Abstract
Objective:
To assess the clinical and immunologic findings in children with voltage-gated potassium channel (VGKC)-complex antibodies (Abs).
Methods:
Thirty-nine of 363 sera, referred from 2 pediatric centers from 2007 to 2013, had been reported positive (>100 pM) for VGKC-complex Abs. Medical records were reviewed retrospectively and the patients' condition was independently classified as inflammatory (n = 159) or noninflammatory (n = 204). Positive sera (>100 pM) were tested/retested for the VGKC-complex Ab–positive complex proteins LGI1 and CASPR2, screened for binding to live hippocampal neurons, and 12 high-titer sera (>400 pM) tested by radioimmunoassay for binding to VGKC Kv1 subunits with or without intracellular postsynaptic density proteins.
Results:
VGKC-complex Abs were found in 39 children, including 20% of encephalopathies and 7.6% of other conditions (p = 0.001). Thirty children had inflammatory conditions and 9 had noninflammatory etiologies but titers >400 pM (n = 12) were found only in inflammatory diseases (p < 0.0001). Four sera, including from 2 children with coexisting NMDA receptor Abs and one with Guillain-Barré syndrome and Abs to both LGI1 and CASPR2, bound to hippocampal neurons. None of the sera bound detectably to VGKC Kv1 subunits on live HEK cells, but 4 of 12 >400 pM sera immunoprecipitated VGKC Kv1 subunits, with or without postsynaptic densities, extracted from transfected cells.
Conclusion:
Positive VGKC-complex Abs cannot be taken to indicate a specific clinical syndrome in children, but appear to be a nonspecific biomarker of inflammatory neurologic diseases, particularly of encephalopathy. Some of the Abs may bind to intracellular epitopes on the VGKC subunits, or to the intracellular interacting proteins, but in many the targets remain undefined.
Voltage-gated potassium channel (VGKC)-complex antibody (Ab), identified by a radioimmunoprecipitation assay, can be associated with CNS diseases in adults and children. The VGKC complex includes Kv1 subunits and other cell surface proteins such as LGI1 (leucine-rich, glioma inactivated), CASPR2 (contactin-associated protein 2), and contactin-2; other membrane proteins include a disintegrin, and metalloproteinase 22 and 23 (ADAM22 and 23), whereas the membrane-associated guanylate kinases (MAGUK), which include postsynaptic density (PSD) proteins 93 and 95,1 are intracellular. When the Abs are identified by cell-based assays (CBAs) for the individual proteins, LGI1 Abs are most common in adult limbic encephalitis,2,3 and CASPR2 Abs in patients with peripheral nerve hyperexcitability3,4 and the rare Morvan syndrome.5 However, it has become clear that not all VGKC-complex Abs can be accounted for by Abs binding to these proteins.6 This is particularly the case in children whose titers are usually <400 pM even when they have limbic encephalitis.7,8 Moreover, the VGKC-complex Ab–associated clinical phenotypes are varied and include epilepsies9 and demyelination.10
Because of the lack of clear relationship between VGKC-complex Abs and clinical syndromes in children, we hypothesized that the radioimmunoassay may detect binding to intracellular epitopes on the VGKC subunits themselves and be clinically irrelevant. The aim was to explore the clinical phenotypes of VGKC-complex Abs at different levels in children and to begin to dissect their targets within the VGKC-complex.
METHODS
Between 2008 and 2013, 363 serum samples were sent from the Evelina London Children's Hospital (241) and Great Ormond Street Children's Hospital (122) to the Clinical Neuroimmunology service at the Oxford Radcliffe Hospital Trust requesting VGKC-complex and NMDA receptor (NMDAR) or other Ab tests. Demographic information, clinical features at presentation, discharge and follow-up, and results of laboratory, electrophysiologic, and neuroimaging testing, were compiled (Y.H.) and presented anonymously to 2 pediatric neurologists (R.S., M.L.) who were blinded to the Ab results and classified the patients using the ICD-10 into inflammatory diseases of the central and peripheral nervous system (n = 158) and noninflammatory etiologies (n = 205). No CSFs were available from these children for further analysis.
VGKC-complex radioimmunoprecipitation and CBAs.
VGKC-complex Abs were measured by radioimmunoassay, which involves preparation of rabbit brain digitonin-extracted VGKC complexes, labeling the extract with 125I-dendrotoxin (125I-DTX), and adding 1 and 5 μL of serum, followed by anti-human immunoglobulin G (IgG) to immunoprecipitate the complexes. The results are calculated as picomolar of 125I-DTX precipitated3,11 (normal values <100 pM). Retrospectively, all sera positive for VGKC-complex Abs were tested for Abs to NMDAR, LGI1 and contactin-2 (serum 1:20), and to CASPR2 (serum at 1:100) using CBAs as previously described.3 These depend on binding of IgG to human embryonic kidney (HEK) cells, transfected with complementary DNA encoding the relevant autoantigen. The binding of serum IgG to the surface of the transfected cells is visualized using a fluorescence-labeled secondary Ab. Similar CBAs were used to look for binding to ADAM22 and 23 (serum at 1:100), and for the Kv1 subunits themselves by CBA, using cells cotransfected with DNAs for Kv1.1, 1.2, and 1.6.3,12–14 Commercial Abs to Kv1.1, 1.2, and 1.6 (Alomone Labs, Jerusalem, Israel) were used to confirm the expression of each Kv1 subtype (see results section).
Radioimmunoassay for Abs to VGKC Kv1 subunits.
This was performed to mirror the tissue extraction used for the VGKC-complex Ab assay (see above). HEK cells were seeded at 15 million/flask in 175 cm2 flasks and grown until 40% confluent. They were transfected with a total of 60 μg of complementary DNA (e.g., 10–15 μg each of Kv1.1, 1.2, and 1.6, and the intracellular β2 subunit were cotransfected with or without PSD93 and PSD95), with 30 μL of polyethyleneimine and 25 μL of 20% glucose. After 15 hours, the medium was changed, and at 48 hours posttransfection, the flasks were washed in phosphate-buffered saline and the cells lysed in 3 mL of a 2% digitonin (Calbiochem, Billerica, MA) Tris-buffered (pH 7.12) saline with 5 mM KCl and 1:100 protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). The lysates were gently rotated for 1 hour at 4°C, spun (13,000 rpm for 5 minutes) at 4°C, and labeled with 125I-αDTX (106 cpm/mL). Fifty-microliter aliquots of the extracted proteins were then used in radioimmunoassays by adding 2.5 μL of patient serum and immunoprecipitating with anti-human IgG (RSR Ltd., Cardiff, UK).
Primary mouse hippocampal and mixed neuron cultures.
To identify possible novel Abs to extracellular epitopes, primary neuronal cell cultures were established from neonatal (P1) rat brain.3 The live neurons (days 7–9 for whole brain, days 12–15 for hippocampal neurons) were incubated with patient or control sera (diluted 1:100) at 37°C for 1 hour followed by incubation with appropriate secondary Ab before fixation/permeabilization and incubation with mouse anti-MAP2 (microtubule-associated protein 2) Ab (1:1,000; Invitrogen, Carlsbad, CA) to identify the neurons among the mixed hippocampal cultures.
Statistical analysis.
Statistical analysis was performed using the commercially available software GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA). Nonparametric statistical tests (Mann–Whitney tests) were used for continuous distributions and Fisher exact tests for nominal data.
Standard protocol approvals, registrations, and patient consents.
Ethical approval for further Ab testing on referred samples was from the Oxfordshire Regional Ethical Committee A (07/Q1604/28). Because the data analysis was retrospective and no additional data were collected beyond that required for standard medical care of the patient, a full ethics review under the terms of the Governance Arrangements of Research Ethics Committees in the UK was deemed not necessary by the study team.
RESULTS
Clinical diagnoses of all patients referred for testing.
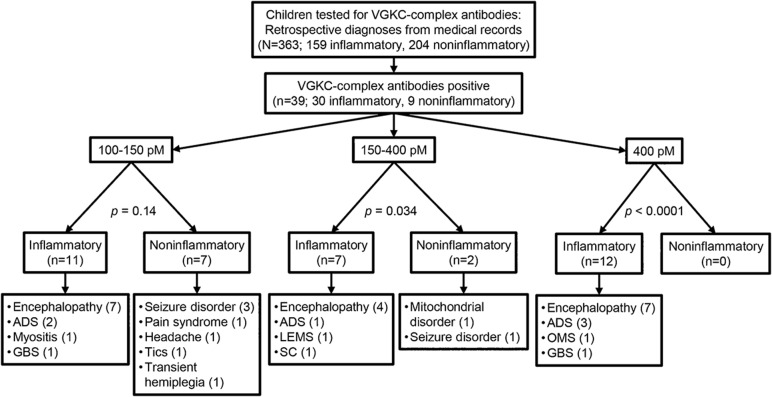
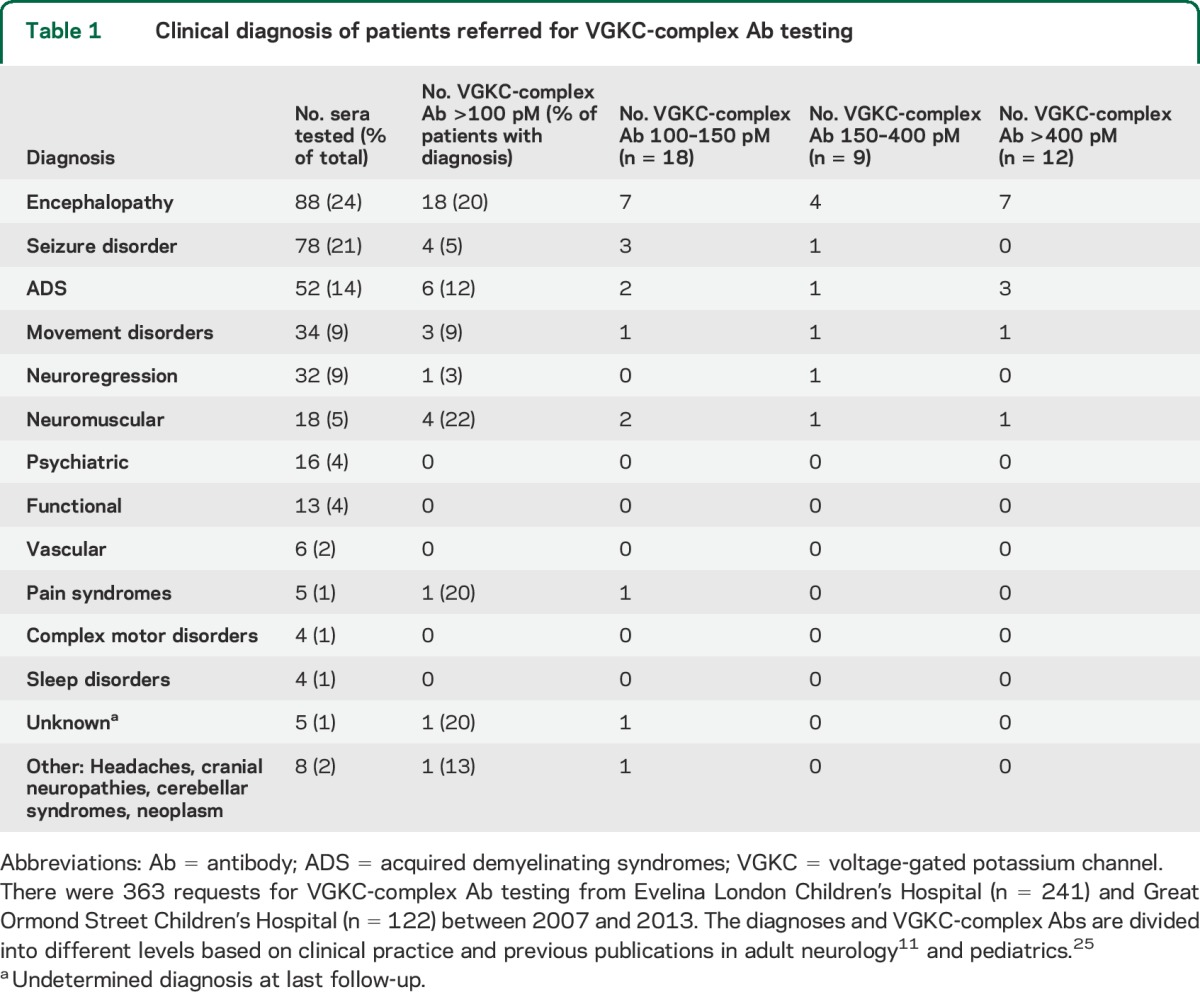
The clinical records for each patient sent for testing were examined and the diagnoses established. Based on the ICD-10, a primary inflammatory condition was identified in 158 patients (67 males, age range 4 months to 18 years, median 8 years) and a noninflammatory diagnosis in 205 patients (98 males, age range 2 months to 18 years, median 9 years). The stratification of the results are shown in figure 1 and summarized in table 1.
Figure 1. Flowchart of samples and results.
ADS = acquired demyelinating syndromes; GBS = Guillain-Barré syndrome; LEMS = Lambert-Eaton myasthenic syndrome; OMS = opsoclonus myoclonus syndrome; SC = Sydenham chorea; VGKC = voltage-gated potassium channel.
Table 1.
Clinical diagnosis of patients referred for VGKC-complex Ab testing
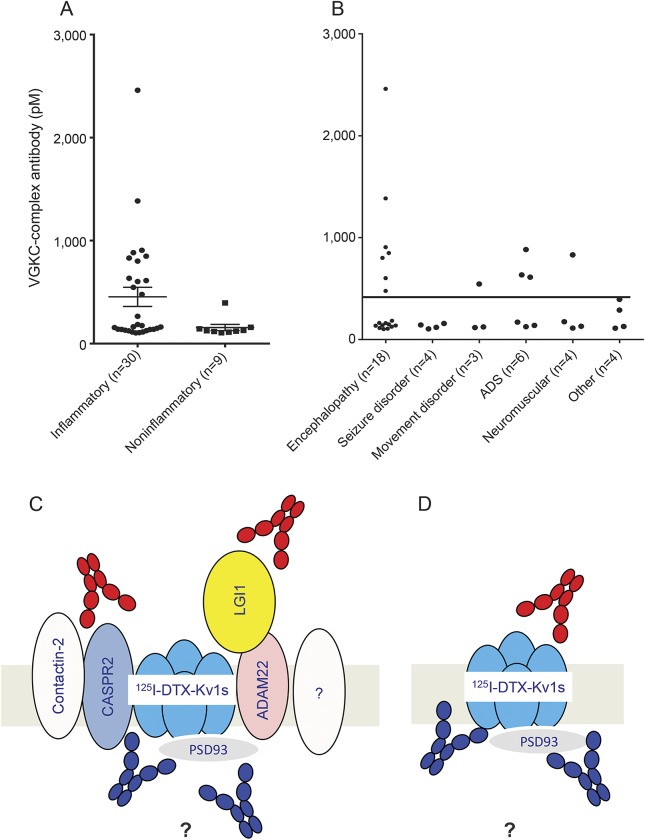
Overall, 30 of the 158 patients in the inflammatory group had VGKC-complex Abs compared with 9 of the 204 in the noninflammatory group (p < 0.0001, Fisher exact test), and the noninflammatory group had significantly lower VGKC-complex titers at presentation (figures 1 and 2A; 105–394 pM, median 125 pM vs 103–2,500 pM, median 169 pM) (p = 0.02, Mann–Whitney test). Twelve of the 158 patients in the inflammatory group had titers >400 pM compared with none of the 205 in the noninflammatory group (p < 0.0001, Fisher exact test).
Figure 2. VGKC-complex Abs.
(A) Patients were classified using the ICD-10 into CNS inflammatory and noninflammatory etiologies. The noninflammatory group had significantly lower VGKC-complex Abs at presentation (105–394 pM, median 125 pM) compared with the inflammatory group (103–2,500 pM, median 169 pM) (p = 0.02, Mann–Whitney test). Bars indicate mean ± SEM. (B) VGKC-complex Abs were associated with a range of different phenotypes, although encephalopathies were most frequent (figure 1). (C) Theoretical representation of the VGKC complex as it sits in the membrane. For radioimmunoprecipitation assays, the complex is extracted from rabbit brain in mild detergent and labeled with 125I-DTX, which binds strongly to VGKC Kv1 subunits. Any antibodies that bind extracellularly (red) to the proteins in the complex, specifically LGI1 and CASPR2, or any novel proteins (?), or those binding to intracellular epitopes (blue), can immunoprecipitate the VGKC complex and give a positive test result. (D) To look specifically for antibodies that bind to the VGKC themselves, the Kv1 subunits (including the β2, not shown) were expressed, with or without the PSDs, extracted, labeled with 125I-DTX, and used in antibody assays. The antibodies that bound in this assay might be mainly against intracellular epitopes (blue). Ab = antibody; ADAM22 = a disintegrin, and metalloproteinase 22; ADS = acquired demyelinating syndromes; CASPR2 = contactin-associated protein 2; 125I-DTX = 125I-dendrotoxin; ICD-10 = International Classification of Diseases, Tenth Revision; LGI1 = leucine-rich, glioma inactivated; PSD93 = postsynaptic density protein 93; VGKC = voltage-gated potassium channel.
Clinical phenotypes of patients with VGKC-complex Abs.
No CSF samples were sent or subsequently available for Ab testing. The diagnoses and titers of the patients positive for VGKC-complex Ab were quite varied (table 1; figure 2B). Although only 3 had limbic encephalitis and one had a typical NMDAR-Ab encephalitis, VGKC-complex Abs were more common in encephalopathies (18/88; 20%) than in other presentations (21/275, 7.6%; p = 0.0014, Fisher exact test). Four additional patients had seizure disorders, 6 had acquired demyelinating syndromes (ADS), 3 had movement disorders, 4 had varied clinical features, and 4 had autoimmune neuromuscular disorders (table 1). Patients were followed up for a median time of 30 months (range 12–63 months).
Noninflammatory group.
All 9 patients who did not have inflammatory disease had VGKC-complex Abs <400 pM (table e-1 on the Neurology® Web site at Neurology.org). Four children were diagnosed with a seizure disorder, one with pyridoxine-dependent epilepsy (compound heterozygote mutation in ALDH7A1). The other 5 patients were diagnosed with headaches, a pain syndrome, a transient hemiplegia, a tic disorder, and one child with mitochondrial cytopathy and POLG mutation (titer 394 pM) who unfortunately died.
Inflammatory group.
VGKC-complex Abs ranging from 100 to 150 pM.
Eleven children had been reported as “low positive” for VGKC-complex Abs (100–150 pM; see details in table e-2). Encephalopathy was the most frequent presentation (n = 7), including one with NMDAR-Ab encephalitis. Others presented with ADS (n = 2) or Guillain-Barré syndrome (GBS) (n = 1). One patient presented with myositis and serum binding strongly to hippocampal neurons in a punctate pattern (data not shown), despite no evidence of CNS involvement. None of these patients' sera were positive for binding to the VGKC-complex proteins LGI1 or CASPR2. Immunotherapy was given in 10 of 11 patients with varying responses to treatments and outcomes.
VGKC-complex Abs ranging from 150 to 400 pM.
Seven patients had Abs in this range and presented with encephalopathy (n = 4) and one each with acute demyelination syndrome (ADS), Lambert-Eaton myasthenic syndrome, and acute Sydenham chorea. The 4 patients who received immunotherapy made a full recovery (see table e-2).
VGKC-complex Abs >400 pM.
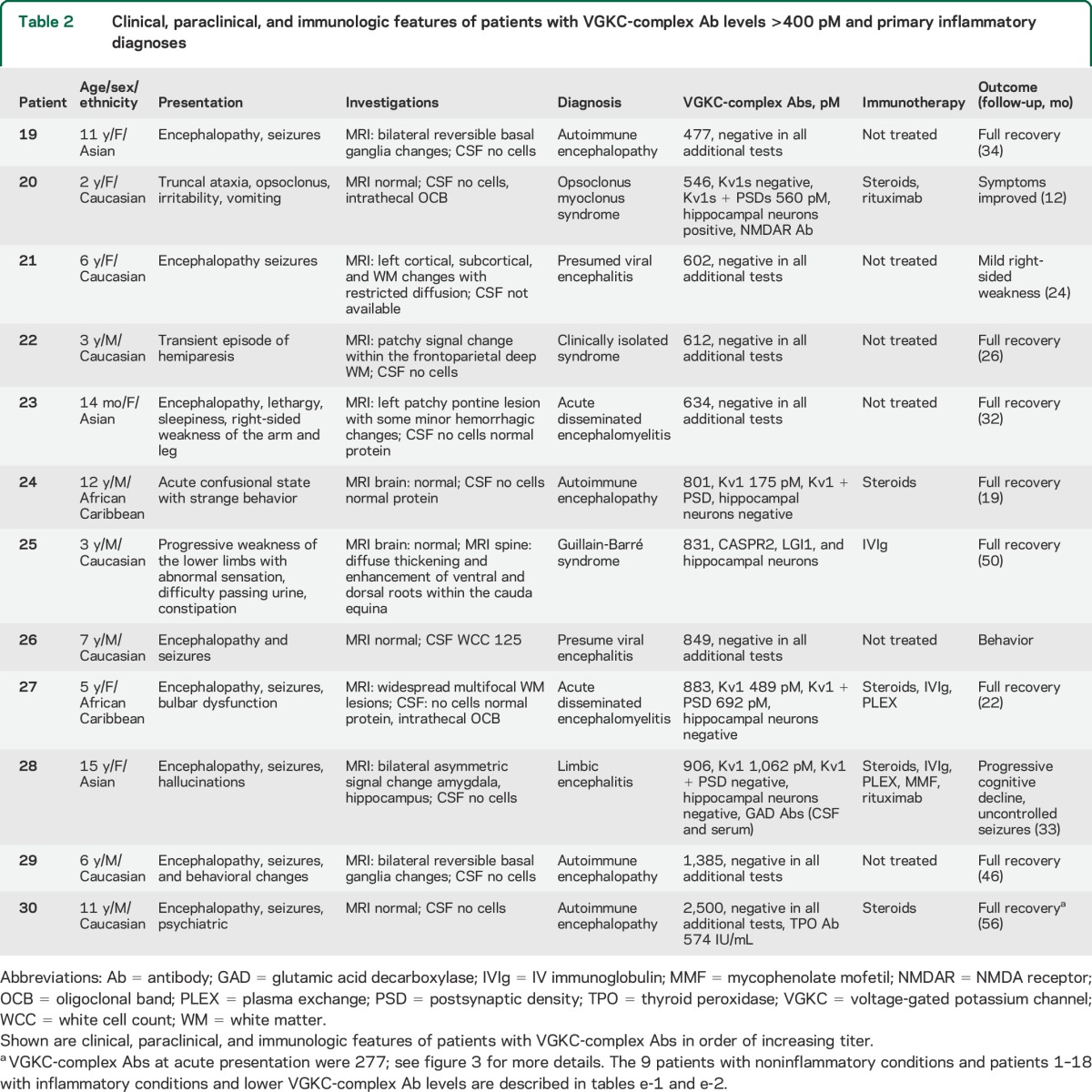
A total of 12 patients had Ab titers >400 pM. They presented with encephalopathy (n = 7), ADS (n = 3), opsoclonus myoclonus syndrome (OMS) (no neuroblastoma found) (n = 1), and GBS (n = 1). Ten of these patients made a good recovery, in 2 cases without immunotherapy (table 2).
Table 2.
Clinical, paraclinical, and immunologic features of patients with VGKC-complex Ab levels >400 pM and primary inflammatory diagnoses
Abs binding to defined components of the VGKC complex.
Only one child (table 2, case 25; VGKC-complex Ab 831 pM), a boy with a diagnosis of GBS without encephalopathy,15 tested positive for the known VGKC-complex–associated proteins, binding to both LGI1 and CASPR2 at titers of 1:500. Autoantibodies to ADAM22 and 23 were not identified in his or any of the other 39 VGKC-complex Ab–positive sera, and there was no detectable binding to live HEK cells expressing Kv1.1, 1.2, and 1.6 either in isolation or when cotransfected with Kvβ2, although all 3 monoclonal Abs bound to the live Kv1-expressing cells (data not shown).
Binding to primary rat hippocampal and mixed neuronal cultures.
Only 4 patients' sera bound to live hippocampal and mixed neuronal cultures. Two of these were also positive for NMDAR Ab (one with encephalopathy and one with OMS) and one was positive for both LGI1 and CASPR2 (described above). The fourth patient was a 13-year-old girl with scleroderma who presented with acute myositis and VGKC-complex Abs of only 112 pM (table e-2, case 3).
Binding to Kv1 subunits with or without PSD93/95.
The components of the VGKC complex are illustrated in figure 2C. The lack of binding to Kv1 transfected cells could be because the Abs that immunoprecipitate VGKC complexes bind to novel complexed proteins or to intracellular determinants on the Kv1s or other components of the complex.
To find whether the Abs bind to the VGKC themselves, Kv1-expressing HEK cells, with or without the intracellular PSDs, were extracted under the same conditions used for extracting brain tissue for VGKC-complex Ab assays (figure 2D). The extracts were labeled with 125I-DTX for testing the 12 sera with high titers (>400 pM) of VGKC-complex Abs. Four of the 12 (33%, cases 20, 24, 27, 28) immunoprecipitated 125I-DTX-Kv1s; 2 patients with encephalitis bound the Kv1 subunits only, the patient with OMS bound the Kv1s when cotransfected with PSD93 and PSD95, and one patient with ADS bound to Kv1s alone or with the PSDs.
Clinical correlation with repeat samples.
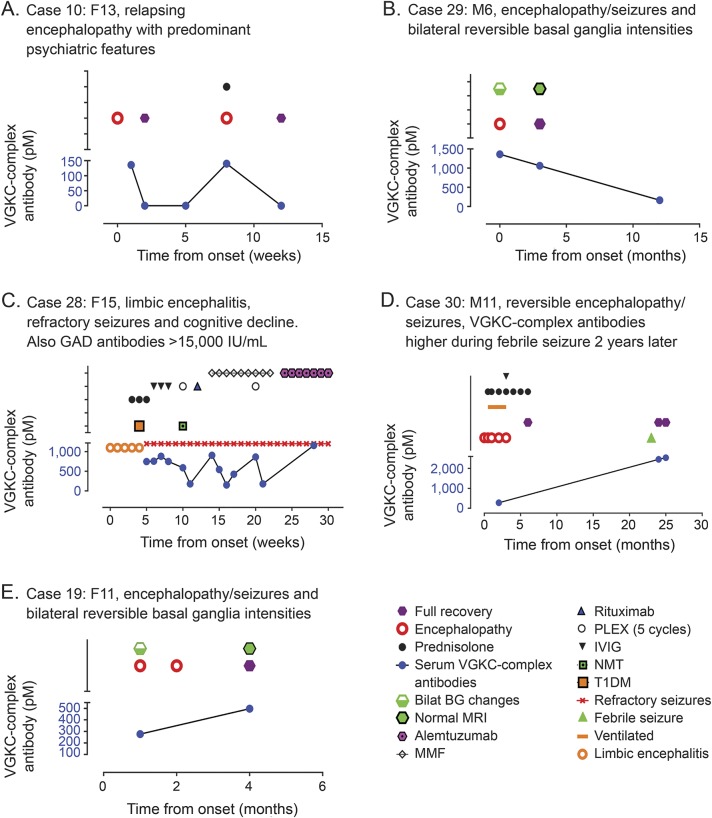
Further samples were available for 9 patients (6 encephalopathy, 3 ADS). VGKC-complex Abs became negative at 3-month follow-up in the 3 patients with ADS who improved (data not shown). A correlation with titer was also seen in 3 of 6 patients with encephalopathies: clinical improvement with Ab reduction in 2 (figure 3, A and B), with increased titer at relapse in one (figure 3A), and persistence of symptoms and Abs in a further patient who had very high titers of glutamic acid decarboxylase (GAD) Abs (>50,000 IU/mL; figure 3C). However, in 3 patients with encephalopathies, there was no temporal correlation with clinical features; the VGKC-complex Ab levels increased when asymptomatic in 2 (figure 3, D and E) and persisted despite complete recovery in one.
Figure 3. VGKC-complex Abs, clinical syndromes, and treatment responses in individual patients.
(A) Case 10 (13-year-old girl) presented with encephalopathy, psychosis, and bradykinesia. She responded to steroids but relapsed 8 months later and required further steroid treatment. (B) Case 29 (6-year-old boy) presented with encephalopathy, predominant seizures, and bilateral basal ganglia changes on MRI with good recovery.23 (C) Case 28 (15-year-old female) presented with limbic encephalitis with auditory and visual hallucination and multiple seizure types. VGKC-complex Abs with associated high GAD Abs fluctuated widely and no clinical improvements. Despite aggressive immunotherapy, she did not respond and continued to experience refractory seizures and gradual cognitive decline. Her VGKC-complex Abs remained persistently elevated. However, very high titers of GAD Abs were present in serum and CSF. (D) Case 30 (11-year-old male) presented with encephalopathy with prolonged seizures and low GCS requiring ventilation for 3 weeks. VGKC-complex Abs were 277 pM and he was treated with IVMP followed by oral prednisolone and made a complete recovery. Two years later, he had a seizure with intercurrent illness; at the time, VGKC-complex Abs were 2,460 pM despite complete clinical recovery without treatment (2 years later). (E) Case 19 (11-year-old female) with reversible MRI basal ganglia intensities and full recovery despite modest increase in VGKC-complex Abs. Ab = antibody; BG = basal ganglia; GAD = glutamic acid decarboxylase; IVIG = IV immunoglobulin; IVMP = IV methylprednisolone; MMF = mycophenolate mofetil; NMT = neuromyotonia; PLEX = plasma exchange; T1DM = type 1 diabetes mellitus; VGKC = voltage-gated potassium channel.
DISCUSSION
The large number of VGKC-complex Abs identified in Oxford, and the growing number in which a specific antigen has not been defined, led to concerns that the Abs might be partly binding to intracellular epitopes and would be of doubtful clinical relevance. The data presented confirm the lack of specific antigens and the probable binding of 4 of 12 high-titer sera to intracellular epitopes on the Kv1 subunits. Nevertheless, they also indicate that VGKC-complex Abs in children are more common in inflammatory conditions than in noninflammatory neurologic conditions, suggesting that they can be a nonspecific biomarker for neuronal inflammation. It is possible that these Abs occur when the nervous system is damaged, perhaps because released epitopes of the VGKC complex are able to induce autoantibodies.
Because of the retrospective nature of this study, with diagnoses established from clinical records, there was no uniformity in reporting of clinical features or time to sampling from first presentation. The children with inflammatory diseases exhibited a range of presentations (figure 2, table 2, tables e-1 and e-2), not all showed a clear response to immunotherapy, and Ab titers did not correlate with the clinical state in 3 of the 9 patients from whom serial samples were available (figure 3). Thus, it is difficult to assess the clinical relevance of the Abs in children. Moreover, although there were 39 (10.7%) positives from 363 patient samples, only 12 had Ab levels >400 pM, a useful cutoff for identifying CNS diseases.6,11
These high-titer VGKC-complex Abs were only present in children with inflammatory diseases as defined by ICD-10, and 7 of the 12 (58%) had encephalopathy. Two of the sera bound to hippocampal neurons, one with Abs to both the VGKC-complex–associated proteins LGI1 and CASPR2, and one with NMDAR-Ab encephalitis. None bound to the membrane VGKC-associated proteins ADAM22 or ADAM23. However, 4 additional sera immunoprecipitated Kv1 subunits extracted from HEK cells, with or without the VGKC clustering proteins PSD93 and PSD95. This suggests that even some of the high VGKC-complex Abs may be binding partly or exclusively to intracellular epitopes that are exposed in detergent extracts, although the relative sensitivities of immunoprecipitation (very high specific activity 125I-DTX) compared to the CBAs (fluorescence detection) is not established.
In this study, the overall positivity was almost double that reported in UK adult patients (5.2%; p = 0.0005, Fisher exact test) but the number of patients with high levels (>400 pM) was not very different (2.1% vs 3.3%).6 The positivity for LGI1 or CASPR2 Abs was very low here (1/39; 3%) compared with 17%6 and 31%16,17 in adult patients, but the range of titers was similar to that reported in 12 children referred to the Mayo Clinic who were positive for VGKC-complex Ab.18
Because only 4 of our 39 positive patients demonstrated binding to the surface of neurons (of which 3 were positive for specific antigens), it seemed likely that some of the Abs were binding to intracellular epitopes on the VGKC, Kv1s, or other complexed proteins. Four sera did bind to the isolated Kv1 subunits and 2 of these, from patients with encephalopathies, only bound to Kv1 subunits in the absence of PSDs. It is possible that the PSD proteins obscure access of some Abs to intracellular Kv1 subunit epitopes (figure 2D). PSD93 and PSD95 are part of the intracellular MAGUKs, which bind the C-terminal consensus sequence known as the PDZ-binding domain of Kv1, and are important for clustering of Kv1 at the juxtaparanodes.1 Axoglial proteins were increased in CSF of pediatric patients with acquired demyelination syndromes,19 which likely reflects neuronal damage; the release of these proteins could stimulate immune responses to epitopes on the VGKCs and other axonal proteins. The VGKC complex appears to be very immunogenic since inhalation of porcine brain tissue antigens led to very high serum VGKC-complex Abs in both humans and mice.20
VGKC-complex Abs are often requested in patients with possible or suspected autoimmune forms of encephalitis, and in highly selected cohorts or isolated case reports, VGKC-complex Abs have been reported in status epilepticus,21 fever-induced refractory epileptic encephalopathy in school-age children,22 seizures and basal ganglia changes,23 and focal epilepsies9 but usually at very low levels. Here, similarly low VGKC-complex Abs (105–158 pM) were identified in 4 of the 80 patients (5%) with seizure disorders but none of these was considered immune-mediated. We did not detect the Abs in developmental and autistic regressions (n = 27), primary psychiatric disorders (n = 15), or functional illness (n = 14) (table 1). Identification of low-titer Abs in primary genetic and neurometabolic disorders suggests a possible secondary inflammatory component, which might, in some cases, benefit from immunotherapy.24
The utility of an Ab as a disease-specific biomarker requires strict clinical inclusion criteria in order to determine the applicability of the test in a clinical setting. Diagnosis of a specific disorder in Ab-positive patients with some, but not all features of a well-defined syndrome, rests on confidence in both the pathogenicity of the Ab and on the rigor of the method. As demonstrated here, identification of VGKC-complex Ab cannot be used as a positive predictive value for clinical management. Nevertheless, although there was no evidence for novel extracellular antigen(s), the association of the VGKC-complex Abs, particularly those at high titer, with inflammatory diseases, and in 20% of encephalopathies, suggests that positive VGKC-complex Abs represent a nonspecific biomarker of neuronal inflammation, without specific diagnostic relevance.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Mrs. Linda Clover for confirming antibody results and Drs. L. Jacobson, S. Irani, and P. Waters for help and advice. The authors are very grateful to all the patients and their respective clinicians from GOSH: Dr. Sarah Aylett, Dr. Sanjay Bhate, Dr. Lucinda Carr, Prof. Helen Cross, Dr. Catherine deVile, Dr. Christin Eltze, Dr. Vijeya Ganesan, Dr. Marios Kaliakatsos, Dr. Robert Robinson, Dr. Prab Prabhakar, and Dr. Sophie Varadkar; and ELCH: Dr. Tammy Hedderly, Dr. Elaine Hughes, Dr. Heinz Jungbulth, Dr. Margaret Kaminska, Dr. Karin Lascelles, Dr. Jean Pierre Lin, Dr. Keith Pohl, Dr. Ruth Williams, and Dr. Elizabeth Wraige.
GLOSSARY
- Ab
antibody
- ADAM22 and 23
cytoskeletal scaffold, disintegrin, and metalloproteinase 22 and 23
- ADS
acquired demyelinating syndromes
- CASPR2
contactin-associated protein 2
- CBA
cell-based assay
- GAD
glutamic acid decarboxylase
- GBS
Guillain-Barré syndrome
- HEK
human embryonic kidney
- 125I-DTX
125I-dendrotoxin
- ICD-10
International Classification of Diseases, Tenth Revision
- IgG
immunoglobulin G
- LGI1
leucine-rich, glioma inactivated
- MAGUK
membrane-associated guanylate kinases
- NMDAR
NMDA receptor
- OMS
opsoclonus myoclonus syndrome
- PSD93 and 95
postsynaptic density proteins 93 and 95
- VGKC
voltage-gated potassium channel
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
The study was designed by Y.H. and A.V., with suggestions from M.L. and C.H. A.V. was responsible for supervising the laboratory studies performed by Y.H., M.R., and B.L. Y.H., R.S., M.L., and C.H. contributed to the evaluation and recording of clinical data. A.V. supervised the study as a whole and contributed to the key message of the manuscript. Y.H. prepared early drafts of the manuscript and all authors contributed to the revisions of the manuscript.
STUDY FUNDING
This work was supported by the Oxford University Clinical Academic Graduate School, Oxford (Y.H.), and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust and the University of Oxford (Y.H. and A.V.).
DISCLOSURE
Y. Hacohen, R. Singh, M. Rossi, and B. Lang report no disclosures relevant to the manuscript. C. Hemingway has received travel grants from Merck Serono and Bayer. M. Lim receives research grants from Action Medical Research, DES Society, GOSH Charity, NIHR, MS Society, SPARKS Charity, and the London Clinical Research Network and Evelina Appeal; has received consultation fees from CSL Behring; received travel grants from Merck Serono; and was awarded educational grants to organize meetings by Novartis, Biogen Idec, Merck Serono, and Bayer. A. Vincent serves/has served on scientific advisory boards for the Patrick Berthoud Trust, the Brain Research Trust, and the Myasthenia Gravis Foundation of America; has received funding for travel and a speaker honorarium from Baxter International Inc. and Biogen Inc.; serves as an associate editor for Brain; receives royalties from the publication of Clinical Neuroimmunology (Blackwell Publishing, 2005) and Inflammatory and Autoimmune Disorders of the Nervous System in Children (Mac Keith Press, 2010); receives/has received research support from the European Union, NIHR Biomedical Research Centre Oxford, EUROIMMUN AG, and the Sir Halley Stewart Trust; and has received Musk antibody royalties and consulting fees from Athena Diagnostics Inc. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Ogawa Y, Oses-Prieto J, Kim MY, et al. ADAM22, a Kv1 channel-interacting protein, recruits membrane-associated guanylate kinases to juxtaparanodes of myelinated axons. J Neurosci 2010;30:1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 2010;9:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain 2010;133:2734–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lancaster E, Huijbers MG, Bar V, et al. Investigations of Caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol 2011;69:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irani SR, Pettingill P, Kleopa KA, et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol 2012;72:241–255. [DOI] [PubMed] [Google Scholar]
- 6.Paterson RW, Zandi MS, Armstrong R, Vincent A, Schott JM. Clinical relevance of positive voltage-gated potassium channel (VGKC)-complex antibodies: experience from a tertiary referral centre. J Neurol Neurosurg Psychiatry 2014;85:625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armangue T, Petit-Pedrol M, Dalmau J. Autoimmune encephalitis in children. J Child Neurol 2012;27:1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hacohen Y, Wright S, Waters P, et al. Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. J Neurol Neurosurg Psychiatry 2013;84:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suleiman J, Wright S, Gill D, et al. Autoantibodies to neuronal antigens in children with new-onset seizures classified according to the revised ILAE organization of seizures and epilepsies. Epilepsia 2013;54:2091–2100. [DOI] [PubMed] [Google Scholar]
- 10.Hacohen Y, Absoud M, Woodhall M, et al. Autoantibody biomarkers in childhood-acquired demyelinating syndromes: results from a national surveillance cohort. J Neurol Neurosurg Psychiatry 2014;85:456–461. [DOI] [PubMed] [Google Scholar]
- 11.Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 2004;127:701–712. [DOI] [PubMed] [Google Scholar]
- 12.Kleopa KA, Elman LB, Lang B, Vincent A, Scherer SS. Neuromyotonia and limbic encephalitis sera target mature Shaker-type K+ channels: subunit specificity correlates with clinical manifestations. Brain 2006;129:1570–1584. [DOI] [PubMed] [Google Scholar]
- 13.Benarroch EE. ADAM proteins, their ligands, and clinical implications. Neurology 2012;78:914–920. [DOI] [PubMed] [Google Scholar]
- 14.Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010;133:1655–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosch RE, Bamford A, Hacohen Y, et al. Guillain-Barré syndrome associated with CASPR2 antibodies: two paediatric cases. J Peripher Nerv Syst 2014;19:246–249. [DOI] [PubMed] [Google Scholar]
- 16.Huda S, Wong SH, Pettingill P, O'Connell D, Vincent A, Steiger M. An 11-year retrospective experience of antibodies against the voltage-gated potassium channel (VGKC) complex from a tertiary neurological centre. J Neurol 2015;262:418–424. [DOI] [PubMed] [Google Scholar]
- 17.Klein CJ, Lennon VA, Aston PA, et al. Insights from LGI1 and CASPR2 potassium channel complex autoantibody subtyping. JAMA Neurol 2013;70:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhamija R, Renaud DL, Pittock SJ, et al. Neuronal voltage-gated potassium channel complex autoimmunity in children. Pediatr Neurol 2011;44:275–281. [DOI] [PubMed] [Google Scholar]
- 19.Dhaunchak AS, Becker C, Schulman H, et al. Implication of perturbed axoglial apparatus in early pediatric multiple sclerosis. Ann Neurol 2012;71:601–613. [DOI] [PubMed] [Google Scholar]
- 20.Meeusen JW, Klein CJ, Pirko I, et al. Potassium channel complex autoimmunity induced by inhaled brain tissue aerosol. Ann Neurol 2012;71:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suleiman J, Brenner T, Gill D, et al. VGKC antibodies in pediatric encephalitis presenting with status epilepticus. Neurology 2011;76:1252–1255. [DOI] [PubMed] [Google Scholar]
- 22.Illingworth MA, Hanrahan D, Anderson CE, et al. Elevated VGKC-complex antibodies in a boy with fever-induced refractory epileptic encephalopathy in school-age children (FIRES). Dev Med Child Neurol 2011;53:1053–1057. [DOI] [PubMed] [Google Scholar]
- 23.Hacohen Y, Wright S, Siddiqui A, et al. A clinico-radiological phenotype of voltage-gated potassium channel complex antibody-mediated disorder presenting with seizures and basal ganglia changes. Dev Med Child Neurol 2012;54:1157–1159. [DOI] [PubMed] [Google Scholar]
- 24.Lim M. Treating inflammation in childhood neurodegenerative disorders. Dev Med Child Neurol 2011;53:298–304. [DOI] [PubMed] [Google Scholar]
- 25.Haberlandt E, Bast T, Ebner A, et al. Limbic encephalitis in children and adolescents. Arch Dis Child 2011;96:186–191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.