Abstract
Objectives:
We used a prospective clinical trial to examine the risks conferred by metabolic syndrome (METS) and diabetes mellitus (DM) to recurrent strokes in the Secondary Prevention of Small Subcortical Strokes (SPS3) study cohort.
Methods:
The SPS3 trial enrolled 3,020 patients with lacunar strokes. Participants were stratified into groups of METS only, DM only, both, or neither using American Heart Association/National Heart, Lung, and Blood Institute and World Health Organization guidelines. Annualized event rates of strokes, myocardial infarction (MI), and all-cause mortality were calculated, and hazard ratios (HRs) referencing the “neither” group were computed, controlling for significantly associated baseline characteristics.
Results:
Among 2,999 participants, 25% had METS only, 6% had DM only, 32% had both conditions, and 37% had neither. Over a median of 3.8 years of follow-up, there were 274 recurrent strokes (240 ischemic, 34 hemorrhagic) and 74 MIs; among the 240 ischemic strokes, 134 (56%) were lacunar. The rates of any recurrent stroke (HR 1.7, 95% confidence interval [CI] 1.3–2.3) or lacunar stroke (HR 2.4, 95% CI 1.5–3.7) were significantly higher for those with concurrent METS and DM compared with those who had neither. Risk of incident MI was higher in participants with DM (HR 2.8, 95% CI 1.1–7.0) or concurrent DM and METS (HR 2.6, 95% CI 1.4–4.9).
Conclusion:
METS and DM were significant comorbid conditions in lacunar stroke patients and they were associated with stroke recurrence. In patients with lacunar infarcts, a vigilant approach to prevent development of DM in those with METS may be a potential strategy to reduce recurrent strokes.
The metabolic syndrome (METS) is an interrelated group of risk factors that confers a higher risk of incident diabetes mellitus (DM) and cardiovascular disease (CVD).1 Several clinical guidelines exist for the diagnosis of METS2 and all identify a combination of insulin resistance, adiposity, dyslipidemia, and elevated blood pressure (BP). The prevalence of METS increases with age,3 and across various populations may range from 20% to 50%.4–6
METS was originally described as a method to identify nondiabetic populations who were at higher risk of developing incident DM and CVD.4 However, this definition has evolved over the past decade to also encompass those with diagnosis of DM. Although the inclusion of patients with DM has been accepted into most major definitions,2,7 this integration has made it difficult to understand the role of METS as an independent risk factor for incident CVD as compared to the risks imparted by DM itself.
Past studies have shown that both DM and METS are associated with higher risk of cardiovascular and cerebrovascular disease but few studies focus on the lacunar stroke subtype.8–11 Lacunar strokes account for approximately 25% of all ischemic strokes, and recurrence risk within 5 years has been reported to be as high as 20%, leading to progressive morbidity.12,13
We compared METS in nondiabetics vs DM as risk factors for recurrent cardiovascular and symptomatic cerebrovascular disease among participants with lacunar strokes from the Secondary Prevention of Small Subcortical Strokes (SPS3) trial. We hypothesized that both METS and DM contribute to the risk of recurrent stroke.
METHODS
The institutional review board–approved SPS3 (NCT00059306) was a randomized, multicenter, clinical trial that enrolled 3,020 patients with symptomatic, MRI-confirmed lacunar strokes from 81 clinical centers in North America, Latin America, and Spain.14 All patients with recent lacunar stroke (within 6 months) and without surgical ipsilateral carotid artery disease or cardioembolic source were randomized, in a 2-by-2 factorial design, to both an antiplatelet (AP) intervention and one of 2 target levels of systolic BP.14
Baseline METS and DM status were determined using American Heart Association/National Heart, Lung, and Blood Institute2 and World Health Organization guidelines,15 respectively. Participants were stratified into 4 groups: METS only, DM only, both, or neither. Annualized event rates of strokes, cardiovascular events, and all-cause mortality were calculated; hazard ratios (HRs) were computed relative to the reference group of participants who had neither risk factor, controlling for significantly associated baseline characteristics.
METS was determined by the presence of ≥3 of the following: prediabetes with fasting blood glucose of 110 to 125 mg/dL; elevated BP (≥130/≥85 mm Hg) or history of hypertension with antihypertensive medication; increased triglyceride level (≥150 mg/dL); reduced high-density lipoprotein level (<40 mg/dL in men and <50 mg/dL in women); and abdominal obesity (waist circumference ≥88 cm in women and ≥102 cm in men). Of the 3,020 enrolled patients, 2,999 (99.3%) had sufficient data for assessment of METS status. Waist circumference data were not available for 1,654 (55%) of the 3,009 patients. Among these 1,654 participants, 1,243 (75%) met the definition for METS because of the presence of ≥3 other component risk factors. For 411 patients without waist circumference data and only 2 additional component risk factors, body mass index ≥30 kg/m2 was used as a proxy for abdominal obesity.16 DM was determined by fasting glucose ≥126 mg/dL, self-reported history of diabetes, or use of hypoglycemic therapy.
Participants who met criteria for METS but did not meet criteria for DM were categorized into the METS only group. Those who met criteria for DM but not METS were categorized into the DM only group. Participants with “both” concurrently fulfilled criteria for METS and DM. Those in the “neither” group did not meet criteria for either condition.
Covariates.
Dyslipidemia was defined by self-report of dyslipidemia and/or treatment with lipid-lowering drug. Regular alcohol use was defined as ≥7 alcoholic drinks a week; patients with alcohol abuse by history were excluded from enrollment. Regular exercise was defined as exercise ≥3 times a week. Ischemic heart disease was defined as any confirmed history of myocardial infarction (MI), definite/atypical angina, or revascularization procedure.17 White matter hyperintensities (WMH) were evaluated visually using the age-related white matter changes score by 4 independent readers who were blinded to clinical information.18
Outcomes.
Recurrent stroke was defined by the presence of a focal neurologic deficit persisting for more than 24 hours ascertained by clinical evaluation with supplemental noncontrast head CT or brain MRI. MI events were defined by ECG and cardiac enzyme criteria.14 All events were adjudicated by a central committee.
Statistical analysis.
Baseline characteristics of the study population were compared across the 4 groups (neither, DM only, METS only, and both) using χ2 tests of association for categorical variables and analysis of variance for continuous variables. Rates were computed for each group as the number of events divided by the total follow-up time for that group, and this was annualized. Cox proportional hazards models were used to determine the relative stroke rates using the “neither” group as reference in the adjusted models displayed as HRs. The proportional hazards assumption was examined in the model of the primary events. Outcomes examined included any recurrent strokes, lacunar strokes, hemorrhagic strokes, MIs, and all-cause mortality. Interactions between the 4-category METS/DM variable and each of the AP and BP arms were examined for the primary outcome.
RESULTS
Of the 3,020 participants enrolled at baseline, 2,999 (99%) had available data to determine METS and DM status. Hypertension was the most prevalent METS component observed in 90% of participants, followed by low high-density lipoprotein (56%), abdominal obesity (45%), and elevated triglycerides (44%), while prediabetes was relatively uncommon (12%).
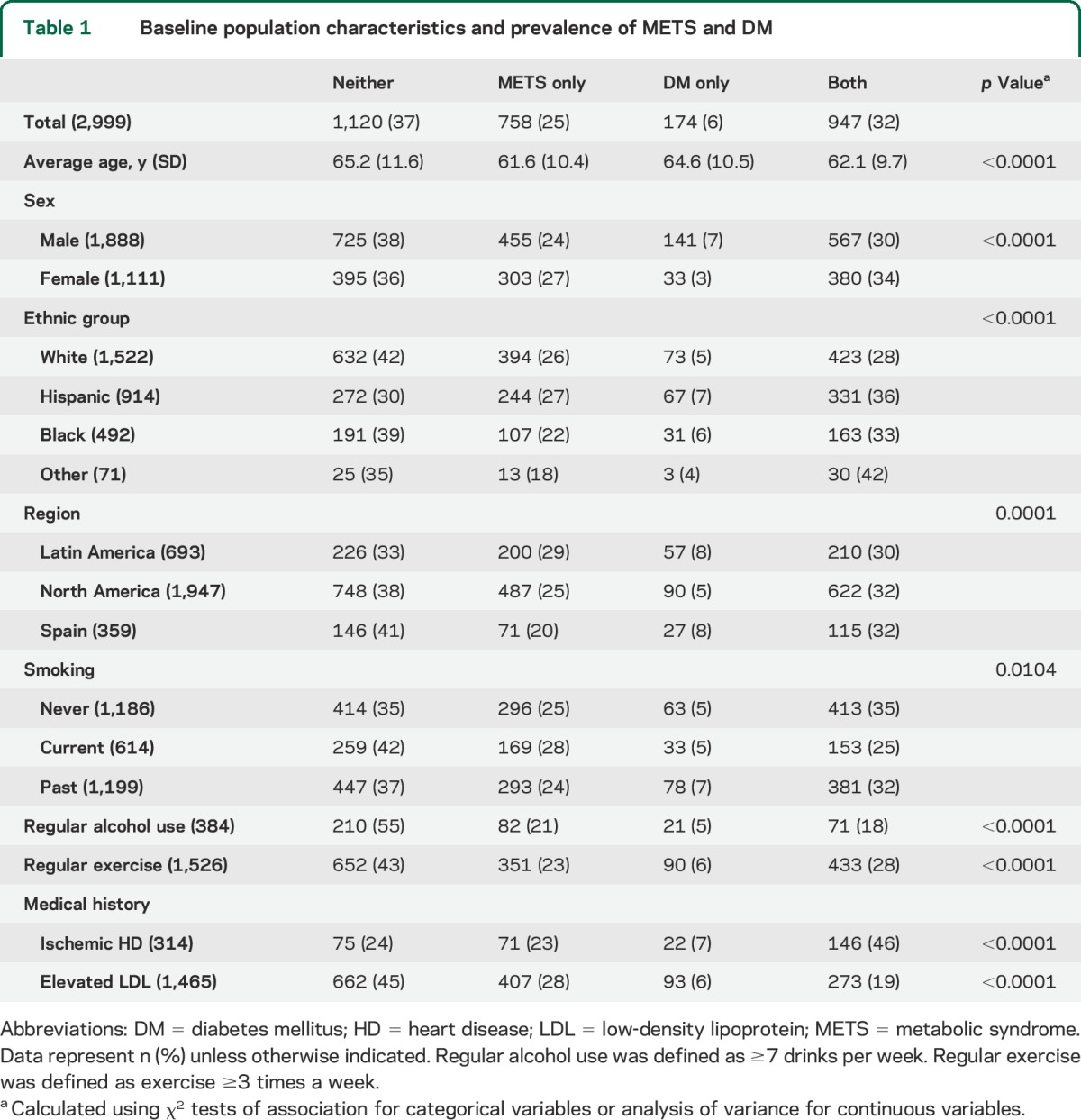
Overall, 25% of the participants met criteria for METS. Only 6% met criteria for DM without METS. The presence of concurrent METS and DM was common with a prevalence of 32%. The remaining 37% participants (“neither” group) did not meet criteria for METS or DM (table 1).
Table 1.
Baseline population characteristics and prevalence of METS and DM
Study sample.
The baseline characteristics across the 4 study groups are provided in table 1. We observed significant regional differences between Latin America, North America, and Spain such that participants from Spain had lower prevalence of traditional stroke risk factors with 41% of participants having neither DM nor METS. Active smokers were less likely than former or nonsmokers to have concurrent DM and METS and were more likely to have neither condition. Participants with regular alcohol intake (≥7 drinks/wk) were also more likely to have neither risk factor (55%) compared to the study average (37%). Those with DM and METS were more likely to report a history of ischemic heart disease compared with other participants.
Baseline lacunar infarct characteristics.
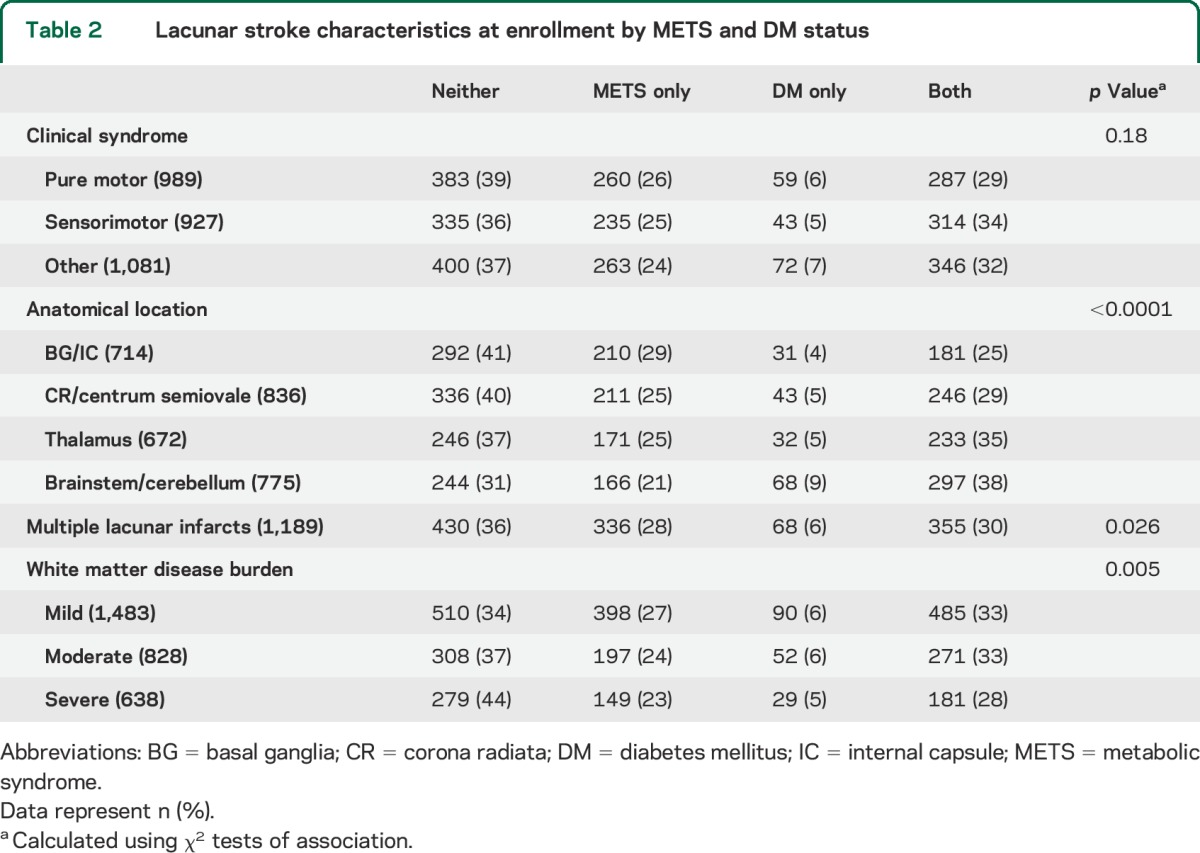
Pure motor lacunar stroke was the single most common clinical syndrome in 33% (989/2,997) followed by sensorimotor stroke in 31% (927/2,997); the remaining 36% (1,081/2,997) of participants had clinical stroke syndromes such as ataxic hemiparesis, dysarthria/clumsy-hand syndrome, and hemiballismus (table 2). A significant number of patients had multiple lacunar infarcts noted on MRI at enrollment (39%). Twenty-five percent of participants in the “neither” group had severe WMH compared with only 19% of participants with both DM and METS (table 2).
Table 2.
Lacunar stroke characteristics at enrollment by METS and DM status
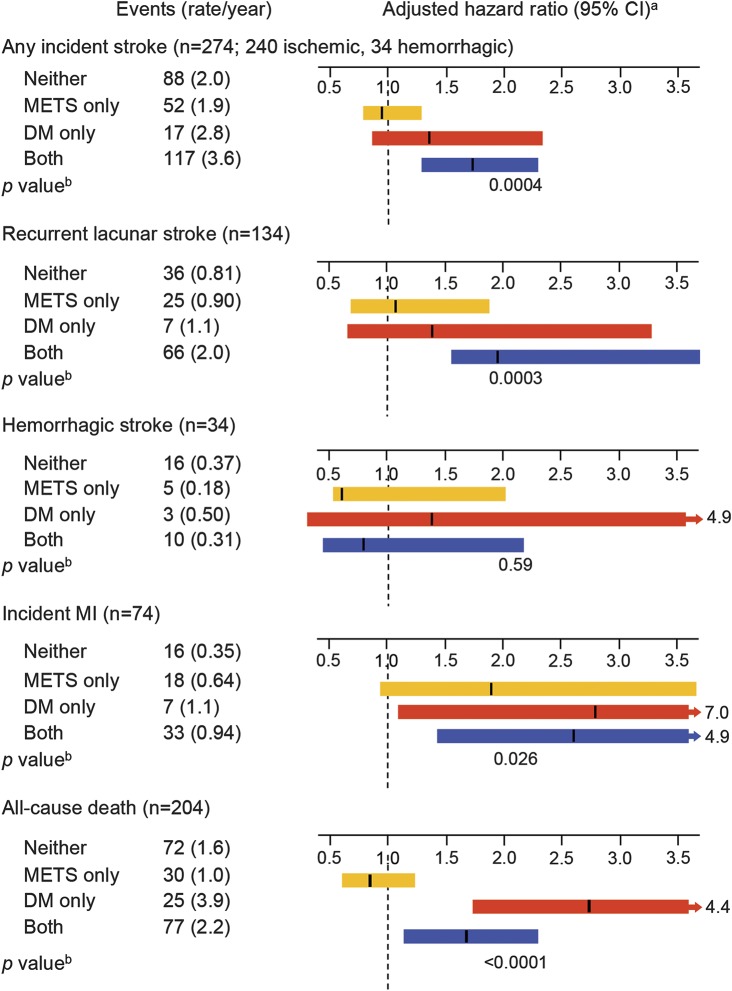
Over a median of 3.8 years of follow-up, there were 274 recurrent strokes, of which 240 were ischemic and 34 were hemorrhagic (figure). Of the 240 ischemic strokes, 134 (56%) were lacunar. Participants with concurrent DM and METS had significantly higher annual rate of any recurrent stroke (all strokes, including ischemic and hemorrhagic, HR 1.7, 95% confidence interval [CI] 1.3–2.3) and lacunar stroke (HR 2.4, 95% CI 1.5–3.7) relative to those with neither. We did not observe an association with hemorrhagic stroke for any of the groups.
Figure. Event rates and hazard ratios by METS and DM status.
CI = confidence interval; DM = diabetes mellitus; METS = metabolic syndrome; MI = myocardial infarction. aControlled for all variables significant in tables 1 and 2. bCalculated by Wald test for overall differences.
METS alone did not confer a significantly increased risk of incident MI although participants with DM only or DM with concurrent METS did have increased annual event rates (HR 2.8, 95% CI 1.1–7.0 and HR 2.6, 95% CI 1.4–4.5, respectively). A similar result was found for all-cause death with DM (alone or with METS) showing an increased risk (HR 2.7, 95% CI 1.7–4.4, and HR 1.6, 95% CI 1.2–2.4, respectively). There were no differences in terms of single vs dual AP therapy (table e-1 on the Neurology® Web site at Neurology.org) or BP target (table e-2) regarding stroke or MI risk reduction in any of the DM/METS groups.
DISCUSSION
The SPS3 study comprised patients with recent, MRI-proven lacunar stroke, all of whom were managed with AP and BP therapy. With combined medical therapy, participants with METS only had less risk of stroke recurrence compared with those who had METS and DM. Participants with DM (with or without METS) were also more likely to have incident MI.
Lacunar strokes comprise approximately one-quarter9 of all ischemic strokes. Without medical intervention, they are characterized by a high rate of recurrence leading to increasing disability.12 The SPS3 study is the largest study of lacunar stroke patients within a multiethnic and international population of more than 3,000 participants. It is distinctive as it defines the presence of suspected recent lacunar stroke via requisite MRI rather than relying solely on clinical criteria, which are less specific.19 The large study size and the rigor in identifying and enrolling patients have allowed us to gain important insights into the underlying risk factors and effectiveness of treatment in patients with lacunar strokes.
METS and DM were found to be frequent comorbid conditions in those with lacunar strokes. In this cohort, 37% had neither DM nor METS, and 32% had both (DM and METS). Although a significant number of patients had METS only (25%), it was quite rare (6%) for participants to have DM in the absence of METS; this matches our observation that those with METS are at high risk of developing DM and that DM is less likely to occur in the absence of associated risk factors.
The overall rate of symptomatic recurrent stroke (ischemic and hemorrhagic) in this cohort was 8%, which was considerably lower than prior epidemiology-based studies of lacunar disease with reported recurrence rates of nearly 20%.12,13 The low recurrence rate seen in the SPS3 trial was similar to results from other randomized controlled trials assessing the efficacy of secondary prevention,20,21 providing strong evidence that appropriate medical therapy can lower the overall risk of lacunar stroke recurrence.
The risk of any recurrent stroke and specifically, lacunar stroke, for patients with either METS or DM was not significantly higher than in those with neither condition. Patients with concurrent DM and METS had a higher rate of recurrent strokes (both lacunar and any ischemic stroke) despite undergoing similar medical treatment. DM alone showed a trend suggestive of increased risk of recurrent lacunar stroke but did not reach statistical significance, which may be attributable to small sample size as DM rarely occurred alone, and almost always occurred in conjunction with METS. The physiology underlying the increased risk of recurrent ischemic stroke in diabetics with METS may be attributable to the increased prevalence of intracranial stenosis in this population,22 which we were not able to examine in this study.
The relationship between METS and DM regarding incident MI differed from that for incident stroke. For those with METS only, the rate of MI was not significantly different from the “neither” group (HR 1.9, 95% CI 0.94–3.8); however, for those with DM, whether with METS (HR 2.6, 95% CI 1.4–4.9) or without (HR 2.8, 95% CI 1.1–7.0), the risk of MI was significantly higher. DM has been linked to a higher burden of underlying coronary artery disease23,24 and a chronic prothrombotic state25 that may be the underlying reason for the increased risk of MI in diabetics.
In the SPS3 population, the risk of recurrent lacunar stroke for participants with METS appears to be reduced by combined AP therapy and BP control. Medical therapy seemed less effective for MI and recurrent stroke prevention in those with concurrent METS and DM. Neither dual AP nor lower BP target provided additional benefit in stroke or MI risk reduction in any of the groups (tables e-1 and e-2).
Although SPS3 represents an ideal patient population in which to examine underlying pathophysiology of lacunar strokes, there may be some limitations to the generalizability of the data to the entire ischemic stroke population. Patients with multiple stroke subtypes, such as those with cortical or large subcortical strokes, or ipsilateral carotid disease were excluded from the study. This selection bias may be the underlying reason why active smokers (table 1) were less likely than former/nonsmokers to have concurrent DM and METS and why those with neither risk factor had more severe WMH than those with concurrent DM and METS (table 2). There was also a relative lack of ethnically Asian participants (1.4% of total enrolled), which may limit applicability to this population, particularly since Asians tend to have differences in stroke risk factors compared with other ethnicities.26,27 In addition, this is a post hoc analysis of a randomized controlled trial, and as such, there may be residual confounding in the relationships between METS, DM, and recurrent events.
Nevertheless, the results from our study suggest that it would be important for clinicians to identify patients at high risk of developing DM (such as those with METS). Initiating preventive strategies may prove to be an effective measure to stroke and MI prevention in patients with METS who are at risk of developing DM. These strategies need to be examined in further clinical trials.
Supplementary Material
GLOSSARY
- AP
antiplatelet
- BP
blood pressure
- CI
confidence interval
- CVD
cardiovascular disease
- DM
diabetes mellitus
- HR
hazard ratio
- METS
metabolic syndrome
- MI
myocardial infarction
- SPS3
Secondary Prevention of Small Subcortical Strokes
- WMH
white matter hyperintensities
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Study concept and design: Helena Lau, Shuhan Zhu, Leslie A. McClure, Oscar Benevente, Aleksandra Pikula. Acquisition of data: Leslie A. McClure, Helena Lau, Carole White, Oscar Benevente. Analysis and interpretation of data: Leslie A. McClure, Shuhan Zhu, Helena Lau, Jose R. Romero, Carole White, Viken Babikian, Thanh Nguyen, Oscar Benavente, Carlos S. Kase, Aleksandra Pikula. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Leslie A. McClure. Study supervision: Aleksandra Pikula.
STUDY FUNDING
This work was supported by the NIH/NHLBI (National Heart, Lung, and Blood Institute) and the National Institute of Neurological Disorders and Stroke (NINDS) (U01 NS38529). The content is solely the responsibility of the authors and does not necessarily represent the official views of NHLBI or NIH/NINDS.
DISCLOSURE
S. Zhu reports no disclosures relevant to the manuscript. L. McClure was supported by a grant from the NIH/NINDS. H. Lau, J. Romero, C. White, V. Babikian, T. Nguyen, O. Benavente, C. Kase, and A. Pikula report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal. Diabetes Care 2005;28:2289–2304. [DOI] [PubMed] [Google Scholar]
- 2.Grundy S, Cleeman J, Daniels S, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 3.Ford E, Giles W, Dietz W. Prevalence of the metabolic syndrome among US adults. J Am Med Assoc 2002;287:356–359. [DOI] [PubMed] [Google Scholar]
- 4.Cull C, Jensen C, Retnakaran R, Holman R. Impact of the metabolic syndrome on macrovascular and microvascular outcomes in type 2 diabetes mellitus: United Kingdom Prospective Diabetes Study 78. Circulation 2007;116:2119–2126. [DOI] [PubMed] [Google Scholar]
- 5.Ballantyne C, Hoogeveen R, McNeill A, et al. Metabolic syndrome risk for cardiovascular disease and diabetes in the ARIC Study. Int J Obes 2008;32:S21–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boden-Albala B, Sacco R, Lee H, et al. Metabolic syndrome and ischemic stroke risk: Northern Manhattan Study. Stroke 2008;39:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grundy S, Brewer H, Cleeman J, Simith S, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004;109:433–438. [DOI] [PubMed] [Google Scholar]
- 8.Dekker J, Girman C, Rhodes T, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation 2005;112:666–673. [DOI] [PubMed] [Google Scholar]
- 9.Jackson C, Sudlow C. Are lacunar strokes really different? A systematic review of differences in risk factor profiles between lacunar and nonlacunar infarcts. Stroke 2005;36:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurl S, Laukkanen J, Niskanen L, et al. Metabolic syndrome and the risk of stroke in middle-aged men. Stroke 2006;37:806–811. [DOI] [PubMed] [Google Scholar]
- 11.Najarian R, Sullivan L, Kannel W, Wilson P, D'Agostino R, Wolf P. Metabolic syndrome compared with type 2 diabetes mellitus as a risk factor for stroke. Arch Intern Med 2006;166:106–111. [DOI] [PubMed] [Google Scholar]
- 12.Samuelsson M, Soderfeldt B, Olsson G. Functional outcome in patients with lacunar infarction. Stroke 1996;27:842–846. [DOI] [PubMed] [Google Scholar]
- 13.Kolominsky-Rabas P, Weber M, Gefeller O, Neundoerfer B, Heuschmann P. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 2001;32:2735–2740. [DOI] [PubMed] [Google Scholar]
- 14.Benavente O, White C, Pearce L, et al. The Secondary Prevention of Small Subcortical Strokes (SPS3) study. Int J Stroke 2011;6:164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation [online]. 2006. Available at: http://www.who.int/diabetes/publications. Accessed February 1, 2015. [Google Scholar]
- 16.Klein S, Allison D, Heymsfield S, et al. Waist circumference and cardiometabolic risk: a consensus statement from Shaping America's Health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care 2007;30:1647–1652. [DOI] [PubMed] [Google Scholar]
- 17.White C, Szychowski J, Roldan A, et al. Clinical features and racial/ethnic differences among the 3020 participants in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial. J Stroke Cerebrovasc Dis 2013;22:764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahlund L, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001;32:1318–1322. [DOI] [PubMed] [Google Scholar]
- 19.Lee L, Kidwell C, Alger J, Starkman S, Saver J. Impact on stroke subtype diagnosis of early diffusion-weighted magnetic resonance imaging and magnetic resonance angiography. Stroke 2000;31:1081–1089. [DOI] [PubMed] [Google Scholar]
- 20.Bousser M, Amarenco P, Chamorro A, Fisher M, Ford I. Terutroban versus aspirin in patients with cerebral ischaemic events (PERFORM): a randomised, double-blind, parallel-group trial. Lancet 2011;377:2013–2022. [DOI] [PubMed] [Google Scholar]
- 21.Sacco R, Diener H, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med 2008;359:1238–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palacio S, McClure L, Benavente O, Bazan C, Pergola P, Hart R. Lacunar strokes in patients with diabetes mellitus: risk factors, infarct location, and prognosis: the Secondary Prevention of Small Subcortical Strokes Study. Stroke 2014;45:2689–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kornowski R, Mintz G, Lansky A, et al. Paradoxic decreases in atherosclerotic plaque mass in insulin-treated diabetic patients. Am J Cardiol 1998;81:1298–1304. [DOI] [PubMed] [Google Scholar]
- 24.Goraya T, Leibson C, Palumbo P, et al. Coronary atherosclerosis in diabetes mellitus: a population-based autopsy study. J Am Coll Cardiol 2002;40:946–953. [DOI] [PubMed] [Google Scholar]
- 25.Knobler H, Savion N, Shenkman B, Kotev-Emeth S, Varon D. Shear-induced platelet adhesion and aggregation on subendothelium are increased in diabetic patients. Thromb Res 1998;90:181–190. [DOI] [PubMed] [Google Scholar]
- 26.Kim B, Kim J. Ischemic stroke subtype classification: an Asian viewpoint. J Stroke 2014;4:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimaru K, McHenry L, Toole J. Cerebral angiographic and clinical differences in carotid system transient ischemic attacks between American Caucasian and Japanese patients. Stroke 1984;15:56–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.