta-analyses, were underpowered to appropriately address the question.9,10
Given new recently published studies on the topic, including larger cohorts, we performed an updated systematic review and meta-analysis to assess whether the presence of CMBs on prethrombolysis MRI scans of patients with acute ischemic stroke is associated with an increased risk of symptomatic ICH.
METHODS
Search strategy and selection criteria.
We searched PubMed between January 1, 1995, and October 6, 2014, using the following search terms: “micro(-)bleed*,” or “micro(-)h(a)emorrhag*, or “gradient-echo,” or “susceptibility-weighted” in association with “thromboly*” or “tPA,” or “tissue plasminogen activator.” Reference lists from all included articles, review papers on the topic, and the authors' own files were also searched for relevant studies. Case reports were excluded and articles not published in English were translated where needed. Two authors (A.C. and D.W.) identified potentially relevant studies, resolving any uncertainties with a third author (A.S.).
Studies were eligible for inclusion if they had (1) defined and assessed symptomatic ICH risk (the outcome of interest) in patients with acute ischemic stroke treated with thrombolysis, and (2) quantified this risk in relation to the presence of CMBs on pretreatment MRI scans.
Data extraction.
Two authors (A.C. and A.S.) reviewed all articles selected as potentially relevant and extracted data independently. For each study, we extracted information on study design, number and characteristics of participants (including mean age and sex), blood-sensitive MRI parameters used, thrombolytic treatment and dosage, duration of follow-up, number of participants with at least one CMB, and number of participants with the outcome of interest (symptomatic ICH clearly defined according to standard criteria). Disagreements were resolved by discussion and consensus.
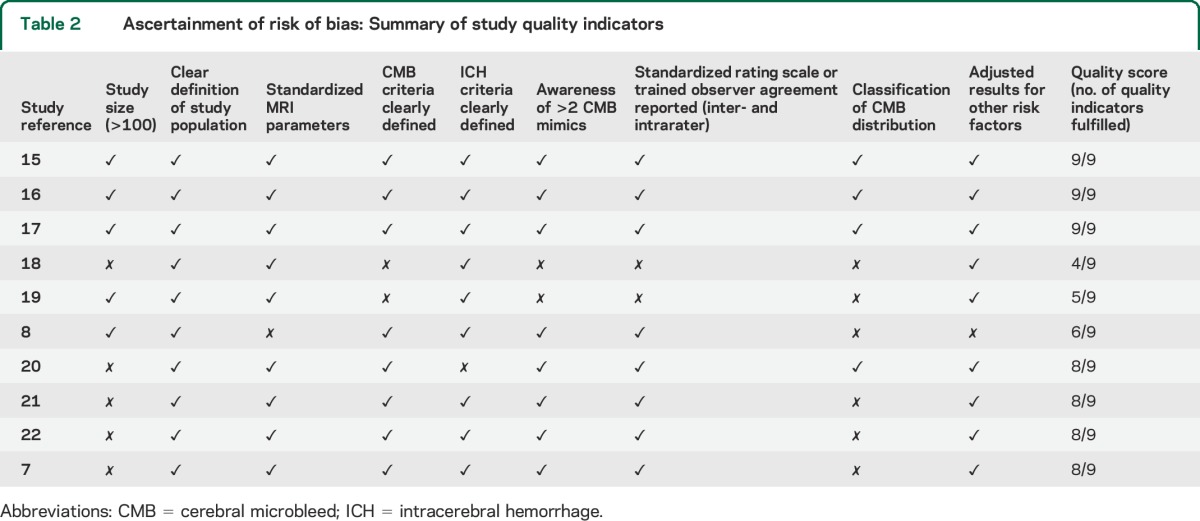
All included studies were critically appraised against a checklist of key quality indicators that we developed,9–11 with reference to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement and the ideal characteristics for a study of CMBs12 (study sample size, clearly defined CMB criteria, clear definition of the study population, standardized MRI parameters, ICH criteria clearly defined, awareness of >2 CMB mimics, standardized rating scale or trained observer agreement [inter- and intrarater], classification of CMB distribution, and adjusted results for other risk factors).
Statistical analysis.
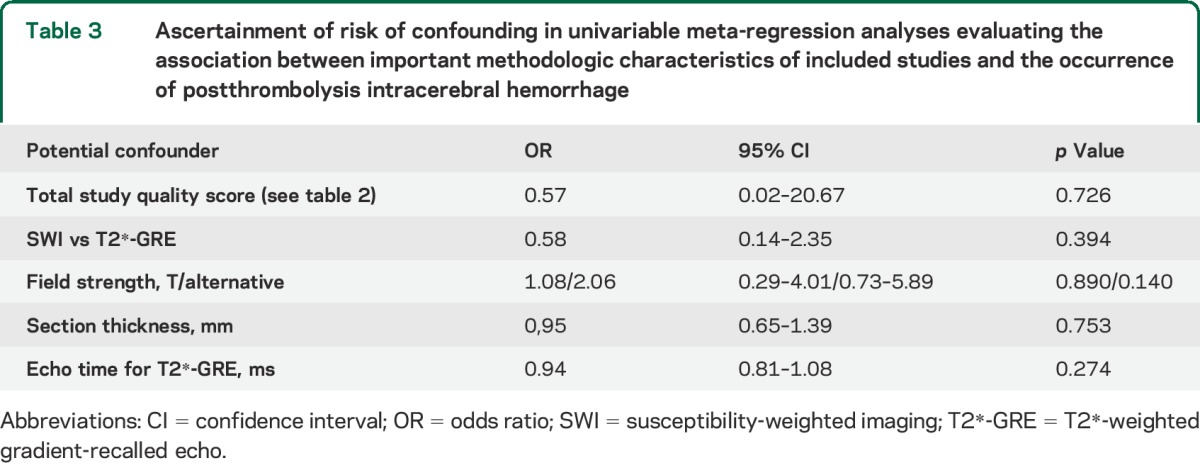
Because of the relatively small number of studies and outcome events, we used a fixed-effects model. We quantified the strength of the association between CMBs and ICH using odds ratios (ORs) and their corresponding 95% CIs, with the inverse variance method for weighting. To account for methodologic variability in the route of thrombolysis treatment among the included studies, a subgroup analysis was performed including only studies that provided relevant data on patients treated only with IV tPA. We assessed statistical heterogeneity using I2 statistics and also visually through inspection of the forest plot. We explored publication bias with funnel plots. We used meta-regression to explore whether certain baseline characteristics of the included patient populations could have affected our results. We also performed fixed-effect univariable meta-regression analyses to evaluate whether certain methodologic characteristics of the studies (including key quality indicators and MRI sequence parameters, i.e., SWI vs T2*-weighted gradient-recalled echo, echo time, field strength, and slice thickness) could be confounders in the relationship between CMBs and ICH. As a sensitivity analysis, we investigated the influence of each study on the overall meta-analyses estimates (using the “metaninf” command) and inspected the results graphically with meta-analyses estimates computed, omitting one study in each turn. We repeated all analyses using random-effects models. Meta-analyses were performed using Stata 11.2 (StataCorp LP, College Station, TX). We prepared this report with reference to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses)13 and MOOSE (Meta-analysis of Observational Studies in Epidemiology)14 guidelines.
RESULTS
Ten studies including a total of 2,028 patients met our inclusion criteria and were pooled in meta-analysis (figure 1).7,8,15–22 A summary of the characteristics of included studies, methodologic key issues, and quality indicators are noted in tables 1 and 2 as well as in tables e-1 and e-2 on the Neurology® Web site at Neurology.org. There was no evidence of publication bias in the funnel plot. From inspection of each of the studies, the CMB (+) vs CMB (−) groups were not significantly different in basic characteristics (including age, sex, or stroke severity) except that in 2 studies, higher age was associated with CMBs.8,17 The crude prevalence of CMBs on pretreatment MRI scans was 472 of 2,028 (23.3%).
Figure 1. Study selection.

Flowchart of literature search and study selection.
Table 1.
Characteristics and methodologic aspects of included studies

Table 2.
Ascertainment of risk of bias: Summary of study quality indicators
Symptomatic ICH occurred in 5% (95% CI: 4.1%–6.2%) of the entire population. Among patients with CMBs, 40 of 472 (8.5%; 95% CI: 6.1%–11.4%) experienced a symptomatic ICH after thrombolysis compared with 61 of 1,556 patients (3.9%; 95% CI: 3.0%–5.0%) without CMBs. The OR of ICH across all pooled studies was 2.26 (95% CI: 1.46–3.49; p < 0.0001) (figure 2). Eight studies, including 1,704 patients (n = 401 with CMBs), provided data on patients treated with IV thrombolysis only.8,15–19,21,22 Pooled analysis of these studies demonstrated OR for the presence of CMBs and the development of symptomatic ICH to be 2.87 (95% CI: 1.76–4.69; p < 0.0001) (figure 2). These results remained consistent when only the 4 largest studies (including >100 patients)8,15–17 were pooled in meta-analysis.
Figure 2. Forest plots of CMB presence and risk of postthrombolysis symptomatic ICH.
Meta-analysis of the association between symptomatic ICH risk in patients with acute ischemic stroke treated with thrombolysis, in relation to the presence of CMBs on pretreatment MRI scans. Pooled analysis results in all studies/entire study population (A) and in studies providing data on patients treated with IV thrombolysis only (B). CI = confidence interval; CMB = cerebral microbleed; ICH = intracerebral hemorrhage; OR = odds ratio.
No significant heterogeneity was noted between studies (that provided relevant data) according to age, sex, hypertension, or initial stroke severity (NIH Stroke Scale score) for any of the outcomes (all p values >0.1). In further meta-regression analyses, none of the key methodologic characteristics of included studies (i.e., total quality score as presented in table 2 and MRI parameters) reached statistical significance for the association with postthrombolysis ICH occurrence (table 3). In all pooled analyses, the results were of similar effect size when exploring the influence of each individual study on the overall meta-analysis summary estimates (data not shown). All analyses (including the IV thrombolysis only subanalysis) were consistent using a random-effects model.
Table 3.
Ascertainment of risk of confounding in univariable meta-regression analyses evaluating the association between important methodologic characteristics of included studies and the occurrence of postthrombolysis intracerebral hemorrhage
DISCUSSION
Our updated meta-analysis in more than 2,000 patients with acute ischemic stroke shows that CMB presence on pretreatment MRI scans is associated with an approximate doubling of the risk of symptomatic ICH following thrombolytic treatment. These results remained consistent in predefined subgroup pooled analyses including only patients treated with IV rtPA.
CMBs may heighten the risk of thrombolysis-related ICH either as the direct source of the ICH or, more likely, as a general marker of hemorrhage-prone pathologic state due to severe small vessel disease. It seems plausible that small vessel disease (including cerebral amyloid angiopathy and hypertensive arteriopathy), causing the blood vessel walls to become brittle and fragile, may interact with other factors that potentially increase bleeding risk after rtPA, such as upregulation of matrix metalloproteinases, disruption of the blood-brain barrier, hyperglycemia, and hypertension,2,23 lowering the threshold for postthrombolysis ICH.24 The relationship of cerebral small vessel disease with an increased risk of developing ICH after thrombolysis is also supported by studies showing an association between moderate to severe leukoaraiosis (another neuroimaging correlate of microangiopathy) and postthrombolysis ICH.24 However, these studies did not adjust for presence of concurrent CMBs, and the extent to which leukoaraiosis in itself can be used as a reliable predictor of ICH is questionable, since it lacks pathologic specificity and may reflect mainly ischemic aspects of microangiopathy.24 Compared with leukoaraiosis, which can also be assessed on CT, CMBs, which can only be detected on MRI, may be a more specific marker of a bleeding-prone form of small vessel disease.5 Indeed, the role of CMBs as reliable predictors of ICH risk in other clinical scenarios outside acute stroke and thrombolysis (including incident and recurrent spontaneous ICH) is supported by recent data.25–28 Furthermore, higher rates of future spontaneous ICH have been observed in patients with CMBs treated with aspirin.29,30 It is important to note that patients with moderate to severe leukoaraiosis appear to still obtain clinical benefit from IV rtPA despite the increased risk of symptomatic ICH.31 Accordingly, in the absence of comparative data demonstrating lack of functional benefit from thrombolysis in CMB-positive acute ischemic stroke patients vs those without CMBs, our results do not justify withholding IV rtPA from otherwise eligible candidates solely on the basis of CMB presence on MRI.
A small number of autopsy cases also support a direct role of preexisting small vessel pathology, particularly cerebral amyloid angiopathy, in some cases of thrombolysis-related ICH.32 In a small PET study using Pittsburgh compound B to detect cerebral β-amyloid burden, cortical Pittsburgh compound B retention was higher among patients with thrombolysis-related parenchymal hemorrhages compared with thrombolysed acute stroke patients without hemorrhage and normal controls.33 Finally, an increased risk of ICH was associated with IV thrombolysis in cerebral amyloid angiopathy transgenic mice, which display the typical findings of human amyloid angiopathy.9,34,35 Given this circumstantial evidence suggesting a link between thrombolysis-related ICH and cerebral amyloid angiopathy, multiple strictly lobar CMBs (a characteristic marker of the disease)36 may be of particular prognostic value, but, limited by the available data, we were unable to address this question in our meta-analysis. It should be noted that preexisting cerebral small vessel disease pathology might be specifically related with remote postthrombolysis ICH distinct from the acute infarct area.37,38
Several methodologic aspects of the included studies and limitations of our analyses deserve consideration. First, the MRI parameters used varied between studies, and this is likely to affect the prevalence of CMBs: it has been demonstrated that among others, field strength, echo time, and the use of SWI have a significant effect on CMB detection.39 For example, the relative low prevalence of CMBs in the 2002 study by Kidwell et al.7 may be explained by the short echo time and low field strength used. Second, slightly different definitions for postthrombolysis ICH and different follow-up strategies were used. However, most definitions included ICH (both hemorrhagic transformation and parenchymal hemorrhage) that was likely to be clinically relevant and associated with clinical deterioration. Third, different thrombolysis protocols were used across the cohorts, but we accounted for this methodologic heterogeneity by presenting a sensitivity analysis including only patients treated with IV tPA. It is reassuring that studies that included both IV and intraarterial thrombolysis patients did not find any significant difference in the occurrence of hemorrhagic complications.20,22 Although published data suggest further increased symptomatic ICH rates in patients with higher CMB counts,10,17 the studies overall have not systematically reported on ICH risk in relation to CMB number and anatomical distribution using uniform definitions, precluding any meaningful pooled analysis without individual patient data available. Finally, there is a clear possibility of selection bias, since not all acute stroke patients undergo MRI, and such patients were excluded from all study cohorts. The limitations highlighted above would tend to bias our analysis toward a null result (no group differences between patients with vs without CMBs), suggesting that CMB burden, or a certain CMB cutoff, may in fact be a stronger predictor of postthrombolysis ICH than we have been able to demonstrate here.10
Although our analysis does show that the presence of CMBs on pretreatment MRI increases the risk of early symptomatic ICH after thrombolysis, these results should be treated with caution and considered preliminary and hypothesis-generating. Despite our best efforts, differences in key methodologic aspects in the available studies might still be confounding the relationship under investigation. Since MRI is often not the first-line routine imaging modality, our results cannot yet be translated into clinical practice. In addition, data are limited on CMBs and interventional endovascular treatments in acute stroke, especially without prior IV thrombolysis; only 2 of the studies included in our meta-analysis included such data. Based on present evidence, detecting CMBs should not prevent thrombolytic treatment, given the clear benefit of this treatment on longer-term outcomes in large randomized controlled trials. Moreover, the extra information about ICH risk provided by MRI needs to be carefully balanced against any potential to delay thrombolysis by using pretreatment MRI protocols. Our study, however, raises the question of whether the balance of risk vs benefit may not favor intervention in certain patient subgroups, for example elderly individuals with known cognitive impairment who might be more likely to harbor multiple or lobar CMBs and could potentially be targeted for MRI. Data presented here thus reinforce the need to further evaluate CMBs in individual patient meta-analyses and large multicenter studies, not only for the risk of postthrombolysis early symptomatic ICH, but also for long-term functional outcome.
Supplementary Material
GLOSSARY
- CI
confidence interval
- CMB
cerebral microbleed
- ICH
intracerebral hemorrhage
- OR
odds ratio
- rtPA
recombinant tissue plasminogen activator
- SWI
susceptibility-weighted imaging
Footnotes
Supplemental data at Neurology.org
Editorial, page 925
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. A. Charidimou and Dr. Z. Fox. A. Charidimou: study concept and design, systematic review, data extraction, data analysis, write-up. A. Shoamanesh: study concept, systematic review, data extraction, critical revisions. D. Wilson: systematic review, data extraction, critical revisions. Q. Gang: translation of non-English papers, critical revisions. Z. Fox: statistical analysis, critical revisions. H.R. Jäger: critical revisions. O.R. Benavente: critical revisions. D.J. Werring: study concept and design, critical revisions, funding.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
A. Charidimou received research support from the Greek State Scholarship Foundation, the Stroke Association, and the British Heart Foundation. A. Shoamanesh reports no disclosures relevant to the manuscript. D. Wilson is funded by the Stroke Association and the British Heart Foundation. Q. Gang is supported by a UCL Impact Studentship and the Chinese Scholarship Council. Z. Fox reports no disclosures relevant to the manuscript. R. Jäger has received research support from the Samantha Dickson Brain Tumour Trust and the Brain Research Trust. O. Benavente reports no disclosures relevant to the manuscript. D. Werring receives research support from the Stroke Association and the British Heart Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;384:1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derex L, Nighoghossian N. Intracerebral haemorrhage after thrombolysis for acute ischaemic stroke: an update. J Neurol Neurosurg Psychiatry 2008;79:1093–1099. [DOI] [PubMed] [Google Scholar]
- 3.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 4.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- 5.Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol 1999;20:637–642. [PMC free article] [PubMed] [Google Scholar]
- 6.Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis 2011;32:528–534. [DOI] [PubMed] [Google Scholar]
- 7.Kidwell CS, Saver JL, Villablanca JP, et al. Magnetic resonance imaging detection of microbleeds before thrombolysis: an emerging application. Stroke 2002;33:95–98. [DOI] [PubMed] [Google Scholar]
- 8.Fiehler J, Albers GW, Boulanger JM, et al. Bleeding Risk Analysis in Stroke Imaging Before Thrombolysis (BRASIL): pooled analysis of T2*-weighted magnetic resonance imaging data from 570 patients. Stroke 2007;38:2738–2744. [DOI] [PubMed] [Google Scholar]
- 9.Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and the risk of intracerebral haemorrhage after thrombolysis for acute ischaemic stroke: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2013;84:277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoamanesh A, Kwok CS, Lim PA, Benavente OR. Postthrombolysis intracranial hemorrhage risk of cerebral microbleeds in acute stroke patients: a systematic review and meta-analysis. Int J Stroke 2013;8:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charidimou A, Fox Z, Werring DJ. Do cerebral microbleeds increase the risk of intracerebral hemorrhage after thrombolysis for acute ischemic stroke? Int J Stroke 2013;8:E1–E2. [DOI] [PubMed] [Google Scholar]
- 12.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain 2007;130:1988–2003. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 15.Yan SQ, Wan JP, Guo Y, Lou M. Impact of cerebral microbleeds on outcomes of acute ischemic stroke treated with intravenous thrombolysis [in Chinese]. Zhejiang Da Xue Xue Bao Yi Xue Ban 2014;43:20–27. [DOI] [PubMed] [Google Scholar]
- 16.Gratz PP, El-Koussy M, Hsieh K, et al. Preexisting cerebral microbleeds on susceptibility-weighted magnetic resonance imaging and post-thrombolysis bleeding risk in 392 patients. Stroke 2014;45:1684–1688. [DOI] [PubMed] [Google Scholar]
- 17.Dannenberg S, Scheitz JF, Rozanski M, et al. Number of cerebral microbleeds and risk of intracerebral hemorrhage after intravenous thrombolysis. Stroke 2014;45:2900–2905. [DOI] [PubMed] [Google Scholar]
- 18.Moriya Y, Takahashi W, Kijima C, et al. Predictors for hemorrhagic transformation with intravenous tissue plasminogen activator in acute ischemic stroke. Tokai J Exp Clin Med 2013;38:24–27. [PubMed] [Google Scholar]
- 19.Kimura K, Aoki J, Shibazaki K, Saji N, Uemura J, Sakamoto Y. New Appearance of extraischemic microbleeds on T2*-weighted magnetic resonance imaging 24 hours after tissue-type plasminogen activator administration. Stroke 2013;44:2776–2781. [DOI] [PubMed] [Google Scholar]
- 20.Kim HS, Lee DH, Ryu CW, et al. Multiple cerebral microbleeds in hyperacute ischemic stroke: impact on prevalence and severity of early hemorrhagic transformation after thrombolytic treatment. AJR Am J Roentgenol 2006;186:1443–1449. [DOI] [PubMed] [Google Scholar]
- 21.Kakuda W, Thijs VN, Lansberg MG, et al. Clinical importance of microbleeds in patients receiving IV thrombolysis. Neurology 2005;65:1175–1178. [DOI] [PubMed] [Google Scholar]
- 22.Derex L, Nighoghossian N, Hermier M, et al. Thrombolysis for ischemic stroke in patients with old microbleeds on pretreatment MRI. Cerebrovasc Dis 2004;17:238–241. [DOI] [PubMed] [Google Scholar]
- 23.Mazya M, Egido JA, Ford GA, et al. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: Safe Implementation of Treatments in Stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke 2012;43:1524–1531. [DOI] [PubMed] [Google Scholar]
- 24.Pantoni L, Fierini F, Poggesi A. Thrombolysis in acute stroke patients with cerebral small vessel disease. Cerebrovasc Dis 2014;37:5–13. [DOI] [PubMed] [Google Scholar]
- 25.van Etten ES, Auriel E, Haley KE, et al. Incidence of symptomatic hemorrhage in patients with lobar microbleeds. Stroke 2014;45:2280–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and recurrent stroke risk: systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke 2013;44:995–1001. [DOI] [PubMed] [Google Scholar]
- 27.Lovelock CE, Cordonnier C, Naka H, et al. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke 2010;41:1222–1228. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke 2004;35:1415–1420. [DOI] [PubMed] [Google Scholar]
- 29.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 2010;75:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong KS, Chan YL, Liu JY, Gao S, Lam WW. Asymptomatic microbleeds as a risk factor for aspirin-associated intracerebral hemorrhages. Neurology 2003;60:511–513. [DOI] [PubMed] [Google Scholar]
- 31.Demchuk AM, Khan F, Hill MD, et al. Importance of leukoaraiosis on CT for tissue plasminogen activator decision making: evaluation of the NINDS rt-PA Stroke Study. Cerebrovasc Dis 2008;26:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarron MO, Nicoll JA. Cerebral amyloid angiopathy and thrombolysis-related intracerebral haemorrhage. Lancet Neurol 2004;3:484–492. [DOI] [PubMed] [Google Scholar]
- 33.Ly JV, Rowe CC, Villemagne VL, et al. Cerebral beta-amyloid detected by Pittsburgh compound B positron emission topography predisposes to recombinant tissue plasminogen activator-related hemorrhage. Ann Neurol 2010;68:959–962. [DOI] [PubMed] [Google Scholar]
- 34.Winkler DT, Biedermann L, Tolnay M, et al. Thrombolysis induces cerebral hemorrhage in a mouse model of cerebral amyloid angiopathy. Ann Neurol 2002;51:790–793. [DOI] [PubMed] [Google Scholar]
- 35.Reuter B, Grudzenski S, Chatzikonstantinou E, et al. Thrombolysis in experimental cerebral amyloid angiopathy and the risk of secondary intracerebral hemorrhage. Stroke 2014;45:2411–2416. [DOI] [PubMed] [Google Scholar]
- 36.Dierksen GA, Skehan ME, Khan MA, et al. Spatial relation between microbleeds and amyloid deposits in amyloid angiopathy. Ann Neurol 2010;68:545–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazya MV, Ahmed N, Ford GA, et al. Remote or extraischemic intracerebral hemorrhage—an uncommon complication of stroke thrombolysis: results from the Safe Implementation of Treatments in Stroke–International Stroke Thrombolysis Register. Stroke 2014;45:1657–1663. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed N, Wahlgren N, Grond M, et al. Implementation and outcome of thrombolysis with alteplase 3–4.5 h after an acute stroke: an updated analysis from SITS-ISTR. Lancet Neurol 2010;9:866–874. [DOI] [PubMed] [Google Scholar]
- 39.Charidimou A, Krishnan A, Werring DJ, Rolf Jäger H. Cerebral microbleeds: a guide to detection and clinical relevance in different disease settings. Neuroradiology 2013;55:655–674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



